Background
The SMART study was a large randomized clinical trial that investigated continuous versus interrupted antiretroviral therapy (ART) in both HIV monoinfected and HIV/viral hepatitis coinfected individuals. Overall, participants randomized to the ART interruption strategy had a 2.6 fold increased risk of the combined end-point of opportunistic disease and all-cause mortality [1]. We have recently reported the findings of a sub-study within SMART that showed that hepatitis coinfected participants randomized to the ART interruption arm were at much higher risk of non-AIDS death, but not opportunistic diseases, than the HIV monoinfected participants [2]. Hepatitis coinfected individuals constituted 17% of all participants enrolled in SMART, but almost half of all non-AIDS deaths occurred in this population. However, factors that place the coinfected population at increased risk of non-AIDS death when interrupting ART have not been well defined.
Coinfected persons are at increased risk of hepatic fibrosis progression compared with HCV and HBV monoinfected persons [3,4]. ART has been shown to be beneficial in coinfected persons in reducing the rates of progression of liver disease [5–7]. ART interruption therefore has the potential to destabilize chronic hepatitis both in the short term, through increasing hepatic inflammation, and in the long term by contributing to accelerating fibrosis and clinical outcomes. Consequently, we hypothesized that persons with higher baseline levels of hepatic fibrosis would be at particularly increased risk for development of hepatic and other non-AIDS related outcomes after treatment interruption. Since liver biopsies were not routinely performed in the SMART study, we used plasma hyaluronic acid (HA) as a surrogate marker of liver fibrosis. HA is a glycosaminoglycan, which is produced by different connective tissue cells. In the liver, HA is mostly synthesized by hepatic stellate cells and cleared from the circulation by the hepatic sinusoids. The level of HA has been correlated with the stage of liver fibrosis in both HBV [8,9] and HCV monoinfection [10] as well as in HIV/HBV and HIV/HCV coinfection [11–13]. HA has also been predictive for the development of hepatic complications in patients with HCV-related cirrhosis [14] and HIV/viral hepatitis coinfection [15,16]. While HA has been comparable with other surrogate markers such as APRI in predicting fibrosis level and outcomes, an additional benefit of using HA over other non-invasive surrogate markers, is that it does not include platelet counts, which may be unduly influenced by treatment interruption.
The unique design of the SMART study further enabled us to investigate the influence of treatment interruption, CD4+ count, and HIV-RNA on liver fibrosis progression in both hepatitis coinfected persons and in matched HIV monoinfected controls by measuring changes in HA during follow-up. We hypothesized that fibrosis, as measured by HA level, would increase in interrupting ART as compared with those continuing ART, in hepatitis coinfected, but not HIV monoinfected, individuals.
Methods
Participants and study design
The design and data collection methods of the SMART trial have previously been reported. Briefly, the SMART study was a randomized clinical trial that compared two antiretroviral treatment strategies in a cohort of participants over 13 years of age with confirmed HIV-1 infection and CD4+ counts >350 cells/µl at the time of screening. The participants were randomized to either the drug conservation (DC) strategy, where participants deferred ART until the CD4+ count decreased to less than 250 cells/µL. ART was initiated or resumed until the CD4 cell count reached 350 cells/µL and then suspended again. Cycles of ART were based on those CD4+ counts or the presence of HIV-related symptoms or if the percentage of CD4+ lymphocytes dropped below 15%. The other study group was viral suppression (VS), and stipulated that participants in that group should initiate or continue ART in an uninterrupted manner with the goal of maximal viral suppression. The choice of antiretroviral agents and combinations in both groups was based on clinician/participant preference. The primary endpoint of the SMART study was the composite outcome of development of a new or recurrent opportunistic disease or death from any cause, and the secondary end-point was a composite of major cardiovascular, renal, and hepatic diseases (cirrhosis with or without hepatic decompensation). Because of safety risk in the DC group, all SMART participants randomized to this group (except those who had remained ART naïve) were advised to restart ART in January 2006. All participants were then followed for an additional 18 months [17]. This study includes data through the end of follow-up.
Hepatitis status
Screening for hepatitis B and C in the SMART study has been described [2]. All participants had been investigated for serological evidence of hepatitis B or C infection and participants positive for anti-HCV antibodies had baseline plasma samples analyzed for HCV-RNA in a central laboratory, using a branched DNA assay (VERSANT HCV RNA 3.0) with a lower level of detection of 615 IU/mL. All participants positive at baseline for HCV-RNA (denoted HCV+) and/or HBsAg (denoted HBV+) and with available plasma samples were included in the study.
Controls
A control group of HIV monoinfected participants matched 1:1 on randomization date (+/− 6 months), gender, age (+/− 5 years), treatment group (DC vs. VS), history of alcohol abuse and number of follow-up plasma samples available (controls ≥ cases), was included. We were unable to match on the above criteria for 11 out of the 677 cases. For these, randomization date criteria were removed and age criteria were expanded to +/− 12 years.
Hyaluronic acid
HA was measured in stored plasma samples at baseline and at months 6, 12 (coinfected only) and 24 during follow-up using a commercial enzyme linked binding protein assay (Corgenix, Colorado, USA) with a HA range in a healthy population between 0–75 ng/mL. In statistical analyses 75 ng/mL was chosen a priori as cut-off for baseline HA patient categories. Each HA level was measured in duplicate according to the manufacturers specifications.
Data collection and follow up
Prior to randomization, the following participant information was collected: ART history, nadir CD4+ count and highest viral load, 3 previous laboratory results for CD4+ count and percentage and HIV viral load. Participants were seen at 1 month and every 2 months during Year 1 and every 4 months in Year 2 onward. Alanine aminotransferase and aspartate aminotransferase and platelet levels were not collected routinely in all participants and therefore could not be used for constructing surrogate markers for comparative purposes with HA. Liver biopsies were only performed at the discretion of the treating physician.
Statistical analysis
Baseline characteristics were compared between coinfected and monoinfected controls using Pearson Chi-square test for categorical variables and Wilcoxon rank test for continuous variables. Risk of events at 36 months was estimated using Kaplan-Meier methods. Time to event methods (Kaplan-Meier survival curves, log-rank tests and Cox proportional hazards models) were used to compare event rates between treatment groups and baseline HA categories (> 75 ng/mL vs. ≤ 75 ng/mL). To determine whether the DC versus VS hazard ratios for events varied by baseline HA category, expanded Cox models with interaction terms were considered. Cox models were also carried out to examine predictors for events, including treatment group, baseline HA categories, age, gender, race, prior AIDS, baseline RNA, baseline CD4+, nadir CD4+, baseline ART status, history of alcohol abuse and HBV+ status. Additionally, stepwise Cox models were performed including only treatment group, baseline HA level, age, time-updated CD4+ and time-updated HA level. Changes in HA level were compared between DC and VS groups using Wilcoxon rank test.
Results
Baseline characteristics
Out of 5472 participants enrolled in the SMART study from January 2002 – January 2006, 675 were HBV+ or HCV+ and had specimens available for analysis. 110 (16.3%) were HBV+, 553 (81.9%) were HCV+ and 12 (1.8%) were both HBV+ and HCV+. Table 1 shows the baseline characteristics of the coinfected participants and their matched HIV monoinfected controls according to randomization group. Compared with the controls, the coinfected group was more likely to be of black race (48.4 vs. 34.1%) and less likely to be ART naïve (2.4 vs. 4.3%) and on treatment with ART (79.3 vs. 85.8%) and had longer median time since initiation of ART (7 vs. 6 years). For the coinfected individuals the median (IQR) baseline CD4+ count (cells/µL) was 580 (460 – 736) while the proportion of individuals with suppressed HIV-RNA (≤400 copies/mL) was 64.1%. The corresponding numbers were not significantly different in the HIV monoinfected controls. For the remaining baseline parameters, the two groups were also similar.
Table 1.
Baseline characteristics of included cases and controls according to randomization group
| HBV and/or HCV coinfected |
HIV monoinfected controls |
||||||
|---|---|---|---|---|---|---|---|
| DC (n=347) |
VS (n=328) |
Total (n=675) |
DC (n=347) |
VS (n=327) |
Total (n=674) |
P-value* | |
| HBV infection (%) | 17.6 | 14.9 | 16.3 | 0 | 0 | 0 | NA |
| HCV infection (%) | 81.0 | 82.9 | 81.9 | 0 | 0 | 0 | NA |
| HBV and HCV infection (%) | 1.4 | 2.1 | 1.8 | 0 | 0 | 0 | NA |
| Age, median years (IQR) | 45 (40, 51) | 45 (41, 50) | 45 (40, 50) | 44 (40, 51) | 44 (40, 50) | 44 (40, 51) | NA |
| Female sex (%) | 26.5 | 24.4 | 25.5 | 26.5 | 24.5 | 25.5 | NA |
| Black race (%) | 48.7 | 48.2 | 48.4 | 33.1 | 35.2 | 34.1 | <0.0001 |
| History of alcohol abuse (%) | 25.9 | 25.0 | 25.5 | 25.9 | 24.8 | 25.4 | NA |
| Intravenous drug use as mode of HIV transmission (%) | 49.9 | 52.1 | 51.0 | 2.0 | 3.4 | 2.7 | <0.0001 |
| Baseline CD4+ count, median cells/µL (IQR) | 599 (462, 759) | 566 (459, 702) | 580 (460, 736) | 567 (464, 800) | 599 (476, 823) | 583 (470, 803) | 0.17 |
| Nadir CD4+ count, median cells/µL (IQR) | 232 (131, 343) | 248 (145, 379) | 240 (134, 361) | 248 (131, 375) | 240 (150, 350) | 244 (148, 358) | 0.80 |
| HIV RNA ≤ 400 copies/mL (%) | 67.6 | 60.4 | 64.1 | 65.1 | 69.4 | 67.2 | 0.24 |
| Prior diagnosis of AIDS (%) | 30.0 | 29.0 | 29.5 | 29.1 | 26.6 | 27.9 | 0.52 |
| Antiretroviral therapy naïve (%) | 1.2 | 3.7 | 2.4 | 5.8 | 2.8 | 4.3 | 0.05 |
| On antiretroviral therapy (%) | 81.8 | 76.5 | 79.3 | 84.1 | 87.5 | 85.8 | 0.002 |
| Time since initiation of ART, median years (IQR) | 7 (5, 9) | 6 (4, 9) | 7 (4, 9) | 5 (3, 8) | 7 (4, 9) | 6 (3, 9) | 0.001 |
| Hyaluronic acid level, median ng/mL (IQR) | 29.0 (16.4, 54.1) | 30.9 (18.2, 59.1) | 29.7 (17.3, 55.9) | 18.8 (11.0, 30.2) | 18.9 (11.8, 29.7) | 18.9 (11.5, 30.2) | <0.0001 |
| Hyaluronic acid >75 ng/mL (%) | 17.6 | 18.9 | 18.2 | 3.2 | 1.5 | 2.4 | <0.0001 |
DC: drug conservation, VS: viral suppression, IQR: interquartile range, ART: antiretroviral therapy
p-value compares HCV/HBV coinfected participants to HIV monoinfected participants. (NA: not applicable)
Changes in CD4+ and HIV- RNA levels
The median follow-up was 33 months for the coinfected group and 35 months for the controls. For coinfected participants in the DC group, the median (IQR) CD4+ count had decreased from 599 (462 – 759) at baseline to 442 (329 – 567), 415 (314 – 544) and 418 (314 – 556) cells/µL at month 6, 12 and 24 of follow-up, respectively. In the VS group the corresponding follow-up levels were 595 (446 – 732), 551 (432 – 748) and 576 (434 – 775) cells/µL. By month 6, 25.8% and 66.3% of the coinfected participants had HIV-RNA ≤400 copies/mL in the DC and VS groups, respectively. The CD4+ counts and proportion of individuals with HIV-RNA ≤400 copies/mL during follow-up were similar when comparing the coinfected group with the HIV monoinfected controls (results not shown).
Clinical outcomes
Among coinfected participants 52 (31 in DC, 21 in VS) died from non-AIDS causes. A breakdown of the causes of death according to randomized treatment group and by baseline HA level is shown in Table 2. Of note, a wide range of causes of death were observed even among those with elevated baseline levels of HA, suggesting that excess mortality in this group was not simply a result of hepatic failure alone. Twenty-nine (24 in DC, 5 in VS) developed an opportunistic disease,21 (15 in DC, 6 in VS) developed a major liver event (17 cirrhosis, four liver-related deaths), 7 developed renal events (5 in DC, 2 in VS), 21 developed cardiovascular disease (13 in DC, 8 in VS) and 21 developed non-AIDS cancer (11 in DC, 10 in VS).
Table 2.
Cause of death by baseline hyaluronic acid (HA) level and treatment group in coinfected persons.
| Baseline HA ≤ 75 ng/mL | Baseline HA > 75 ng/mL | |||
|---|---|---|---|---|
| DC (n=286) | VS (n=266) | DC (n=61) | VS (n=62) | |
| Non-AIDS cancer | 0 | 4 | 2 | 0 |
| CVD | 2 | 2 | 1 | 0 |
| Infection | 2 | 2 | 2 | 1 |
| Hepatic | 1 | 1 | 1 | 1 |
| Renal | 0 | 0 | 3 | 1 |
| COPD | 0 | 1 | 0 | 0 |
| CNS disease | 1 | 0 | 0 | 0 |
| Substance abuse | 4 | 3 | 0 | 0 |
| Accident/violent/suicide | 2 | 2 | 0 | 0 |
| Unknown | 4 | 1 | 6 | 2 |
| Total | 16 | 16 | 15 | 5 |
DC: drug conservation, VS: viral suppression, CVD: cardiovascular disease, COPD: chronic obstructive pulmonary disease, CNS: central nervous system
The vast majority of events occurred among HCV coinfected participants. Only 3 non-AIDS deaths (none which were liver related) and 1 liver event occurred among HBV coinfected participants indicating that hepatic flares from interrupting HBV active ART were not contributory to the excess mortality observed.
Only 12 (7 in DC, 5 in VS) HIV monoinfected participants died from non-AIDS causes, while 27 (18 in DC, 9 in VS) developed an opportunistic disease and none developed a major liver event.
HA as a predictor of development of clinical events in coinfected participants
At baseline the median (IQR) HA levels in the DC and VS groups were 29.0 (16.4 – 54.1) ng/mL and 30.9 (18.2 – 59.1) ng/mL; p=0.20, respectively. 17.6% in the DC group and 18.9% in the VS group had a baseline HA level higher than the upper level of normal (>75 ng/mL). The median (IQR) HA levels were lower for the HBV+ than for the HCV+ participants, 18.9 (12.3 – 34.2) ng/mL vs. 32.9 (19.7 – 61.1) ng/mL; p<0.001.
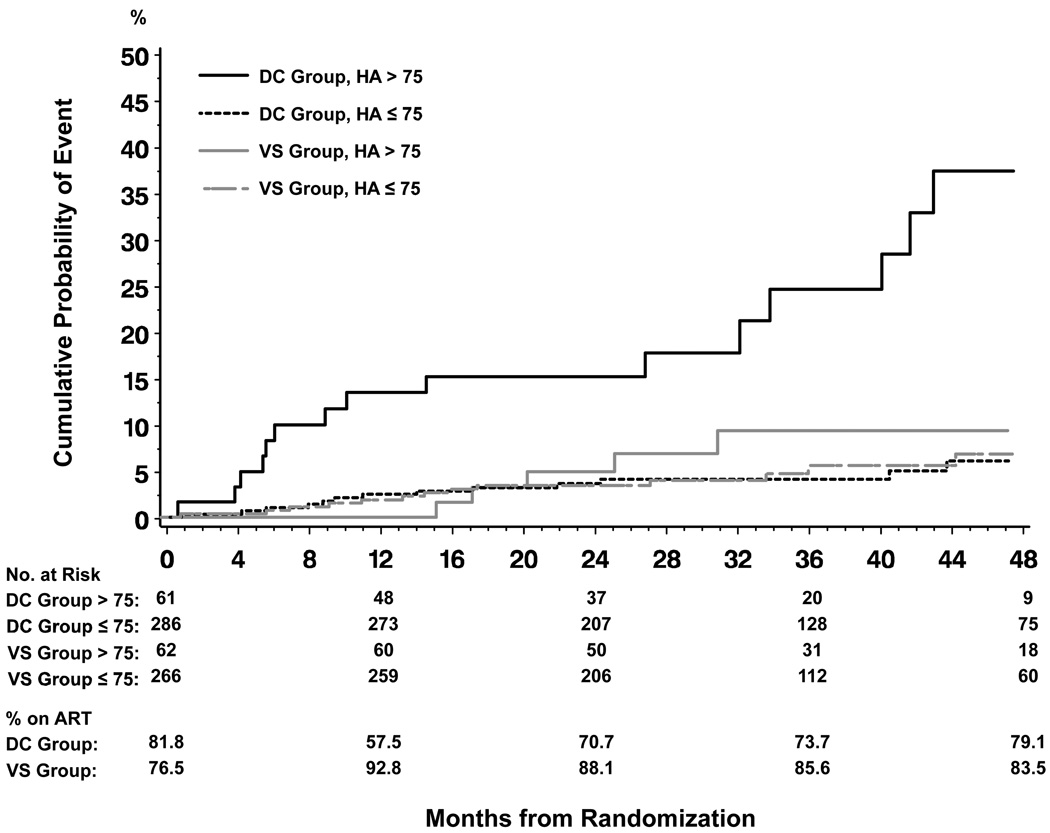
For the 52 coinfected individuals who died from non-AIDS causes the median baseline (IQR) HA level was 54.7 (29.4 – 161.0) ng/mL. The group with baseline HA level >75 ng/mL who were randomized to the DC group had a cumulative risk of non-AIDS death of 24.6% after 36 months of follow-up (95% CI 14.2–40.6) compared with a risk of only 9.3% (95% CI 3.9–21.3) for participants randomized to the VS group with a baseline HA level >75 ng/mL (p=0.005). For participants with a baseline HA level ≤75 ng/mL the cumulative risk of non-AIDS death was far lower at 4.1% (95% CI 2.3–7.3) and 4.7% (95% CI 2.6–8.6) when randomized to the DC and VS group, respectively (p=0.76), Figure 1. The DC/VS hazard ratio (HR) for non-AIDS death in coinfected participants with a baseline HA level >75 ng/mL was (HR 3.8, 95% CI 1.4–10.6, p=0.009), while for those with HA level ≤75 ng/mL it was (HR 0.9, 95% CI 0.4–1.8, p=0.76). P-value for interaction was 0.02.
Figure 1. Cumulative probability of non-AIDS death according to randomization group and baseline hyaluronic acid (HA) (ng/mL) level in coinfected participants.
DC: drug conservation, VS: viral suppression
Log rank test: p<0.0001 (overall), p<0.0001 (DC + HA >75 ng/mL vs. 3 other groups), p=0.85 (3 groups other than DC + HA >75 ng/mL)
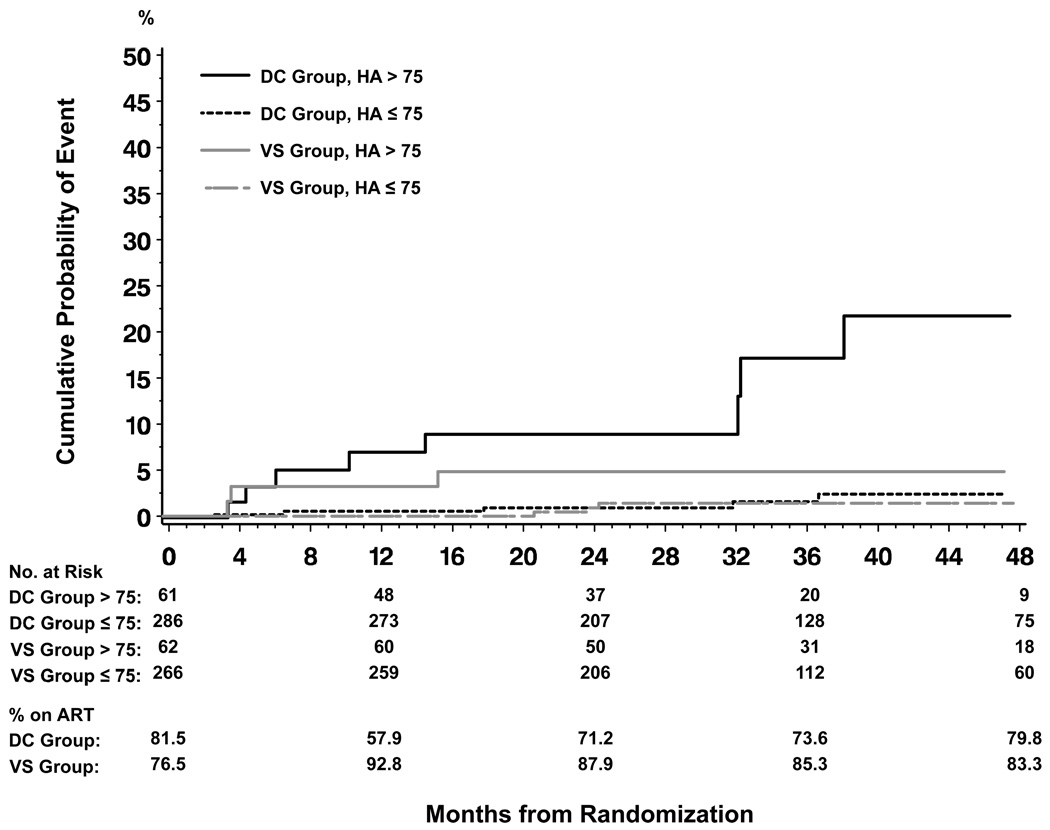
The cumulative risk of experiencing a liver-related event (liver-related death or cirrhosis) when randomized to the DC group with a baseline HA level >75 ng/mL was 17.3% after 36 months of follow-up (95% CI 8.0–35.1), while it was 4.8% (95% CI 1.6–14.3) for individuals in the VS group with baseline HA >75 ng/mL (p=0.06). For individuals with baseline HA ≤75 ng/mL the cumulative risk of a liver-related event were 1.8% (95% CI 0.6–4.8) and 1.4% (95% CI 0.5–4.3) when randomized to the DC and VS group, respectively (p=0.24), Figure 2. For liver events the DC/VS hazard ratio in coinfected participants with a baseline HA level >75 ng/mL was 3.3, 95% CI 0.9–12.6, p=0.08, while for those with HA level ≤75 ng/mL it was 2.2, 95% CI 0.6–8.5, p=0.26, p-value for interaction 0.63.
Figure 2. Cumulative probability of liver-related death or development of cirrhosis according to randomization group and baseline hyaluronic acid (HA) (ng/mL) level in coinfected participants.
DC: drug conservation, VS: viral suppression
Log rank test: p<0.0001 (overall), p<0.0001 (DC + HA >75 ng/mL vs. 3 other groups), p=0.20 (3 groups other than DC + >HA 75 ng/mL)
The cumulative risks of developing either non-AIDS cancer, a major cardiovascular event or opportunistic disease did not differ significantly when stratified according to baseline HA level and randomization group (results not shown).
Predictors of time to clinical events in coinfected participants
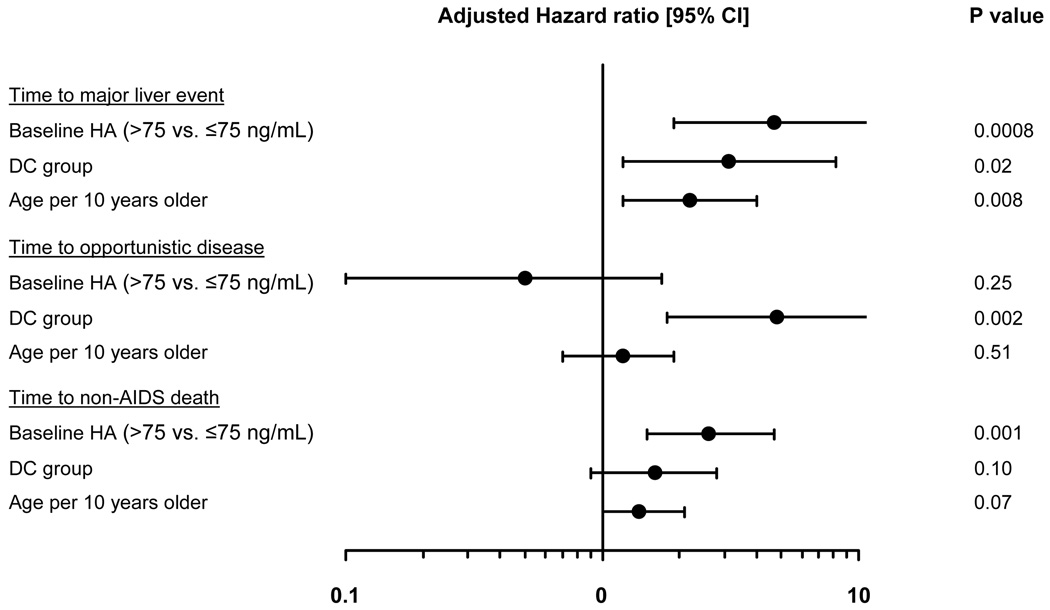
In an analysis where baseline HA was modelled as a dichotomous variable, HA (>75 vs. ≤75 ng/ml) was the only significant predictor of “time to non-AIDS death” (hazard ratio [HR] 2.6; 95% CI 1.5–4.7; p=0.001), Figure 3. HA (together with treatment group and age per 10 years older) was also a significant predictor of time to a major liver-related event (HR 4.7, 95% CI 1.9 – 11.5; p=0.0008), but not opportunistic disease.
Figure 3. Predictors of development of clinical events in coinfected participants.
HA: hyaluronic acid, DC: drug conservation, VS: viral suppression
Predictors included in the models are treatment group, baseline HA categories, age gender, race, prior AIDS, baseline HIV-RNA, baseline CD4+, nadir CD4+, baseline ART status, history of alcohol abuse and HBV+ status.
In a stepwise multivariate regression model using stepping criterion of 0.05, time-updated HA remained in the model for both "time to non-AIDS death" and “time to a major liver-related event”. Hazard ratios for latest HA (>75 vs. ≤75 ng/ml) were 2.9 (95% CI 1.7–5.1; p=0.0002) and 7.5 (95% CI 2.9–19.5; p<0.0001), respectively.
Changes in hyaluronic acid levels during follow-up
By month 6 the DC group had a significant increase in median (IQR) HA level from baseline compared to the VS group 10.1 (−5.9 – 29.1) ng/mL versus 5.0 (−10.2 – 22.5) ng/mL; p=0.04. However, this difference in HA levels between DC and VS decreased subsequently and was not statistically significant during the remaining follow-up. Limiting the analysis to participants on ART at baseline (175 DC and 147 VS), the median (IQR) change at 6 months was similar 10.3 (−5.2 – 26.7) for DC versus 5.5 (−10.2 – 26.9) ng/mL for VS; p=0.14. The difference at month 6 remained significant when censoring participants in the DC group after the first ART re-initiation (p=0.01) and remained borderline significant (p=0.057) when excluding HBV+. In the HBV+ group alone there was no differences in change in HA levels between DC and VS during follow-up (data not shown).
Compared with the coinfected participants the median HA levels were considerably lower in HIV monoinfected controls at all time-points, with no significant differences between the DC and VS groups. The median (IQR) HA baseline levels were 18.8 (11.0 – 30.2) ng/mL and 18.9 (11.8 – 29.7) ng/mL; p=0.88 for the DC and VS groups respectively. Only 3.2% in DC and 1.5% in VS had a baseline HA level higher than 75 ng/mL. The increase from baseline to month 24 in median (IQR) HA level was only 3.4 (−5.6 – 12.9) ng/mL in the DC group and 3.1 (−3.8 – 14.2) ng/mL in the VS group; (p=0.26).
Discussion
In the SMART study, we have previously shown that, compared with HIV monoinfected participants, viral hepatitis coinfected participants were at much higher risk of non-AIDS death, but not opportunistic disease, when randomized to the ART interruption arm. However, we could not identify factors that placed the coinfected population at increased risk of non-AIDS death when interrupting ART [2].
In clinical practice, as well as in clinical trials, except in cases of established cirrhosis, the degree of hepatic impairment present in coinfected persons is seldom known as liver biopsies and other non-invasive evaluations such as FibroScan are not routinely performed in most centers. As this was also the case in the SMART study, we instead investigated liver impairment as a risk factor for clinical events in patients interrupting ART by using plasma hyaluronic acid as a surrogate marker of liver fibrosis.
Having a baseline HA above the upper level of normal (>75 ng/mL) was a surprisingly strong risk factor for development of non-AIDS death for coinfected participants in the DC group. Those randomized to the DC group with an elevated baseline HA level had a 24.6% risk of non-AIDS death after 36 months of follow-up, whereas the risk of non-AIDS death in the DC group with baseline HA <75 ng/mL was only 4.1%. In the VS group the risk was similarly low and independent of baseline HA level. Similar trends, though less striking, were seen for the liver-related outcomes (cirrhosis and liver-related death) although the numbers of these events were smaller. Since the majority of non-AIDS deaths were believed not to be liver-related, this strong association is particularly intriguing.
Although patients are no longer likely to be counselled to interrupt ART, in clinical practice, they will continue to do so for a variety of reasons. The strong association observed between the degree of liver fibrosis and risk for clinical events suggests that an evaluation of fibrosis stage is warranted in coinfected persons so to be able to counsel these patients about their risk of interrupting treatment and to select those for closer monitoring when interruptions occur. Additionally, our results suggest that continuous ART among coinfected persons with advanced fibrosis is an important strategy for preventing death from a variety of non-AIDS causes.
The pathogenic mechanisms are, however, not clear. Advanced liver fibrosis has been shown to be associated with endotoxemia and both higher plasma levels of pro-inflammatory cytokines (such as TNF-α and interleukin-6) and low-grade disseminated intravascular coagulation with increased levels of D-dimer and other markers of coagulation and fibrinolysis [18,19]. In another sub-study of SMART, elevated baseline levels of both interleukin-6 and D-dimer were found to be strongly related to all-cause mortality. Levels of both markers also increased significantly from study entry to month 1 in a random sample of DC group participants compared to the VS group [20]. It is therefore plausible that ART interruptions could exacerbate the underlying pro-inflammatory and hypercoagulable state associated with advanced liver disease and initiate or accelerate multiple pathogenic processes and contribute to the increased risk of non-AIDS death seen in the DC group of the SMART study. In future studies we plan to further explore these potential associations.
Since the excess mortality seen in the coinfected population randomized to the ART interruption strategy was not entirely due to liver disease, it is uncertain whether episodic ART, compared with continuous ART in the SMART study, increased the progression of liver fibrosis in hepatitis coinfected individuals. Observational studies have shown that the rate of liver fibrosis progression is accelerated in persons coinfected with HIV and HBV or HCV, compared to HIV monoinfected persons, and the rate of progression has been correlated with lower CD4+ cell counts suggesting that impaired immunity related to HIV may be contributory [7]. In order to indirectly investigate this relationship we measured changes in HA during follow-up and used a matched HIV monoinfected control group. Coinfected participants had higher levels of HA at baseline and during follow-up than the HIV monoinfected control group. For the coinfected the change in median HA from baseline to month 24 was only 8.3 ng/mL and 4.7 ng/mL in the DC and VS group, respectively. The small statistical significant difference in HA level between DC and VS groups of coinfected observed only at month 6 is unlikely to reflect any difference in fibrosis development, but could reflect an acute inflammatory reaction after interrupting ART or could have been a chance finding. It is also possible that any changes in HA over time were mitigated by cycles of re-initiation of ART in participants randomized to the DC arm. Overall our findings suggest that progression of liver fibrosis in most coinfected individuals is fairly slow and not influenced by the differences in ART exposure and CD4+ cell count and HIV viral load in the two randomization groups over the relatively short period of observation.
This study has some limitations. The difference between the DC and VS in absolute number of non-AIDS deaths, although statistical significant, was only ten. Although all end-points have been rigorously ascertained by an independent end-point review committee, the cause of death could not be determined in ten and three cases in the DC and VS groups, respectively, which could have led us to underestimate the number of liver-related deaths.
Furthermore, plasma HA has been shown to increase after food intake with concentrations peaking 75–90 minutes post-prandially [21,22]. As participants in the SMART study were not required to be fasting before blood was drawn, this effect might to some extent explain the fluctuations in HA over time we saw in some individuals, but due to the randomized design of the SMART study this and other confounders should not affect the overall conclusions. Unfortunately we were not able to compare HA with other simple indices of fibrosis like APRI and FIB-4 since liver-enzymes were not routine collected from all participants. Lastly, we were not able to assess the cumulative risk of non-AIDS death in the control group since only 16 HIV monoinfected participants had a baseline HA >75 ng/mL.
In conclusion, whereas the SMART study showed that ART interruptions in general are harmful due to increased risk of opportunistic disease and all-cause death, we have now shown that treatment interruption carries a very serious risk of non-AIDS death for HIV/hepatitis coinfected individuals with advanced fibrosis (as measured by an elevated HA). However, in most coinfected individuals progression of liver disease seems to be slow and not influenced by differences in ART exposure or CD4+ cell counts and HIV viral loads observed over the relatively short period of follow-up in the SMART study.
Acknowledgements
We would like to acknowledge the SMART participants, the SMART study team (see N Engl J Med, 2006:355:2294–2295 for list of investigators), and the INSIGHT Executive Committee.
Financial support:
Supported by grants from the NIAID (U01AI042170 and U01AI46362).
Footnotes
Conflict of interest statement:
No member of the writing group for this report has any financial or personal relations with people or organizations that could inappropriately affect this work, although most members of the group have, at some stage in the past, received funding from a variety of pharmaceutical companies for research, travel grants, speaking engagements, or consultancy fees
Reference List
- 1.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 2.Tedaldi E, Peters L, Neuhaus J, et al. Opportunistic disease and mortality in patients coinfected with hepatitis B or C virus in the strategic management of antiretroviral therapy (SMART) study. Clin Infect Dis. 2008;47:1468–1475. doi: 10.1086/593102. [DOI] [PubMed] [Google Scholar]
- 3.Benhamou Y, Bochet M, Di MV, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 4.Thio CL, Seaberg EC, Skolasky R, Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–1926. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 5.Mocroft A, Soriano V, Rockstroh J, et al. Is there evidence for an increase in the death rate from liver-related disease in patients with HIV? AIDS. 2005;19:2117–2125. doi: 10.1097/01.aids.0000194799.43799.ea. [DOI] [PubMed] [Google Scholar]
- 6.Qurishi N, Kreuzberg C, Luchters G, et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet. 2003;362:1708–1713. doi: 10.1016/S0140-6736(03)14844-1. [DOI] [PubMed] [Google Scholar]
- 7.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 8.Montazeri G, Estakhri A, Mohamadnejad M, et al. Serum hyaluronate as a non-invasive marker of hepatic fibrosis and inflammation in HBeAg-negative chronic hepatitis B. BMC Gastroenterol. 2005;5:32. doi: 10.1186/1471-230X-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng MD, Lu LG, Mao YM, et al. Prediction of significant fibrosis in HBeAg-positive patients with chronic hepatitis B by a noninvasive model. Hepatology. 2005;42:1437–1445. doi: 10.1002/hep.20960. [DOI] [PubMed] [Google Scholar]
- 10.Halfon P, Bourliere M, Penaranda G, et al. Accuracy of hyaluronic acid level for predicting liver fibrosis stages in patients with hepatitis C virus. Comp Hepatol. 2005;4:6. doi: 10.1186/1476-5926-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottero J, Lacombe K, Guechot J, et al. Performance of 11 biomarkers for liver fibrosis assessment in HIV/HBV co-infected patients. J Hepatol. 2009;50:1074–1083. doi: 10.1016/j.jhep.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Larrousse M, Laguno M, Segarra M, et al. Noninvasive diagnosis of hepatic fibrosis in HIV/HCV-coinfected patients. J Acquir Immune Defic Syndr. 2007;46:304–311. doi: 10.1097/qai.0b013e3181520502. [DOI] [PubMed] [Google Scholar]
- 13.Sanvisens A, Serra I, Tural C, et al. Hyaluronic acid, transforming growth factor-beta1 and hepatic fibrosis in patients with chronic hepatitis C virus and human immunodeficiency virus co-infection. J Viral Hepat. 2009 Jul;16(7):513–518. doi: 10.1111/j.1365-2893.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- 14.Guechot J, Serfaty L, Bonnand AM, Chazouilleres O, Poupon RE, Poupon R. Prognostic value of serum hyaluronan in patients with compensated HCV cirrhosis. J Hepatol. 2000;32:447–452. doi: 10.1016/s0168-8278(00)80396-7. [DOI] [PubMed] [Google Scholar]
- 15.Nunes D, Fleming C, Offner G, et al. Noninvasive Markers of Liver Fibrosis Are Highly Predictive of Liver-Related Death in a Cohort of HCV-Infected Individuals With and Without HIV Infection. Am J Gastroenterol. 2010 Jun;105(6):1346–1353. doi: 10.1038/ajg.2009.746. [DOI] [PubMed] [Google Scholar]
- 16.Peters L, Mocroft A, Soriano V, et al. Abstract 821 at the 16th Conference on Retroviruses and Opportunistic Infections; February 8–11 2009; Montreal, Canada. [Google Scholar]
- 17.El Sadr WM, Grund B, Neuhaus J, et al. Risk for opportunistic disease and death after reinitiating continuous antiretroviral therapy in patients with HIV previously receiving episodic therapy: a randomized trial. Ann Intern Med. 2008;149:289–299. doi: 10.7326/0003-4819-149-5-200809020-00003. [DOI] [PubMed] [Google Scholar]
- 18.Lee FY, Lu RH, Tsai YT, et al. Plasma interleukin-6 levels in patients with cirrhosis. Relationship to endotoxemia, tumor necrosis factor-alpha, and hyperdynamic circulation. Scand J Gastroenterol. 1996;31:500–505. doi: 10.3109/00365529609006772. [DOI] [PubMed] [Google Scholar]
- 19.Violi F, Ferro D, Basili S, et al. Association between low-grade disseminated intravascular coagulation and endotoxemia in patients with liver cirrhosis. Gastroenterology. 1995;109:531–539. doi: 10.1016/0016-5085(95)90342-9. [DOI] [PubMed] [Google Scholar]
- 20.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong CS, Gibson PR. Effects of eating on plasma hyaluronan in patients with cirrhosis: its mechanism and influence on clinical interpretation. J Gastroenterol Hepatol. 1998;13:1218–1224. [PubMed] [Google Scholar]
- 22.Idobe Y, Murawaki Y, Ikuta Y, Koda M, Kawasaki H. Post-prandial serum hyaluronan concentration in patients with chronic liver disease. Intern Med. 1998;37:568–575. doi: 10.2169/internalmedicine.37.568. [DOI] [PubMed] [Google Scholar]