Abstract
Our previous work suggested that treatment of cells with hyperosmotic media during 2D passaging primes cells for cartilage tissue engineering applications. Here, we used label-free proteomic profiling to evaluate the effects of control and hyperosmotic treatment environments on the phenotype of multipotent adipose-derived stem cells (ASCs) cultivated with a chondrogenic growth factor cocktail. Spectra were recorded in a data-independent fashion at alternate low (precursor) and high (product) fragmentation voltages (MSE). This method was supplemented with data mining of accurate mass and retention time matches in precursor ion spectra across the experiment. The results indicated a complex cellular response to osmotic treatment, with a number of proteins differentially expressed between control and treated cell groups. The roles of some of these proteins have been documented in the literature as characteristic of the physiological states studied, especially aldose reductase (osmotic stress). This protein acted as a positive control in this work, providing independent corroborative validation. Other proteins, including 5’-nucleotidase and transgelin, have been previously linked to cell differentiation state. This study demonstrates that label-free profiling can serve as a useful tool in characterizing cellular responses to chondrogenic treatment regimes, recommending its use in optimization of cell priming protocols for cartilage tissue engineering.
Keywords: Label-free, proteomics, data mining, adipose, stem cells, chondrocytes, cartilage
INTRODUCTION
Osteoarthritis (OA), the destruction of articular cartilage that covers the surfaces of our mobile joints (e.g., knee and hip), costs an estimated $128 billion in annual healthcare and job-related losses in the United States. The poor healing capacity of articular cartilage has led to intense research toward development of cell-based therapies for cartilage repair, including efforts to identify cell sources with potential clinical relevance for cartilage tissue engineering. Challenges associated with these sources include donor site availability and capability of cells to produce levels of extracellular matrix sufficient for the survival of engineered tissues upon implantation in the knee joint environment. Cell expansion and priming with chemical or physical factors are two interleaved approaches often attempted to address these challenges.1 In these strategies, culturing of cells on a two-dimensional (2D) tissue culture dish provides a platform for increasing cell number (expansion) as well as an opportunity for applying chemical and physical stimuli (priming) that can induce differentiation of cells towards a desired lineage, e.g., a chondrogenic lineage. Cells subjected to expansion and priming have previously yielded more robust tissue production when subsequently seeded in 3D scaffolds that are supportive of the chondrogenic phenotype.2, 3
Methods for accurately and rapidly assessing the impact of 2D priming techniques on cell cartilage tissue generation in subsequent 3D culture are currently lacking. Comparison of RNA levels expressed in cells during 2D culture in control and experimental conditions may capture only transient phenomena. Furthermore, RNA levels may not correlate well with production of functional tissue proteins, given protein synthetic capability and the extensive trafficking and post-translational modification pathways that cartilage tissue proteins must undergo before secretion from the cell. Additionally, one-by-one evaluation of the efficacy of each 2D treatment condition, by comparison of tissue component elaboration in subsequent 3D cultures, requires a large investment of time and resources in both the 2D expansion and 3D culture stages of the experiment. Therefore, an ability to identify during 2D expansion the experimental treatments that best improve cells’ capacity to form functional engineered cartilage tissues in subsequent 3D culture would enhance the rapid optimization of cartilage tissue engineering protocols.
Proteomics techniques could allow the rapid assessment of the influence of 2D treatment conditions on cell protein, rather than RNA, production at early stages of cell culture.4 However, in spite of this potential, few studies have applied modern proteomic technologies to the biological assessment of chondrocytes and chondrogenic potential.5–11 Studies using human cell sources, as well as proteomics techniques that provide quantitative information with multiple replicates, are particularly scant. Of the large-scale protein profiling strategies currently available, label-free protein profiling has attracted interest due to its simplicity of implementation and its potential to support complex experimental designs. Increased reproducibility of chromatography at nanoliter per minute flow rates, coupled with increased sensitivity and resolution of mass spectrometers, have reinforced the attractiveness and feasibility of the label-free protein profiling approach. In a variant of the method, spectra are recorded at alternately low (precursor) and high (product) fragmentation voltages, in a data-independent process called MSE acquisition,12 allowing high data density and predictable data acquisition. The conventional approach to protein identification and quantification employs data-dependent acquisition (DDA). Published findings from a number of groups indicate that MSE is effective for a wide range of biological systems including microbial cells,12 mammalian tissues,13 tumor samples,14 stem cell knockout cell lines,15 and many other applications.16–21
Therefore, we describe here our use of label-free proteomics to detect reproducible alterations in chondrocyte precursor cell phenotype upon application of a potential chondrogenic stimulus in 2D cell culture. We prepared proteomic samples from multipotent ASCs that had been expanded in 2D in control or physiologically-relevant hyperosmotic culture environments22 that included a chondrogenic growth factor cocktail. We chose the hyperosmotic stimulus based upon our laboratory’s previous success in applying physiologic stimuli in cartilage tissue engineering applications and upon preliminary data indicating that expansion of human chondrocytes in media of higher osmolarity increases cells’ expression of a gene (aggrecan) related to production of cartilage tissue (unpublished data). Additionally, we chose ASCs as a clinically-relevant allogeneic and autologous cell type that is advantageous in donor tissue accessibility and abundance relative to other multipotent cell types, due to the prevalence of elective cosmetic liposuction procedures. The results of this study provide an initial assessment of the feasibility of using proteomics to evaluate the effects of 2D cell priming treatments on cell phenotype, working toward our ultimate goal of using this technology to rapidly optimize such treatments for 3D cartilage engineering applications.
MATERIALS AND METHODS
Cell Culture
ASC medium was prepared from a 1:1 ratio of high-glucose (4.5 g/L) DMEM:DMEM/F12 containing 3.25 mM L-glutamine (Invitrogen) by the addition of 1% penicillin-streptomycin (Invitrogen), 0.1% gentamycin (Invitrogen), 0.5% Fungizone (Invitrogen), 100 nM dexamethasone (Sigma), and 10% FBS (Invitrogen). Control and treatment osmotic media were prepared from a base medium consisting of high-glucose (4.5 g/L) DMEM containing 4 mM L-glutamine (Invitrogen), 10 mM each HEPES, BES, and TES buffers (Mediatech), 1x each MEM and non-essential amino acids (Mediatech), 1% penicillin/streptomycin (Invitrogen), and 5% FBS (Atlantic Biologicals). This base medium was diluted with deionized water to 300 mOsM (control medium), from which 400 mOsM media (treatment medium) was prepared by the addition of NaCl (biotechnology grade, Sigma). Both osmotic media were further supplemented with growth factor cocktail (1 ng/mL TGF-β3, 10 ng/mL PDGF, 5 ng/mL FGF-2, and 5 ng/mL EGF), a chondrogenic cocktail similar to that used previously in our laboratory.23
Twice-passaged human ASCs isolated from the abdominal fat of a 42-year-old female patient were obtained in a cryopreserved state from Dr. Kacey Marra, at the University of Pittsburgh (IRB-exempt). Cells were thawed and expanded to ~90% confluence for two additional passages in tissue culture-treated flasks containing ASC medium. At passage five, cells were split into flasks containing either control or treatment media (plating density 1.4×10−2 cells/cm2). Cells were grown until the control group reached ~90% confluence (3 days). Due to slight differences in the cell doubling rate between osmotic groups at each passage, treatment group cells were at ~80% confluence when control group cells were at ~90% confluence. Cells were then subjected to an additional passage in media of the same osmolarity at the same density (8 days). Upon confluence at passage six, cells were plated for a final passage (three dishes per osmotic group) at 6×10−3 cells/cm2 and maintained in the same osmotic media used in previous passages. Once the control group had reached 90% confluence (10 days), cells from each dish were harvested as follows for proteomics analysis, yielding three unique biological replicate samples per osmotic culture condition (control and treatment).
Sample Preparation
Cells were washed three times with 10 mL of ice cold PBS. Careful attention was paid to the cell washing procedure, to rid the cell samples of excess BSA and other proteins from the culture media. Cells were lysed in 0.3% SDS, Tris-buffered saline with 1% protease inhibitor cocktail (Sigma), precipitated using methanol/chloroform,24 then dissolved in 0.1% Rapigest (Waters Corp.). Dithiothreitol (6 mM) was added; the solution was sonicated in a bath sonicator for 5 minutes and then boiled for five minutes. Approximate protein content in each sample was estimated with the Bradford Protein Assay (Biorad). Cysteines were alkylated with iodoacetamide. Proteins were digested with trypsin (6 ng/μL (#V511A, Promega Corp.) in 50 mM NH4HCO3) and 50 fmol of a digest of yeast alcohol dehydrogenase was added as an internal detection control.
Chromatography and Mass Spectrometry
Three chromatograms were recorded for each of six biological replicates (three isotonic, three hypertonic), yielding 18 chromatograms. Prior to analytical separation on a NanoAcquity UPLC (Waters Corp.), peptides were trapped on a Symmetry C18 Trap column, 5 μm particles, 180 μm × 20 mm (Waters Corp.), for 1 minute at 15 μL/minute in 1% solvent B (0.3% formic acid in acetonitrile)/99% solvent A (0.3% formic acid, aqueous). Peptides were analyzed in a 120-minute chromatogram on a 75 μm ID × 10 cm reverse phase 1.7 μm particle diameter bridged ethyl hybrid (BEH) C18 column at a flow rate of 300 nL/minute. For the analytical separation, Solvent B was increased in a 90-minute linear gradient between 3 and 40%, and post-gradient cycled to 95% B for 7 minute, followed by post-run equilibration at 3% B. Spectra were recorded in V-positive mode with a Synapt quadrupole-time-of-flight mass spectrometer (Waters Corp). Source settings included extraction cone at 3.5 V, sampling cone at 24 V, and source temperature 80 °C. Collision energy was held at 6V for low energy scan and ramped from 15–35 V for the high energy scan with a collision gas flow (Ar) of 1.5 mL/minute. Alternate 0.6 s scans at low and high energy were recorded for the range between 100 and 1990 m/z. A reference sprayer was operated at 300 nL/minute to produce a lockmass spectrum with Glu-1-Fibrinopeptide B (m/z 785.8426) every 30 s.
Data Analysis
Spectra were analyzed with ProteinLynx Global Server (Vers. 2.4, RC7) (Waters Corp.) and searched against a database of human protein sequences (reviewed canonical sequences with isoforms) from UniProt release 15.5 (July 7, 2009). The database also contained sequences for yeast alcohol dehydrogenase, porcine trypsin, bovine serum proteins (BSA, serotransferrin, fibrinogen alpha chain, fibrinogen beta chain and fibrinogen gamma-B). This database was comprised of 34,602 protein sequences (20,005,831 amino acid residues). Data mining and statistical analysis was performed with the Elucidator Protein Expression Data Analysis System, Version 3.3 (3.3.0.1.SP3_CRE52.21) (Rosetta Biosoftware). Proteins were identified with a PeptideTeller predicted error (false positive rate) of 1% and a calculated decoy error rate of 0.2% (see Keller et al.25). Ratio P-values for differential expression were calculated by the Elucidator program using the xdev parameter.26, 27 The P-values calculated are not from an analysis of variance, but instead derive from an application of an error model developed for large scale microarray data as adapted for proteomics within the Elucidator program. Analysis of results was also enhanced by use of the DAVID28 and KEGG29 resources for pathway analysis.
The data associated with this manuscript may be downloaded from ProteomeCommons.org Tranche using the following hashes.
-
A spreadsheet with details on detected peptides can be accessed with this hash:
keRkXr0LAKZduC5IMYhwlEywWoYgrkctqxInmcbWUDnop6ZJEtkzJU5n2Fo6Oo/UK70kdtvzaGgeV 8N0t2mW4B+1qV4AAAAAAAACUw==
-
All 18 raw data files may be accessed with this hash:
zJqhLDUH1FRNpLaHO6Wqo+E34i9xmuR7K1q8RBKa+zcaQFZv3L2PfHygfiWY2fRjCo4I7o0k3uhz 43xRtq2fknKwLOsAAAAAAACRZQ==
These hashes may be used to prove exactly what files were published as part of this manuscript’s data set, and the hash may also be used to check that the data has not changed since publication.
RESULTS
MSE technology identified and quantified proteins in whole cell lysates of six separate cultures (biological replicates of three control and three experimental treatments). Principal component analysis revealed good reproducibility for the technical replicates for each biological replicate (Figure 1). It also revealed clear separation in the protein abundance patterns between control and experimental treatments.
Figure 1.
Principal component analysis of Z-score transformed intensity data processed by the Elucidator program for all 18 LC/MS Chromatograms in this experiment. Each data point represents a chromatogram. Data from control cells grown in 300 mOsM media are all found on the low end of the first principal component axis. Data from the 400 mOsM grown cells (treated) are grouped on the higher end of that axis, suggesting an overall global effect of the osmolarity treatment. Replicate LC/MS runs (of like color) are relatively close together in most cases indicating excellent reproducibility of chromatography and mass spectrometry.
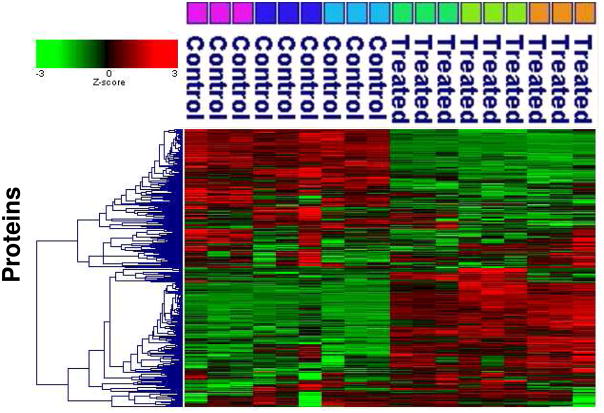
Hierarchical clustering revealed that treatment of cells with media of higher osmolarity resulted in increased abundance of some proteins and decreased abundance of others (Figure 2). This figure represents intensity data transformed via Z-score, which may magnify small differences in protein abundance. No statistical or protein identification filters were applied to improve reliability at this level of the analysis or in the expanded graphic covering the entire dataset (Supporting Data Figure 1). Thus, important overall trends in the data are apparent, but individual proteins are evaluated based on other factors (see below). Figures 1 and 2 show overall good reproducibility of replicate LC/MS runs. Figure 2 also shows less variability in the mass spectrometric measurement than in the biological variability, thus suggesting that the effects of treatment were an important factor in this experiment.
Figure 2.
Agglomerative hierarchical cluster of Z-score transformed intensity data processed by the Elucidator program for all 18 LC/MS chromatograms in this experiment. Proteins identified in each chromatogram are labeled as control (300 mOsM) or treated (400 mOsM). Groups of three identically-colored boxes above the results of each LC/MS run indicate the results for three replicate LC/MS runs for each biological replicate (three biological replicates of control (300 mOsM) or treated (400 mOsM) cells). Z-score coloration indicates protein abundance in the sample (red indicates higher abundance, green indicates lower abundance and black equal abundance). The cluster suggests many proteins were downregulated at 400 mOsM (upper part of cluster) and many proteins upregulated at 400 mOsM (lower part of cluster). It should be emphasized that there was no filtering applied for statistical significance, fold change or the relative reliability of the protein identification. Z-score transformation as presented emphasizes small differences.
A total of 294 proteins were detected with a peptide error (false positive rate) of 1% and a calculated decoy error rate of 0.2% (see list in Supporting Data Table 1). Of those proteins, 185 proteins were identified based on two or more peptides. There were 113,595 peptide hits (matched spectra) to the forward database including multiple detections of the same peptide at the same or different charge states. A total of 2,251 unique peptide sequences were identified. Overall, the average mass error for these peptides was 7.0 ppm. Average protein sequence coverage was 19.9%.
Three examples of protein expression patterns obtained for the two treatment conditions are shown in Figure 3. Glyceraldehyde-3-phosphate dehydrogenase exhibited largely equal expression between control and treatment samples. This was seen in the MS signal for a single feature from an isotopic cluster for each of two peptides (LISWYDNEFGYSNR and GALQNIIPASTGAAK), illustrating the lack of demonstrable difference in relative abundance between samples from control and treated cultures. Also, an MS/MS spectrum derived from each of these peptides extracted from the high collision energy scan is shown illustrating y- and b-series of fragment ions. In contrast, aldose reductase exhibited a significant increase (3.5-fold) in its abundance in the treatment group, as reflected in MS features from two peptides and in increased relative expression values calculated for the individual LC/MS chromatograms. Corresponding prominent y-ion series in MSE-derived spectra for two peptides support the identification of this protein. Finally, an example of a protein, 5’-nucleotidase (5NTD), with decreased abundance (0.6-fold) in medium of higher osmolarity is also illustrated in Figure 3. As for two other proteins, example MS features from two peptides, and decreased relative expression values and y- and b-ion series in MSE-derived spectra, are illustrated.
Figure 3.
Example relative quantification data and supporting identification data for a protein of equal unchanged abundance (upper) panel, a protein with increased abundance (middle panel) and one with decreased abundance (lower panel) as a result of treatment (400 mOsM). Relative abundance (%) plots show the results for three replicate LC/MS runs for each biological replicate (three biological replicates of control (300 mOsM) or treated (400 mOsM). For each panel, example graphics for two peptides are shown, including a single component of an isotopic cluster (feature plotted at relative % abundance) generated by the Elucidator program (charge state indicated), and a derived ms/ms spectrum generated by the PLGS program from high collision energy scan of MSE data. Red peaks are y-ions, blue peaks are b-ions, and green are modified ions (e.g. loss of NH3) in the MSE scans. The feature peak in each case is an overlay of aligned MS spectra (the most intense from an isotopic cluster representing a single charge state) of matching m/z and retention time for the 18 LC/MS runs in the experiment. Below the graphics is a table representing relative expression of these proteins in (millions of) relative units. Nine replicates for control and treated are shown. They are ordered sequentially in groups of three representing replicate LC/MS runs of the same biological replicate sample. Glyceraldehyde-3-phosphate dehydrogenase had a ratio of treated/control 1.0 (P = n.s.), ALDR had an abundance ratio of 3.5 (P < 10−45) and 5NTD had an abundance ratio of 0.62 (P < 10−41). Abundance ratio P-values were calculated within Elucidator as described previously.26
Analogous differences were recorded for additional proteins from this dataset that were selected based on the magnitude of their fold-change in abundance (+/−1.5-fold ratio), significant P-value (< 0.01), ProteinTeller probability (≥0.98) and number of unique peptide sequences supporting both the protein identification and protein quantification (≥2) (Figure 4). The 26 proteins meeting these criteria were selected from the 294 proteins detected by the Elucidator program (see Supporting Data, Figure 1 and Table 1). A hierarchical agglomerative cluster of Z-score transformed intensities along with average intensities for control and treated cells, fold-changes and P-value are summarized for the 26 proteins in Figure 4.
Figure 4.
Expression data for selected proteins in agglomerative hierarchical cluster of Z-score transformed intensity data processed by the Elucidator program for all 18 LC/MS chromatograms in this experiment. These proteins have at least 1.5-fold response to the treatment, and are represented by at least two peptides for both identification and quantification at ratio P-values as calculated by Elucidator.26 See text for other acceptance criteria. Mean relative abundance of each protein in control and treated as well as abundance ratio (as calculated by Elucidator) are listed (see Supporting Data Table 1 for individual abundance intensities for each replicate and each protein). Cluster coloration indicates protein abundance in the sample (red indicates higher abundance, green indicates lower abundance and black, unchanged abundance). Some proteins were upregulated at 400 mOsM (upper part of cluster) and some were downregulated at 400 mOsM (lower part of cluster). See Supporting Data Figure 1 for graphic that includes all proteins in an agglomerative hierarchical cluster of Z-score transformed intensity data processed by the Elucidator program. Elucidator identified fibronectin as the (shorter) isoform 3, based on the peptides actually detected (the algorithm seeks to provide the simplest interpretation of the data). However, no spectra were annotated to any unique peptides ascribed to isoform 3, thus the protein is listed here as the canonical isoform (P02751).
Table 1 provides a listing of gene ontology (GO) terms derived from the Uniprot database (www.uniprot.org)30 along with Refseq summary descriptions from the NCBI database (www.ncbi.nlm.nih.gov) to provide a summary description of the proteins from Figure 4. Based solely on this summary of functional information, we can surmise that proteins of increased abundance in the treatment group are regulators of cell proliferation, migration and apoptosis (ATP synthase alpha mitochondrial, pyruvate kinase M1/M2, superoxide dismutase (SOD), reticulon-4, S100-A6, zyxin) and glycolysis (pyruvate kinase R/L and transketolase). Aldose reductase, known for its activity in reducing glucose to sorbitol in response to osmotic challenges, was increased in abundance by higher osmolarity treatment of cells. Of the set of proteins detected at lower abundance in treated cells, those related to collagen biosynthesis and binding and the extracellular matrix (fibronectin, pro-collagen, lysine 2-oxoglutarate 5-dioxygenase 2 (PLOD2), Prolyl 4-hydroxylase subunit alpha-1 and alpha-2 (P4HA1 and P4HA2)) are particularly intriguing, due to the function of chondrocyte lineage cells in generating and binding ECM.
Table 1.
Gene Ontology (GO) terms associated with proteins listed in Figure 4 (listed here in hierarchical order that they appear in Figure 4). GO terms were downloaded from the Uniprot database. For some proteins, summary information (in parentheses at end of entry) is also included from Refseq database (NCBI).
| Uniprot Accession number and Protein Name (boldface), GO terms and Refseq description (in parentheses) |
|---|
| Increased Abundance |
| P25705 (ATPA_HUMAN): ATP synthase subunit alpha, mitochondrial - ATP binding; MHC class I protein binding; embryonic development; eukaryotic cell surface binding; hydrogen ion transporting ATP synthase activity, rotational mechanism; lipid metabolic process; mitochondrial ATP synthesis coupled proton transport; mitochondrial matrix; negative regulation of endothelial cell proliferation; plasma membrane; proton-transporting ATPase activity, rotational mechanism |
| Q99879 (H2B1M_HUMAN): Histone H2B type F-S - DNA binding; nucleosome; nucleosome assembly; nucleus |
| P14618 (KPYM_HUMAN): Pyruvate kinase isozymes M1/M2 - ATP binding; cytosol; glycolysis; magnesium ion binding; nucleus; potassium ion binding; programmed cell death; protein binding; pyruvate kinase activity |
| P15121 (ALDR_HUMAN): Aldose reductase - aldehyde reductase activity; carbohydrate metabolic process; cytosol; electron carrier activity; extracellular space; oxidation reduction; protein binding; response to stress. (This gene encodes a member of the aldo/keto reductase superfamily, which consists of more than 40 known enzymes and proteins. This member catalyzes the reduction of a number of aldehydes, including the aldehyde form of glucose, and is thereby implicated in the development of diabetic complications by catalyzing the reduction of glucose to sorbitol.) |
| P14618-2 (KPYM_HUMAN): Isoform M1 of Pyruvate kinase isozymes M1/M2 - ATP binding; cytosol; glycolysis; magnesium ion binding; nucleus; potassium ion binding; programmed cell death; protein binding; pyruvate kinase activity. (This protein has been found to bind Opa protein, a bacterial outer membrane protein involved in gonococcal adherence to and invasion of human cells, suggesting a role of this protein in bacterial pathogenesis.) |
| P52895 (AK1C2_HUMAN): Aldo-keto reductase family 1 member C2 - 3-alpha-hydroxysteroid dehydrogenase (A-specific) activity; bile acid binding; cytoplasm; digestion; oxidation reduction; prostaglandin metabolic process; steroid metabolic process; trans-1,2-dihydrobenzene-1,2-diol dehydrogenase activity. (This gene encodes a member of the aldo/keto reductase superfamily, which consists of more than 40 known enzymes and proteins. These enzymes catalyze the conversion of aldehydes and ketones to their corresponding alcohols using NADH and/or NADPH as cofactors.) |
| P04179 (SODM_HUMAN): Superoxide dismutase [Mn], mitochondrial - age-dependent response to reactive oxygen species; manganese ion binding; mitochondrial matrix; negative regulation of neuron apoptosis; oxygen homeostasis; protein binding; protein homotetramerization; regulation of transcription from RNA polymerase II promoter; release of cytochrome c from mitochondria; removal of superoxide radicals; superoxide dismutase activity; vasodilation by acetylcholine involved in regulation of systemic arterial blood pressure |
| P40939 (ECHA_HUMAN): Trifunctional enzyme subunit alpha, mitochondrial - 3-hydroxyacyl-CoA dehydrogenase activity; acetyl-CoA C-acetyltransferase activity; enoyl-CoA hydratase activity; fatty acid beta-oxidation multienzyme complex; long-chain-3-hydroxyacyl-CoA dehydrogenase activity; mitochondrial nucleoid; oxidation reduction |
| P61026 (RAB10_HUMAN): Ras-related protein Rab-10 - GTP binding; plasma membrane; protein binding; protein transport; small GTPase mediated signal transduction |
| P29401 (TKT_HUMAN): Transketolase - cytosol; metabolic process; metal ion binding; protein binding; transketolase activity. (This gene encodes a thiamine-dependent enzyme which plays a role in the channeling of excess sugar phosphates to glycolysis in the pentose phosphate pathway.) |
| P30613 (KPYR_HUMAN): Pyruvate kinase isozymes R/L - ATP binding; glycolysis; magnesium ion binding; potassium ion binding; pyruvate kinase activity. (The protein encoded by this gene is a pyruvate kinase that catalyzes the transphosphorylation of phohsphoenolpyruvate into pyruvate and ATP, which is the rate-limiting step of glycolysis.) |
| Q9NQC3 (RTN4_HUMAN): Reticulon-4 - apoptosis; integral to endoplasmic reticulum membrane; negative regulation of anti-apoptosis; negative regulation of axon extension; nuclear envelope; plasma membrane; regulation of apoptosis; regulation of cell migration |
| Q99536 (VAT1_HUMAN): Synaptic vesicle membrane protein VAT-1 homolog - cytoplasm; integral to membrane; oxidation reduction; oxidoreductase activity; zinc ion binding. (The protein encoded by this gene is an abundant integral membrane protein of cholinergic synaptic vesicles and is thought to be involved in vesicular transport.) |
| Q16695 (H31T_HUMAN): Histone H3.1 - DNA binding; nucleoplasm; nucleosome; nucleosome assembly; protein binding |
| P06703 (S10A6_HUMAN): Protein S100-A6 - S100 beta binding; axonogenesis; calcium ion binding; calcium-dependent protein binding; cytosol; extrinsic to internal side of plasma membrane; nuclear envelope; perinuclear region of cytoplasm; positive regulation of fibroblast proliferation; protein homodimerization activity; ruffle; signal transduction; tropomyosin binding |
| Q15942 (ZYX_HUMAN): Zyxin - cell adhesion; cell-cell adherens junction; cell-cell signaling; cytoplasm; focal adhesion; integral to plasma membrane; interspecies interaction between organisms; nucleus; protein binding; signal transduction; stress fiber; zinc ion binding. (Zyxin may function as a messenger in the signal transduction pathway that mediates adhesion-stimulated changes in gene expression and may modulate the cytoskeletal organization of actin bundles.) |
| Decreased Abundance |
| P02751 (FINC_HUMAN): Fibronectin - ER-Golgi intermediate compartment; acute-phase response; angiogenesis; cell migration; collagen binding; extracellular matrix structural constituent; fibrinogen complex; heparin binding; peptide cross-linking; platelet alpha granule lumen; proteinaceous extracellular matrix; regulation of cell shape; substrate adhesion-dependent cell spreading |
| P19105 (ML12A_HUMAN): Myosin regulatory light chain 12A - calcium ion binding; motor activity; myosin complex; protein binding |
| P42224 (STAT1_HUMAN): Signal transducer and activator of transcription 1-alpha/beta - I-kappaB kinase/NF-kappaB cascade; activation of caspase activity; calcium ion binding; cytoplasm; hematopoietin/interferon-class (D200-domain) cytokine receptor signal transducer activity; interspecies interaction between organisms; nucleolus; protein binding; regulation of transcription, DNA-dependent; response to virus; transcription factor activity; transcription from RNA polymerase II promoter; tyrosine phosphorylation of STAT protein |
| O00469-2 (PLOD2_HUMAN): Procollagen-lysine,2-oxoglutarate 5-dioxygenase 2 - L-ascorbic acid binding; iron ion binding; oxidation reduction; oxidoreductase activity, acting on single donors with incorporation of molecular oxygen, incorporation of two atoms of oxygen; procollagen-lysine 5-dioxygenase activity; protein binding; protein modification process; response to hypoxia; rough endoplasmic reticulum membrane. (The enzyme (cofactors iron and ascorbate) catalyzes the hydroxylation of lysyl residues in collagen-like peptides. The resultant hydroxylysyl groups are attachment sites for carbohydrates in collagen and thus are critical for the stability of intermolecular crosslinks.) |
| P21589 (5NTD_HUMAN): 5’-nucleotidase - 5’-nucleotidase activity; DNA metabolic process; anchored to membrane; membrane fraction; nucleotide binding; plasma membrane. (The enzyme is used as a marker of lymphocyte differentiation.) |
| P40261 (NNMT_HUMAN): Nicotinamide N-methyltransferase - cytoplasm; nicotinamide N-methyltransferase activity. (N-methylation is one method by which drug and other xenobiotic compounds are metabolized by the liver.) |
| O15460 (P4HA2_HUMAN): Prolyl 4-hydroxylase subunit alpha-2 - L-ascorbic acid binding; electron carrier activity; endoplasmic reticulum lumen; iron ion binding; oxidation reduction; oxidoreductase activity, acting on single donors with incorporation of molecular oxygen, incorporation of two atoms of oxygen; procollagen-proline 4-dioxygenase activity; protein binding. (This gene encodes a component of prolyl 4-hydroxylase, a key enzyme in collagen synthesis. In collagen and related proteins, prolyl 4-hydroxylase catalyzes the formation of 4-hydroxyproline that is essential to the proper three-dimensional folding of newly synthesized procollagen chains.) |
| P13674 (P4HA1_HUMAN): Prolyl 4-hydroxylase subunit alpha-1 - L-ascorbic acid binding; endoplasmic reticulum lumen; iron ion binding; mitochondrion; oxidation reduction; oxidoreductase activity, acting on single donors with incorporation of molecular oxygen, incorporation of two atoms of oxygen; procollagen-proline 4-dioxygenase activity. (This gene encodes a component of prolyl 4-hydroxylase, a key enzyme in collagen synthesis. In collagen and related proteins, prolyl 4-hydroxylase catalyzes the formation of 4-hydroxyproline that is essential to the proper three-dimensional folding of newly synthesized procollagen chains.) |
| Q9Y696 (CLIC4_HUMAN): Chloride intracellular channel protein 4 - actin cytoskeleton; cell junction; cellular response to calcium ion; chloride channel complex; cytoplasmic vesicle membrane; cytosol; keratinocyte differentiation; microtubule organizing center; microvillus; mitochondrion; negative regulation of cell migration; nuclear matrix; soluble fraction; voltage-gated chloride channel activity |
| Q01995 (TAGL_HUMAN): Transgelin - actin binding; cytoplasm; muscle organ development. (The protein encoded by this gene is a transformation and shape-change sensitive actin cross-linking/gelling protein found in fibroblasts and smooth muscle. Its expression is down-regulated in many cell lines, and this down-regulation may be an early and sensitive marker for the onset of transformation. A functional role of this protein is unclear.) |
DISCUSSION
The results of this study indicate that proteomics can serve as a useful tool for assessing cartilage precursor cell response to chondrogenic treatment conditions. Proteomics data not only confirmed previously reported cellular responses to osmotic stress, but also described a complex cell differentiation response to the treatment condition. In our study, both control and treatment groups were subjected to a chondrogenic growth factor cocktail during cell expansion. Therefore, the observed effects on cell protein expression may be attributed to the superposition of osmotic effects on growth factor effects.
There were several alterations in the ASC proteome that were consistent with previous reports on the effects of osmotic stress on protein expression. Aldose reductase (ALDR), a protein increased in expression under treatment conditions (Figure 3, Figure 4), has long been known to mount a primary response to osmotic stress through catalyzing the reduction of glucose to sorbitol, a compatible osmotic solute accumulated at high osmotic pressure.31–33 In our previous work with cultured mouse kidney cells, a 27-fold increase in abundance accompanied a shift in media osmolarity from 300 to 900 mOsM.34 However, a gel-based study on chondrocytes undergoing osmotic stress failed to detect any ALDR.35 It is likely that ALDR was obscured by some other, more abundant protein that migrated similarly on the gels (a common occurrence in gel-based proteomics). The failure to detect ALDR also may have resulted from differences in the processing of biological samples or in the application of osmotic treatment. Consistent with the notion of ALDR involvement in osmotic response are the results of another study, wherein ALDR was not detected as part of the proteome of mouse cartilage not specifically subjected to osmotic stress.36 The upregulation of ALDR in the present work serves as an important positive control, indicating cell proteomic response to the applied osmotic treatment and providing essential independent corroborative validation of the proteomics methods used.
A known side effect of the increased abundance and activity of ALDR is the depletion of NADPH, a cofactor for the reduction of glutathione, whose depletion increases the level of oxidative stress.32 In the present study, increased oxidative stress may have contributed to the increased levels of superoxide dismutase (SOD) that were observed in the treatment group (Figure 4). Another member of the aldo-keto reductase family, aldo-keto reductase family 1 member C2 (AKR1C2), was also more abundant in the experimental treatment group than in the control group. AKR1C2 also plays a role in the cellular response to oxidative stress.37 AKR1C2 is also highly expressed in adipose tissue, where it functions in androgen metabolism.38 Thus, AKR1C2 may be indicative of the tissue from which adipocytes are derived (see discussion of adipose tissue below) or it may contribute to an antioxidative response associated with osmotic stress. Interestingly, the promoters of both ALDR and AKR1C2 share the phorbol ester response (AP-1) element,39 supporting the coordinated regulation of these proteins observed in the present work. In addition to its role in osmotic regulation ALDR also shares with AKR1C2 a direct role in detoxification of lipid aldehydes during oxidative stress.39
The proteomic data analysis detailed above confirms ASCs’ capacity to sense and respond to osmotic stress conditions by altering their protein expression. However, evaluation of the possible influence of osmotic stress on cell differentiation and protein composition indicates that the cellular response is complex, defying a simple interpretation. Comparison of protein expression of cells in our experiment with proteomes of differentiated chondrocytes9 and adipose tissue40 (the latter containing the undifferentiated cells used in this study) suggests that cells in our treatment group expressed several proteins that resembled those found at high levels in adipose tissue. Alternately, cells in our control group expressed several proteins reliably detected in the chondrocyte proteome. Six proteins that were most abundantly expressed in cells in the treatment group (ATP synthase subunit alpha, pyruvate kinase isozymes M1/M2 (canonical sequence), AKR1C2, SOD, trifunctional enzyme subunit alpha, transketolase) were also among the most abundant 10% of proteins expressed in adipose tissue (based on spectral counts only), as measured in a recent study by Xie et al.40
In contrast, none of the proteins of higher abundance in the control group was found among the most abundant 10% of proteins in adipose tissue.40 In fact, many of the high abundance proteins in the control group (e.g., fibronectin, Signal Transducer and Activator of Transcription 1 (STAT1), 5NTD, P4HA1, P4HA2) were not detected at all in the 1493 proteins identified by Xie et al.40
While no quantitative measure of individual proteins was made in the chondrocyte proteome study, several of the proteins detected at higher abundance in our control group cells (fibronectin, 5NTD, and nicotinamide N-methyltransferase (NNMT), and P4HA1) were also reliably detected in the chondrocyte proteome (reliability based on the number of peptides).9 NNMT has also been demonstrated to exist only in low levels in adipose tissue,40 and STAT1 has been shown to be intimately involved in controlling differentiation of chondrocytes.41, 42
The proteomic results presented here suggest that treatment of cells with media of higher osmolarity does not lead to greater expression of a chondrogenic proteome; instead, it may even decrease expression of chondrogenic proteins. However, further examination of the proteins detected in our study suggests a more complex differentiation response to treatment with higher osmolarity. For example, isoforms of pyruvate kinase, upregulated in our treatment group (Figure 4), have been found in many cartilage-related tissue and cell types including mouse cartilage36 and cultured chondrosarcoma cells.10 While some amount of pyruvate kinase is expected in all glycolysis-dependent cells, several studies have found positive correlation between cell expression of pyruvate kinase isoforms upregulated in our treatment group and maturity of the cell differentiation state.43,44 Transgelin, which decreased in expression in our treated cells (Figure 4), has been detected in neonatal, but not postnatal, mouse cartilage,36 indicating that this protein may serve as a marker of undifferentiated cells. The lower expression of this protein in the treatment group of our study may therefore reflect a more differentiated state in this group. Furthermore, global analysis of the data using DAVID and KEGG indicates changes in treated cell expression of extracellular matrix component-related proteins (fibronectin, collagen VI subunit alpha, PLOD2, P4HA1 and P4HA2) and of proteins involved in the pathway leading to focal adhesions (integrin β1, zyxin, vinculin, myosin regulatory light chain 12A (MLC)) (Supporting Data, Table 1). This analysis suggests that the osmotic treatment may induce a cellular state characterized by low motility that is more similar to that of chondrocyte cells (Supporting Data, Figure 2). The influence of hyperosmotic treatment on cell aggrecan protein expression, of interest based on our previous finding of increased chondrocyte aggrecan gene expression under similar treatments, could not be assessed here, since the sample preparation methods used were incompatible with processing insoluble polymers.45
The results of this study demonstrate that proteomics is clearly useful in identifying treatment-induced alterations in cell differentiation-related proteins. The use of label-free proteomic profiling in this study allowed for quantitative assessment of the cellular protein levels for multiple biological and technical replicates. These methods improve upon previous proteomics studies that required selection of specific protein spots on 2D gels for quantification or that provided only lists of detected proteins without allowing their quantitation. The proteomics approach was particularly useful in our study of osmotic treatment-related alterations in cartilage precursor cell phenotype, as it allowed for identification of osmolarity-sensitive proteins whose precursor gene expression would not have been detected in traditional assays focused only on extracellular matrix protein metabolism as a marker of cell differentiation.
The complex nature of the cell differentiation-related protein response observed in this study motivates further studies to determine whether higher-osmolarity treatment guides ASCs toward a phenotype capable of robust engineered cartilage production in subsequent 3D culture. It has been previously demonstrated that early phenotypic changes of cells subjected to differentiation regimes may not translate directly into engineered cartilage constructs with improved functional properties.46 Future studies will therefore assess the correlation of protein expression in 2D culture under osmotic and other chondrogenic priming regimes with cell tissue production in subsequent 3D culture, identifying proteomic biomarkers that can accurately serve as benchmarks for rapid optimization of 2D priming protocols. While ASCs were selected for the present work due to their advantages as a cell source for tissue engineering, the proteomics-based approach explored here could be easily expanded to assess the influence of chondrogenic priming regimes on other potential cell sources for cartilage tissue engineering.
Supplementary Material
Acknowledgments
This work was supported by NIH grants AR46568, AR46568-10S1 (BIRT), and AR52871, and an NSF graduate fellowship (ESO). We also thank the Department of Biological Sciences, Offices of the Vice President for Arts and Sciences, Executive Vice President for Research, Alexandr Gornstein, David Madigan (Columbia University); Philip Andrews, Mark Gjukich (University of Michigan); New York State Stem Cell Science Board (NYSTEM); Scott Geromanos (Waters Corp.); Cindy Chepanoske (Ceiba Solutions); Kacey Marra (University of Pittsburgh); Kevin Blackburn (NC State U.); and Mark Burke and Sarah Schwartz (David H. Murdock Research Institute).
Footnotes
SUPPORTING INFORMATION AVAILABLE. The proteins detected in this study are listed in the spreadsheet Supporting Data Table 1 and include individual abundance intensities for each replicate of each protein and each treatment. P-values, ProteinTeller probability and Peptide Count Score indicate reliability of protein identification and the number of peptides supporting each identification. Other data in the spreadsheet includes percentage sequence coverage. Gene names and gene product ontological and functional annotations were downloaded from the Uniprot database (when available for a particular protein). Supporting Data Figure 1 is a graphic that includes all proteins in an agglomerative hierarchical cluster of Z-score transformed intensity data processed by the Elucidator program in this experiment. Supporting Data Figure 2 was derived by searching differentially expressed proteins in the DAVID and KEGG databases leading to illustration of the role of several proteins from this study relating to the extracellular matrix. Arrows pointing up indicate proteins higher in abundance in treated samples and those pointing down indicate lower abundance in treated samples. This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
Lewis M. Brown, Email: Lewis.Brown@biology.columbia.edu.
Clark T. Hung, Email: cth6@columbia.edu.
References
- 1.Estes BT, Diekman BO, Guilak F. Monolayer cell expansion conditions affect the chondrogenic potential of adipose-derived stem cells. Biotechnol Bioeng. 2008;99(4):986–995. doi: 10.1002/bit.21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, Ateshian GA, Hung CT. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-B3. Osteoarthritis Cartilage. 2007;15(9):1025–1033. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selmi TA, Verdonk PCM, Chambat P, Dubrana F, Potel JF, Barnouin L, Neyret P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Jt Surg. 2008;90(5):597–604. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- 4.Lammi MJ, Hayrinen J, Mahonen A. Proteomic analysis of cartilage- and bone-associated samples. Electrophoresis. 2006;27(13):2687–701. doi: 10.1002/elps.200600004. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz-Romero C, Lopez-Armada MJ, Blanco FJ. Proteomic characterization of human normal articular chondrocytes: a novel tool for the study of osteoarthritis and other rheumatic diseases. Proteomics. 2005;5(12):3048–59. doi: 10.1002/pmic.200402106. [DOI] [PubMed] [Google Scholar]
- 6.Lambrecht S, Verbruggen G, Verdonk PC, Elewaut D, Deforce D. Differential proteome analysis of normal and osteoarthritic chondrocytes reveals distortion of vimentin network in osteoarthritis. Osteoarthritis Cartilage. 2008;16(2):163–73. doi: 10.1016/j.joca.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Romero C, Calamia V, Mateos J, Carreira V, Martinez-Gomariz M, Fernandez M, Blanco FJ. Mitochondrial dysregulation of osteoarthritic human articular chondrocytes analyzed by proteomics: a decrease in mitochondrial superoxide dismutase points to a redox imbalance. Mol Cell Proteomics. 2009;8(1):172–89. doi: 10.1074/mcp.M800292-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz-Romero C, Calamia V, Rocha B, Mateos J, Fernandez-Puente P, Blanco FJ. Hypoxia Conditions Differentially Modulate Human Normal and Osteoarthritic Chondrocyte Proteomes. Journal Of Proteome Research. 2010;9(6):3035–3045. doi: 10.1021/pr901209s. [DOI] [PubMed] [Google Scholar]
- 9.Lambrecht S, Dhaenens M, Almqvist F, Verdonk P, Verbruggen G, Deforce D, Elewaut D. Proteome characterization of human articular chondrocytes leads to novel insights in the function of small heat-shock proteins in chondrocyte homeostasis. Osteoarthritis and Cartilage. 2010;18(3):440–446. doi: 10.1016/j.joca.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Piltti J, Hayrinen J, Karjalainen HM, Lammi MJ. Proteomics of chondrocytes with special reference to phosphorylation changes of proteins in stretched human chondrosarcoma cells. Biorheology. 2008;45(3–4):323–35. [PubMed] [Google Scholar]
- 11.Wilson R, Belluoccio D, Little CB, Fosang AJ, Bateman JF. Proteomic Characterization of Mouse Cartilage Degradation In Vitro. Arthritis and Rheumatism. 2008;58(10):3120–3131. doi: 10.1002/art.23789. [DOI] [PubMed] [Google Scholar]
- 12.Silva JC, Denny R, Dorschel C, Gorenstein MV, Li GZ, Richardson K, Wall D, Geromanos SJ. Simultaneous qualitative and quantitative analysis of the Escherichia coli Proteome - A sweet tale. Molecular & Cellular Proteomics. 2006;5(4):589–607. doi: 10.1074/mcp.M500321-MCP200. [DOI] [PubMed] [Google Scholar]
- 13.Ma D, Chan MK, Lockstone HE, Pietsch SR, Jones DNC, Cilia J, Hill MD, Robbins MJ, Benzel IM, Umrania Y, Guest PC, Levin Y, Maycox PR, Bahn S. Antipsychotic Treatment Alters Protein Expression Associated with Presynaptic Function and Nervous System Development in Rat Frontal Cortex. Journal Of Proteome Research. 2009;8(7):3284–3297. doi: 10.1021/pr800983p. [DOI] [PubMed] [Google Scholar]
- 14.Xu DM, Suenaga N, Edelmann MJ, Fridman R, Muschel RJ, Kessler BM. Novel MMP-9 Substrates in Cancer Cells Revealed by a Label-free Quantitative Proteomics Approach. Molecular & Cellular Proteomics. 2008;7(11):2215–2228. doi: 10.1074/mcp.M800095-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambery A, Vissers JPC, Langridge JI, Lonardo E, Minchiotti G, Ruvo M, Parente A. Qualitative and Quantitative Proteomic Profiling of Cripto(−/−) Embryonic Stem Cells by Means of Accurate Mass LC-MS Analysis. Journal Of Proteome Research. 2009;8(2):1047–1058. doi: 10.1021/pr800485c. [DOI] [PubMed] [Google Scholar]
- 16.Lo Turco EG, Souza G, Garcia JS, Ferreira CR, Eberlin MN, Bertolla RP. Effect of endometriosis on the protein expression pattern of follicular fluid from patients submitted to controlled ovarian hyperstimulation for in vitro fertilization. Human Reproduction. 2010;25(7):1755–1766. doi: 10.1093/humrep/deq102. [DOI] [PubMed] [Google Scholar]
- 17.Rower C, Vissers JPC, Koy C, Kipping M, Hecker M, Reimer T, Gerber B, Thiesen HJ, Glocker MO. Towards a proteome signature for invasive ductal breast carcinoma derived from label-free nanoscale LC-MS protein expression profiling of tumorous and glandular tissue. Analytical and Bioanalytical Chemistry. 2009;395(8):2443–2456. doi: 10.1007/s00216-009-3187-9. [DOI] [PubMed] [Google Scholar]
- 18.Patel VJ, Thalassinos K, Slade SE, Connolly JB, Crombie A, Murrell JC, Scrivens JH. A Comparison of Labeling and Label-Free Mass Spectrometry-Based Proteomics Approaches. Journal Of Proteome Research. 2009;8(7):3752–3759. doi: 10.1021/pr900080y. [DOI] [PubMed] [Google Scholar]
- 19.Chambery A, Colucci-Damato L, Vissers JPC, Scarpella S, Langridge JI, Parente A. Proteomic Profiling of Proliferating and Differentiated Neural mes-c-myc A1 Cell Line from Mouse Embryonic Mesencephalon by LC-MS. Journal Of Proteome Research. 2009;8(1):227–238. doi: 10.1021/pr800454n. [DOI] [PubMed] [Google Scholar]
- 20.Cheng FY, Blackburn K, Lin YM, Goshe MB, Williamson JD. Absolute Protein Quantification by LC/MSE for Global Analysis of Salicylic Acid-induced Plant Protein Secretion Responses. Journal Of Proteome Research. 2009;8(1):82–93. doi: 10.1021/pr800649s. [DOI] [PubMed] [Google Scholar]
- 21.Blackburn K, Mbeunkui F, Mitra SK, Mentzel T, Goshe MB. Improving Protein and Proteome Coverage through Data-Independent Multiplexed Peptide Fragmentation. Journal Of Proteome Research. 2010;9(7):3621–3637. doi: 10.1021/pr100144z. [DOI] [PubMed] [Google Scholar]
- 22.Oswald ES, Chao PHG, Bulinski JC, Ateshian GA, Hung CT. Dependence of Zonal Chondrocyte Water Transport Properties on Osmotic Environment. Cellular and Molecular Bioengineering. 2008;1(4):339–348. doi: 10.1007/s12195-008-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bian L, Fong JV, Lima EG, Stoker AM, Ateshian GA, Cook JL, Hung CT. Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Eng Part A. 2010;16(5):1781–90. doi: 10.1089/ten.tea.2009.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wessel D, Flugge UI. A Method For The Quantitative Recovery Of Protein In Dilute-Solution In The Presence Of Detergents And Lipids. Analytical Biochemistry. 1984;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 25.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74(20):5383–92. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 26.Dai HY, Meyer M, Stepaniants S, Ziman M, Stoughton R. Use of hybridization kinetics for differentiating specific from non-specific binding to oligonucleotide microarrays. Nucleic Acids Research. 2002;30(16):e86. doi: 10.1093/nar/gnf085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weng L, Dai H, Zhan Y, He Y, Stepaniants SB, Bassett DE. Rosetta error model for gene expression analysis. Bioinformatics. 2006;22(9):1111–21. doi: 10.1093/bioinformatics/btl045. [DOI] [PubMed] [Google Scholar]
- 28.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009 Jan;37(1):1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Research. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain E, Bairoch A, Duvaud S, Phan I, Redaschi N, Suzek BE, Martin MJ, McGarvey P, Gasteiger E. Infrastructure for the life sciences: design and implementation of the UniProt website. Bmc Bioinformatics. 2009;10:136. doi: 10.1186/1471-2105-10-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of Cell Volume Regulation in Vertebrates. Physiological Reviews. 2009;89(1):193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 32.Del Corso A, Cappiello M, Mura U. From a dull enzyme to something else: Facts and perspectives regarding aldose reductase. Current Medicinal Chemistry. 2008;15(15):1452–1461. doi: 10.2174/092986708784638870. [DOI] [PubMed] [Google Scholar]
- 33.Ko BCB, Ruepp B, Bohren KM, Gabbay KH, Chung SSM. Identification and characterization of multiple osmotic response sequences in the human aldose reductase gene. Journal of Biological Chemistry. 1997;272(26):16431–16437. doi: 10.1074/jbc.272.26.16431. [DOI] [PubMed] [Google Scholar]
- 34.Klawitter J, Rivard CJ, Brown LM, Capasso JM, Almeida NE, Maunsbach AB, Pihakaski-Maunsbach K, Berl T, Leibfritz D, Christians U, Chan L. A metabonomic and proteomic analysis of changes in IMCD3 cells chronically adapted to hypertonicity. Nephron Physiol. 2008;109(1):1–10. doi: 10.1159/000129074. [DOI] [PubMed] [Google Scholar]
- 35.Koo J, Kim KI, Min BH, Lee GM. Differential Protein Expression in Human Articular Chondrocytes Expanded in Serum-Free Media of Different Medium Osmolalities by DIGE. Journal Of Proteome Research. 2010;9(5):2480–2487. doi: 10.1021/pr100136q. [DOI] [PubMed] [Google Scholar]
- 36.Wilson R, Diseberg AF, Gordon L, Zivkovic S, Tatarczuch L, Mackie EJ, Gorman JJ, Bateman JF. Comprehensive Profiling of Cartilage Extracellular Matrix Formation and Maturation Using Sequential Extraction and Label-free Quantitative Proteomics. Molecular & Cellular Proteomics. 2010;9(6):1296–1313. doi: 10.1074/mcp.M000014-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lou H, Du SY, Ji Q, Stolz A. Induction of AKR1C2 by phase II inducers: Identification of a distal consensus antioxidant response element regulated by NRF2. Molecular Pharmacology. 2006;69(5):1662–1672. doi: 10.1124/mol.105.019794. [DOI] [PubMed] [Google Scholar]
- 38.Blouin K, Veilleux A, Luu-The V, Tchernof A. Androgen metabolism in adipose tissue: Recent advances. Molecular and Cellular Endocrinology. 2009;301(1–2):97–103. doi: 10.1016/j.mce.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 39.Penning TM, Drury JE. Human aldo-keto reductases: Function, gene regulation, and single nucleotide polymorphisms. Arch Biochem Biophys. 2007;464(2):241–50. doi: 10.1016/j.abb.2007.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie XT, Yi ZP, Bowen B, Wolf C, Flynn CR, Sinha S, Mandarino LJ, Meyer C. Characterization of the Human Adipocyte Proteome and Reproducibility of Protein Abundance by One-Dimensional Gel Electrophoresis and HPLC-ESI-MS/MS. Journal Of Proteome Research. 2010;9(9):4521–4534. doi: 10.1021/pr100268f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao LP, Naganawa T, Obugunde E, Gronowicz G, Ornitz DM, Coffin JD, Hurley MM. Stat1 controls postnatal bone formation by regulating fibroblast growth factor signaling in osteoblasts. Journal of Biological Chemistry. 2004;279(26):27743–27752. doi: 10.1074/jbc.M314323200. [DOI] [PubMed] [Google Scholar]
- 42.Krejci P, Prochazkova J, Bryja V, Jelinkova P, Pejchalova K, Kozubik A, Thompson LM, Wilcox WR. Fibroblast growth factor inhibits interferon gamma-STAT1 and interleukin 6-STAT3 signaling in chondrocytes. Cellular Signalling. 2009;21(1):151–160. doi: 10.1016/j.cellsig.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staal GEJ, Rijksen G. Regulation of pyruvate kinase in normal and pathological conditions. In: Beitner R, editor. Regulation of carbohydrate metabolism. I. CRC Press; Boca Raton: 1985. pp. 143–159. [Google Scholar]
- 44.Imamura K, Noguchi T, Tanaka T. Regulation of isozyme patterns of pyruvate kinase in normal and neoplastic tissues. In: Staal G, van Veelen C, editors. Markers of human neuroectodermal tumors. CRC Press; Boca Raton, FI: 1986. pp. 191–222. [Google Scholar]
- 45.Zaia J, Liu BS, Boynton R, Barry F. Structural analysis of cartilage proteoglycans and glycoproteins using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal Biochem. 2000;277(1):94–103. doi: 10.1006/abio.1999.4379. [DOI] [PubMed] [Google Scholar]
- 46.Huang AH, Farrell MJ, Mauck RL. Mechanics and mechanobiology of mesenchymal stem cell-based engineered cartilage. J Biomech. 2010;43(1):128–36. doi: 10.1016/j.jbiomech.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.