Abstract
Vanadium-dependent nitrogenase was previously shown in a small-scale reaction to catalyze reductive catenation of CO to C2H4, C2H6, C3H6 and C3H8. Here, we report the identification of additional hydrocarbon products, α-C4H8, n-C4H10 and CH4, in a scaled-up reaction featuring 20 milligrams of vanadium-iron protein, the catalytic component of vanadium nitrogenase. Additionally, we show that the more common molybdenum-dependent nitrogenase can generate the same hydrocarbons from CO, although CH4 was not detected. The identification of CO as a substrate for both molybdenum- and vanadium-nitrogenases strengthens the hypothesis that CO-reduction is an evolutionary relic of the function of the nitrogenase family; moreover, the comparison between the CO-reducing capacities of the two nitrogenases suggests that the identity of heterometal at the active cofactor site impacts the efficiency and product distribution of this reaction.
Nitrogenase enzymes catalyze the reduction of dinitrogen (N2) to ammonia (NH3), a key step in the global nitrogen cycle (1). The molybdenum (Mo)- and vanadium (V)-dependent nitrogenases are two homologous members of this enzyme family, distinguished primarily by the heterometals (Mo or V) at their respective cofactor centers (FeMoco or FeVco) (2). Both nitrogenases are composed of a specific reductase (nifH- or vnfH-encoded Fe protein) and a catalytic component (nifDK-encoded MoFe protein or vnfDGK-encoded VFe protein). Additionally, both enzymes presumably form a functional complex between the two component proteins during catalysis (3), in which electrons are transferred in an adenosine triphosphate (ATP)-dependent process from the reductase to the cofactor site of the catalytic component, where substrate reduction eventually occurs.
The homology between Mo- and V-nitrogenases renders their substrate profiles highly similar (4); yet, the two nitrogenases react differently with carbon monoxide (CO). In a small-scale reaction (5, 6), V nitrogenase catalyzes reductive catenation of CO to ethylene (C2H4), ethane (C2H6) and propane (C3H8); in contrast, these products have not been detected in the same reaction catalyzed by the wild-type Mo nitrogenase, although a very recent report has indicated that the Valα70-substituted Mo nitrogenase variants can catalyze the formation of low molecular-weight hydrocarbon products from CO (7–9). The ability of V nitrogenase to catalyze hydrocarbon formation from CO is somewhat analogous to the capacities of late transition metal catalysts in the industrial Fischer-Tropsch process (10). However, the enzymatic reaction uses H+—instead of H2—for hydrocarbon formation (11), and it requires the participation of both components of the enzyme, the hydrolysis of ATP, and the supplies of electron sources (7). Thus, V nitrogenase-based hydrocarbon formation likely involves the reductive protonation of CO, a process that parallels the nitrogenase-based NH3 formation pathway, which involves the reductive protonation of N2.
A fourth hydrocarbon product of V nitrogenase catalysis—propylene (C3H6)—can be detected in the standard reaction upon the substitution of H2O by D2O (11). One possible explanation for this effect is a stalling of the reaction by D2O and the consequent accumulation of intermediates that are normally turned over quickly into other products. This observation leads to the speculation that other hydrocarbon products may have remained unidentified due to their minor yields. In this scenario, a sufficiently scaled-up reaction would allow us to identify these products either directly in H2O or following the substitution of H2O by D2O.
Indeed, when we scaled up the reaction by 130-fold, C3H6, along with C2H4, C2H6 and C3H8, was readily detected in the H2O-based reactions using either 12CO (Fig. S1B and C, traces 1) or 13CO (Fig. S1B and C, traces 2) as the substrate (12). Moreover, we identified three additional products in the large-scale, H2O-based assays. Gas chromatography-mass spectroscopy (GC-MS) analyses revealed the identities of these products as methane (CH4), α-butylene (C4H8) and n-butane (C4H10), which displayed m/z ratios of 16.032, 56.064 and 58.080, respectively, in the reaction of 12CO (Fig. S1A and D, traces 1) and the expected mass shifts of +1, +4 and +4, respectively, on substituting 13CO for 12CO (Fig. S1A and D, traces 2). This complete set of products was also detected in the D2O-based reactions with the mass shifts expected for the D/H substitution (Fig. S1A-D, traces 3 and 4).
Unexpectedly, when V nitrogenase was replaced by Mo nitrogenase in the scaled-up reaction, the same two- and three-carbon products were detected in the H2O-based reaction (Fig. S2B and C). Thus, like its V counterpart, Mo nitrogenase used CO and H+ as the carbon and hydrogen sources for hydrocarbon formation. Furthermore, the deuterium effect documented for C3H6 in the small-scale V nitrogenase reaction (11) was duplicated for α-C4H8 and n-C4H10 in the scaled-up Mo nitrogenase reaction. These four-carbon products, which were not detected in the H2O-based reaction (Fig. S2D, traces 1 and 2), could be clearly identified in the D2O-based reaction (Fig. S2D, traces 3 and 4). In contrast, the one-carbon product (CH4) remained unidentified even upon the substitution of H2O by D2O (Fig. S2A, traces 3 and 4), suggesting that CH4 (if any) was formed at an extremely low yield in the Mo nitrogenase-catalyzed reaction (13).
The observation of CO-reduction by Mo nitrogenase comes as a big surprise, as CO has never been shown to be a substrate of Mo nitrogenase; instead, it has long been regarded as a potent inhibitor for the reduction of all known substrates of Mo nitrogenase except H+ (1). The presence of CO suppresses the ability of Mo nitrogenase to turn over substrates other than H+ and, consequently, the electrons which are normally used for the reduction of these substrates are routed toward the concomitant reduction of H+. The fact that CO inhibits the V nitrogenase-catalyzed H+-reduction by ~76% suggests a re-routing of electrons from H+-reduction to CO-reduction, an argument supported by the formation of hydrocarbon products from CO in this reaction (4, 7); in contrast, CO exerts a minimal impact on the H+-reduction by Mo nitrogenase, indicating that an almost untraceable quantity of electrons (14) are diverted from H+-reduction to CO-reduction, thereby limiting the ability of Mo nitrogenase to generate hydrocarbons from CO.
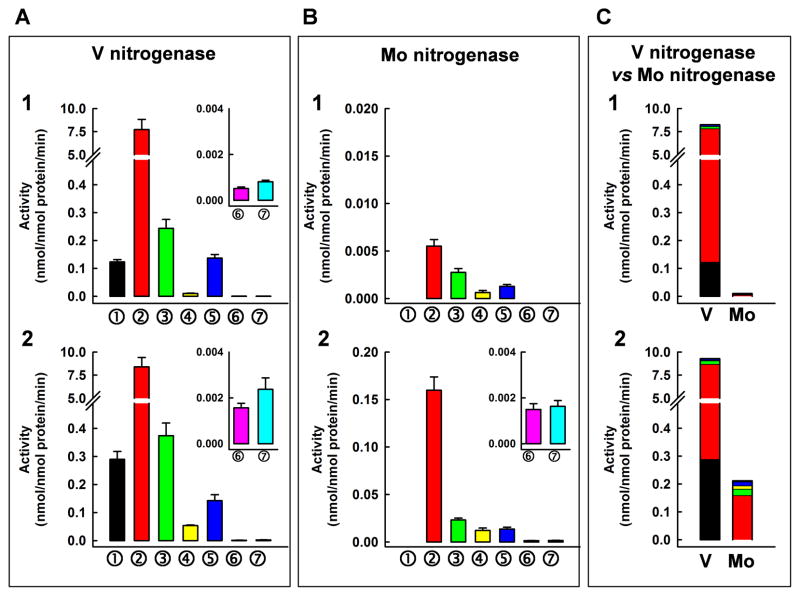
Consistent with this hypothesis, quantitative GC analyses show that Mo nitrogenase (Fig. 1B) is considerably less efficient than its V counterpart (Fig. 1A) in hydrocarbon formation, and the total specific activity of product formation by the former is only ~0.1% and ~2%, respectively, of that by the latter in H2O- and D2O-based reactions (Fig. 1C). The disparate CO-reducing activities of V- and Mo-nitrogenase parallel the differential capacities of synthetic V- and Mo-compounds to reductively couple two CO moieties into functionalized acetylene ligands (15), suggesting that V (a group VB transition metal) is superior to Mo (a group VIB transition metal) in this particular type of reaction. The substitution of H2O by D2O, however, has a much more dramatic effect on the reaction catalyzed by Mo nitrogenase than that catalyzed by its V counterpart, leading to an increase in the specific activity of total product formation by ~21-fold in the case of the former (Fig. 1C, Mo, 2 vs. 1) and by only ~12% in the case of the latter (Fig. 1C, V, 2 vs. 1). In both cases, the increase in hydrocarbon formation is accompanied by a ~30% decrease in D2 evolution, suggesting a possible shift of electrons from D+-reduction toward CO-reduction (16).
Fig. 1.
Specific activities for individual product formation catalyzed by (A) V- and (B) Mo-nitrogenase and (C) comparison of total activities for hydrocarbon formation by the two nitrogenases in the presence of (1) H2O or (2) D2O. Legend: ➀ CH4; ➁ C2H4; ➂ C2H6; ➃ C3H6; ➄ C3H8; ➅ α-C4H8; ➆ n-C4H10. Expanded scales of α-C4H8 and n-C4H10 formation are shown as insets. Specific activities were determined by quantitative GC analyses and calculated based on the formation of products (see Fig. S3) in the first 10 min of reaction (linear range). Data are presented as mean ± SD (N = 5).
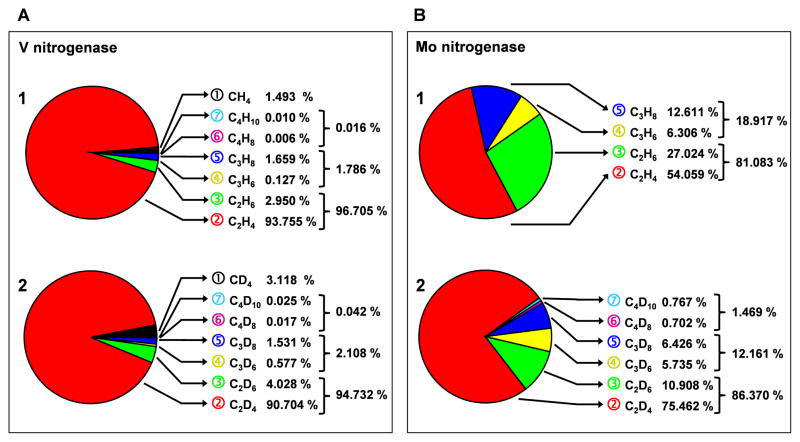
A closer examination of the product distributions of V- and Mo-nitrogenases reveals additional features that set the reactions catalyzed by the two nitrogenases apart (Fig. 2; Table S1). Compared to V nitrogenase (Fig. 2A, 1), Mo nitrogenase (Fig. 2B, 1) displays a much lower C2H4/C2H6 ratio in its product profile; moreover, Mo nitrogenase generates a lower percentage of two-carbon products, and rather seems to shift the H2O-based reaction toward the formation of C3H6 and C3H8. In the presence of D2O, however, Mo nitrogenase shifts the product distribution back toward two-carbon products; additionally, there is an appreciable increase in the C2H4/C2H6 ratio of its product profile (Fig. 2B, 2). Contrary to Mo nitrogenase, V nitrogenase is less affected by the deuterium effect; nevertheless, it shows a small decrease in the C2H4/C2H6 ratio of its product profile and a slight shift toward the formation of three- and four-carbon products—an opposite trend compared to its Mo counterpart—in the D2O-based reaction (Fig. 2A, 2). Strikingly, these opposite changes in D2O render a closer resemblance between the product profiles of V- and Mo-nitrogenases (Fig. 2A vs. B, 2), pointing to possible similarities between the CO-reducing mechanisms of these two nitrogenases.
Fig. 2.
Distributions of hydrocarbons formed by (A) V- and (B) Mo-nitrogenases in the presence of (1) H2O or (2) D2O. The total amounts of hydrocarbons detected in V (A)- and Mo (B)-based reactions were set as 100%, respectively, and the percentages of individual products were determined accordingly for each nitrogenase. The alkene/alkane ratios of V- and Mo-nitrogenases are summarized in Table S1.
One common trait shared by the reactions catalyzed by V- and Mo-nitrogenases is the overwhelmingly predominant formation/release of C2H4 (Fig. 1 and 2). The fact that CH4—the one-carbon product that could result directly from the reductive protonation of CO—is observed in such lower abundance than C2H4 suggests that the formation of a C-C bond between CO derivatives is relatively rapid. The observation that C2H6—the two-carbon product that could originate from the reductive protonation of C2H4—drops sharply in quantity relative to C2H4 indicates that the release of C2H4 is favored over the further reduction of this product. Furthermore, the huge discrepancy between the amounts of two- and three-/four-carbon products points to a potential competition between the formation of two-carbon products (particularly C2H4) and the extension of the carbon chain from the same two-carbon intermediate. The preferred C2H4 formation catalyzed by nitrogenases likely reflects a dominant impact of kinetic factors that guide the overall product distribution, resulting in a drastically decreased yield of hydrocarbon formation beyond two-carbon-length.
A second conserved feature between the reactions catalyzed by the two nitrogenases is the formation of products in the following rank: CH2=CH2≫CH3–CH3>CH3–CH2–CH3>CH2=CH2–CH3 >CH3–CH2–CH2–CH3>CH2=CH2–CH2–CH3 (Fig. 1 and 2). As such, except for the two-carbon products, both nitrogenases display a preference toward the formation of saturated, three- or four-carbon alkanes rather than their corresponding, unsaturated alkenes, suggesting a possible mechanistic switch following the formation of the first C-C bond. One plausible account for the reaction beyond the two-carbon stage involves the formation of alkene prior to a fast protonation step that converts alkene to alkane. Such a hypothesis is supported by two lines of evidence. First, Mo nitrogenase displays a product profile of higher three- and four-carbon alkene/alkane ratios than its V counterpart (Table S1), which could be explained by its limited access to electrons for CO-reduction that slows down the coupled electron/proton addition to the unsaturated C=C bonds of alkenes. Second, both V- and Mo-nitrogenases show increased three- and four-carbon alkene/alkane ratios in the presence of D2O (Table S1), which may reflect a stalling effect of deuterium on the protonation step (16).
The discrepancy between the CO-reducing capacities of Mo- and V-nitrogenases suggests a prevailing impact of heterometal chemistry on the efficiency of the reaction; at the same time, the merging of the product profiles of the two nitrogenases in D2O points to a critical role of coupled electron/proton tunneling in the mechanism of this particular reaction. The discovery that Mo nitrogenase is capable of utilizing CO as a substrate—albeit at a considerably lower rate than its V counterpart—more broadly supports the hypothesis that CO-reduction is an evolutionary relic of the function of this enzyme family. The parallelism between the nitrogenase-catalyzed CO- and N2-reduction—demonstrated further by the ability of V nitrogenase to form CH4, an analogous product of NH3—strengthens the theory that the ancestral nitrogenase may represent an evolutionary link between carbon and nitrogen cycles on earth (7). In a practical vein, the industrial Fischer-Tropsch process uses H2—a costly syngas component—for CO-reduction and shows a tendency toward excessive formation of CH4—a low-value product (17) whereas the nitrogenase-based reaction utilizes H+ for hydrocarbon formation and favors the formation of C-C bond over the cleavage of C-O bond. These features make nitrogenase an attractive template for cost-efficient hydrocarbon formation that is directed toward C-C coupling and carbon chain extension. Borrowing a trick or two from this ancient enzyme family, perhaps an efficient strategy could be developed in the future for controlled fuel production from CO?
Supplementary Material
Acknowledgments
We thank Prof. D. C. Rees and Dr. N. Dalleska of Caltech (Pasadena) for help on the GC-MS analysis. This work was supported by Herman Frasch Foundation grant 617-HF07 (M.W.R.) and National Institutes of Health grant GM 67626 (M.W.R.).
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
References and Notes
- 1.Burgess BK, Lowe DJ. Chem Rev. 1996;96:2983. doi: 10.1021/cr950055x. [DOI] [PubMed] [Google Scholar]
- 2.Hales B. Avd Inorg Biochem. 1990;8:165. [PubMed] [Google Scholar]
- 3.Schindelin H, Kisker C, Schlessman JL, Howard JB, Rees DC. Nature. 1997;387:370. doi: 10.1038/387370a0. [DOI] [PubMed] [Google Scholar]
- 4.Lee CC, Hu Y, Ribbe MW. Proc Natl Acad Sci U S A. 2009;106:9209. doi: 10.1073/pnas.0904408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.A small-scale nitrogenase reaction typically features 0.15 mg VFe or MoFe protein, the catalytic component of V- or Mo-nitrogenase. Such an assay condition was established empirically some 30 years ago (6) and has since been used as the conventional scale of in vitro nitrogenase assays in the field.
- 6.Burgess BK, Jacobs DB, Stiefel EI. Biochim Biophys Acta. 1980;614:196. doi: 10.1016/0005-2744(80)90180-1. [DOI] [PubMed] [Google Scholar]
- 7.Lee CC, Hu Y, Ribbe MW. Science. 2010;329:642. doi: 10.1126/science.1191455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Based on the retention time on a gas chromatograph column, the low molecular-weight hydrocarbon products formed by the Valα70-substituted Mo nitrogenase variants have been assigned as methane, ethylene, ethane, propylene and propane (9).
- 9.Yang ZY, Dean DR, Seefeldt LC. J Biol Chem. 2011;286:19417. doi: 10.1074/jbc.M111.229344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rofer-DePoorter CK. Chem Rev. 1981;81:447. [Google Scholar]
- 11.Lee CC, Hu Y, Ribbe MW. Angew Chem Int Ed Engl. 2011;50:5545. doi: 10.1002/anie.201100869. [DOI] [PubMed] [Google Scholar]
- 12.Materials and methods are detailed in supporting material online.
- 13.Given the detection threshold of CH4 (0.0007 nmol/nmol protein/min) and the activity of V nitrogenase in CH4 production (0.12 nmol/nmol protein/min), the Mo nitrogenase is at least 170-fold less active than its V counterpart in catalyzing the formation of CH4 from CO.
- 14.Only 0.04% of electrons can be traced in the hydrocarbon products formed in the Mo nitrogenase-catalyzed reaction.
- 15.Carnahan EC, Protasiewicz JD, Lippard SJ. Acc Chem Res. 1993;26:90. [Google Scholar]
- 16.The effect of deuterium on nitrogenase-catalyzed CO-reduction is likely multifaceted. Apart from the inverse kinetic isotope effects (i.e., kH/kD < 1) of D2O that favor the formation of deuterated products (18, 19), other solvent effects of D2O, such as those affecting the protein conformation, the interaction between proteins, and the network of hydrogen bonds, have been observed (20–23).
- 17.Khodadadi AA, Hudgins RR, Silveston PL. Can J Chem Engin. 1996;74:695. [Google Scholar]
- 18.Kurtz KA, Fitzpatrick PF. J Am Chem Soc. 1997;119:1155. [Google Scholar]
- 19.Churchill DG, Janak KE, Wittenberg JS, Parkin G. J Am Chem Soc. 2003;125:1403. doi: 10.1021/ja027670k. [DOI] [PubMed] [Google Scholar]
- 20.Karsten WE, Lai CJ, Cook PF. J Am Chem Soc. 1995;117:5914. [Google Scholar]
- 21.Morgan TV, et al. Biochemistry. 1990;29:3077. doi: 10.1021/bi00464a026. [DOI] [PubMed] [Google Scholar]
- 22.Cassman M. Arch Biochem Biophys. 1974;165:60. doi: 10.1016/0003-9861(74)90141-6. [DOI] [PubMed] [Google Scholar]
- 23.Sheu SY, Schlag EW, Selzle HL, Yang DY. J Phys Chem A. 2008;112:797. doi: 10.1021/jp0771668. [DOI] [PubMed] [Google Scholar]
- 24.Glasoe PK, Long FA. J Phys Chem. 1960;64:188. [Google Scholar]
- 25.Gavini N, Burgess BK. J Biol Chem. 1992;267:21179. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.