Abstract
Background
Individuals with vascular disease or risk factors have substantially higher rates of cognitive decline, yet little is known on means of maintaining cognition in this group.
Methods
We examined the relation between physical activity and cognitive decline in participants of the Women’s Antioxidant Cardiovascular Study (WACS), a cohort of women with prevalent vascular disease or ≥3 coronary risk factors. Recreational physical activity was assessed at baseline (1995–1996) and every two years thereafter. In 1998–2000, participants aged ≥65 years underwent a telephone cognitive battery including five tests of global cognition, verbal memory, and category fluency (n=2809). Tests were administered three additional times over 5.4 years. We used multivariable-adjusted generalized linear models for repeated measures to compare the annual rates of cognitive score changes across levels of total physical activity and on walking, as assessed at WACS baseline.
Results
We found a significant trend (p-trend<0.001) of slower rates of cognitive decline with increasing energy expenditure. Compared to the bottom quintile of total physical activity, significant differences in rates of cognitive decline were observed from the fourth quintile (p=0.04 for fourth quintile, p<0.001 for fifth quintile) or the equivalent of daily 30-minute walks at a brisk pace. This difference was equivalent to the difference in cognitive decline observed for women who were younger by 5–7 years. Walking was also strongly related to slower rates of cognitive decline (p-trend=0.003).
Conclusions
Regular physical activity, including walking, was associated with better preservation of cognitive function in older women with vascular disease or risk factors.
Older individuals with cardiovascular disease (CVD) or coronary risk factors have substantially increased risks of cognitive impairment. Not only are the domains traditionally associated with vascular health (e.g., executive function) affected, but so are general cognition and episodic memory1, 2. Additionally, the prevalence of CVD and risk factors has dramatically increased due to population aging and a decline in cardiovascular mortality with improved treatments3. Yet, little is known about strategies, especially behavioral approaches, to preserve cognition in this group.
Accumulating evidence suggests a protective effect of physical activity on cognition4–12; but so far, most human studies were of generally healthy populations. Because physical activity has been linked with reductions in disease progression among those with CVD or vascular risk factors13, the potential benefits of physical activity on cognition could be important in this group. Thus, we utilized data from the Women’s Antioxidant Cardiovascular Study to examine the relation of physical activity to cognitive decline in nearly 3000 older women.
METHODS
Parent cohort
The Women’s Antioxidant Cardiovascular Study (WACS) began in 1995–1996 (“baseline”) among 8171 women, as a 2×2×2 randomized placebo-controlled trial of vitamin E, vitamin C, and beta-carotene supplementation for CVD secondary prevention14. Eligible participants were female health professionals, aged ≥40 years, with ≥3 coronary risk factors (i.e., parental history of premature myocardial infarction (MI), diabetes, hypertension, hyperlipidemia, and body mass index ≥30 kg/m2) or CVD (i.e., MI, stroke, symptomatic angina pectoris, transient cerebral ischemia, or revascularization procedures such as percutaneous transluminal angioplasty, coronary artery bypass graft, carotid endarterectomy, or peripheral artery surgery). In 1998, a fourth arm for B vitamin supplementation was added among 5442 women15. Until 2005, participants completed annual questionnaires on compliance, side effects, health and lifestyle, and clinical endpoints. None of the supplements were associated with cardiovascular disease recurrence14, 15 or cognitive change16, 17.
Cognitive subcohort
From 1998–2000, we assessed cognitive function using telephone interviews among participants aged ≥65 years. Of the 3170 eligible women, 190 were unreachable, 156 declined participation, and 2824 (95% of contacted women) completed the initial assessment. Participants received three follow-up assessments at two-year intervals until 2005; 93% completed at least one follow-up assessment, and 81% completed at least three assessments. For the fourth assessment, 24% were not contacted as only a short interval had passed between their third interview and the trial end. We excluded 15 participants with prevalent Parkinson’s disease who likely had cognitive impairment and also did not engage in regular physical activity. Thus, the analyses included 2809 women. This study was approved by the institutional review board of Brigham and Women’s Hospital, Boston, MA.
Physical activity assessment
At baseline and biennially thereafter, women were asked about the average weekly time spent during the past year on walking or hiking; jogging (speed <10-minute miles); running (10-minute miles or faster); bicycling, including use of stationary machines; aerobic exercise, aerobic dance, or use of exercise machines; lower-intensity exercise, including yoga, stretching or toning; tennis, squash or racquetball; and lap-swimming. We also inquired about the usual pace of walking (3.2 km/h [2.0 mph; easy pace], 3.2–4.7 km/h [2.0–2.9 mph; normal pace], 4.8–6.3 km/h [3.0–3.9 mph; brisk pace], or ≥6.4 km/h [≥4.0 mph; very brisk pace]) and the number of flights of stairs climbed daily (0, 1–2, 3–4, 5–9, 10–14, ≥15). We assigned each activity a metabolic equivalent value (MET), where one MET is proportional to the energy expended while sitting quietly18. MET values were 12 for running; 8 for stair-climbing; 7 for jogging, racquet sports, lap-swimming and bicycling; 6 for aerobic exercise, dance or use of exercise machines; and 4 for yoga, stretching or toning. MET values for walking varied by pace, from 2.5 METs for easy to 4.5 METs for very brisk pace. For each activity, we estimated the energy expended in MET-hours/week, by multiplying its MET value by the duration.
In a validation study in comparable female participants in a large cohort study19, the physical activity responses given two years apart were reasonably correlated (r=0.6), given the expected true changes that might occur. Moreover, the physical activity recalled for the previous year correlated with activity based on past-week recalls (r=0.8) and activity diaries during the year (r=0.6).
Cognitive Assessment
Cognitive function was assessed by telephone, using five cognitive tests. General cognition was evaluated with the Telephone Interview of Cognitive Status (TICS)20, a telephone adaptation of the Mini-Mental State Examination (range: 0 to 41 points). Verbal memory was assessed with the TICS 10-word list (immediate and delayed recalls) and the East Boston Memory Test (immediate and delayed recalls)21. Women were asked to name as many animals as possible in one minute in a test of category fluency22.
Our primary outcome was the rate of change in the global composite score, computed as the mean of the z-scores from all cognitive tests. As secondary outcomes, we considered the changes in TICS score, verbal memory composite score (mean of the verbal memory z-scores), and category fluency score. Verbal memory is among the best predictors of Alzheimer disease23, and category fluency partly measures executive function, which is associated with vascular dementia24, 25. To derive the composite scores for participants who did not complete all tests (only 0.5% of participants), we used the means of the z-scores from the available relevant tests.
In a validation study of 61 women, the global composite score from the brief telephone-administered assessment correlated strongly with the total score from an extensive in-person interview of cognition (r=0.8). In a reliability study among 35 high-functioning, educated women, the correlation between TICS scores administered twice 31 days apart was 0.7. Among 88 older female health professionals, who were demographically similar to WACS participants, poor performance in the TICS and in the verbal memory composite score were respectively associated with significant 8 and 12 fold increases in subsequent dementia diagnoses6. Thus, extensive evidence supports the validity of our telephone cognitive instrument.
Covariates
We considered several potential confounding factors plausibly linked with both cognitive decline and physical activity. Basic models included age at initial cognitive assessment and education. Multivariable models also included the WACS randomization assignments as well as numerous lifestyle and health variables listed in Table 1, with covariate specifications in the footnote of Table 2.
Table 1.
Age and age-standardized baseline characteristics of WACS cognitive cohort by quintiles of total physical activity (n=2809)
| Quintiles of total physical activity | p-value* | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Total energy (mean MET-hours per week) | 0.6 | 2.9 | 7.4 | 15.4 | 38.9 | |
| Energy expended on walking (mean MET-hours per week) | 0.2 | 1.6 | 3.4 | 6.7 | 13.7 | |
| Age at initial cognitive assessment (y): mean±SD (range) | 72.8±4.8 (66.1–91.3) | 72.3±4.3 (66.1–90.1) | 72.7±4.2 (66.2–90.5) | 72.4±4.0 (66.1–90.7) | 72.6±4.0 (66.1–87.5) | 0.27 |
| Highest attained education (%) | ||||||
| Licensed practical or vocational nurse/associate’s degree | 33 | 27 | 24 | 24 | 26 | |
| Registered nurse/Bachelor’s degree | 59 | 64 | 63 | 65 | 61 | 0.003 |
| Master’s degree/Doctoral degree | 8 | 9 | 13 | 11 | 12 | |
| Married as marital status (%) | 54 | 60 | 61 | 60 | 60 | 0.03 |
| Alcohol intake (g/d) | 3.3 | 3.3 | 3.7 | 3.9 | 4.8 | 0.02 |
| Use of multivitamin supplements (%) | 26 | 27 | 28 | 34 | 32 | 0.009 |
| Current smoking status (%) | 16 | 13 | 7 | 5 | 8 | <0.001 |
| Current postmenopausal hormone therapy (%) | 37 | 39 | 36 | 40 | 40 | 0.40 |
| Body mass index (kg/m2) | 30.5 | 29.1 | 28.6 | 27.8 | 27.3 | <0.001 |
| Use of aspirin exceeding 10 days in the previous month (%) | 42 | 42 | 46 | 47 | 52 | 0.002 |
| Use of non-steroidal anti-inflammatory drug exceeding 10 days in the previous month (%) | 27 | 22 | 24 | 24 | 20 | 0.16 |
| History of lung disease (%) | 24 | 16 | 17 | 12 | 15 | <0.001 |
| History of joint pain or swelling (%) | 59 | 53 | 55 | 55 | 55 | 0.005 |
| History of arthritis (%) | 62 | 59 | 58 | 55 | 55 | 0.09 |
| History of depression (%) | 17 | 17 | 15 | 14 | 12 | 0.09 |
| History of myocardial infarction (%) | 22 | 18 | 21 | 23 | 22 | 0.27 |
| History of stroke (%) | 10 | 8 | 9 | 9 | 7 | 0.36 |
| History of revascularization surgery (%) | 21 | 19 | 20 | 22 | 24 | 0.20 |
| History of angina (%) | 41 | 44 | 44 | 46 | 48 | 0.21 |
| History of transient ischemic attack (%) | 17 | 15 | 16 | 14 | 14 | 0.33 |
| History of diabetes (%) | 19 | 21 | 18 | 16 | 13 | 0.01 |
| History of hypertension (%) | 82 | 79 | 81 | 74 | 72 | <0.001 |
| History of hyperlipidemia (%) | 73 | 72 | 75 | 75 | 77 | 0.16 |
From chi-squared tests or analysis of variance depending on the type of variable (categorical or numeric)
Table 2.
Adjusted differences (95% confidence intervals) in annual rates of cognitive change in various cognitive scores over four assessments by quintiles of total physical activity at baseline, WACS cognitive cohort (n=2809)
| Quintiles of total physical activity; median (range) in MET-hours per week | p-trend | |||||
|---|---|---|---|---|---|---|
| 1 0.4 (<1.7) |
2 2.9 (1.7–4.6) |
3 7.5 (4.7–10.6) |
4 15.2 (10.7–21.2) |
5 32.7 (>21.2) |
||
| Global cognitive score (n=2809) | ||||||
| Basic-adjusted model† | Reference | 0.00 (−0.01,0.02) | 0.02 (0.00,0.04) | 0.02* (0.001,0.04) | 0.03* (0.01,0.05) | <0.001 |
| Multivariable-adjusted model‡ | Reference | 0.00 (−0.01,0.02) | 0.02 (0.00,0.04) | 0.02* (0.001,0.04) | 0.03* (0.01,0.05) | <0.001 |
| TICS (n=2809) | ||||||
| Basic-adjusted model† | Reference | −0.02 (−0.11,0.07) | 0.05 (−0.04,0.15) | 0.07 (−0.02,0.16) | 0.09 (0.00,0.18) | 0.02 |
| Multivariable-adjusted model‡ | Reference | −0.01 (−0.10,0.08) | 0.05 (−0.04,0.15) | 0.07 (−0.02,0.17) | 0.09 (−0.01,0.18) | 0.03 |
| Verbal memory score (n=2809) | ||||||
| Basic-adjusted model† | Reference | 0.00 (−0.02,0.02) | 0.01 (−0.01,0.03) | 0.03* (0.01,0.05) | 0.03* (0.01,0.05) | <0.001 |
| Multivariable-adjusted model‡ | Reference | 0.00 (−0.02,0.02) | 0.01 (−0.01,0.03) | 0.02* (0.001,0.05) | 0.03* (0.01,0.05) | <0.001 |
| Category fluency (n=2804) | ||||||
| Basic-adjusted model† | Reference | 0.04 (−0.08,0.17) | 0.05 (−0.08,0.17) | 0.02 (−0.10,0.15) | 0.07 (−0.05,0.20) | 0.40 |
| Multivariable-adjusted model‡ | Reference | 0.05 (−0.08,0.18) | 0.05 (−0.08,0.18) | 0.02 (−0.11,0.15) | 0.08 (−0.05,0.21) | 0.40 |
Significant at the α=0.05 level
Adjusted on age (years) and education (three categories as defined in Table 1)
Further adjusted on marital status (married, divorced, widowed, single), alcohol intake (abstainer, 0.1–0.9g/day, ≥10g/day), use of multivitamin supplements (no, yes), smoking status (never, past, current), body mass index (quartiles), postmenopausal hormone therapy use (never, past, current), aspirin use exceeding 10 days in the previous month (no, yes), non-steroidal anti-inflammatory drug use exceeding 10 days in the previous month (no, yes), history of depression (no, yes), lung disease (no, yes), joint pain or swelling (no, yes), arthritis (no, yes), cardiovascular profile at baseline (myocardial infarction, stroke, revascularization procedures, symptomatic angina pectoris, transient cerebral ischemia, no CVD), diabetes (no, yes), hypertension (no, yes on treatment, yes without treatment), hyperlipidemia (no, yes on treatment, yes without treatment), and randomization trial assignment for vitamin E (placebo, active), vitamin C (placebo, active), beta-carotene (placebo, active), and folate (not included, placebo, active)
Statistical Analysis
Physical activity
We examined quintiles of total energy expended on all activities and quartiles of energy expended on walking (walking had a narrower distribution of energy expenditure), which was the most common activity. Before the first cognitive assessment, physical activity was assessed at randomization and again after 24 months. To reduce the impact of any recent changes in activity due to health or cognitive status (i.e., “reverse causation” bias), our main analyses were based on the energy expenditures from the baseline questionnaire, which was assessed a mean 3.5 years before the first cognitive assessment. However, given our interest in longer-term consistent physical activity levels, in secondary analyses, we also examined the average energy expenditures from reports on baseline and 24-month questionnaires. Furthermore, we examined associations among a subset of women whose activity remained stable (i.e., in the same or adjacent quintile at baseline and at 24-months).
When examining walking for exercise, we controlled for the energy expended in other activities as potential confounders. To isolate the effects of walking, we also conducted a stratified analysis in women who only reported walking or engaging in low-intensity exercises for their activities (n=1387).
Statistical models
We used general linear models for repeated measures with random intercepts and slopes with an unstructured covariance matrix to estimate the association of physical activity with the annual rate of cognitive change. We tested for linear trends across activity categories by testing a continuous variable in which participants were assigned the median of the category. We used Wald tests for statistical testing. Models were fitted by maximum likelihood method using the SAS software (SAS release 9.1, SAS Institute Inc., Cary, NC).
Because age, education, depression, diabetes, hypertension or cardiovascular profile could modify the association between physical activity and cognitive decline, we evaluated three-way interaction terms of time, physical activity level, and the potential effect modifier in separate multivariable-adjusted models. We conducted an analysis excluding women who had the worst cognitive function at the initial assessment (defined as the worst 10% of the distribution) to reduce any potential bias due to less healthy behaviors or worse reporting in this group.
RESULTS
The mean time from the first activity assessment to initial cognitive assessment was 3.5 years (range 3.1–4.7), and the mean time from the initial to the last cognitive assessments was 5.4 years (range 4.0–6.1). Participants lost to follow-up tended to be older and less active at baseline.
Walking accounted for about half of the total expenditure (Table 1). Women with greater physical activity had lower mean body mass index, were more likely to consume alcohol and less likely to smoke or report lung disease, diabetes or hypertension. Physical activity was not associated with cardiovascular disease.
Total physical activity
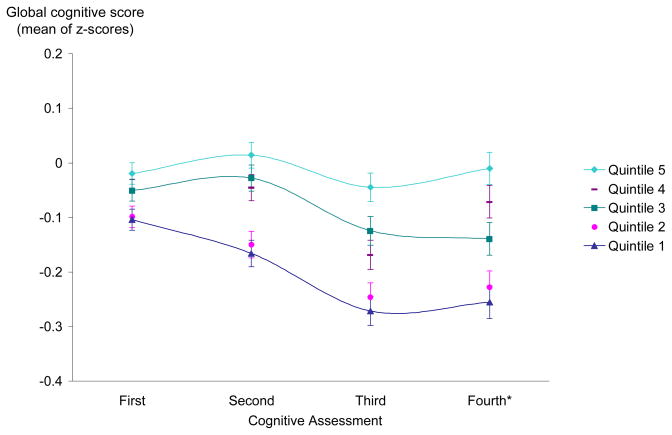
More active women tended to have better scores over time (Figure 1), and the difference in performance by activity level widened over time. In both the basic- and multivariable-adjusted models, greater physical activity was significantly associated with slower declines in the global score (p-trend<0.001), TICS (p-trend=0.03), and verbal memory score (p-trend<0.001), but not associated with the category fluency score (Table 2). We found statistically significant differences in global cognitive decline beginning with women in the fourth and fifth quintiles of total energy expenditures (p=0.04 for fourth quintile, p<0.001 for fifth quintile), which was equivalent to walking 30 minutes or more every day at a brisk pace (i.e., 3.5 mph or 5.5km/h). Because mean differences in cognitive decline can be difficult to interpret, we compared the estimates for physical activity and cognitive decline to that for age and cognitive decline, thus using the effect of age on cognitive decline as a “benchmark” for interpretation. The mean differences in rates of decline between the fourth and first quintiles of activity was equivalent to the mean differences we found for women 5 years apart in age, and the difference between the fifth and the first was equivalent to 7 years of age. That is, the apparent cognitive benefits with physical activity were equivalent to being cognitively younger by 5–7 years.
Figure 1.
Mean adjusted global cognitive score during cognitive follow-up (1998–2005) by quintile of total physical activity as assessed at baseline (n=2809)
*For the fourth assessment, 24% of participants were not contacted as only a short interval had passed between their third interview and the parent trial end; thus there is more statistical variation for this assessment.
In alternate analyses, when we considered the average of the two physical activity measures prior to the first cognitive evaluation, the associations were very similar and somewhat stronger, notably for the category fluency test: comparing the fifth quintile to the first quintile, the difference in annual decline was 0.03 standard units (95% CI: 0.01, 0.05) for the global score (p-trend <0.001); 0.12 points (0.03, 0.22) for the TICS score (p-trend: 0.003); 0.03 standard units (0.01, 0.05) for the verbal memory score (p-trend <0.001); and 0.12 points (−0.01, 0.25) for the category fluency score (p-trend: 0.09). In addition, among the subset of 1971 women with stable activity levels on the two assessments before cognitive evaluations, results were also similar: comparing the fifth quintile to the first quintile, the difference in annual decline was 0.04 standard units (0.01, 0.06) for the global score (p-trend <0.001); 0.13 points (0.02, 0.25) for the TICS score (p-trend: 0.002); 0.04 standard units (0.01, 0.06) for the verbal memory score (p-trend <0.001); and 0.14 points (−0.02, 0.30) for the category fluency score (p-trend: 0.17).
Walking
In models adjusted for other physical activities, we found significantly slower rates of decline in the global, verbal memory, and category fluency scores with greater levels of walking (p-trend of 0.003, 0.01 and 0.03, respectively) (Table 3). However, significant associations were observed with the last quartile only (with a minimum of daily 30-minute walks), indicating a possible threshold effect. The difference in rates of cognitive decline between the fourth and first quartiles of walking was equivalent to the differences found for women 5 years apart in age.
Table 3.
Adjusted differences (95% confidence intervals) in annual rates of cognitive change in various cognitive scores over four assessments by quartiles of walking at baseline, WACS cognitive cohort (n=2809)
| Quartile of walking; median (range) in MET-hours per week | p-trend | ||||
|---|---|---|---|---|---|
| 1 0 (<0.5) |
2 1.0 (0.5–1.9) |
3 3.1 (2.0–7.4) |
4 15.0 (>7.4) |
||
| Global cognitive score (n=2809) | |||||
| Basic-adjusted model† | Reference | 0.01 (−0.01,0.03) | 0.00 (−0.01,0.02) | 0.03* (0.01,0.04) | 0.002 |
| Multivariable-adjusted model‡ | Reference | 0.01 (−0.01,0.02) | 0.00 (−0.02,0.02) | 0.02* (0.01,0.04) | 0.003 |
| TICS (n=2809) | |||||
| Basic-adjusted model† | Reference | 0.02 (−0.06,0.10) | −0.06 (−0.14, 0.03) | 0.06 (−0.03,0.14) | 0.10 |
| Multivariable-adjusted model‡ | Reference | 0.02 (−0.07,0.10) | −0.06 (−0.15, 0.03) | 0.05 (−0.03,0.14) | 0.09 |
| Verbal memory score (n=2809) | |||||
| Basic-adjusted model† | Reference | 0.01 (−0.01,0.02) | 0.00 (−0.02,0.02) | 0.02* (0.01,0.04) | 0.005 |
| Multivariable-adjusted model‡ | Reference | 0.00 (−0.02,0.02) | 0.00 (−0.02,0.02) | 0.02* (0.001,0.04) | 0.01 |
| Category fluency (n=2804) | |||||
| Basic-adjusted model† | Reference | 0.04 (−0.07,0.15) | 0.04 (−0.08,0.16) | 0.12* (0.01,0.24) | 0.03 |
| Multivariable-adjusted model‡ | Reference | 0.05 (−0.06,0.16) | 0.05 (−0.07,0.17) | 0.13* (0.02,0.25) | 0.03 |
Significant at α=0.05 level
Adjusted on age (years) and education (three categories as defined in Table 1)
Multivariable-adjusted models included as covariates all those indicated in the footnote of Table 2 + energies expended on other types of physical activity (as continuous variables): jogging, running, bicycling, aerobic exercise/dance/exercise machines, lower intensity exercise (yoga/stretching/toning), racquet sports, swimming, stair climbing
Furthermore, in the restricted group of 1387 women who did not engage in any vigorous activity, the associations with walking remained similar, although the results were of borderline significance. For example, the difference in mean decline for the global score was 0.02 standard units (0.00, 0.05) (p-trend = 0.08) when contrasting the highest to the lowest quartiles of walking.
Effect modification and further stratified analyses
We observed no significant interactions with the variables we tested as potential effect modifiers (i.e., age, education, depression status, cardiovascular profile, diabetes, and hypertension). Models limited to women in the top 90th percentile of global score at first cognitive interview (i.e., women with preserved cognitive function) yielded similar results as the primary analyses, with a p for trend across quintiles of total physical activity of 0.004 for both global cognitive and verbal memory scores.
COMMENT
In this large prospective study of women with preexisting CVD or vascular risk factors at higher risk of cognitive decline, greater physical activity was associated with substantially slower cognitive decline. Being in the two highest quintiles of physical activity was cognitively equivalent to being 5 to 7 years younger in age. Importantly, the association with total physical activity was not restricted to women engaged in vigorous exercise; higher levels of walking were significantly related to less cognitive decline.
Strong evidence supports the hypothesis that physical activity, including walking, may prevent cognitive decline in generally healthy older adults4–12. However, studies of those at increased risk of cognitive impairment have been scarce. Some small clinical trials in participants with general cognitive impairments or complaints9, 11 or with severe congestive heart failure26 reported improvement or preservation of cognition in the physically active groups, but overall, the definitions of “high-risk” have been heterogeneous (e.g., general cognitive complaints rather than specific causes of cognitive complaints), yielding some inconsistent and inconclusive findings5, 7, 27, 28. Thus, our findings provide important population-based long-term data that should be confirmed.
In our study, associations with change in category fluency were less consistent than those for memory and general cognition; this was also observed in a cohort of healthy women6. Category fluency partially measures executive function, which is known to be affected by vascular disease24, 25. One could speculate that the indirect vasculoprotective effects are weaker than the direct neuroprotective effects in preserving cognition, and thus, the domain most affected by vascular factors, such as executive function, may be less influenced than other domains. The potential differential associations with various cognitive domains are poorly understood. In addition, short-term intervention studies11, 29–31 and large observational cohorts4, 6, 10, 12 have revealed the possibility of differential effects with sex and different types of activities. These are all important issues that need evaluation in future studies.
Our study had several strengths. Four repeated cognitive assessments with high response rates were completed, maximizing information and minimizing biases due to loss to follow-up. Extensive health-related information was available, which allowed us to address confounding by baseline health status. Moreover, physical activity levels showed little variability according to baseline vascular disease, indicating low chance of confounding by severity of cardiovascular condition.
Some limitations should be considered. First, a telephone cognitive assessment might lack validity. However, both reliability and validity studies of our telephone instrument have provided convincing evidence of its utility to evaluate cognitive function in an epidemiologic study. Second, there may have been some misclassification of physical activity levels that were based on self-report. However, the physical activity questionnaire has been shown to reliably estimate physical activity levels19, and misclassification would have led to biases towards the null. Most importantly, physical activity was assessed in late adulthood and may not reflect long-term exercise levels; it is also possible that inactivity/sedentary lifestyle may reflect pre-existing cognitive impairment rather than a risk factor for its future development32. We addressed this possible bias in several ways; we imposed a mean 3.5-year lag between report of physical activity and the initial cognitive assessment, and we conducted several secondary analyses among women whose activity levels were stable over the two assessments before the cognitive assessment and among women at the top 90th percentile at the first cognitive interview (i.e., after excluding those more likely to have reduced physical activity or have more errors in reporting activity due to cognitive impairment). We confirmed that overall, higher levels of physical activity were consistently and significantly associated with less cognitive decline. We were unable to adjust for other potential confounders (e.g., chronic kidney disease or other psychiatric disorders); thus, some residual confounding by other health or lifestyle factors is a possibility, and the results should be interpreted with appropriate caution. Finally, our study population, which was composed of female health professionals with vascular conditions, may not allow for direct generalizeability of the results to the general aging population. However, given that the majority of today’s elderly have prevalent cardiovascular disease (affecting over 70% of those aged ≥65 years in the US3), our study provides some important evidence, which should be confirmed in other studies, of a modifiable risk factor for reducing cognitive impairment in this growing segment of the population.
Various biologic mechanisms may explain the positive relationship between physical activity and cognitive health33. Exercise may directly preserve neuronal structures by stimulating brain-derived neurotrophic factor and neuronal growth34, possibly providing reserve against cognitive decline and dementia35. It may also have indirect effects by strengthening the underlying systems that support brain plasticity36, 37 and helping to sustain the brain’s vascular health38 by beneficially influencing cardiovascular risk factors, promoting endothelial function, improving glucose and insulin regulation, and ensuring adequate cerebral perfusion39. Furthermore, physical activity reduces inflammation, which is higher in those with vascular disease40, 41 and impairs both systemic and brain-specific growth factor signaling. Physical activity may also improve psychological well-being42, which, in turn, may protect against decline in cognitive functioning43.
In summary, we found clear and strong associations between greater physical activity and reduced cognitive decline in this population of women with vascular disease or coronary risk factors. If confirmed in future studies, given the growing number of older persons with vascular disease or risk factors and their higher risk of cognitive impairment, physical activity recommendations could yield substantial public health benefits.
Acknowledgments
Funding/Support: This work was supported by the National Institutes of Health (HL046959, AG15933) and the American Heart Association. Dr Vercambre is supported by the Fondation Bettencourt-Schueller for her postdoctoral fellowship
Role of the Sponsor: The American Heart Association financially supported the study but was not involved in any of the following: design and conduct of the study, collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript.
Footnotes
Dr Kang had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Vercambre, Kang, Grodstein.
Acquisition of data: Kang, Grodstein, Manson.
Analysis and interpretation of data: Vercambre, Kang, Grodstein, Stampfer, Manson.
Drafting of the manuscript: Vercambre.
Critical revision of the manuscript for important intellectual content: Kang, Grodstein, Stampfer, Manson.
Statistical analysis: Vercambre.
Obtained funding: Kang, Grodstein.
Study supervision: Kang.
Financial Disclosures: None reported.
Additional contribution: We are grateful to the investigators, staff and participants of the WACS cognitive substudy.
References
- 1.de la Torre JC. How do heart disease and stroke become risk factors for Alzheimer’s disease? Neurol Res. 2006;28(6):637–644. doi: 10.1179/016164106X130362. [DOI] [PubMed] [Google Scholar]
- 2.Reitz C, Tang M-X, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. A summary risk score for the prediction of Alzheimer Disease in elderly persons. Arch Neurol. 2010;67(7):835–841. doi: 10.1001/archneurol.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart Disease and Stroke Statistics--2009 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 4.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161(14):1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 5.Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33(5):772–777. doi: 10.1097/00005768-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292(12):1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 7.Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161(7):639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 8.Cassilhas RC, Viana VA, Grassmann V, et al. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc. 2007;39(8):1401–1407. doi: 10.1249/mss.0b013e318060111f. [DOI] [PubMed] [Google Scholar]
- 9.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 10.Middleton LE, Mitnitski A, Fallah N, Kirkland SA, Rockwood K. Changes in cognition and mortality in relation to exercise in late life: a population based study. PLoS One. 2008;3(9):e3124. doi: 10.1371/journal.pone.0003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67(1):71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etgen T, Sander D, Huntgeburth U, Poppert H, Forstl H, Bickel H. Physical activity and incident cognitive impairment in elderly persons: the INVADE study. Arch Intern Med. 2010;170(2):186–193. doi: 10.1001/archinternmed.2009.498. [DOI] [PubMed] [Google Scholar]
- 13.Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2000;(4):CD001800. doi: 10.1002/14651858.CD001800. [DOI] [PubMed] [Google Scholar]
- 14.Cook NR, Albert CM, Gaziano JM, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women’s Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167(15):1610–1618. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299(17):2027–2036. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang JH, Cook N, Manson J, Buring JE, Albert CM, Grodstein F. A trial of B vitamins and cognitive function among women at high risk of cardiovascular disease. Am J Clin Nutr. 2008;88(6):1602–1610. doi: 10.3945/ajcn.2008.26404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang JH, Cook NR, Manson JE, Buring JE, Albert CM, Grodstein F. Vitamin E, vitamin C, beta carotene, and cognitive function among women with or at risk of cardiovascular disease: The Women’s Antioxidant and Cardiovascular Study. Circulation. 2009;119(21):2772–2780. doi: 10.1161/CIRCULATIONAHA.108.816900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 20.Brandt J, Folstein MF. Telephone Interview for Cognitive Status: Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- 21.Scherr PA, Albert MS, Funkenstein HH, et al. Correlates of cognitive function in an elderly community population. Am J Epidemiol. 1988;128:1084–1101. doi: 10.1093/oxfordjournals.aje.a115051. [DOI] [PubMed] [Google Scholar]
- 22.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 23.Small BJ, Fratiglioni L, Viitanen M, Winblad B, Backman L. The course of cognitive impairment in preclinical Alzheimer disease: three- and 6-year follow-up of a population based sample. Arch Neurol. 2000;57:839–844. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien JT. Vascular cognitive impairment. Am J Geriatr Psychiatry. 2006;14(9):724–733. doi: 10.1097/01.JGP.0000231780.44684.7e. [DOI] [PubMed] [Google Scholar]
- 25.Desmond DW. The neuropsychology of vascular cognitive impairment: is there a specific cognitive deficit? J Neurol Sci. 2004;226(1–2):3–7. doi: 10.1016/j.jns.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Tanne D, Freimark D, Poreh A, et al. Cognitive functions in severe congestive heart failure before and after an exercise training program. Int J Cardiol. 2005;103(2):145–149. doi: 10.1016/j.ijcard.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 27.Ploughman M, McCarthy J, Bosse M, Sullivan HJ, Corbett D. Does treadmill exercise improve performance of cognitive or upper-extremity tasks in people with chronic stroke? A randomized cross-over trial. Arch Phys Med Rehabil. 2008;89(11):2041–2047. doi: 10.1016/j.apmr.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Devore EE, Kang JH, Okereke O, Grodstein F. Physical Activity Levels and Cognition in Women With Type 2 Diabetes. Am J Epidemiol. 2009;170(8):1040–1047. doi: 10.1093/aje/kwp224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008;(3):CD005381. doi: 10.1002/14651858.CD005381.pub3. [DOI] [PubMed] [Google Scholar]
- 30.Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance Training and Executive Functions: A 12-Month Randomized Controlled Trial. Arch Intern Med. 2010;170(2):170–178. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klusmann V, Evers A, Schwarzer R, et al. Complex mental and physical activity in older women and cognitive performance: a 6-month randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2010;65(6):680–688. doi: 10.1093/gerona/glq053. [DOI] [PubMed] [Google Scholar]
- 32.Solfrizzi V, Capurso C, D’Introno A, et al. Lifestyle-related factors in predementia and dementia syndromes. Expert Rev Neurother. 2008;8(1):133–158. doi: 10.1586/14737175.8.1.133. [DOI] [PubMed] [Google Scholar]
- 33.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11(8):342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3(6):343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 36.Burns JM, Cronk BB, Anderson HS, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71(3):210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson PD, Buchner D, Pina IL, et al. Exercise and Physical Activity in the Prevention and Treatment of Atherosclerotic Cardiovascular Disease: A Statement From the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107(24):3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 39.Rosano C, Newman AB. Cardiovascular disease and risk of Alzheimer’s disease. Neurol Res. 2006;28(6):612–620. doi: 10.1179/016164106X130407. [DOI] [PubMed] [Google Scholar]
- 40.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 41.Hamer M, Stamatakis E. Physical activity and risk of cardiovascular disease events: inflammatory and metabolic mechanisms. Med Sci Sports Exerc. 2009;41(6):1206–1211. doi: 10.1249/MSS.0b013e3181971247. [DOI] [PubMed] [Google Scholar]
- 42.Vance DE, Wadley VG, Ball KK, Roenker DL, Rizzo M. The effects of physical activity and sedentary behavior on cognitive health in older adults. J Aging Phys Act. 2005;13(3):294–313. doi: 10.1123/japa.13.3.294. [DOI] [PubMed] [Google Scholar]
- 43.Beaudreau SA, O’Hara R. The association of anxiety and depressive symptoms with cognitive performance in community-dwelling older adults. Psychol Aging. 2009;24(2):507–512. doi: 10.1037/a0016035. [DOI] [PMC free article] [PubMed] [Google Scholar]