Abstract
An ultra-sensitive liquid chromatography tandem mass spectrometry (LC-MS/MS) assay was developed and validated to facilitate the assessment of clinical pharmacokinetics of nucleotide analogs from lysed intracellular matrix. The method utilized a strong anion exchange isolation of mono-(MP), di-(DP), and tri-phosphates (TP) from intracellular matrix. Each fraction was then dephosphorylated to the parent moiety yielding a molar equivalent to the original nucleotide analog intracellular concentration. The analytical portion of the methodology was optimized in specific nucleoside analog centric modes (i.e. tenofovir (TFV) centric, zidovudine (ZDV) centric), which included desalting/concentration by solid phase extraction and detection by LC-MS/MS. Nucleoside analog MP-, DP-, and TP- determined on the TFV centric mode of analysis include TFV, lamivudine (3TC), and emtricitibine (FTC). The quantifiable linear range for TFV was 2.50 to 2000 fmol/sample, and that for 3TC/FTC was 0.10 to 200 pmol/sample. Nucleoside analog MP-, DP-, and TP- determined on the ZDV centric mode of analysis included 3TC and ZDV. The quantifiable linear range for 3TC was 0.10 to 100 pmol/sample, and 5.00 to 2000 fmol/sample for ZDV. Stable labeled isotopic internal standards facilitated accuracy and precision in alternative cell matrices, which supported the intended use of the method for MP, DP, and TP determinations in various cell types. The method was successfully applied to clinical research samples generating novel intracellular information for TFV, FTC, ZDV, and 3TC nucleotides. This document outlines method development, validation, and application to clinical research.
Keywords: Clinical Pharmacology, Analytical Methods, Nucleoside Analogs, Intracellular Pharmacology, LC-MS/MS
1. Introduction
Nucleoside analogs (NA) are used for the treatment of major viral infections in humans such as human immunodeficiency virus (HIV), hepatitis viruses B and C, and Human Herpes viruses. They are also used for certain cancers and as immunosuppressants. NAs possess unique pharmacology in that the active moiety is the intracellular phosphates formed by cellular or virally-encoded enzymes [1, 2]. The intracellular NA-phosphates are ion-trapped in the cell, which creates a pharmacokinetic profile that differs from the parent NA in plasma [3].
In an effort to understand anti-HIV NA cellular pharmacology in vivo, previous methodologies have been developed to quantify NA-triphosphate (TP), the active drug form, in human peripheral blood mononuclear cells (hPBMC) of clinical research volunteers [4–10]. The data that were generated support dosing strategies currently used in HIV-infected patients, but each method had limitations in terms of sensitivity, specificity, and other capabilities (e.g. single analyte, only the TP, etc). The following document describes the development and validation of a highly sensitive and comprehensive approach to quantify nucleotide analogs from lysed cellular matrices to support clinical pharmacology research for NAs.
2. Methods
2.1 Chemicals and Materials
The following chemicals were acquired from the stated manufacturers: NIH AIDS Research & Reference Reagent Program, Germantown, MD, USA; tenofovir (TFV, MW=287.2, as anhydrous base), lamivudine, (3TC, MW= 229.3), emtricitabine (FTC, MW= 247.2), zidovudine (ZDV, MW= 267.2); Moravek Biochemicals, Inc, Brea, CA, USA; tenofovir isotopic internal standard (13C5TFV-iso, MW= 292.2); tenofovir monophosphate (TFV-MP, MW= 367.2 for free acid), tenofovir diphosphate (TFV-DP, MW= 447.2 for free acid), emtricitabine-isotopic internal standard (15N2,13C1 FTC-iso, MW=250.2), emtricitabine-5′-triphoshate (FTC-TP, MW=487.2 for free acid), zidovudine isotopic internal standard (15N2,13C1 ZDV-iso, MW= 270.2); Sierra Bioresearch, Tucson, AZ, USA; lamivudine-5′-triphosphate (3TC-TP, MW= 469.3 for free acid), and zidovudine-5′-triphosphate (ZDV-TP, MW= 507.2); Martex Inc, Minnetonka, MN, USA; lamivudine-isotopic internal standard (15N2,13C1 3TC-iso, MW= 232.3); and Sigma Chemical, St. Louis, MO, USA; natural nucleosides (adenosine, 2′deoxyadenosine, cytidine, 2′deoxycytidine, guanosine, 2′deoxyguanosine, thymidine, uridine [A, dA, C, dC, G, dG, T, U]) and the nucleotide counterparts (-MP, -DP, and –TP).
The following analytical grade reagents were acquired from the stated manufacturers; methanol, 2-propanol, formic acid, glacial acetic acid, potassium chloride, and ammonium acetate; Fisher Scientific, Fairlawn, NJ, USA; dichloromethane, sodium acetate, acid phosphatase; Sigma Aldrich Chemical, St. Louis, MO, USA and acetonitrile; JT Baker, Phillipsburg, NJ, USA. Ultrapure (UP) water was prepared in house from deionized water with a Barnstead Nanopure System (Thermo Fisher Scientific, Waltham, MA, USA). Consumables included Waters Sep-Pak Accell Plus QMA Cartridge, 3cc (500mg), Waters Corporation, Milford, MA, USA and Phenomenex Strata-X 33μm Polymeric Reversed Phase Cartridge 200mg/3mL, Phenomenex, Inc., Torrance, CA, USA and blood products for lysed cellular matrix (Bonfils, Denver CO, USA).
2.2 Analytical Approach
2.2.1 Lysed Cellular Matrix
Intracellular NA-phosphate concentrations were measured from various cellular types that had been processed using isolation procedures specifically developed for the type of cell to be analyzed. Isolation procedures included red blood cell (RBC) removal with RBC lysis media (Gibco, Invitrogen, Carlsbad, CA, USA) unless the processing procedure already precluded RBC, such as flow cytometry. This was an essential step since MP, DP, and TP anabolites of some NAs such as TFV and RBV are found at significant levels in RBC [11, 12]. Once cell samples were isolated, purified, and counted, the cells were lysed with 0.5mL cold 70:30 methanol:ultrapure water (v:v) and stored at −80°C. It is this lysed cellular matrix (70:30) that was analyzed with this procedure.
Blank hPBMC were harvested from leukocyte reduction filters and lysed in 70:30 at a concentration of 10×10^6 cells/mL. This lysed cellular matrix was used for quality control preparation and for extraction matrix for the calibration curve.
2.2.2 Preparation of standards, Internal Standard, and QCs
Standard preparation stocks were created at 1 mg/mL concentrations in ultrapure water (UP water) for each individual parent NA from reference powder. Combined preparation stocks of the parent NAs were prepared at concentrations of 500, 50, and 5 pmol/μL, which were further diluted in UP water to create the final working standard solutions. The working standard concentrations ranged from 0.1 to 200 pmol/sample for FTC and 3TC and 1 to 2000 fmol/sample for TFV and ZDV. Sample was defined as 20 μL working stock added to 2mL 1M KCL resulting from blank lysed cellular matrix carried through the strong anion exchange and dephosphorylation process (described below). Combined isotopic internal standard (iso) working stocks were prepared in UP water at concentrations of 50 fmol/μL for TFV-iso and ZDV-iso and at concentrations of 0.5 pmol/μL for 3TC-iso and FTC-iso. Standard and internal standard solutions were stored at 4°C.
Individual Quality Control (QC) Preparation Stocks were prepared from NA-triphosphate (NA-TP) reference standard in pmol/μL by dissolving in ultrapure water. It was necessary to perform quality assurance procedures on these NA-TP stocks received from the manufacturers to assess both purity and potency, as described previously [6, 13]. Initial NA-TP QC preparation stocks of 1 and 50 pmol/μL were prepared in UP water and stored at −80°C. Combined QCs were prepared by appropriate dilution with blank lysed cellular matrix (10million cells/mL) in 25mL volumetric flasks. Five sets of QCs were prepared for validation; three sets of low QCs: Concentration of QC Low1: TFV-DP 2.5 fmol/sample; 3TC-TP 0.265 pmol/sample; and FTC-TP 0.25 pmol/sample. QC Low2: TFV-DP and ZDV-TP 5 fmol/sample; 3TC-TP 0.53 pmol/sample and FTC-TP 0.5 pmol/sample. QC Low3: TFV-DP and ZDV-TP 15 fmol/sample; 3TC-TP 1.59 pmol/sample and FTC-TP 1.5 pmol/sample. QC Med: TFV-DP and ZDV-TP 150 fmol/sample; 3TC-TP 15.9 pmol/sample and FTC-TP 15.0 pmol/sample. QC High: TFV-DP, and ZDV-TP 1500 fmol/sample; 3TC-TP 159 pmol/sample and FTC-TP 150 pmol/sample. The volume extracted for each QC above was 0.2mL. This volume was defined as sample. QCs were stored at −80°C.
2.2.3 Extraction Overview
The following were loaded onto appropriately prepared Waters QMA Solid Phase Extraction (SPE) cartridges (washed with 2mL ultrapure water, 1.5mL 1M KCl, and 2mL 5mM KCl): Blank lysed cellular matrix (0.2mL) to be used for each calibration standard and each of two blanks, QCs (0.2mL) and unknowns (predetermined volume; typically 2 million cells but not limited to this cell count). Washes utilized centrifugation (100×g; 2 minutes). The MP elution was accomplished with 5mL of 75mM KCL; elution of DP was accomplished with 7mL of 90mM KCL; and the final TP elution was accomplished with a single 2mL wash with 1M KCL. Each elution was set aside for dephosphorylation.
These isolated MP, DP, and TP NA fractions were then treated with 100μL of acid phosphatase in 1M sodium acetate, pH 5 (4–5 units), to dephosphorylate to their corresponding parent NA. The mixture was vortexed, covered with parafilm, and incubated for 60 minutes at 37°C in water bath. After incubation, tubes were allowed to equilibrate at ambient temperature for approximately 15 minutes. Working standard solutions (20μL) were added to the blank hPBMC samples and internal standard working stock solution (20μL) was added to all tubes except the blank without IS. The Phenomenex Strata-X SPE was then used to de-salt and concentrate the samples. First, the Strata-X SPE cartridges were prepared with three washes: 2.0mL methanol then 2×2.0mL 15mM ammonium acetate. After sample application, cartridges were washed with 2.0mL of 10mM ammonium acetate followed by cartridge drying. The second and final wash was with 0.5mL dichloromethane followed by cartridge drying. The analytes were eluted with two methanol applications of 0.5mL each. Samples were dried for 25 minutes under nitrogen at 40°C in a Zymark TurboVap (Zymark Corp., Hopkinton, MA, USA). Final reconstitution was with 100μL UP water. The sample was vortex mixed and transferred to a 150 μL low volume insert. 30μL were injected onto the liquid chromatography tandem mass spectrometry (LC-MS/MS) system.
2.2.4 LC-MS/MS Instrumentation and Conditions
A Thermo Scientific TSQ Vantage® triple quadrupole mass spectrometer was coupled with a HESI II® probe. The LC was an Thermo Scientific Accela® pump, Thermo Scientific, San Jose, CA, an Eppendorf CH30/CH50 (Heater/Controller) Hauppauge, NY, and CTC Analytics HTC PAL® autosampler, Zwingen, Switzerland. The Thermo TSQ Quantum Vantage used a 100 μL Sample Syringe, 50 μL PEEK Loop, and data were captured with Xcalibur™ 2.0.7 SP1. A Synergi Polar RP 2.5 μM, 100A, 2.0×100mm, Phenomenex, Inc, Torrance, CA, USA. analytical column was used for chromatographic separations. Two mobile phase conditions, ZDV centric and TFV centric, were used to optimize chromatographic separations and detection in order to achieve the most sensitive determination of analytes in each mode. The TFV centric mode contained 2% acetonitrile and 0.1% formic acid in ultrapure water at an isocratic flow of 250μl/min. The ZDV centric mode contained 6% 2-propanol and 0.1% acetic acid in ultrapure water at an isocratic flow of 200μl/min. For the TFV centric mode, the column temperature was approximately 40°C, sample temperature approximately 20°C, injection volume 30μL, and the run time was 8 minutes. Each injection was followed by a strong and weak needle wash (0.1% Formic Acid in 50% Acetonitrile: 50% UP water, and 10% Methanol: 90% UP water, respectively). The source was operated in the positive ionization mode. The spray voltage was 3500 V, vaporizer temperature 380°C, sheath gas (nitrogen) 40 arbitrary units, aux gas (nitrogen) 5 arbitrary units, capillary temperature 225°C, chromfilter peak width 20.0 s, collision gas (argon) pressure 1.0 mTorr, experiment type SRM (HSRM) peak width Q1: 0.2 FWHM, Q3: 0.7 FWHM, scan width 0.002 m/z, scan time 0.600 s, and centroid data type. These same settings were used for the ZDV centric mode except the source utilized polarity switching (3TC utilized positive ionization and ZDV utilized negative ionization). The run time for the ZDV centric mode was 12 minutes. SRM monitoring conditions for each mode are shown in Table 1.
Table 1.
SRM MS/MS Monitoring Settings
| TFV Centric
| |||||||
|---|---|---|---|---|---|---|---|
| Parent(m/z) | Product(m/z) | CE (V) | Start (min) | Stop (min) | Lens (V) | Polarity | NA |
| 230.033 | 112.048 | 15 | 3.2 | 5.0 | 48 | + | 3TC |
| 233.033 | 115.048 | 15 | 3.2 | 5.0 | 48 | + | 3TC-IS |
| 248.100 | 130.000 | 15 | 5.1 | 8.0 | 45 | + | FTC |
| 251.100 | 133.000 | 15 | 5.1 | 8.0 | 45 | + | FTC-IS |
| 288.044 | 176.108 | 25 | 1.5 | 3.1 | 130 | + | TFV |
| 293.044 | 181.108 | 25 | 1.5 | 3.1 | 130 | + | TFV-IS |
|
ZDV Centric
| |||||||
| Parent(m/z) | Product(m/z) | CE (V) | Start (min) | Stop (min) | S-Lens (V) | Polarity | NA |
|
| |||||||
| 230.033 | 112.048 | 15 | 2.0 | 4.6 | 48 | + | 3TC |
| 233.033 | 115.048 | 15 | 2.0 | 4.6 | 48 | + | 3TC-IS |
| 266.071 | 223.151 | 11 | 7.0 | 11.8 | 60 | -- | ZDV |
| 269.071 | 226.151 | 11 | 7.0 | 11.8 | 60 | -- | ZDV-IS |
| Reset to | |||||||
| 300 | 150 | 10 | 11.9 | 12.0 | 50 | + | ESI+ |
3 Validation Results
3.1 Separation and isolation of MP, DP, and TP from intracellular matrix
Endogenous nucleotides, G/dG, A/dA, C/dC, U, and T, were utilized to validate the isolation of MP, DP, and TP fractions from the QMA strong anion exchange cartridges. Select NAs (TFV, TFV-MP, TFV-DP and entecavir (ETV) -MP and ETV-TP) that were available in sufficient quantities were used to verify the results. Unextracted MP, DP, and TP were spiked into the elution matrix at corresponding elution volumes. The mean (n=3) response of extracted endogenous MP, DP, and TP were compared to the (mean) unextracted response and expressed as percent recovered. Different lots of QMA (n=3) were utilized. Analysis of the fractions was performed using ion pairing UV methodology. Acceptance criteria were a mean extraction efficiency of 85–115% for each MP, DP, and TP fraction.
The MP fractions were shown to elute with 5mL of 75mM KCl, DP fractions with 7mL of 90mM KCl, and TP fractions with 2mL of 1M KCl. The recoveries were between 88.7% to 102% and precision was within 3.9% for all determinations indicative of a precise isolation methodology showing consistency between lots of QMA cartridges.
3.2 Dephosphorylation
Acid phosphatase crude stock was received as 2500 units and working stock was prepared by diluting crude stock (200–250 units) to a total volume of 5mL with 1M sodium acetate, pH 5. Acid phosphatase working stock was stored at 4°C. Dephosphorylation was validated using endogenous AMP, ADP, and ATP as well as NAs TFV, TFV-MP, TFV-DP, ETV-MP, and ETV-TP. These solutions were treated with acid phosphatase working stock for 0, 30, 60, and 120 minutes controlled at 37°C in a water bath. Analysis was performed with ion pairing ultraviolet detection (UV) methodology. Peak area response of the resulting parent A, TFV, and ETV was compared to a 100% molar equivalent peak injected for each analyte (prepared in equivalent matrix and volume) for recovery determination of the parent from the MP, DP, and TP fractions. Acceptance criteria was >85% recovery. The ion pairing method also allowed for monitoring and detection of any remaining MP, DP, or TP form in the respective timed samples above. Acceptance criteria were no detectable nucleotide forms remaining in the dephosphorylated tested sample. It was found that dephosphorylation was complete after 30 minutes of incubation time for each analyte tested. At least 85% of the parent counterpart was recovered and there was no evidence of MP, DP, or TP moieties at the injections occurring 30 minutes or later.
3.3 Desalting and concentration: Matrix effect, Recovery, and Process Efficiency
The matrix effect, recovery, and process efficiency of parent NA from isolated TP hPBMC matrix on the Strata X SPE optimized procedure was determined at three concentrations: 25.0/2.50, 250/25.0 and 1000/100 (fmol per sample for TFV and ZDV, and pmol per sample for 3TC and FTC, respectively) [14]. Five different lots of hPBMC were processed in triplicate through the QMA and dephosphorylation process for preparation of each concentration level. Peak response from extracted (Set 3) preparations (Strata-X SPE) was compared to neat samples prepared in UP water, which is the reconstitution matrix (Set 1), and post extraction spiked (Set 2) blank preparations (Strata-X SPE). ME was determined by comparing the NA response from Sets 1 and 2. RE was determined by comparing the response from Sets 2 and 3, and PE was determined by comparing the response from Sets 1 and 3. Finally, unweighted linear regression slopes were generated for each of the 5 different lots of hPBMC extracted based upon the three concentrations tested and peak area ratio. Acceptance criteria for effects due to matrix were less than 5%CV on the slope measurements for each analyte from the extracted (Set 3) samples. The ME, RE, and PE results are shown in Table 2, section A. The ME ranged from 63% to 78%, RE from 63% to 103%, and PE from 40% to 68% among the analytes. The slopes ranged from 1.1% to 1.8% among the analytes indicating a lack of significant matrix effect.
Table 2.
Validation Summary Data.
| TFV Centric Mode of Analysis | ZDV Centric Mode of Analysis | ||||
|---|---|---|---|---|---|
| TFV/TFV-DP | 3TC/3TC-TP | FTC/FTC-TP | 3TC/3TC-TP | ZDV/ZDV-TP | |
| SECTION A | |||||
| Matrix Effect, Recovery, Process Efficiency | |||||
| Analyte/Analyte-IS | |||||
| Matrix Effect | 65%/69% | 78%/78% | 66%/77% | 65%/63% | 71%/76% |
| Recovery | 82%/76% | 72%/73% | 103%/87% | 67%/63% | 93%/86% |
| Process Efficiency | 52%/52% | 56%/57% | 68%/68% | 44%/40% | 66%/65% |
| Matrix Effect Slope | |||||
| Precision (n=5 hPBMC lots) | 1.8% | 1.3% | 1.5% | 1.1% | 1.8% |
|
| |||||
| SECTION B | |||||
| Back Calculated Calibration Standards | |||||
| Calibration Standard Range | 2.50–2000 fmol/sample | 0.100–200 pmol/sample | 0.100–200 pmol/sample | 0.100–200 pmol/sample | 5.00–2000 fmol/sample |
| Interassay Accuracya (n=5) | −4.3% to 3.4% | −3.7% to 7.0% | −2.4% to 7.4% | −4.8% to 3.7% | −5.0% to 3.3% |
| Interassay Precision (n=5) | 1.5% to 12.0% | 0.9% to 4.4% | 1.0% to 8.5% | 1.5% to 12.6% | 1.3% to 11.3% |
| Slope Mean (n=5) | 6.354E-04 | 1.263E-01 | 9.491E-02 | 1.260E-01 | 1.462E-03 |
| Slope Precision (n=5) | 2.9% | 0.9% | 1.9% | 4.7% | 2.6% |
| Coefficient of Determination (r^2) Mean (n=5) | 0.9994 | 0.9998 | 0.9996 | 0.9990 | 0.9993 |
| Quality Control Accuracya and Precision | |||||
| Quality Control Low 1 | 2.50 fmol/sample | 0.265 pmol/sample | 0.250 pmol/sample | 0.265 pmol/sample | Not Available |
| Interassay Accuracy | −3.6% | −2.1% | −0.5% | −5.0% | |
| Interassay Precision | 16.9% | 3.6% | 6.7% | 3.3% | |
| Interassay (n) | 24 | 25 | 25 | 30 | |
| Intraassay Accuracy (n=5) | −12.9% to 6.1% | −4.9% to 0.7% | −5.9% to 10.7% | −8.4% to −1.5% | |
| Intraassay Precision (n=5) | 6.8% to 23.6% | 1.7% to 4.9% | 0.5% to 3.8% | 1.0% to 2.6% | |
| Quality Control Low 2 | 5.00 fmol/sample | 0.530 pmol/sample | 0.500 pmol/sample | 0.530 pmol/sample | 5.00 fmol/sample |
| Interassay Accuracy | −1.9% | −0.3% | −0.3% | −1.3% | −1.2% |
| Interassay Precision | 14.9% | 2.7% | 5.8% | 1.7% | 19.6% |
| Interassay (n) | 25 | 25 | 25 | 30 | 23 |
| Intraassay Accuracy (n=5) | −11.3% to 9.4% | −1.8% to 2.8% | −5.3% to 8.8% | −2.3% to 0.2% | 17.2% to 10.3% |
| Intraassay Precision (n=5) | 6.2% to 14.8% | 0.9% to 3.9% | 2.2% to 3.4% | 1.3% to 2.6% | 8.1% to 32.6% |
| Quality Control Low 3 | 15.0 fmol/sample | 1.59 pmol/sample | 1.50 pmol/sample | 1.59 pmol/sample | 15.0 fmol/sample |
| Interassay Accuracy | −2.4% | 1.8% | 0.0% | 3.8% | −1.6% |
| Interassay Precision | 8.4% | 1.5% | 3.4% | 2.5% | 9.0% |
| Interassay (n) | 25 | 25 | 25 | 30 | 25 |
| Intraassay Accuracy (n=5) | −6.6% to 1.8% | 1.0% to 3.4 % | −2.8% to 5.2% | 1.6% to 6.3% | −6.2% to 0.9% |
| Intraassay Precision (n=5) | 5.8% to 11.2% | 0.9% to 1.6% | 1.0% to 2.7% | 1.0% to 2.9% | 6.2% to 13.1% |
| Quality Control Medium | 150 fmol/sample | 15.9 pmol/sample | 15.0 pmol/sample | 15.9 pmol/sample | 150 fmol/sample |
| Interassay Accuracy | −6.9% | 3.9% | −0.9% | 6.5% | −2.3% |
| Interassay Precision | 5.2% | 1.4% | 3.5% | 3.8% | 4.3% |
| Interassay (n) | 25 | 25 | 25 | 30 | 25 |
| Intraassay Accuracy (n=5) | −12.3% to −2.5% | 3.0% to 4.9% | −5.2% to 3.2% | 2.2% to 13.6% | −5.0% to −0.5% |
| Intraassay Precision (n=5) | 1.1% to 4.8% | 0.9% to 1.7% | 0.7% to 1.9% | 0.8% to 1.9% | 1.6% to 5.7% |
| Quality Control High | 1500 fmol/sample | 159 pmol/sample | 150 pmol/sample | 159 pmol/sample | 1500 fmol/sample |
| Interassay Accuracy | −6.7% | 5.4% | −0.3% | 8.0% | −2.1% |
| Interassay Precision | 5.3% | 1.6% | 3.3% | 4.8% | 2.8% |
| Interassay (n) | 25 | 25 | 25 | 30 | 25 |
| Intraassay Accuracy (n=5) | −13.6% to −1.5% | 3.9% to 7.4% | −3.7% to 3.9% | 3.3% to 16.3% | −6.4% to −0.3% |
| Intraassay Precision (n=5) | 0.9% to 3.5% | 0.7% to 1.5% | 0.8% to 2.0% | 0.3% to 6.1% | 1.0% to 2.2% |
|
| |||||
| SECTION C | |||||
| Alternative Matrices Accuracya and Precision | |||||
| Low Concentration Tested | 25.0 fmol/sample | 2.50 pmol/sample | 2.50 pmol/sample | 2.50 pmol/sample | 25.0 fmol/sample |
| hPBMC MP Fraction | |||||
| Accuracy | 19.1% | −2.5% | −3.1% | 2.2% | 2.0% |
| Precision | 7.4% | 0.8% | 1.1% | 0.4% | 8.3% |
| hPBMC DP Fraction | |||||
| Accuracy | 2.4% | −0.5% | −2.1% | 2.9% | 2.0% |
| Precision | 4.5% | 1.2% | 2.0% | 1.5% | 10.5% |
| RBC MP Fraction | |||||
| Accuracy | 3.3% | −1.4% | −1.5% | 2.5% | −4.7% |
| Precision | 9.3% | 1.8% | 2.0% | 1.9% | 1.6% |
| RBC DP Fraction | |||||
| Accuracy | 6.3% | −1.9% | −2.9% | 1.9% | 1.0% |
| Precision | 10.8% | 1.4% | 0.7% | 0.6% | 10.4% |
| RBC TP Fraction | |||||
| Accuracy | 2.0% | −3.0% | −1.3% | 0.8% | 5.5% |
| Precision | 7.0% | 0.9% | 0.6% | 0.8% | 5.6% |
| High Concentration Tested | 1000 fmol/sample | 100 pmol/sample | 100 pmol/sample | 100 pmol/sample | 1000 fmol/sample |
| hPBMC MP Fraction | |||||
| Accuracy | 4.6% | 1.3% | −1.6% | 3.5% | −3.2% |
| Precision | 1.0% | 0.8% | 0.9% | 0.5% | 0.9% |
| hPBMC DP Fraction | |||||
| Accuracy | 5.4% | 2.1% | −1.5% | 2.4% | −2.8% |
| Precision | 2.7% | 2.2% | 1.9% | 0.9% | 1.6% |
| RBC MP Fraction | |||||
| Accuracy | 5.4% | 1.8% | −1.1% | 2.9% | −1.9% |
| Precision | 1.8% | 0.5% | 0.6% | 0.5% | 1.7% |
| RBC DP Fraction | |||||
| Accuracy | 6.0% | 2.2% | −0.8% | 2.8% | −1.9% |
| Precision | 1.6% | 0.2% | 0.8% | 0.3% | 1.5% |
| RBC TP Fraction | |||||
| Accuracy | 2.4% | 0.3% | −0.1% | 2.0% | −2.3% |
| Precision | 1.2% | 0.5% | 0.4% | 0.3% | 1.4% |
|
| |||||
| SECTION D | |||||
| hPBMC Cell Number Accuracya and Precision | |||||
| Concentration tested (n=3) | 100 fmol/sample | 10.0 pmol/sample | 10.0 pmol/sample | 10.0 pmol/sample | 100 fmol/sample |
| 0.1 million cells extracted | |||||
| Accuracy | 1.6% | 1.9% | −0.2% | 4.0% | 1.2% |
| Precision | 5.5% | 1.8% | 0.8% | 0.7% | 1.9% |
| 1.0 million cells extracted | |||||
| Accuracy | −0.3% | 2.2% | −0.7% | 4.0% | 0.2% |
| Precision | 2.9% | 0.8% | 0.3% | 0.3% | 5.2% |
| 10 million cells extracted | |||||
| Accuracy | 1.5% | 0.6% | −1.1% | 2.3% | 1.3% |
| Precision | 4.2% | 0.7% | 0.4% | 0.8% | 3.9% |
|
| |||||
| SECTION E | |||||
| Conditional Stability | |||||
| Freeze/Thaw Cycles (n=4cycles) | |||||
| QC Low 3 %Diff vs Control | −1.6% | 0.0% | 0.3% | −0.2% | 12.9% |
| QC High %Diff vs Control | −0.1% | 0.2% | 0.6% | −4.4% | 1.4% |
| 24Hour Room Temperature | |||||
| QC Low 3 %Diff vs Control | 5.8% | 0.9% | 0.8% | 2.1% | 9.4% |
| QC High %Diff vs Control | 1.7% | 0.9% | 2.1% | 0.0% | 2.1% |
| 1 Month Extracted Sample- Autosampler (20C) | |||||
| QC Medium %Diff vs Control | 3.9% | 1.1% | 0.3% | −1.2% | −0.3% |
| Long Term Stability | |||||
| −80C Lysed Intracellular Matrix | 30 Months | Not Available | Not Available | Not Available | 18 Months |
Accuracy is expressed as % difference from nominal
3.4 Accuracy and Precision
Accuracy and precision were determined by replicate analysis (n=5) of each of the QC levels described above (n=5) in at least five separate analytical runs. The analytical runs were injected on both the TFV centric and ZDV centric modes of analysis. Acceptance criteria were ±15% for both accuracy (compared to nominal as % difference) and precision determinations at all concentrations except at the lower limits of quantitation. While ±20% is typically allowed at LLOQs, a main goal for this assay was to allow for analysis of rare cellular samples with potentially extremely low cell counts therefore, ±25% was allowed at the LLOQ While this allowance adds potentially 5% more variability and inaccuracy to the LLOQ it minimizes the potential loss of research data from these rare cellular samples.
Standard curves for all analytes were found to be best fit by linear regression with 1/concentration weighting. In the TFV centric mode, standard curves were linear between 2.5 and 2000 fmol/sample for TFV and between 0.1 and 200 pmol/sample for 3TC and FTC. TFV at 1 fmol/sample could not be reliably determined. In the ZDV centric mode, 3TC was linear in the same range, and ZDV was linear from 5 to 2000 fmol/sample. ZDV at 1 and 2.5 fmol/sample could not be reliably determined. One ZDV run was disqualified due to ZDV contamination, which was traced back to a KCl preparation for that run. The 3TC data from the run were included for analysis. Standard performance is shown in Table 2, section B. Accuracy was within ±7.4% and precision ≤12.6% for back-calculated standards. The CV for slopes and the R2 were ≤ 4.7% and ≥ 0.9990, respectively. One ZDV calibrator (STD 5.00fmol/sample) was excluded from validation run 1, as it was outside the acceptance criteria. All other calibrators met acceptance criteria.
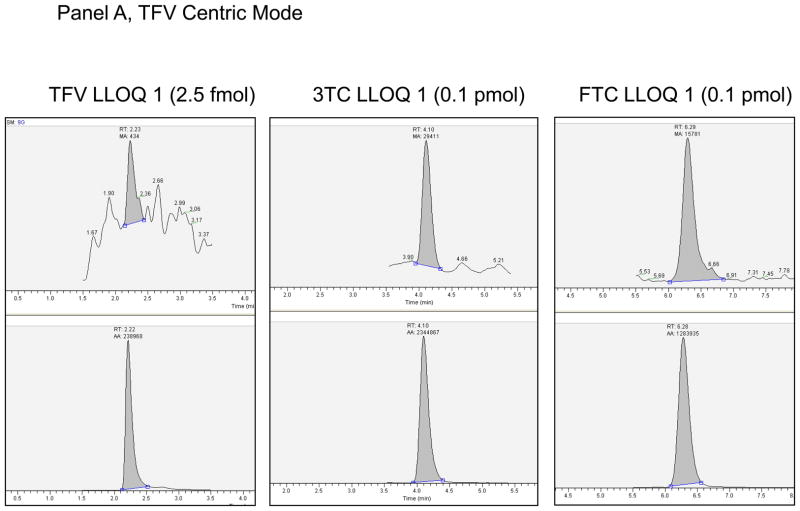
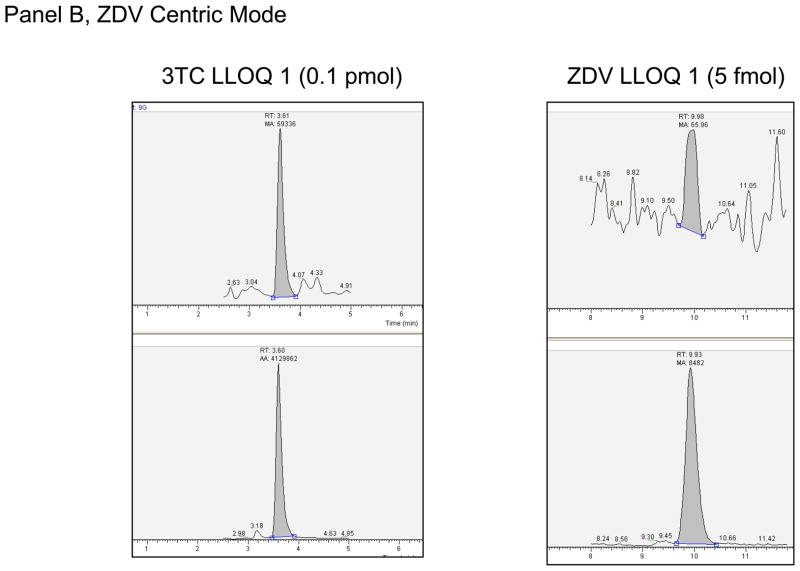
The intraassay and interassay accuracy and precision based upon the QCs are shown in Table 2, section B. The greatest mean interassay percent deviation was 8.0% for the 3TC 159 fmol/sample in the ZDV-centric analysis. The greatest mean interassay %CV was 19.6% for the ZDV 5 fmol/sample (LLOQ), and the highest non-LLOQ %CV was 14.9% for the TFV 5 fmol/sample. The maximum intraassay percent deviation was −17.2% for ZDV 5 fmol/sample (LLOQ), and 16.8% for the 3TC 159 fmol/sample on run 4 of the ZDV centric mode of analysis, all other non-LLOQs were within ±13.6%. The maximum intraassay %CV was 32.6% for the ZDV 5 fmol/sample (LLOQ) on run 1 of the ZDV centric mode of analysis, 23.6% for the TFV-DP 2.5fmol/sample (LLOQ), and 14.4% for the non-LLOQ samples. The ZDV centric mode of analysis had one run for each of 3TC-TP and ZDV-TP that did not meet intraassay acceptance criteria as described above. The QH for 3TC-TP on run 4 had a deviation from nominal of +16.3% (1.2% CV, n=5). The other 5 runs for the QH were within ±9.3% deviation. As discussed in Discussion below, the upper limit of quantitation for 3TC-TP on the ZDV centric mode of analysis will be 100 fmol/sample. The LLOQ for ZDV-TP for Run 1 had a precision determination of 32.6%. The other 4 runs were ≤ 18.2%, such that 80% of the runs passed criteria for the ZDV-TP LLOQ. Based upon assay performance the following LLOQs were defined: For 3TC and FTC, 0.10 pmol/sample; for ZDV, 5.00 fmol/sample; and for TFV, 2.5 fmol/sample. Typical chromatographs at the reported LLOQ are shown in Figure 1.
Figure 1.
Panel A. LLOQ 1 chromatographs for TFV Centric Mode of analysis. Top panel is analyte, bottom the corresponding internal standard. Y-axis is relative abundance to 100%. Panel B. LLOQ 1 chromatographs for ZDV Centric Mode of analysis. Top panel is analyte, bottom the corresponding internal standard. Y-axis is relative abundance to 100%.
3.5 Alternative matrices
The alternative matrices for this method were considered hPBMC MP and DP fractions and RBC MP, DP, TP fractions isolated from lysed intracellular matrix with the QMA SPE process. Calibration standards were spiked into blank TP hPBMC fractions isolated from the QMA SPE process (as described above). The MP and DP fractions contained different elution salt concentration and volume and RBC were a different cellular type. The accuracy and precision for the alternative matrix testing was generated from 3 different lots of hPBMC and RBC to generate a triplicate analysis. The QMA fractions were dephosphorylated and spiked at two concentration levels with parent NA working standards (STD G- 25.0/2.50 and STD E- 1000/100; for TFV, ZDV/3TC, FTC respectively). Acceptance criteria were ±15% for both accuracy (compared to nominal) and precision determinations.
The alternative matrices results are presented in Table 2, section C. The accuracy across all matrices for all compounds ranged from −4.7% to 6.3% except for TFV at the low concentration (25.0 fmol/sample) in hPBMC MP matrix where the accuracy was +19.1%. The precision (%CV) for these determinations was within 10.8% for all compounds in all matrices. The TFV result from hPBMC MP matrix at the low concentration was outside the acceptable limit of ±15%, but passed the hPBMC DP at the low concentration, and both the hPBMC MP and DP at the high concentration as well as all RBC MP, DP, and TP matrices. Taken together, these data support the method’s ability to accurately and precisely determine parent NAs from alternative matrices.
3.6 Specificity
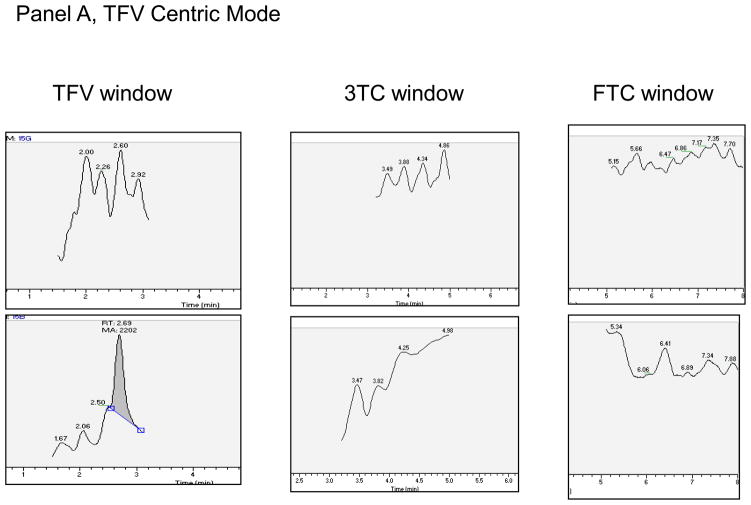
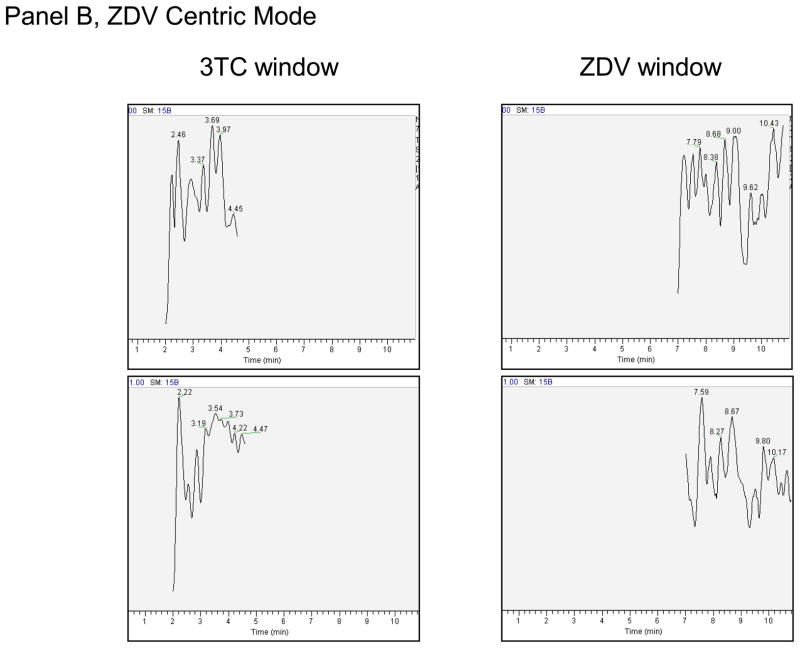
Specificity was determined by injecting blank hPBMC MP, DP, and TP, and RBC MP, DP, and TP (n=6 lots each) and monitoring for analytes. The high standard (A) with no IS and blank with IS were used to evaluate cross-talk between NA and NA-IS. Carry over was evaluated by injecting blank water after the cross-talk samples to check for signal in the blank injection. Additionally, each analytical run contained a blank and blank with internal standard sample to monitor specific response within each run. A lack of response was demonstrated in NA analyte windows for the 6 lots of hPBMC MP, DP, and TP and RBC MP, DP, or TP. Typical chromatographs are shown in Figure 2. No significant cross-talk or carry over was observed between or among NA and NA-IS.
Figure 2.
Panel A. Blank/blank chromatographs for TFV Centric Mode of analysis. Top panel is analyte, bottom the corresponding internal standard. Y-axis is relative abundance to 100%. Expected retention times for analyte/analyte-IS are: TFV/TFV-IS (2.15 minutes), 3TC/3TC-IS (3.71 minutes), FTC/FTC-IS (5.74 minutes). The peak at 2.60 minutes in the TFV-IS window was apparent in all runs. Panel B. Blank/blank chromatographs for ZDV Centric Mode of analysis. Top panel is analyte, bottom panel the corresponding internal standard. Y-axis is relative abundance to 100%. Expected retention times for analyte/analyte-IS are 3TC/3TC-IS (3.39 minutes). ZDV/ZDV-IS (9.29 minutes).
3.7 Effect of cell number
The ability to quantitate NA in low cell numbers or high cell numbers is an important consideration for this method due to the types of tissues and cell populations for which the method is intended. The number of cells assayed can also be controlled to allow for results to fall within the acceptable reportable ranges for the different analytes. The accuracy and precision of TFV and ZDV (100 fmol/sample) and 3TC and FTC (10 pmol/sample) were determined from lysed intracellular matrix containing 0.1, 1.0, and 10 million cells. Acceptance criteria were ±15% for both accuracy (compared to nominal) and precision determinations. The results are shown in Table 2, section D. The mean accuracy for all analytes for all cell number experiments ranged from −1.1% to 4.0%. The precision was within ± 5.5%.
3.8 Stability of analytes
Conditional NA-TP and parent NA stabilities were determined by assessing freeze/thaw stability, room temperature stability, and extracted sample stability. QL3 and QH were subjected to four freeze/thaw cycles. In a separate experiment, QL3 and QH were subjected to room temperature for 24 hours prior to extraction. The samples were then carried through the QMA and Strata X extractions and run in three replicates. The mean response was compared to the nominal concentration for QL3 and QH and also compared to fresh QL3 and QH controls run in triplicate that did not undergo treatment conditions. Extracted sample stability was assessed by retaining five replicate QM samples from one of the accuracy/precision validation runs in the autosampler for 1 month which was maintained at 20°C. The retained samples were injected with an independent run that occurred 1 month later. Mean response was compared to both nominal and mean response from freshly prepared QM that was not subjected to the test condition.
Table 2, section E shows results from conditional stability experiments. The range of deviations from control for all the tested conditions was −4.4% to 12.9%.
Long term stability of parent NA preparation stocks and working stocks in water stored at 4°C was assessed by comparing the peak responses of current stock solutions to the peak responses from stock solutions that had been in storage for various lengths of time. 3TC solutions at all concentrations tested were shown to be stable for up to 5.86 years. All FTC solutions were stable for at least 2.17 years, and all ZDV and TFV solutions were stable for up to 5.88 and 4.68 years, respectively.
Long term stability of NA-TP preparation and working stocks (at −70°C in water) were taken into account with purity/potency determinations prior to QC preparation for use in the assay. Repeated purity assessments for FTC-TP were 78% at baseline and 78% at 4.7 years. The same for 3TC-TP stock solutions were initially 94% and 97% 2.2 years later. Purity/potency for current NA-TP lots include: ZDV-TP 94.8% pure and 46.8% potent yielding a correction factor of 0.444, FTC-TP 95.8% pure and 31% potent for a correction factor of 0.297, 3TC-TP 92.9% pure and 84.4% potent for correction factor of 0.782, and TFV-DP 93.7% pure and 108.3% potent for a correction factor of 1.015. The NA-TP QC preparation stocks were adjusted with the determined correction factor.
Long term stability data for TFV-DP and ZDV-TP in lysed intracellular matrix (−80°C) have been compiled from quality controls for respective single analyte assays previously utilized [6, 13]. TFV-DP was tracked over 30 months for QM (750 fmol/sample) and QH (7500 fmol/sample) and both were within ±13.9% of nominal. QL (150 fmol/sample) was followed to 18 months and was within −2.9% of nominal. There was not sufficient QL volume remaining to accurately/precisely test at 30 months. ZDV-TP was tracked over 18 months. ZDV-TP (QL=100 fmol/sample; QH=3000 fmol/sample) results were within ±12.8% from nominal except for QM (600 fmol/sample) at the 18 month interval where it was −16.6%. Additional long term stability data for 3TC-TP and FTC-TP will be generated. Taken together, these experiments support stability of analytes in storage, and under various conditions commonly encountered in laboratories.
3.9 Clinical application
The method was used to analyze hPBMC and RBC samples containing ZDV-phosphates and 3TC-phosphates (hPBMC) or TFV-phosphates and FTC-phosphates (hPBMC and RBC) in either HIV-negative or HIV infected subjects [15–17]. The samples arose from IRB approved protocols, and all subjects gave written informed consent. Subjects received standard NA doses, and provided blood samples on one or more study visits. Typically, eight mL of blood was drawn into a heparin Cell Preparation Tube at various time points following the dose. Blood was centrifuged and hPBMC and RBC were harvested and counted with a manual hemocytometer or Countess Automated Cell Counter (Invitrogen, Carlsbad, CA, USA). Cells were lysed with ice cold 70% methanol and stored at −80°C until analysis.
The method has been applied to > 100 hPBMC samples to quantify TFV-DP/FTC-TP and > 700 hPBMC samples to quantify ZDV-TP/3TC-TP. For the ZDV centric mode, two million cells were typically assayed, although as few as 100,000 have been assayed with success. Average (range) values in one study with 43 subjects (599 samples) were 33 fmol/106 cells (2.63 to 198 fmol/106 cells) for ZDV-TP and 4.8 pmol/106 cells (0.15 to 37 pmol/106 cells) for 3TC-TP. ZDV- and 3TC-MP and DP were measured in approximately 200 samples. The values for ZDV-MP were 441 fmol/106 cells (25.9 to 4369 fmol/106 cells), which were approximately 10-fold higher than ZDV-TP. ZDV-DP was 37.5 fmol/106 cells (2.97 to 238 fmol/106 cells), similar to ZDV-TP. The values for 3TC-MP and 3TC-DP were 3.0 pmol/106 cells (0.15 to 18.3 pmol/106 cells) and 3.3 pmol/106 cells (0.14 to 11.6 pmol/106 cells), respectively, both slightly lower than 3TC-TP [16].
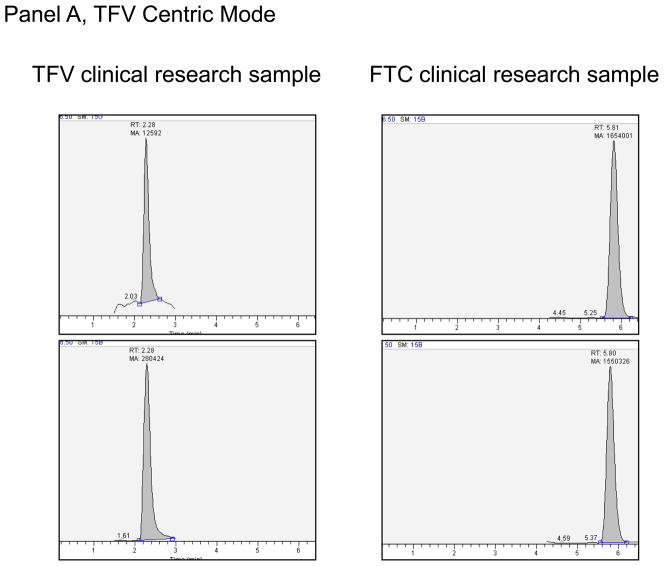
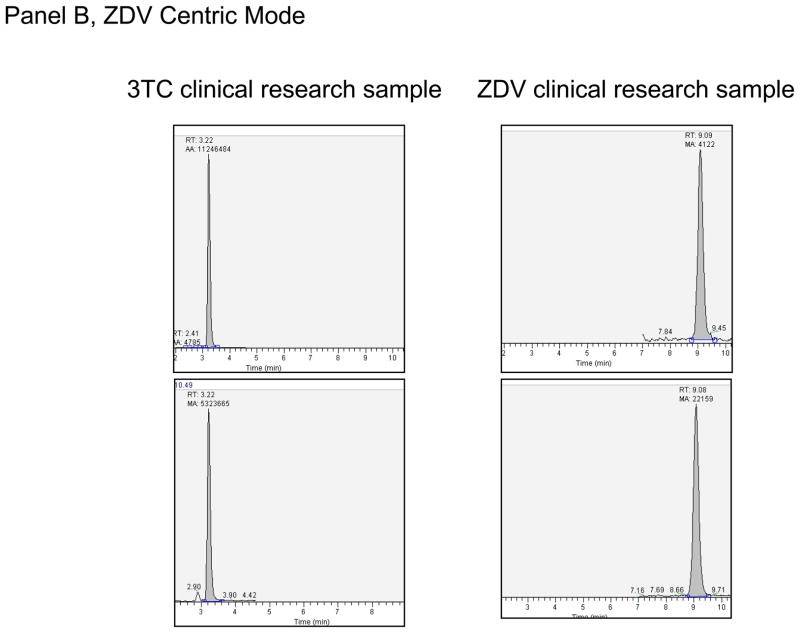
For the TFV centric mode, two million cells were typically assayed, although as few as 100,000 have been assayed with success. One study of hPBMC from a single blood draw in 10 participants showed an average (range) TFV-DP and FTC-TP of 92 (41 – 171) fmol/106 and 6.6 (2.0 – 10.7) pmol/106 cells respectively. In an another study, TFV, TFV-MP, TFV-DP, FTC-MP, FTC-DP, and FTC-TP were all evaluated at a single time point in paired RBC (2 million cells extracted) and hPBMC (1.35 to 4.7 million cells extracted) from five subjects. In hPBMC, the average (range) TFV, TFV-MP, TFV-DP were 11.4 (below the limit of quantitation (BLQ) to 19.4), 16.6 (2.7 to 28.1), and 53.3 (3.7 to 100) fmol/106 cells. The same values in the paired RBC were 6.4 (3.0 to 12.7), 99.8 (29.1 to 171), and 91 (21.2 to 184) fmol/106 cells. For FTC, the average (range) FTC-MP, FTC-DP, FTC-TP in hPBMC were 1.2 (BLQ to 1.7), 6.0 (BLQ to 8.1), and 4.7 (BLQ to 6.2) pmol/106 cells. FTC-DP and FTC-TP were not detectable in the paired RBC samples, but FTC-MP was detectable in two samples (0.27 and 0.09 pmol/106 cells). One of the five subjects was likely non-adherent, with BLQ FTC nucleotides in hPBMC and low or BLQ TFV nucleotides in RBC and PBMCs. Typical chromatographs from clinical research samples are shown in Figure 3.
Figure 3.
Panel A. Extracted clinical research sample for TFV Centric Mode of analysis for hPBMC-TP. This sample had a TFV-DP of 44.8 fmol/10^6 Cells and FTC-TP of 6.20 pmol/10^6 cells. Panel B. Extracted clinical research sample for ZDV Centric Mode of analysis for hPBMC-TP. This sample had a ZDV-TP of 64.5 fmol/10^6 Cells and 3TC-TP of 8.80 pmol/10^6 cells.
4 Discussion
At the onset of assay development, several main goals were set so as to best support the research direction envisioned for the NA clinical pharmacology field: Attainment of ultrahigh sensitivity with the ability for simultaneous measurement of multiple analytes; the ability to quantify the MP, DP and TP moeities; and an ability to assay 70:30 lysate from various alternative cell matrices. Each of these goals was met with the present methodology. The method is 10- to 20-fold more sensitive than the previous methods that used a similar approach, or different analytical techniques [6, 9, 13, 18–20]. The enhanced sensitivity arises from the state-of-the-art analytical equipment available (ThermoScientific Vantage), the optimization of the desalting and concentration procedure, and the creation of the two centric analytical modes, TFV centric for TFV, 3TC, and FTC, and ZDV centric for 3TC and ZDV. While 3TC was included in both modes, it was observed that the response for 3TC in the ZDV centric mode was approaching the typical non-linear or ionization saturation point seen with electrospray ionization in LC-MS/MS between the STD B (100 pmol/sample) and STD A (200 pmol/sample). Thus, although 3TC passed at 200 pmol/sample in the ZDV centric mode, an upper limit of quantitation (ULOQ) of 100 pmol/sample will be used for this mode compared with the TFV centric mode (ULOQ of 200 pmol/sample).
A critical step in attaining the increased sensitivity was maximizing the process efficiency for TFV and ZDV, which require detection in the fmol/sample range. During development, injection of neat standards afforded measurable signal down to the 1 fmol/sample for TFV, which corresponded to 0.30 fmol of TFV on column. However, this level could not be achieved in extracted samples because of low or variable process efficiency. Therefore, a goal process efficiency of 50% or more was set in order to attain the desired quantitation level of the NAs in the fmol range (a target of at least 2.5 fmol/sample for TFV). Attempts to meet this goal using the previous Waters Oasis HLB cartridge (3cc-60mg-30uM) SPE that included trifluoroacetic acid to aid in TFV recovery were not successful [6]. Added to the difficulty was developing a procedure that could be applied to multiple NAs for a desired simultaneous methodology. The former desalting/concentration SPE (Oasis HLB) method utilized for TFV and ZDV differed for the separate methods [6, 13]. While trifluoracetic acid was necessary for TFV retention on the HLB, it was not optimal for other potential NAs, which could be targeted in the future such as dideoxyadenosine (ddA) due to instability in acidic conditions. The previous ZDV method utilized water washes on the HLB, but this method did not retain TFV sufficiently. Other SPEs from different manufacture’s and with different sorbents and sizes were then tested including, JT Baker BakerBond H2O-philic DVB, Biotage Evolute ABN, Isolute MFC 18, C18 (EC), C18, Varian Bond Elut (C18), and Phenomenex C18, Strata-X. The SPE cartridges were screened for optimal retention of TFV, the most polar and difficult analyte to retain due to its phosphonate group. Once the Phenomenex Strata-X was determined to retain TFV the best using a non acidic medium, a delicate balance was needed between aqueous and salt removal and maintaining an adequate recovery of TFV. Rezk et al. utilized Bond Elut C18 cartridges to extract TFV and FTC from plasma and found that increasing ionic strength of ammonium acetate washes helped in recovery of TFV [21]. However, TFV retention to the Bond Elut SPE when applied in the salt elution matrix from the QMA process was not successful. The wash strategy with ammonium acetate was also attempted on the Strata-X, but increased retention was not observed. This is likely due, in part, to the already high ionic strength of the QMA solution that was applied to the desalting/concentration SPE cartridge.
Hydrophobic organic washes (0.5mL) were then tested in an effort to strip aqueous/salt from the SPE by density interaction, ideally with minimal elution of the NAs. The solvents tested were MTBE, hexane, dichloromethane, and dichlorethane. Of these, dichlormethane lowered the observed matrix effect compared to no solvent wash, and also decreased drying time of the final eluate by about 50%, supporting that the solvent decreased the amount of aqueous/salt in the final eluate. Dichloromethane only slightly lowered the RE of the polar NAs such that the goal overall process efficiency of 50% or more for the NAs in the fmol range could be met with the Phenomenex Strata-X SPE methodology.
The final step in obtaining the desired sensitivity for a simultaneous NA method was developing suitable chromatography. The goal was to develop conditions optimal for all NAs with one analytical column and one mobile phase condition. It was soon realized that the same obstacles that were encountered in the desalting/concentration step with chemical differences between the NAs would also be encountered in the chromatographic and MS detection development. This led to the division of the analysis into a TFV centric mode and ZDV centric mode. The major difference between the TFV centric mode and ZDV centric mode was the mobile phase components. The mobile phase was optimized for TFV signal which required formic acid as the modifier in order to be ionized in the esi positive mode and to be adequately retained on the analytical column. This posed a problem for ZDV analysis since it required a low concentration of acetic acid for optimal ionization in the esi negative mode. It was desired to utilize a single analytical column for both modes of analysis. A number of analytical columns were tested for optimal retention and peak shape of TFV, while allowing for adequate chromatographic performance of the other NAs as well. A Phenomenex Polar-RP, 2.5uM 2×100mm analytical column gave the best overall performance which could be utilized for both the TFV and ZDV centric modes of analysis. Gradient and isocratic elution were also tested, but an isocratic flow gave the best overall performance.
The second main goal was to quantify the MP and DP, as well as the TP from multiple NA analytes, which was eventually accomplished by optimizing a QMA procedure for comprehensive separation and purification of MP, DP, and TPs. During development, one other approach was also tested, a weak anion exchange methodology, which utilized volatile salt solutions at varying pH for MP, DP, and TP purification. It was discovered that a salt gradient worked better than changing the pH of the solutions. The approach showed promise utilizing salt gradients of ammonium acetate, however it was too imprecise to proceed to validation.
Ultimately, the method that worked best was a modification of a QMA strong anion exchange procedure that was previously utilized for separate TFV-DP and ZDV-TP assays [6, 13]. However, these previous procedures were developed to optimize TP recovery, not to yield purified MP and DP fractions. Volatile salt solutions were not efficient in exchanging with the strong anion exchange packing of the QMA, thus modifications were made to the previously used KCl concentrations and volumes to isolate MP, DP, and TP fractions. During development, it was observed that G and dG compounds required the highest volume for elution (eluted most difficultly) and A and T compounds eluted with the lowest volume (eluted most easily). Therefore, these analytes bracketed the recovery of all other analytes and were therefore used to refine the isolation conditions for validation.
The final main goal was to create a method that was accurate and precise in alternative cellular matrices. This goal was ultimately met through the availability and use of stable labeled internal standards. It should be noted that not all possible alternative cellular matrices were available for testing, in this case only hPBMC and RBC MP, DP, and TP elutions, which were chosen to be representative of cellular matrices that might be encountered in the research field. The matrix analyzed or applied to the QMA was the 70:30 MeOH:UP water, which lyses or perforates cellular membranes thereby releasing the intracellular material into a lysate regardless of cell type. The consistent accuracy and precision in the RBC and PBMC matrix, as well as the different salt concentration/volume matrices of the MP, DP, and TP elutions provide evidence that the method can be applied to alternative cellular matrices. Extracted blank hPBMC TP fractions were utilized for the calibration curves for this method. Preliminary data for NAs for which stable labeled ISs were not available (eg ETV, CBV, ddA) showed that pairing with one of the existing stable labeled ISs worked well for TP fractions, but fell short in the MP and DP alternative matrices. Thus, matching stable labeled internal standards were shown to be necessary for accurate and precise quantification using alternative cellular matrices.
The method was successfully applied to clinical research samples of RBC and hPBMC. The recovered concentrations were in agreement with previous results from other methods, which reported typical values for TFV-DP, FTC-TP, ZDV-TP, and 3TC-TP of approximately 80–160, 1000–4000, 30–150, and 3000–12000 fmol/106 cells [3, 8, 9, 18, 19, 22–28]. The observed results for MP and DP were also similarly comparable to previous reports, where available [9, 11, 23, 26, 29]. The first characterization of FTC nucleotides in RBC showed very low levels of FTC-MP in two of five samples. This is consistent with a previous report that described low levels of 3TC-TP, a close analog of FTC, in RBC [11]. The first characterization of TFV and TFV-MP in paired hPBMC and RBC shows a different profile in the two cell types. In hPBMC, TFV and TFV-MP were similar in concentration, whereas TFV-DP was about 3 to 4-fold higher. In RBC, TFV-MP and TFV-DP were similar in concentration, whereas TFV was about 15-fold lower. FTC-MP and DP showed potential differences compared with its close analog, 3TC in hPBMC. 3TC-MP and DP were similar in concentration, whereas 3TC-TP was about 2-fold higher. However, FTC-DP and FTC-TP were similar in concentration, whereas FTC-MP was about 4-fold lower. Thus, these novel data show the potential value of this new assay to test for distinct cellular pharmacology profiles according to cell type.
It is important to note that certain assumptions were made during method development, and steps were taken to test these assumptions in validation. The first such assumption was that endogenous nucleotides could be used to define the QMA isolation of the MP, DP, and TP fractions for the NAs to be assessed in the method. The endogenous nucleotides were commercially available in MP, DP, and TP forms at relatively low costs, which was not the case for the NAs of interest for this method. Two available NAs were used to verify the results obtained from the endogenous nucleotides. One of these was the MP, DP, and TP forms of TFV which was an analyte validated and analyzed with this methodology and the other was the MP and TP forms of entecavir (ETC) which was only used for verification of the QMA process. These two NAs were chosen because there were sufficient quantities available in the laboratory to perform the necessary work. Importantly, the TP forms of all NA analytes (QCs) were used to assess accuracy and precision in the validation and to verify performance for assay maintenance. Second, an assumption was made that the MP and DP determinations were accurate and precise for the method based upon validated QMA recovery and alternative matrices data. NA-MP and DPs for QCs were either unavailable or were very expensive. However, the MP, DP, and TP fractions were dephosphorylated to the parent NA prior to quantitation and the MP and DP alternative matrices were included in the validation. The data showed that the NAs of interest could be accurately and precisely determined from the MP and DP dephosphorylated fractions.
This is one of the main advantages of indirect methodologies in general, that it allows for quantitation of MP, DP and TP fractions of NAs even if reference standards do not exist for the nucleotide forms. Furthermore, the NAs are generally more stable than their nucleotide counterparts, which lessens concern about QC/standard instability. Additionally, the NAs are amenable to traditional reversed phase LC methodologies and do not require specialized ion pairing agents or ion exchange techniques that can limit sensitivity of nucleotides in MS detection due to ion suppression. The NAs also possess distinct precursor/product transitions where the product with highest MS signal is unique. This is not the case for NA-TP where the highest product ion is often the non-unique phosphates shared by all nucleotides.
However, along with advantages, indirect methods also have some disadvantages. For example, the current method would require separate injections for the TFV centric and ZDV centric modes of analysis to cover all the analytes presented in the validation. However, most current drug regimens would require only one mode of analysis because they pair either 3TC-ZDV or TFV-FTC. Another possible disadvantage is that one extracted sample through the QMA process becomes three fractions (MP, DP, and TP), which increases by 3-fold the number of injections required on the LC-MS/MS to quantitate the MP, DP and TPs. Although indirect methodologies such as this one are viewed as labor intensive, the laboratory has developed an efficient system that pairs lab technologists who specialize in each separation phase of the assay (QMA/Strata-X). The process divides into approximately 2 × 2 hour extraction segments to complete the assay.
In conclusion, a new methodology was developed and validated that possesses ultrahigh sensitivity, the ability to simultaneously measure multiple analytes; the ability to quantify the MP and DP, as well as the TP moieties, and the ability to accurately and precisely assay various cell matrices.
Acknowledgments
We wish to thank the NIH AIDS Research and Reference Reagent Program for the antiretroviral drugs used for assays; Tracy King and Courtney V. Fletcher, Pharm. D. for previous studies; the study personnel, research staff, and nursing who assisted with the clinical protocols; and the subjects who participated in the clinical studies.
Funding Source: This work was supported by grants from NIH; S10 RR23442 (PLA), U01 AI84735 (PLA), R01 AI64029 (PLA), K23 DK82321 (JJK), and the Colorado Clinical Translational Sciences Institute (1UL1 RR025780).
Abbreviations
- NA
nucleoside analog
- MP
monophosphate
- DP
diphosphate
- TP
triphosphate
- IS
internal standard
- PAR
Peak Area Ratio
- hPBMC
human peripheral blood mononuclear cells
- SPE
Solid Phase Extraction
- RBC
red blood cells
- LC
liquid chromatography
- LLOQ
lower limit of quantitation
- CV
coefficient of variation
- A
adenosine
- dA
2′deoxyadenosine
- G
guanosine
- dG
2′deoxyguanosine
- C
cytidine
- dC
2′deoxycytidine
- T
thymidine
- U
uridine
- TFV
tenofovir
- FTC
emtricitabine
- 3TC
lamivudine
- ETV
entecavir
- CBV
carbovir
- ddA
dideoxyadenosine
- QC
quality control
- QL
low quality control
- QM
medium quality control
- QH
high quality control
- QMA
Waters strong anion exchanger cartridge
- ME
matrix effect
- RE
recovery
- PE
process efficiency
- STD
standard
- ULOQ
upper limit of quantification
- fmol
femtomole
- pmol
picomole
- HPLC
high pressure liquid chromatography
- UPLC
ultra performance liquid chromatography
- MS/MS
tandem mass spectrometry
- FWHM
full width at half maximum, HIV, human immunodeficiency virus
- HCV
hepatitis C virus
- HBV
hepatitis B virus
Footnotes
Conflict of Interest: None to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kakuda TN. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin Ther. 2000;22:685–708. doi: 10.1016/S0149-2918(00)90004-3. [DOI] [PubMed] [Google Scholar]
- 2.Cihlar T, Ray AS. Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after zidovudine. Antiviral Res. 2010;85:39–58. doi: 10.1016/j.antiviral.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Anderson PL, Kiser JJ, Gardner EM, Rower JE, Meditz A, Grant RM. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J Antimicrob Chemother. 2011;66:240–50. doi: 10.1093/jac/dkq447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez JF, Rodriguez JL, Santana J, Garcia H, Rosario O. Simultaneous quantitation of intracellular zidovudine and lamivudine triphosphates in human immunodeficiency virus-infected individuals. Antimicrob Agents Chemother. 2000;44:3097–3100. doi: 10.1128/aac.44.11.3097-3100.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robbins BL, Tran TT, Pinkerton FH, Jr, Akeb F, Guedj R, Grassi J, Lancaster D, Fridland A. Development of a new cartridge radioimmunoassay for determination of intracellular levels of lamivudine triphosphate in the peripheral blood mononuclear cells of human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 1998;42:2656–2660. doi: 10.1128/aac.42.10.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King T, Bushman L, Kiser J, Anderson PL, Ray M, Delahunty T, Fletcher CV. Liquid chromatography-tandem mass spectrometric determination of tenofovir-diphosphate in human peripheral blood mononuclear cells. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;843:147–156. doi: 10.1016/j.jchromb.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 7.Becher F, Pruvost A, Goujard C, Guerreiro C, Delfraissy JF, Grassi J, Benech H. Improved method for the simultaneous determination of d4T, 3TC and ddl intracellular phosphorylated anabolites in human peripheral-blood mononuclear cells using high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2002;16:555–565. doi: 10.1002/rcm.605. [DOI] [PubMed] [Google Scholar]
- 8.Barry M, Wild M, Veal G, Back D, Breckenridge A, Fox R, Beeching N, Nye F, Carey P, Timmins D. Zidovudine phosphorylation in HIV-infected patients and seronegative volunteers. AIDS. 1994;8:F1–5. doi: 10.1097/00002030-199408000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Jansen RS, Rosing H, Kromdijk W, ter Heine R, Schellens JHM, Beijnen JH. Simultaneous quantification of emtricitabine and tenofovir nucleotides in peripheral blood mononuclear cells using weak anion-exchange liquid chromatography coupled with tandem mass spectrometry. Journal of Chromatography B. 878:621–627. doi: 10.1016/j.jchromb.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Kuklenyik Z, Martin A, Pau CP, Holder A, Youngpairoj AS, Zheng Q, Cong ME, Garcia-Lerma JG, Heneine W, Pirkle JL, Barr JR. On-line coupling of anion exchange and ion-pair chromatography for measurement of intracellular triphosphate metabolites of reverse transcriptase inhibitors. Journal of Chromatography B. 2009;877:3659–3666. doi: 10.1016/j.jchromb.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Durand-Gasselin L, Da Silva D, Benech H, Pruvost A, Grassi J. Evidence and possible consequences of the phosphorylation of nucleoside reverse transcriptase inhibitors in human red blood cells. Antimicrob Agents Chemother. 2007;51:2105–2111. doi: 10.1128/AAC.00831-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiser JJ, Bushman LR, Anderson PL, Tise S, Klein B, Rower JE, Zheng JH, Everson GT. Ribavirin mono-, di-, and tri-phosphate concentrations in the peripheral blood mononuclear and red blood cells of Hepatitis C virus infected persons [abstract 01] Reviews in Antiviral Therapy and Infections Diseases. 2010;5:3. [Google Scholar]
- 13.King T, Bushman L, Anderson PL, Delahunty T, Ray M, Fletcher CV. Quantitation of zidovudine triphosphate concentrations from human peripheral blood mononuclear cells by anion exchange solid phase extraction and liquid chromatography-tandem mass spectroscopy; an indirect quantitation methodology. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;831:248–257. doi: 10.1016/j.jchromb.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 14.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 15.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapia M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernandez T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallas EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV. Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. N Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson PL, Rower J, Meditz A, Gardner EM, Predhomme J, Klein B, Zheng JH, Bushman L. The cellular pharmacology of zidovudine and lamivudine according to HIV-status and gender (abstract O-6) Reviews in Antiviral Therapy & Infectious Diseases. 2011:8. [Google Scholar]
- 17.Anderson PL, Lama JR, Buchbinder S, Guanira-Carranza JV, Montoya-Herrera O, Casapia M, Bragg L, Bushman L, Glidden DV, Grant R. 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. 2011. (abstract LB96) [Google Scholar]
- 18.Hawkins T, Veikley W, St Claire RL, 3rd, Guyer B, Clark N, Kearney BP. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J Acquir Immune Defic Syndr. 2005;39:406–411. doi: 10.1097/01.qai.0000167155.44980.e8. [DOI] [PubMed] [Google Scholar]
- 19.Robbins BL, Poston PA, Neal EF, Slaughter C, Rodman JH. Simultaneous measurement of intracellular triphosphate metabolites of zidovudine, lamivudine and abacavir (carbovir) in human peripheral blood mononuclear cells by combined anion exchange solid phase extraction and LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;850:310–317. doi: 10.1016/j.jchromb.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Pruvost A, Negredo E, Theodoro F, Puig J, Levi M, Ayen R, Grassi J, Clotet B. Pilot pharmacokinetic study of human immunodeficiency virus-infected patients receiving tenofovir disoproxil fumarate (TDF): investigation of systemic and intracellular interactions between TDF and abacavir, lamivudine, or lopinavir-ritonavir. Antimicrob Agents Chemother. 2009;53:1937–1943. doi: 10.1128/AAC.01064-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezk NL, Crutchley RD, Kashuba ADM. Simultaneous quantification of emtricitabine and tenofovir in human plasma using high-performance liquid chromatography after solid phase extraction. Journal of Chromatography B. 2005;822:201–208. doi: 10.1016/j.jchromb.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Pruvost A, Theodoro F, Agrofoglio L, Negredo E, Benech H. Specificity enhancement with LC-positive ESI-MS/MS for the measurement of nucleotides: application to the quantitative determination of carbovir triphosphate, lamivudine triphosphate and tenofovir diphosphate in human peripheral blood mononuclear cells. Journal of mass spectrometry: JMS. 2008;43:224–233. doi: 10.1002/jms.1294. [DOI] [PubMed] [Google Scholar]
- 23.Moore KH, Barrett JE, Shaw S, Pakes GE, Churchus R, Kapoor A, Lloyd J, Barry MG, Back D. The pharmacokinetics of lamivudine phosphorylation in peripheral blood mononuclear cells from patients infected with HIV-1. AIDS. 1999;13:2239–2250. doi: 10.1097/00002030-199911120-00006. [DOI] [PubMed] [Google Scholar]
- 24.Anderson PL, Zheng JH, King T, Bushman LR, Predhomme J, Meditz A, Gerber J, Fletcher CV. Concentrations of zidovudine- and lamivudine-triphosphate according to cell type in HIV-seronegative adults. AIDS. 2007;21:1849–1854. doi: 10.1097/QAD.0b013e3282741feb. [DOI] [PubMed] [Google Scholar]
- 25.Moore JD, Acosta EP, Johnson VA, Bassett R, Eron JJ, Fischl MA, Long MC, Kuritzkes DR, Sommadossi JP. Intracellular nucleoside triphosphate concentrations in HIV-infected patients on dual nucleoside reverse transcriptase inhibitor therapy. Antivir Ther. 2007;12:981–986. [PubMed] [Google Scholar]
- 26.Aweeka FT, Rosenkranz SL, Segal Y, Coombs RW, Bardeguez A, Thevanayagam L, Lizak P, Aberg J, Watts DH. The impact of sex and contraceptive therapy on the plasma and intracellular pharmacokinetics of zidovudine. AIDS. 2006;20:1833–1841. doi: 10.1097/01.aids.0000244202.18629.36. [DOI] [PubMed] [Google Scholar]
- 27.Anderson PL, Rower JE. Zidovudine and Lamivudine for HIV Infection. Clin Med Rev Ther. 2010;2:a2004. [PMC free article] [PubMed] [Google Scholar]
- 28.Wang LH, Begley J, St Claire RL, 3rd, Harris J, Wakeford C, Rousseau FS. Pharmacokinetic and pharmacodynamic characteristics of emtricitabine support its once daily dosing for the treatment of HIV infection. AIDS Res Hum Retroviruses. AIDS Res Hum Retroviruses. 2004;20:1173–1182. doi: 10.1089/aid.2004.20.1173. [DOI] [PubMed] [Google Scholar]
- 29.Wattanagoon Y, Na Bangchang K, Hoggard PG, Khoo SH, Gibbons SE, Phiboonbhanakit D, Karbwang J, Back DJ. Pharmacokinetics of zidovudine phosphorylation in human immunodeficiency virus-positive thai patients and healthy volunteers. Antimicrob Agents Chemother. 2000;44:1986–1989. doi: 10.1128/aac.44.7.1986-1989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]