Abstract
Object
Quality improvement techniques are being implemented in many areas of medicine. In an effort to reduce the ventriculoperitoneal shunt infection rate, a standardized protocol was developed and implemented at 4 centers of the Hydrocephalus Clinical Research Network (HCRN).
Methods
The protocol was developed sequentially by HCRN members using the current literature and prior institutional experience until consensus was obtained. The protocol was prospectively applied at each HCRN center to all children undergoing a shunt insertion or revision procedure. Infections were defined on the basis of CSF, wound, or pseudocyst cultures; wound breakdown; abdominal pseudocyst; or positive blood cultures in the presence of a ventriculoatrial shunt. Procedures and infections were measured before and after protocol implementation.
Results
Twenty-one surgeons at 4 centers performed 1571 procedures between June 1, 2007, and February 28, 2009. The minimum follow-up was 6 months. The Network infection rate decreased from 8.8% prior to the protocol to 5.7% while using the protocol (p = 0.0028, absolute risk reduction 3.15%, relative risk reduction 36%). Three of 4 centers lowered their infection rate. Shunt surgery after external ventricular drainage (with or without prior infection) had the highest infection rate. Overall protocol compliance was 74.5% and improved over the course of the observation period. Based on logistic regression analysis, the use of BioGlide catheters (odds ratio [OR] 1.91, 95% CI 1.19–3.05; p = 0.007) and the use of antiseptic cream by any members of the surgical team (instead of a formal surgical scrub by all members of the surgical team; OR 4.53, 95% CI 1.43–14.41; p = 0.01) were associated with an increased risk of infection.
Conclusions
The standardized protocol for shunt surgery significantly reduced shunt infection across the HCRN. Overall protocol compliance was good. The protocol has established a common baseline within the Network, which will facilitate assessment of new treatments. Identification of factors associated with infection will allow further protocol refinement in the future.
Keywords: hydrocephalus, quality improvement, shunt, infection, standardized protocol
Infection of ventriculoperitoneal shunts continues to be a source of morbidity for children with hydrocephalus and a frustrating problem for clinicians. In large databases the procedural infection rate has been reported as approximately 8%–10%.7,8,14 Shunt infection is an important contributor to the cost of care in pediatric hydrocephalus.2 Treatment of infection requires hospital admission, surgical removal of the device, inpatient intravenous antibiotic therapy for a variable period of time,1,16 and implantation of a new shunt system. Typical hospitalizations last 7–21 days.9 Despite these measures, recurrent infection is common.9,10 Clearly, prevention of infection is essential, and many techniques have been promoted to minimize the risk.
Recent work in other areas of medicine and surgery has demonstrated beneficial results using quality improvement methodology. This methodology involves the development and application of standardized, stepwise protocols for common health care processes, measurement of compliance, and observation of the effect on outcome. Single-center studies using these methods have suggested benefits in a number of areas. Less commonly, investigators have applied standardized protocols across multiple centers. The National Association of Children’s Hospitals and Related Institutions (NACHRI) demonstrated a 43% reduction in central line infections using two “practice bundles”—one for insertion and another for maintenance.11 The WHO-sponsored study on surgical errors found that application of a standardized preoperative checklist effectively reduced mortality rates in a broad range of surgical procedures across multiple international centers.6
To our knowledge, the application of standardized preoperative protocols across multiple centers has not been conducted in neurosurgery. Our goal was to apply these quality improvement methodologies to CSF shunt surgery in an effort to reduce the 6-month infection rate.
Methods
Study Setting
The HCRN is a collaboration of pediatric neurosurgical centers conducting systematic investigations in the management of pediatric hydrocephalus. In an effort to reduce the risk of shunt infection, a quality improvement methodology was adopted by HCRN centers. Prior to implementation of the standardized protocol, the centers and surgeons had used a variety of perioperative techniques to try to prevent infection. There was substantial variation from one center to another and from one surgeon to another. The HCRN centers agreed to develop a standardized multistep protocol to try to reduce the infection rate across the Network.
Quality Improvement Protocol Development and Implementation
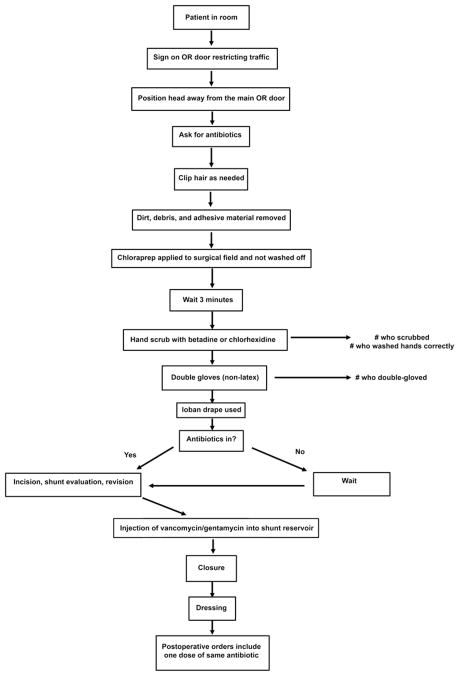
The protocol was developed by HCRN members using the current literature3,4,12 and prior institutional experience (Fig. 1). The protocol steps were discussed during biweekly Network conference calls and at biannual Network meetings. The details were sequentially revised until consensus was obtained. The protocol was reviewed by representatives from the infectious diseases and pharmacy departments and the neurosurgical operating room at each center. The 11-step protocol was instituted at each center with the approval of the local quality assurance committee, and the data were collected locally. Institutional review board approval was obtained to pool the data for the purposes of this analysis.
Fig. 1.
Diagram showing the HCRN shunt surgery protocol. In the step requiring the surgeon to ask for antibiotics, the surgeon requests that intravenous cefazolin (30 mg/kg) be given before making the first incision. In patients allergic to cephalosporins, vancomycin (15 mg/kg) is used. When the patient’s hair is clipped, the hair is removed using clippers in the region of the incision as per the surgeon’s usual practice. The shunt equipment selected for shunt insertion or revision is selected by the surgeon, except that antibiotic-impregnated shunts were not allowed. After the shunt procedure and prior to closure, 1 ml (10 mg/ml) of vancomycin mixed with 2 ml (2 mg/ml) of gentamicin is injected into the shunt reservoir with a 25-gauge needle (or smaller). In patients with a prior adverse reaction to vancomycin, the gentamicin is given alone. For this study, procedures in which vancomycin was not used were counted as noncompliant.
Entry Criteria
All children at each HCRN center were entered into the study when they underwent a shunt insertion or shunt revision operation, including ventriculoperitoneal, ventriculoatrial, ventriculopleural, arachnoid cyst, subdural, and lumboperitoneal shunts and shunts replaced after treatment of infection. Patients whose first presentation was with a shunt infection were entered into the study after their infections were treated, at the time their shunts were being replaced. Children receiving EVDs, Ommaya reservoirs, ventricular access devices, and subgaleal shunts were not included at the time of those procedures but were eligible if they later underwent a procedure listed above.
Procedures were classified as shunt insertion (insertion of a shunt in a child who had not had one previously), shunt revision (surgery in which a child entered the operating room with all shunt equipment previously implanted and left the operating room with all shunt equipment implanted), shunt insertion after external ventricular drainage (not infected), and shunt insertion after treatment of infection. All repeated procedures of any type were recorded.
Outcome Variable
Patients were evaluated for infection at the time of routine clinical follow-up, emergency room visits, or hospital admissions. Evaluation followed the surgeons’ usual clinical practice. The primary end point for the study was shunt infection, defined as: 1) identification of organisms on culture or Gram stain from CSF, wound swab, or pseudocyst fluid; 2) shunt erosion (defined as wound breakdown with visible shunt hardware); 3) abdominal pseudocyst (even in the absence of positive cultures); or 4) positive blood cultures in a child with a ventriculoatrial shunt.
Data Collection
For protocol compliance, a flow sheet outlining the steps (Fig. 1) was used for data collection at each center. As a member of the HCRN, each center has a full-time research coordinator whose responsibility it is to collect the data prospectively. In many of the cases, the coordinator also observed the shunt operations and reminded surgeons to comply with the protocol. Compliance with each step was recorded. The hand-washing technique was recorded as follows: if all personnel participating in the operation performed a formal scrub, then the hand-washing technique was compliant for that operation. If any individual participating in the operation used antiseptic cream (instead of doing a formal scrub), then the hand-washing technique was recorded as noncompliant for that procedure.
In addition, nonprotocol factors that could potentially affect the infection risk were also identified and documented. These included the use of antibiotic-impregnated sutures for wound closure,13 use of BioGlide catheters (Medtronic, Inc.) in the shunt system, preoperative chlorhexidine hair washing, and no-touch surgical technique. Surgery using a no-touch technique meant that the surgical team manipulated the shunt equipment with sterile instruments as much as possible rather than with their gloved hands.
Data Analysis
Data were stored locally and then aggregated for this analysis. Data on shunt infection rates for the 12 months preceding institution of the protocol were obtained from each center. These data were obtained from prospective departmental databases, hospital infection control records, and retrospective review of medical records. At all centers the preprotocol infection data were based on the results of CSF cultures, with positive cultures leading to a diagnosis of infection.
Categorical outcomes were compared with a Pearson chi-square test or Fisher exact test of proportions. Network-wide and center-specific monthly infection rates were plotted on control charts. Logistic regression was performed to assess for associations between protocol and nonprotocol factors and infection within 6 months of surgery. Specifically, factors showing at least a weak association with infection (p < 0.2) in univariate analysis were considered for entry into regression models that adjusted for within-patient correlation (as often the same patient was seen multiple times during the period of this study) using generalized estimating equations. One model included only components of the shunt protocol, whereas a second model tested the association of the protocol as well as additional nonprotocol factors with infection. Analysis was performed using commercially available statistical software (SPSS version 17.0, SAS version 9.2).
Results
Twenty-one surgeons at 4 centers performed 1571 shunt procedures using the protocol from June 1, 2007, to February 28, 2009. The 4 participating centers were Primary Children’s Medical Center, University of Utah; Children’s Hospital of Alabama, University of Alabama at Birmingham; Hospital for Sick Children, University of Toronto; and Texas Children’s Hospital, Baylor College of Medicine.
For this analysis, follow-up monitoring closed on August 31, 2009. The 1571 procedures were performed in 1004 children. The median patient age at surgery was 5.2 years overall, 20.5 weeks for shunt insertion, and 8.9 years for shunt revision. The most common procedure was shunt revision (61%), followed by shunt insertion (26%), insertion after external ventricular drainage (6%), and insertion after infection (6%; Table 1). Infection occurred after 89 (5.7%) of the 1571 procedures. Procedure specific infection rates were significantly different (p = 0.009; Table 1), with a high value of 10.8% for shunt insertion after treatment of an infection and a low value of 4.3% after a shunt revision.
TABLE 1.
Type of shunt procedure*
| Variable | Insertion | Revision | Insertion After EVD | Insertion After Infection | Total |
|---|---|---|---|---|---|
| Center A | 152 (28) | 331 (62) | 28 (5) | 24 (4) | 535 |
| Center B | 144 (27) | 305 (57) | 45 (8) | 37 (7) | 531 |
| Center C | 76 (26) | 192 (66) | 9 (3) | 12 (4) | 289 |
| Center D | 43 (20) | 135 (63) | 18 (8) | 20 (9) | 216 |
| total procedures | 415 (26) | 963 (61) | 100 (6) | 93 (6) | 1571 |
| procedure-specific infections | 29 (7.0) | 41 (4.3) | 9 (9.0) | 10 (10.8) |
Data represent numbers of patients (%).
Protocol procedures were performed by 21 surgeons, each of whom completed between 1 and 161 procedures. There were 15 surgeons who performed 40 or more procedures. These 15 surgeons accounted for 1451 (92.4%) of the procedures (Table 2). Surgeon-specific infection rates varied (0%–12.9%) and did not appear to correlate with surgeon procedure volume (Table 2).
TABLE 2.
Infection rates according to surgeon procedure volume
| Procedures Per Surgeon | No. of Surgeons | Total Procedures | Infection Percentage |
|---|---|---|---|
| >120 | 5 | 728 | 5.4 |
| 80–120 | 3 | 306 | 6.9 |
| 40–79 | 7 | 417 | 5.3 |
| <40 | 6 | 120 | 5.8 |
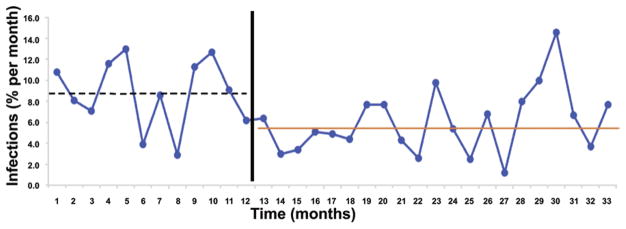
The Network-wide infection rate while the protocol was in place (5.7%) was significantly lower (p = 0.0028) than the preprotocol infection rate of 8.8% (79 infections/896 procedures) and represents an absolute risk reduction of 3.2% and a relative risk reduction of 36%. Center-specific infection rates using the protocol were 4.3%, 4.8%, 5.1%, and 7.7% (p = 0.09). Center-specific infection rates were lower when using the protocol, versus preprotocol, at 3 of the 4 study centers (Table 3, Fig. 2).
TABLE 3.
Center-specific infection rates
| Center | Preprotocol Infections (%) | Infections Using Protocol (%) | Absolute Shunt Infection Risk Reduction (%) ± 95% CI | Relative Shunt Infection Risk Reduction (%) |
|---|---|---|---|---|
| A | 40/316 (12.7) | 23/535 (4.3) | 8.4 ± 2.1 | 66 |
| B | 14/197 (7.1) | 41/531 (7.7) | −0.6 ± 2.2 | −9 |
| C | 14/221 (6.3) | 14/289 (4.8) | 1.5 ± 2.1 | 24 |
| D | 11/162 (6.8) | 11/216 (5.1) | 1.7 ± 2.5 | 25 |
| entire network* | 79/896 (8.8) | 89/1571 (5.7) | 3.2 ± 1.1 | 36 |
Significant reduction in Network-wide infection rate after introduction of the protocol (p = 0.0028).
Fig. 2.
Graph comparing mean monthly shunt infection rate before and after protocol implementation. The infection rate before implementation of the protocol (first 12 months) was 8.8% (dashed line); after implementation, it was 5.7% (solid line; chi-square = 8.93, p = 0.0028).
Surgeon compliance with the protocol was recorded. There were 59 procedures for which compliance could not be accurately determined. Among the 1512 procedures with accurate compliance data, there were 1124 (74.5%) in which all 11 steps of the protocol were completed (perfect compliance). In another 306 procedures (20.2%), 10 of the 11 steps were completed. Among the 1430 procedures with at least 10 of the 11 steps followed, there were 80 infections (5.6%). Among the 82 patients in whom 2 or more protocol steps were missed, there were 7 infections (8.5%, p = 0.32).
Among the 306 patients in whom a single component of the protocol was missed, the most commonly missed items were injection of vancomycin and gentamicin or positioning the patient with the operative site away from the main door of the operating room. Procedures in which the patient was not given vancomycin because of a reported allergy were counted as a protocol violation. The proportion of cases with perfect protocol compliance improved over the course of the study and did not vary greatly between centers (overall compliance rates = 79%, 75%, 69%, and 64%). Among the 15 surgeons who performed 40 or more procedures, 10 surgeons had a lower infection rate when they performed perfectly compliant procedures.
The infection rates associated with the various protocol and nonprotocol factors are outlined in Tables 4 and 5. Preoperative patient hair washing with chlorhexidine shampoo, double gloving by all members of the surgical team, and the use of antibiotic-impregnated sutures were associated with a significantly lower risk of infection. The use of BioGlide catheters and the use of antiseptic cream (instead of a formal scrub by all team members) were associated with a significantly higher risk of infection.
TABLE 4.
Individual components of the protocol*
| Variable | Factor Present
|
Factor Absent
|
p Value | ||
|---|---|---|---|---|---|
| No. of Procedures | Infection % | No. of Procedures | Infection % | ||
| sign on OR door to minimize traffic | 1503 | 5.66 | 41 | 9.76 | 0.29 |
| patient position of op site away from door | 1441 | 5.97 | 106 | 2.83 | 0.18 |
| antibiotics received before incision | 1499 | 5.74 | 43 | 6.98 | 0.73 |
| hair clipped, not shaved | 1533 | 5.81 | 10 | 0 | 1.00 |
| ChloraPrep applied by or approved by attending physician | 1497 | 5.74 | 48 | 6.25 | 0.75 |
| wait 3 min to allow ChloraPrep to dry | 1517 | 5.60 | 22 | 4.55 | 1.00 |
| proper hand-washing technique by all team members | 1515 | 5.61 | 20 | 20.0 | 0.025 |
| double gloving by all team members | 1499 | 5.60 | 34 | 14.71 | 0.043 |
| Ioban use | 1528 | 5.76 | 17 | 5.88 | 1.00 |
| vancomycin/gentamicin injection into shunt reservoir | 1414 | 5.80 | 131 | 5.34 | 0.83 |
| dressing | 1511 | 5.82 | 28 | 3.57 | 1.00 |
OR = operating room.
TABLE 5.
Additional nonprotocol factors
| Variable | Factor Present
|
Factor Absent
|
p Value | ||
|---|---|---|---|---|---|
| No. of Procedures | Infections % | No. of Procedure | Infections % | ||
| BioGlide catheters | 494 | 8.3 | 1077 | 4.5 | 0.002 |
| antibiotic-impregnated sutures | 504 | 3.8 | 1067 | 6.6 | 0.026 |
| antibiotic-impregnated shunts | 119 | 4.2 | 1451 | 5.8 | 0.47 |
| no-touch surgical technique | 579 | 4.5 | 992 | 6.4 | 0.12 |
| preop patient hair wash w/chlorhexidine shampoo* | 477 | 3.4 | 620 | 7.4 | 0.004 |
Not included in regression analyses because of the number of procedures with missing data.
Antibiotic sutures were used by a single HCRN center. At that center, there were 19 infections (3.8%) in 504 procedures with antibiotic suture and 4 infections (12.9%) in 31 procedures without antibiotic sutures (p = 0.038). Similarly, BioGlide catheters were only used at one HCRN center. At that center, there were 41 infections (8.3%) in 494 procedures with BioGlide compared with 0 infections (0%) in 37 procedures without BioGlide (p = 0.10).
Several factors showed at least a weak trend (p < 0.2) of association with infection. These were entered into the logistic regression models. In a model that used only components of the shunt protocol (and thus patient positioning, hand-washing technique, and double gloving were potential factors; Table 4), only the hand-washing technique emerged as an independent predictor. The odds ratio of infection after a procedure in which any team member used antiseptic cream was 4.88 (95% CI 1.46–16.27; p = 0.01) compared with a procedure in which the hand-washing technique was performed properly by all members of the surgical team.
A second model was considered using the above protocol factors as well as additional nonprotocol factors showing association with infection (antibiotic-impregnated sutures, BioGlide catheters, preoperative hair washing, and no-touch surgical technique; Table 5). After implementing these factors in a stepwise fashion, the use of BioGlide catheters and the use of an antiseptic cream by any team member emerged as factors independently predictive of infection. Specifically, in the multivariable model, the odds ratio of infection following a procedure in which BioGlide was used relative to a procedure without BioGlide was 1.91 (95% CI 1.19–3.05; p = 0.007). In this same model, the odds ratio of infection if an antiseptic cream was used by any team member (rather than a formal scrub) was 4.53 (95% CI 1.43–14.41; p = 0.01).
Discussion
Within the HCRN, application of a standardized protocol for 1571 CSF shunt surgery procedures reduced the infection rate from 8.8% to 5.7% (p = 0.0028). These infection rates both before and after institution of the protocol are similar to those of other reports in the literature. The effect size (absolute risk reduction of 3.15%, relative risk reduction of 36%) is important but relatively small compared with those reported in some infection studies in the literature. In a randomized single-center study of antimicrobial versus nonantimicrobial sutures for shunt surgery, the infection rate was reduced from 21% to 4.3% with antimicrobial sutures.14 In another single-center randomized trial,5 antibiotic-impregnated shunt material reduced the infection rate from 16.7% to 6%. Both of these impressive results are at least partly related to an unusually high infection rate in the control group. A significant benefit when baseline infection rates are lower, as we describe here, is less commonly reported, possibly because of the large sample size required to detect an effect in this setting.
The preprotocol data were collected from hospital quality improvement records, divisional databases, and/or retrospective chart reviews. From these sources, cultureproven infections were identified. In the prospectively collected data on the protocol, the definition of infection included culture-proven infection, but in addition, shunt erosion (defined as wound breakdown with visible shunt hardware) or abdominal pseudocyst (even in the absence of positive cultures) were counted as infections. The preprotocol data therefore may actually underestimate the infection rate compared with the protocol data.
The observed effect was predominantly from 3 of the 4 participating centers. At the fourth center, the protocol had no apparent impact. The only difference between centers that could be identified among the risk factors for infection was the use of BioGlide shunt catheters. In the regression analyses, BioGlide was associated with a near doubling of the infection risk and was commonly used at the center with no improvement in infection rate. BioGlide snap shunt ventricular catheters have since been recalled from the market for other reasons.15 Continued observation of the infection rate at that center after the recall may further clarify the role of BioGlide in shunt infection.
This study confirmed that the procedure with the highest risk of infection is shunt insertion after treatment of an infection; however, our observed infection rate of 11% in this scenario is substantially lower than the previously reported rates of 24%10 and 26%.9 This disparity may indicate that the protocol is particularly effective for procedures at the highest risk of infection.
To refine the protocol and plan further research, we analyzed factors present during the time window of the protocol to see which were associated with infection. The most important risk factor for infection was the lack of proper hand-washing technique by any member of the surgical team. The proper hand-washing technique for the protocol was a formal surgical scrub with povidoneiodine or chlorhexidine scrub brushes. All institutions had an antiseptic cream available to surgeons. The use of antiseptic cream instead of a formal surgical scrub was a protocol violation and resulted in a 4.5-fold increase in the risk of infection.
Compliance with the protocol increased as the study progressed. The overall compliance of nearly 75% compares favorably with other multistep protocols. In the WHO surgical checklist study,6 compliance with 6 of 19 steps was recorded and full compliance occurred in 34.2% of patients at baseline and rose to 56.7% after implementation of the protocol. A higher rate of compliance was achieved outside of the operating room environment in the NACHRI study of central line infections.11 In the pediatric ICU setting, there was an 84% compliance rate for one of their protocols and 82% for another. In our future work, it will be important to refine the protocol to the smallest number of factors that appear to have an impact on infection because a shorter, simpler protocol is likely to have higher compliance.
Our data were partially collected by the centers’ clinical research coordinators and partially by the surgical team. We did not record the frequency of these two methods of data collection, but a similar approach was used in the WHO study,6 in which only 37.5% of the data were collected by trained observers.
Conclusions
The application of a standardized protocol for shunt surgery across our network has had a number of advantages. We have reduced the Network-wide infection rate by 36%. Our analysis to identify risk factors suggests that the use of antiseptic cream and/or BioGlide catheters increases the probability of infection. Our ability to identify other risk factors may be limited by the high compliance with individual components of the protocol. The main conclusion is that standardization of the procedure and reducing variation by adherence to a common protocol has reduced our network infection rate. In addition, the protocol has established a common baseline that will facilitate assessment of new treatments in the future. This may be accomplished by a repeated iterative process changing one thing at a time or by assessing one factor in a randomized trial format. We now have center- and surgeon-specific data that will allow the identification of outliers. Simply providing surgeons with their own data may improve protocol adherence and further reduce infection rates. Observation of those surgeons with very low infection rates may allow us to identify factors that could be added to the protocol in the future.
Acknowledgments
The authors would like to thank their colleagues who kindly agreed to participate in this HCRN project and allow data collection on their patients for the purpose of this publication: Douglas Brockmeyer, Peter Dirks, James Rutka, Michael Taylor, Leslie Ackacpo-Satchivi, Jeff Blount, Curtis Rozelle, Daniel Curry, Robert Dauser, and Andrew Jea. In addition, this work would not have been possible without the outstanding support of their team of dedicated personnel at each clinical site and the data-coordinating center. The authors thank them for their hard work for HCRN. They would also like to thank Kristin Kraus, Department of Neurosurgery, University of Utah, for her expert editorial assistance.
Abbreviations used in this paper
- EVD
external ventricular drain
- HCRN
Hydrocephalus Clinical Research Network
Appendix
The HCRN consists of the following personnel
Chair: John R. W. Kestle, M.D.1 Investigator Committee: Jay Riva-Cambrin, M.D.,1 John C. Wellons III, M.D.,2 Abhaya V. Kulkarni, M.D.,3 William E. Whitehead, M.D.,4 and Tamara D. Simon, M.D., M.S.P.H.5 Executive Committee: W. Jerry Oakes, M.D.,2 Marion L. Walker, M.D.,1 James M. Drake, M.B.Ch.B.,3 and Thomas G. Luerssen, M.D.4 Clinical Site Coordinators: Tracey Habrock-Bach,1 Chevis Shannon,2 Lindsay O’Connor,3 and Sheila Ryan.4 Data Coordinating Center: Richard Holubkov, Ph.D.,6 Marcie Langley,6 and Jeff Yearley.6
Subsequent to this study, the following have joined the HCRN
Investigator Committee: Samuel Browd, M.D., Ph.D.,7 Mandeep Tamber, M.D., Ph.D.,8 and David Limbrick, M.D., Ph.D.9 Clinical Site Coordinators: Amy Anderson,7 Amita Bey,2 Arlene Luher,8 and Deanna Mercer.9
HCRN Centers
1Primary Children’s Medical Center, University of Utah; 2Children’s Hospital of Alabama, University of Alabama at Birmingham; 3Hospital for Sick Children, University of Toronto; 4Texas Children’s Hospital, Baylor College of Medicine; 5Seattle Children’s Research Institute, University of Washington; 6HCRN Data Coordinating Center, Department of Pediatrics, University of Utah; 7Seattle Children’s Hospital, University of Washington; 8Children’s Hospital of Pittsburgh, University of Pittsburgh; 9St. Louis Children’s Hospital, Washington University in St. Louis.
Footnotes
Disclosure
This work was supported by private philanthropy and National Institute of Neurological Disorders and Stroke grant no. 1RC1NS068943-01. Dr. Simon is supported by award K23NS062900 from the National Institute of Neurological Disorders and Stroke and by funding to the Pediatric Research in Inpatient Settings (PRIS) Network Executive Council provided by the Child Health Corporation of America. Dr. Simon’s work described herein was supported in part by a Primary Children’s Medical Center Innovative Research Grant and the Children’s Health Research Center at the University of Utah.
Author contributions to the study and manuscript preparation include the following. Conception and design: Kestle, Riva-Cambrin, Wellons, Kulkarni, Whitehead, Walker, Oakes, Drake, Luerrsen, Simon. Acquisition of data: Kestle, Riva-Cambrin, Wellons, Kulkarni, Whitehead, Walker, Oakes, Drake, Luerssen. Analysis and interpretation of data: Kestle, Holubkov. Drafting the article: Kestle. Critically revising the article: all authors.
References
- 1.Arthur AS, Whitehead WE, Kestle JRW. Duration of antibiotic therapy for the treatment of shunt infection: a surgeon and patient survey. Pediatr Neurosurg. 2002;36:256–259. doi: 10.1159/000058429. [DOI] [PubMed] [Google Scholar]
- 2.Cochrane D, Kestle J, Steinbok P, Evans D, Heron N. Model for the cost analysis of shunted hydrocephalic children. Pediatr Neurosurg. 1995;23:14–19. doi: 10.1159/000120930. [DOI] [PubMed] [Google Scholar]
- 3.Darouiche RO, Wall MJ, Jr, Itani KM, Otterson MF, Webb AL, Carrick MM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362:18–26. doi: 10.1056/NEJMoa0810988. [DOI] [PubMed] [Google Scholar]
- 4.Drake JM. Editorial. Does double gloving prevent cerebrospinal fluid shunt infection? J Neurosurg. 2006;104 (1 Suppl):3–4. doi: 10.3171/ped.2006.104.1.3. [DOI] [PubMed] [Google Scholar]
- 5.Govender ST, Nathoo N, van Dellen JR. Evaluation of an antibiotic- impregnated shunt system for the treatment of hydrocephalus. J Neurosurg. 2003;99:831–839. doi: 10.3171/jns.2003.99.5.0831. [DOI] [PubMed] [Google Scholar]
- 6.Haynes AB, Weiser TG, Berry WR, Lipsitz SR, Breizat AH, Dellinger EP, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–499. doi: 10.1056/NEJMsa0810119. [DOI] [PubMed] [Google Scholar]
- 7.Kestle J, Drake J, Milner R, Sainte-Rose C, Cinalli G, Boop F, et al. Long-term follow-up data from the Shunt Design Trial. Pediatr Neurosurg. 2000;33:230–236. doi: 10.1159/000055960. [DOI] [PubMed] [Google Scholar]
- 8.Kestle JR, Drake JM, Cochrane DD, Milner R, Walker ML, Abbott R, III, et al. Lack of benefit of endoscopic ventriculoperitoneal shunt insertion: a multicenter randomized trial. J Neurosurg. 2003;98:284–290. doi: 10.3171/jns.2003.98.2.0284. [DOI] [PubMed] [Google Scholar]
- 9.Kestle JR, Garton HJ, Whitehead WE, Drake JM, Kulkarni AV, Cochrane DD, et al. Management of shunt infections: a multicenter pilot study. J Neurosurg. 2006;105 (3 Suppl):177–181. doi: 10.3171/ped.2006.105.3.177. [DOI] [PubMed] [Google Scholar]
- 10.Kulkarni AV, Rabin D, Lamberti-Pasculli M, Drake JM. Repeat cerebrospinal fluid shunt infection in children. Pediatr Neurosurg. 2001;35:66–71. doi: 10.1159/000050393. [DOI] [PubMed] [Google Scholar]
- 11.Miller MR, Griswold M, Harris JM, II, Yenokyan G, Huskins WC, Moss M, et al. Decreasing PICU catheter-associated bloodstream infections: NACHRI’s quality transformation efforts. Pediatrics. 2010;125:206–213. doi: 10.1542/peds.2009-1382. [DOI] [PubMed] [Google Scholar]
- 12.Ragel BT, Browd SR, Schmidt RH. Surgical shunt infection: significant reduction when using intraventricular and systemic antibiotic agents. J Neurosurg. 2006;105:242–247. doi: 10.3171/jns.2006.105.2.242. [DOI] [PubMed] [Google Scholar]
- 13.Rozzelle CJ, Leonardo J, Li V. Antimicrobial suture wound closure for cerebrospinal fluid shunt surgery: a prospective, double-blinded, randomized controlled trial. J Neurosurg Pediatr. 2008;2:111–117. doi: 10.3171/PED/2008/2/8/111. [DOI] [PubMed] [Google Scholar]
- 14.Simon TD, Hall M, Riva-Cambrin J, Albert JE, Jeffries HE, LaFleur B, et al. Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. Clinical article. J Neurosurg Pediatr. 2009;4:156–165. doi: 10.3171/2009.3.PEDS08215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration. [Accessed April 28, 2011];Medical Device Recalls: Medtronic Neurologic Technologies, Innervision Snap Shunt Ventricular Catheter, BioGlide and Snap Shunt Ventricular Catheter, BioGlide. ( http://www.fda.gov/MedicalDevices/Safety/RecallsCorrectionsRemovals/ListofRecalls/ucm126620.htm)
- 16.Whitehead WE, Kestle JR. The treatment of cerebrospinal fluid shunt infections. Results from a practice survey of the American Society of Pediatric Neurosurgeons. Pediatr Neurosurg. 2001;35:205–210. doi: 10.1159/000050422. [DOI] [PubMed] [Google Scholar]