Abstract
Purpose of review
Little consensus exists on the definition of gestational diabetes (GDM), how the condition should diagnosed, and if interventions for mild maternal hyperglycemia are of any benefit to the mother or fetus. Today, after several large multi-center clinical trials, we are closer than ever to a national and international consensus.
Recent findings
Glucose tolerance in pregnancy is a continuum, which has a fundamental link to fetal growth. The relationship between maternal glycemia and adverse outcomes is continuous, with no distinct inflection point for increased risk. As a result, any cutoff for the diagnosis of GDM is somewhat arbitrary. Treatment for GDM, even mild cases, significantly reduces the rate of certain adverse perinatal and maternal outcomes, warranting intervention.
Summary
Clinical guidelines for the diagnosis of GDM are expected to change in the near future provided that recommendations from the International Association of Diabetes and Pregnancy Study Group (IADPSG) are accepted by professional organizations. The criteria for the diagnosis will likely be based on a single 75g, 2-hour oral glucose tolerance test with at least one abnormal value. The proposed threshold values are based on an international consensus regarding risk of adverse pregnancy outcomes. The public health implications for these changes are anticipated to be significant.
Keywords: Gestational diabetes, pregnancy, glucose intolerance, perinatal outcome
Introduction
Increasing numbers of women are being diagnosed with diabetes in pregnancy. Although a fraction of these women may have unrecognized pre-gestational diabetes, the vast majority have gestational diabetes (GDM) or pregnancy-related glucose intolerance. Although both short and long term consequences of GDM have been suspected or observed for the mother and fetus, differences in the practice patterns have contributed to the clinical confusion associated with its diagnosis, associated risks, and treatment benefits. The worldwide impact and incidence of GDM is difficult to determine, as no international standard for screening, diagnosis, or treatment has been accepted. As a result, the call for well-designed, randomized control trials to assess the risks, benefits, and cost-effectiveness of screening and treatment of GDM began in the early 1990’s [1, 2].
Differing practice for Screening and Diagnosis of GDM
Guidelines for screening and diagnosis of GDM vary among many international professional organizations. As recently as 2008, groups such as the U.S Preventative Task Force, the Canadian Task Force on the Periodic Health Examination, and the U.K. National Health Service have stated that not enough evidence existed to either support or oppose screening for GDM[3–5]. Other organizations supported testing, but protocols have varied significantly and thresholds for the diagnosis have been discrepant. For instance, the American College of Obstetricians and Gynecologists (ACOG) recommends screening, which may be universal or based on risk assessment, with a 1-hour 50g glucose challenge test followed by a 3-hour 100g oral glucose tolerance test (OGTT) for screen positive glucose values above 130–140mg/dL[6]. Threshold values for the diagnostic OGTT may be based on either the Carpenter/Coustan[7] or the National Diabetes Data Group (NDDG) criteria[8], the choice of which will alter the sensitivity and specificity of the test. In contrast, the World Health Organization (WHO) supports universal screening with a single 75g, 2-hour OGTT with the diagnosis given for fasting ≥126mg/dL or 2-hour value ≥200mg/dL[9]. The American Diabetes Association states that either the one or two step method may be used[10]. Not surprisingly, clinical practices regarding GDM vary widely within nations and even institutions.
Contributing to the debate is the consideration of the principle goal for selecting diagnostic thresholds. The O’Sullivan criteria[11], established in the U.S. over forty years ago and later adopted by the National Diabetes Data Group, integrated calculated threshold measurements for glucose tolerance tests of pregnant women that were two standard deviations above the mean for pregnant women. The criteria were aimed at identifying those women with an elevated risk for the future development of type 2 diabetes, as opposed to the values predictive of adverse perinatal outcome. WHO recommended OGTT threshold values are based on the criteria used in non-pregnant individuals[9]. In 1997, the Fourth International Workshop Conference on Gestational Diabetes Mellitus stated that the development of diagnostic criteria for GDM based on the relationship between maternal hyperglycemia and perinatal outcomes was unequivocally needed[1].
Does mild maternal hyperglycemia affect perinatal outcome? The HAPO Trial
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) trial was designed to assess the relationship between mild maternal hyperglycemia and adverse pregnancy outcome, and to provide an evidence-driven foundation on which new diagnostic guidelines could be based[12**]. In 2008, the results from this large (>25,000 subjects), prospective, multi-national trial were published. Of note, the findings were consistent throughout the international centers with diverse patient populations. The 2-hour, 75 g OGTT (after an overnight fast) was used to assess degrees of maternal hyperglycemia. The primary outcomes were birth weight >90th percentile for gestational age, primary cesarean section, neonatal hypoglycemia, and cord blood C-peptide level >90th percentile. Secondary outcomes included premature delivery, shoulder dystocia or birth injury, neonatal intensive care admission, hyperbilirubinemia, and preeclampsia. The most significant observation was that no absolute value of maternal hyperglycemia was identified as an inflection point of increased risk of adverse outcome. Instead, the relationship between maternal glycemia and birth weight, umbilical cord C-peptide levels, as well as the all five of the secondary outcomes was continuous and graded (Figure 1). The association with the other primary outcomes (primary cesarean delivery and neonatal hypoglycemia) was weaker. The HAPO investigators accordingly did not propose specific diagnostic thresholds since such values would be, to some extent, arbitrary in nature and required input and acceptance from the international community of experts in the field of diabetes in pregnancy[13].
Figure 1. Frequency of Primary Outcomes across the Glucose Categories.
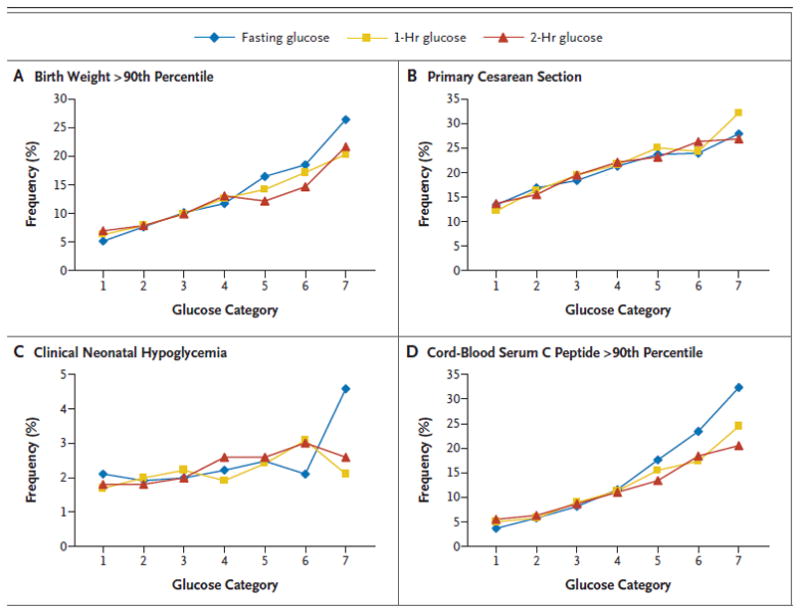
Glucose categories are defined as follows: fasting plasma glucose level — category 1, less than 75 mg per deciliter(4.2 mmol per liter); category 2, 75 to 79 mg per deciliter (4.2 to 4.4 mmol per liter); category 3, 80 to 84 mg per deciliter (4.5 to 4.7 mmol per liter); category 4, 85 to 89 mg per deciliter (4.8 to 4.9 mmol per liter); category 5, 90 to 94 mg per deciliter (5.0 to 5.2 mmol per liter); category 6, 95 to 99 mg per deciliter (5.3 to 5.5 mmol per liter); and category 7, 100 mg per deciliter (5.6 mmol per liter) or more; 1-hour plasma glucose level — category 1, 105 mg per deciliter (5.8 mmol per liter) or less; category 2, 106 to 132 mg per deciliter (5.9 to 7.3 mmol per liter); category 3, 133 to 155 mg per deciliter (7.4 to 8.6 mmol per liter); category 4, 156 to 171 mg per deciliter (8.7 to 9.5 mmol per liter); category 5, 172 to 193 mg per deciliter (9.6 to 10.7 mmol per liter); category 6, 194 to 211 mg per deciliter (10.8 to 11.7 mmol per liter); and category 7, 212 mg per deciliter (11.8 mmol per liter) or more; and 2-hr plasma glucose level — category 1, 90 mg per deciliter (5.0 mmol per liter) or less; category 2, 91 to 108 mg per deciliter (5.1 to 6.0 mmol per liter); category 3, 109 to 125 mg per deciliter (6.1 to 6.9 mmol per liter); category 4, 126 to 139 mg per deciliter (7.0 to 7.7 mmol per liter); category 5, 140 to 157 mg per deciliter (7.8 to 8.7 mmol per liter); category 6, 158 to 177 mg per deciliter (8.8 to 9.8 mmol per liter); and category 7, 178 mg per deciliter (9.9 mmol per liter) or more.
Reprinted with permission from: Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008 May 8;358(19):1991–2002.
Would treatment of mild maternal hyperglycemia affect perinatal outcome?
Another major area of GDM debate surrounds the utility of treating mild maternal hyperglycemia. If interventions offer little or no benefit for the mother or fetus then efforts to create a consensus for diagnosis would be futile. As a result, two major randomized control trials investigated the treatment of mild GDM and associated pregnancy outcomes.
ACHOIS Trial
In the Australian Carbohydrate Intolerance Study (ACHOIS)[14**], 1,000 women with mild maternal hyperglycemia based on a 75g OGTT with a fasting value <140mg/dL (7.8 mmol/L) and 2-hour value between 140–198 mg/dL (7.8–11.0 mmol/L) were randomized to the intervention group or routine care. The intervention consisted of care by a physician, dietitian, daily glucose monitoring, and insulin as needed and was aimed to simulate the care in settings where universal screening and treatment for GDM are available. The routine care group received no additional care. The primary infant outcome was a composite measure of adverse perinatal complications, defined as more than one of the following: death, shoulder dystocia, bone fracture and nerve palsy, neonatal ICU admission, and jaundice requiring phototherapy. Primary maternal outcomes included need for induction of labor and cesarean delivery. In addition, at 6 weeks postpartum, maternal psychological health, as well as general health and well-being were assessed. The rate of serious neonatal adverse complications was lower in the intervention group, although neonatal ICU admission rate was higher in the intervention group. Induction of labor was more common in the intervention group, but the rate of cesarean delivery was not different between groups. Six weeks postpartum, the intervention group had lower rates of depression and higher scores for overall health status.
NICHD MFMU Trial
The National Institute of Child Health and Human Development Maternal Fetal Medicine Unit conducted the second large multi-center, randomized trial for the treatment of mild gestational diabetes[15**]. Mild GDM was defined as 2 abnormal values with a normal fasting glucose (<95mg/dl) on a 3-hour, 100g, OGTT. Over 900 women were randomized to routine care or to treatment with nutritional counseling, diet therapy, and insulin as needed. The primary outcome, a composite of perinatal mortality, hypoglycemia, hyperbilirubinemia, hyperinsulinemia, and birth trauma was not significantly different in the treatment group. However, the control group had significantly higher mean birth weight, neonatal fat mass, and rates of cesarean delivery, shoulder dystocia, and gestational hypertension/pre-eclampsia. In aggregate, these studies show that GDM not only exists, but incurs significant risk of adverse maternal and fetal perinatal outcome. Further, intervention can diminish this risk.
IADPSG Diagnostic Recommendations for Hyperglycemia in Pregnancy: Diagnosis of GDM
The question of how to establish the diagnosis of GDM remained. In March 2010, the International Association of Diabetes and Pregnancy Study Groups (IADPSG) released consensus recommendations for the diagnosis and classification of hyperglycemia in pregnancy [16**]. The IADPSG Consensus panel is affiliated with many international professional organizations, and included 220 delegates representing about 40 different nations. Using the available evidence, the consensus recommendations were intended to be the basis for internationally accepted criteria for the diagnosis and classification of diabetes in pregnancy. This was not an easy task since the relationship between hyperglycemia and adverse outcome is linear, with no clear point at which risk exponentially increases. Mean threshold values were considered for three different specific odds ratios (1.5, 1.75, and 2.0) for increased neonatal body fat, large for gestational age, cord blood C-peptide levels >90th percentile using data from the HAPO trial and the 2-hour, 75g OGTT. Ultimately, the threshold values were chosen for an odds ratio of 1.75, and these were a fasting glucose of 92mg/dl (5.1mmol/l), 1-hour of 180mg/dl (10mmol/l), and 2-hour of 153mg/dl (8.5mmol/l) with only one elevated value needed to make the diagnosis of GDM (Table 1). Further, these values had strong associations with pre-eclampsia and shoulder dystocia and/or birth injury[12].
Table 1. Diagnosis of Hyperglycemia in Pregnancy.
Threshold values for diagnosis of GDM or overt diabetes in pregnancy
| Glucose measure | Glucose concentration threshold * |
Above threshold (%)
|
|
|---|---|---|---|
| mmol/l | mg/dl | Cumulative | |
| FPG | 5.1 | 92 | 8.3 |
| 1-h plasma glucose | 10.0 | 180 | 14.0 |
| 2-h plasma glucose | 8.5 | 153 | 16.1† |
| To diagnose overt in pregnancy
| |||
| Measure of glycemia | Consensus threshold | ||
| FPG‡ | ≥7.0 mmol/l (126 mg/dl) | ||
| A1C‡ | ≥6.5% (DCCT/UKPDS standardized) | ||
| Random plasma glucose | ≥11.1 mmol/l (200 mg/dl) + confirmation§ | ||
One or more of these values from a 75-g OGTT must be equaled or exceeded for the diagnosis of GDM.
In addition, 1.7% of participants in the initial cohort were unblinded because of FPG > 5.8 mmol/l (105 mg/dl) or 2-h OGTT values > 11.1 mmol/l (200 mg/dl), bringing the total to 17.8%.
One of these must be met to identify the patient as having overt diabetes in pregnancy.
If a random plasma glucose is the initial measure, the tentative diagnosis of overt diabetes in pregnancy should be confirmed by FPG or A1C using a DCCT/UKPDS-standardized assay.
Previously published
Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. Mar;33(3):676-82.
Diagnosis of Pregestational Diabetes
In addition, the panel described a two-phase strategy for the evaluation of diabetes in pregnancy (Table 1). The first phase, at the initial prenatal visit, is aimed at revealing unrecognized pre-gestational diabetes using either fasting blood glucose ≥1126mg/dl (7mmol/l), hemoglobin A1C ≥ 6.5%, or random blood glucose ≥ 200mg/dl (11.1mmol/l). Intermediate levels of hyperglycemia in the first trimester can be used to diagnose GDM as well (e.g. fasting glucose ≥92mg/dL, but <126mg/dL). Guidelines for who should be tested in the first trimester were not given and should be made by the provider based on whether the population being served has a high incidence of type 2 diabetes. The second phase, performed between 24–28 weeks’ gestational age, entails the 2-hour 75g OGTT using the threshold values (Table 1).
Public Health Implications
The implementation of the IADPSG recommendations will have major public health implications. Adopting universal testing will be a significant change for many providers around the world. Further, the change in threshold values is expected to substantially increase the prevalence of GDM, although a high percentage of these patients will likely be controlled with diet alone[15]. Further, a large effort to provide education to patients and providers alike will require a considerable increase in resources and personnel to deliver these services. On the other hand, a single diagnostic test with the elimination of a screening phase is more convenient for the patient and the provider, and therefore, may establish earlier diagnosis and treatment. Indeed, having a working international agreement on the diagnosis of GDM will allow for globally consistent care, organized research, and improved patient outcomes.
Using the diverse HAPO trial population for prediction of the impact the new guidelines will have, 16.1% of the population is expected to be diagnosed with GDM (Table 2). If all subjects, including those participants excluded due to glucose values exceeding the predetermined study limits, this number increases to 17.8%. Given the epidemic of obesity and the increasing percentage of reproductive-aged women with type 2 diabetes, the prospect of up to 18% of pregnant women being diagnosed with diabetes in pregnancy should not be surprising. As the distribution curve for body mass index (BMI) shifts to the right and the ‘norm’ for the population increases, normal and healthy may continue to diverge. Similarly, population shifts in maternal glucose tolerance may be underway. As new guidelines are set, standards aimed at differentiating healthy from ‘normal’ are key. Although the threshold valves proposed by the IADPSG are unavoidably arbitrary, at least in part, the foundation for the chosen values is aimed at reducing adverse pregnancy outcomes, rather than conforming to population norms. In addition, the full impact of the proposed guidelines may not be appreciated for decades. Increasing evidence supports a cyclical model for the transgenerational transmission of diabetes and metabolic dysregulation[17]. In utero exposure to mild maternal hyperglycemia and obesity amplifies the risk for the infant of future diabetes and obesity. The impact that clinical interventions may have on long-term risk for the infant has yet to be determined. The ultimate goal for routine diabetes screening in pregnancy is not only to reduce the immediate adverse outcome for mother and child, but also to prevent the adverse long term health consequences for future generations.
Table 2.
Pregnancies Meeting Glucose Thresholds
| Odds Ratio | Glucose Thresholds (mg/dl) | % Subjects ≥ threshold |
|---|---|---|
| 1.5 | 90/167/142 | 20 |
| 1.75 | 92/180/153 | 16.1 |
| 2.0 | 95/191/161 | 8.8 |
Adapted from: Coustan DR, Lowe LP, Metzger BE, Dyer AR. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. Am J Obstet Gynecol. Jun;202(6):654 e1–6.
Conclusion
The current practice for screening, diagnosing, and treating hyperglycemia in pregnancy is variable. This will likely change in the near future if the IADPSG consensus recommendations are accepted by professional organizations. The threshold values for these recommendations were chosen with the aim of reducing adverse perinatal outcome. These values originate from the HAPO trial which showed a clear, linear, graded relationship between maternal hyperglycemia and certain adverse pregnancy outcomes. Large prospective randomized control trials clearly reveal improved perinatal outcome with treatment of mild hyperglycemia. Increasing insight of the immediate and long-term, maternal and neonatal consequences associated with maternal hyperglycemia leads us to strive for improved glycemic control in pregnancy.
Key Points.
A linear, graded relationship between maternal glycemia and perinatal outcome exists, with no distinct inflection point for exponentially increased risk.
Treating mild maternal hyperglycemia improves perinatal outcome.
Currently, there are no national or international consensus guidelines for the screening and diagnosis of GDM.
Soon, the IADPSG recommendations for using a 2 hour 75g OGTT with ≥1 abnormal value will likely become the international protocol for the diagnosis of GDM.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HD063791to F.H.E.) and the Albert Einstein Diabetes Research Center (DK20541). The funders had no role in the decision to publish or preparation of the manuscript.
Footnotes
The authors have no conflicts of interest or financial sponsorship to report.
References
- 1.Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. 1998;21 (Suppl 2):B161–7. [PubMed] [Google Scholar]
- 2.Blank A, Grave GD, Metzger BE. Effects of gestational diabetes on perinatal morbidity reassessed. Report of the International Workshop on Adverse Perinatal Outcomes of Gestational Diabetes Mellitus, December 3–4, 1992. Diabetes Care. 1995;18(1):127–9. doi: 10.2337/diacare.18.1.127. [DOI] [PubMed] [Google Scholar]
- 3.Periodic health examination, 1992 update: 1. Screening for gestational diabetes mellitus. Canadian Task Force on the Periodic Health Examination. CMAJ. 1992;147(4):435–43. [PMC free article] [PubMed] [Google Scholar]
- 4.Screening for gestational diabetes mellitus: U. S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148(10):759–65. doi: 10.7326/0003-4819-148-10-200805200-00008. [DOI] [PubMed] [Google Scholar]
- 5.Committe UK National Screening Committee Policy on gestational diabetes screening in pregnancy. 2006 [updated 2006; cited]; Available from: http://www.screening.nhs.uk/gestational-diabetes.
- 6.ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational diabetes. Obstet Gynecol. 2001;98(3):525–38. [PubMed] [Google Scholar]
- 7.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144(7):768–73. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 8.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28(12):1039–57. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 9.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 10.Bloomgarden ZT. American Diabetes Association 60th Scientific Sessions, 2000: diabetes and pregnancy. Diabetes Care. 2000;23(11):1699–702. doi: 10.2337/diacare.23.11.1699. [DOI] [PubMed] [Google Scholar]
- 11.O’Sullivan JB, Mahan CM. Criteria for the Oral Glucose Tolerance Test in Pregnancy. Diabetes. 1964;13:278–85. [PubMed] [Google Scholar]
- **12.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. This is the first and only large trial to assess the perinatal risk for mild maternal hyperglycemia. Results revealed a continuous, linear, graded relationship between maternal hyperglycemia and adverse perinatal outcome. [DOI] [PubMed] [Google Scholar]
- 13.Coustan DR, Lowe LP, Metzger BE, Dyer AR. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. Am J Obstet Gynecol. 2010;202(6):654, e1–6. doi: 10.1016/j.ajog.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **14.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–86. doi: 10.1056/NEJMoa042973. This is one of the two large randomized control trials looking at the effect of treating mild hyperglycemia in pregnancy. Results showed that serious adverse neonatal complications was lower, and postpartum maternal well-being was improved in the intervention group. [DOI] [PubMed] [Google Scholar]
- **15.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339–48. doi: 10.1056/NEJMoa0902430. This is one of the two large randomized control trials looking at the effect of treating mild hyperglycemia in pregnancy. Treatment reduced risk of fetal overgrowth, shoulder dystocia, cesarean delivery, and hypertensive disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **16.Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–82. doi: 10.2337/dc09-1848. Using the HAPO trial as well as other studies as a basis for their recommendations, an international expert panel came to a consensus regarding new, stricter guidelines for the diagnosis of diabetes in pregnancy, with the goal of reducing adverse perinatal outcome. These threshold values will likely soon be accepted by professional organizations and will result in the diagnosis of GDM in up to 18% of the population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore TR. Fetal exposure to gestational diabetes contributes to subsequent adult metabolic syndrome. Am J Obstet Gynecol. 2010;202(6):643–9. doi: 10.1016/j.ajog.2010.02.059. [DOI] [PubMed] [Google Scholar]


