Abstract
The use of surface versus intramuscular electrodes as well as the effect of electrode targeting on pattern-recognition-based multifunctional prosthesis control was explored. Surface electrodes are touted for their ability to record activity from relatively large portions of muscle tissue. Intramuscular electromyograms (EMGs) can provide focal recordings from deep muscles of the forearm and independent signals relatively free of crosstalk. However, little work has been done to compare the two. Additionally, while previous investigations have either targeted electrodes to specific muscles or used untargeted (symmetric) electrode arrays, no work has compared these approaches to determine if one is superior. The classification accuracies of pattern-recognition-based classifiers utilizing surface and intramuscular as well as targeted and untargeted electrodes were compared across 11 subjects. A repeated-measures analysis of variance revealed that when only EMG amplitude information was used from all available EMG channels, the targeted surface, targeted intramuscular, and untargeted surface electrodes produced similar classification accuracies while the untargeted intramuscular electrodes produced significantly lower accuracies. However, no statistical differences were observed between any of the electrode conditions when additional features were extracted from the EMG signal. It was concluded that the choice of electrode should be driven by clinical factors, such as signal robustness/stability, cost, etc., instead of by classification accuracy.
Index Terms: Electrodes, electromyography, pattern recognition, prosthetics
I. Introduction and Background
For persons with transradial amputation, current clinical prosthetics practice uses surface electromyograms (EMGs) from the wrist flexor and extensor muscle groups to control a small number of movements. Generally, the prosthetic component is driven at a speed that is proportional to the difference in the amplitude of the two EMG signals. This form of control is referred to as “direct control.”
The most frequently implemented myoelectric prosthesis for the transradial population is a single-degree-of-freedom pre-hensor. While it is possible to control other movements (e.g., wrist pronation/supination or wrist flexion/extension), these additional degrees of freedom are often controlled in a serial fashion and require unintuitive movements of the phantom limb to produce both the control signals and the cocontractions necessary to toggle between the different degrees of freedom. While serial control is adequate for a small number of movements, it does not allow for simultaneous control or multiple joints and quickly becomes cumbersome as more degrees of freedom need to be controlled.
Many of the muscles that were used to actuate the hand and wrist are still present after amputation and produce relatively distinct patterns of activity with different movements of the phantom limb. Therefore, rather than use the cumbersome direct control approach for multiple-degree-of-freedom devices, many researchers have attempted to recognize patterns of muscle activity associated with different movements of the phantom limb and link these patterns to movements of the prosthesis. While good offline performance of these controllers has been reported, pattern recognition systems have yet to be implemented commercially.
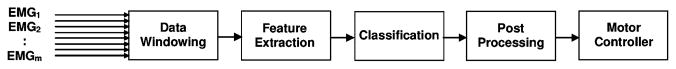
The typical steps of a pattern-recognition-based classifier are shown in Fig. 1. The raw data from the EMG channels are often collected in windows or bins. These windows then undergo some form of signal processing to extract different features from the EMG data. These features can be basic amplitude information or more complex features such as the coefficients of an autoregressive (AR) model. The features are then input into a classifier that compares the features extracted from the current data window to previously collected feature sets extracted for each of the possible movement classes. The movement class that best matches the features from the current window is then selected as the “output class.”
Fig. 1.
Schematic diagram of the typical steps of pattern-recognition-based classifiers.
It is also possible to perform postprocessing techniques such as majority voting to increase the stability and robustness of the class decision stream. Majority voting stipulates that the output of the controller is not simply the most recent class decision but the class that appears the most often in the previous n class decisions. The output of the postprocessing stage dictates which degree of freedom is to be actuated and this signal is then passed into a motor controller that drives the requisite prosthesis component.
Most previous studies have used “classification accuracy” as the metric of success for pattern-recognition-based classifiers. Classification accuracy is defined as the percentage of time that the classifier is able to correctly decipher the intended movement of the user. To maximize classification accuracy, many researchers have examined a variety of different classifiers ranging from AR filters [1] to “evidence accumulation” methods [2], [3], fuzzy logic classifiers [4]–[7], Gaussian mixture matrices [8], hidden Markov models [9], linear discriminant methods [8], [10]–[16], maximum likelihood approaches [17], [18], multiple-hypothesis testing methods [19], nearest-neighbor approaches [18], [20], [21], and a variety of neural-type networks [6], [9], [22]–[39]. In addition, to help the classifiers better interpret the intended movement of the user, researchers have attempted to extract more complex information from the EMG signals. A variety of signal features representing both EMG amplitude and spectral content have been used and most have been shown to increase classification accuracy. Examples of these features include AR model coefficients [1]–[3], [6], [8], [9], [17], [19], [21], [25], [29], [30], [38], time-domain (TD) features [8], [10]–[12], [28], [33], [34], various frequency spectra [10]–[12], [16], [25], [27], wavelet features [10]–[12], [22], [24], and cepstral coefficients [2], [3], [6], [17].
These previous efforts attempted to improve the classification accuracies of multifunctional prosthesis controllers using different feature sets or classifiers; however, nearly all of these efforts used surface EMG recordings. Surface electrodes are advantageous because they are relatively cheap, noninvasive, and have a large pickup area. The large pickup area may be beneficial for pattern-recognition-based classifiers because it allows the electrode to detect activity from muscles other than the muscle located directly beneath the electrode. By extracting features from the surface recordings, it is possible for a classifier to parse out the activity of the different muscles that sum together to produce the recorded surface EMG signal. Detecting activity from many muscles on one channel may increase the amount of information available to the pattern recognition system and allow it to “take advantage of the crosstalk.”
Alternatively, intramuscular EMG may have advantages over surface recordings for prosthesis control. These advantages include the ability to record focally from deep muscles, the ability to provide consistent recording sites as the user changes arm orientation or dons and doffs the prosthesis, and a reduction of crosstalk, which would allow an increase in the number of independent muscle sites for one muscle/one function control or forward dynamic models of the forearm. However, intramuscular EMG has seldom been investigated. Some early work was done with intramuscular EMG to implement direct control using implanted electrodes [40], [41] and the authors are aware of only two groups that have investigated intramuscular EMG for pattern-recognition-based control [42]–[45]. Only Hargrove et al. [42] has compared surface and intramuscular electrodes, recording from 16 untargeted surface (US) and six targeted intramuscular (TI) channels.
As well as almost solely utilizing surface electrodes, previous studies in pattern-recognition-based multifunctional prosthesis control chose to either target the electrodes to specific muscles or use untargeted arrays of electrodes. Studies using only EMG amplitude tended to target their electrodes to specific muscles to increase signal independence [4], [13], [14], [18], [23], [26], [31], [32], [36], [37], [46]–[48]. Only one amplitude—only study did not [20]. Those studies that used additional signal features tended to use untargeted electrode arrays in what the authors perceive as an attempt to capture as much muscle activity as possible. When using additional signal features, most researchers did not target their electrodes to specific muscles [1]–[3], [8]–[11], [19], [21], [27], [28], [30], [33], [35], [49]–[52], but there were a few exceptions [16], [17], [22], [24], [25], [38], [53], [54]. However, the authors are not aware of any study that has attempted to directly compare the use of targeted and untargeted electrodes to determine which method is superior.
Untargeted electrodes are simpler to implement and therefore are preferable for both intramuscular and surface recordings. When considering surface EMG recordings, socket fabrication can be simplified if it is shown that EMG sites for the surface channels simply need to be arranged in an equally spaced array around the forearm instead of targeted over specific muscles. Additionally, while it may be beneficial for increasing signal independence, targeting implantable sensors to specific muscles is not a trivial task. If it can be shown that untargeted intramuscular (UI) EMGs produce similar or better classification accuracies than targeted recordings, the potential need for additional procedures (e.g., ultrasound guidance) to insert the intramuscular electrodes into specific muscles would be eliminated.
Given that relatively little work has been done to examine the effect of either electrode targeting or electrode implantation, the goals of this paper were to compare the classification accuracies of multifunctional prosthesis classifiers that used either surface or intramuscular EMG as well as those that used either targeted and untargeted electrodes. These goals were accomplished by comparing the accuracies resulting from targeted surface (TS), TI, US, and untargeted intramuscular (UI) electrode recordings.
II. Methods
The protocol was approved by the Northwestern University Institutional Review Board and all patients signed informed consent forms. Eleven subjects (mean age 29 ± 8 years) including seven males and four females were enrolled in this study.
A. Hardware
The EMG signals were collected using a 16-channel Noraxon (Scottsdale, AZ) Telemyo 2400 system. The surface EMG electrodes were dual-snap, self-adhesive Ag/AgCl gel electrodes with a recording area diameter of 1 cm and a 2 cm center-to-center spacing (Noraxon, Scottsdale, AZ). The fine wire electrodes were inserted into the forearm using 1.5 in, 25 gauge hypodermic needles (Kendall OEM, Mansfield, MA). The fine wire electrodes were made from 0.002 in. stablohm 800 metal coated with h-poly nylon insulation (California Fine Wire, Grover Beach, CA). Approximately 2–3 mm of the nylon insulation was removed from the ends of the wires. Two wires were contained in each needle. Therefore the exposed portions of the wires were staggered to prevent the fine wires from shorting after insertion into the forearm. The center-to-center spacing of the exposed portions of the wires was approximately 3 mm.
The electrical stimulation used to verify TI site locations was provided by a Digitimer DS7A Constant Current Stimulator (Digitimer Ltd., Hertfordshire, U.K.). Muscle tetanus was achieved using a 200 ms stimulus train that consisted of 200 μs pulses at 30 Hz.
B. Electrode Application and Insertion
The study investigated four different electrode conditions: targeted surface (TS), targeted intramuscular (TI), untargeted surface (US), and untargeted intramuscular (UI). The experiment was divided into two halves, with each half being performed on different days. In one half, TS and UI recordings were collected. Recordings from TI and US electrodes were collected in the other half of the experiment. The half of the experiment that was performed first was randomized among the 11 subjects. Six subjects completed the TI/US condition first while five completed the TS/UI condition first.
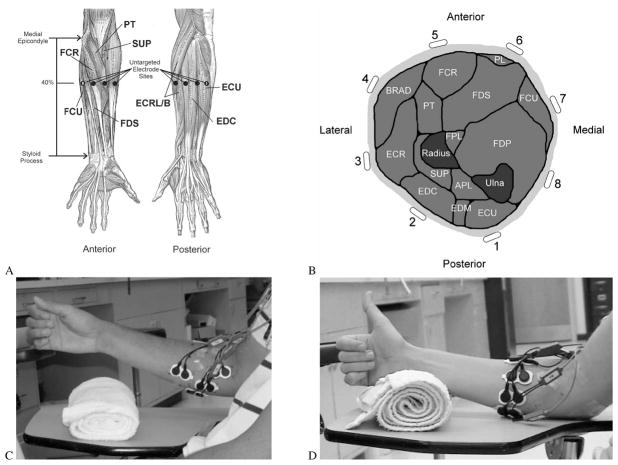
In each half of the experiment, either the surface or the intramuscular electrodes were targeted to specific muscles while the other electrodes were equally spaced around the forearm. Eight muscles from the proximal forearm were chosen for the targeted electrode sites: extensor carpi radialis longus or brevis (ECRL/B), extensor carpi ulnaris (ECU), extensor digitorum communis (EDC), flexor carpi radialis (FCR), flexor carpi ulnaris (FCU), flexor digitorum superficialis (FDS), pronator teres (PT), and supinator (SUP). The anatomical orientation of these muscles is shown in Fig. 2(A) and (B). The US and UI electrode arrays were positioned on the forearm at 40% of the distance from the medial epicondyle of the humerus to the styloid process of the ulna. The eight untargeted electrodes were equally spaced around the circumference of the forearm at this point [Fig. 2(A)], with channel 1 placed just lateral to the ulnar exposure, channel 2 further lateral/anterior to channel 1, and so on around the forearm [Fig. 2(B)]. The array was oriented such that the distance between two electrodes was bisected by the ulnar exposure to avoid attempting to insert an intramuscular electrode into bone. The insertion depth of the UI electrodes was varied based upon the size of the subject’s forearm by dividing the circumference of the forearm at the location of the electrode array by 16. The insertion depths ranged from 1.5 to 2.0 cm. The needle was typically oriented perpendicular to the surface of the forearm but was occasionally inserted at a slight angle to achieve the proper depth without contacting bone.
Fig. 2.
(A) Representation of the anterior and posterior musculature of the left forearm in the anatomical position. The eight muscles that were targeted in this study are indicated. Additionally, the approximate locations of the untargeted sites are indicated by the large black dots. These sites are equally spaced around the circumference of the forearm at 40% of the distance between the medial epicondyle and the styloid process. (B) Cross section of the left forearm musculature, from a superior (proximal) viewpoint looking inferiorly (distally), indicating the approximate locations of the untargeted electrodes. APL: abductor pollicis longus; BRAD: brachioradialis; ECR: extensor carpi radialis; ECU: extensor carpi ulnaris, EDC: extensor digitorum communis; EDM: extensor digiti minimi; FCR: flexor carpi radialis; FCU: flexor carpi ulnaris; FDP: flexor digitorum profundus; FDS: flexor digitorum superficialis; FPL: flexor pollicis longus; PL: palmaris longus; PT: pronator teres; SUP: supinator. (C) Photographs of a forearm with UI (the wires are too fine to see) and TS electrodes. The TS electrodes were placed at the locations that produced the maximum EMG signal during the performance of test movements associated with the target muscle. (D) Photographs of a forearm with TI and US electrodes. Note that the US electrodes are arranged in an equally spaced array around the circumference of the forearm.
The TS sites were located using an approach commonly used in clinical prosthetics practice. The subjects repeatedly produced test movements that were specific to each targeted muscle (e.g., pronation was the test movement for pronator teres). While the subjects repeatedly produced contractions of similar intensity, a test electrode was iteratively moved around the surface of the forearm to find the site that produced the largest EMG signal.
The TI insertion sites were primarily found via palpation. After the fine wires had been inserted, the location of the intramuscular electrodes was verified by electrically stimulating the muscle and observing the induced movement. For example, it was concluded that an electrode was properly located in ECU if wrist extension and ulnar deviation were observed when the muscle was stimulated. The insertion process was repeated until the movement associated with the target muscle was observed. One reason that able-bodied subjects were chosen for this study was that their wrists and hands were still intact, which allowed the use electrical stimulation to verify that the electrodes were properly located. This verification process would not have been possible if persons with amputation were used as subjects as their hands and wrists are no longer present. We believe able-bodied subjects provided a good representation of the data that would be acquired from persons with amputation while allowing verification of the placement of the TI electrodes.
If the desired sites of both a targeted and an untargeted electrode overlapped, the untargeted electrode was moved so that it did not lie on the targeted site but was as close as possible to the originally intended location. An example of the TS electrode locations (the fine wires are too small to see) are shown in Fig. 2(C), and an example of US electrodes are shown in Fig. 2(D). Surface electrode liftoff was a frequent problem in pilot experiments. To address this problem, the forearm was wrapped in Coban after the surface electrodes were applied to ensure that the electrodes retained good contact over the course of the experiment.
C. Data Collection
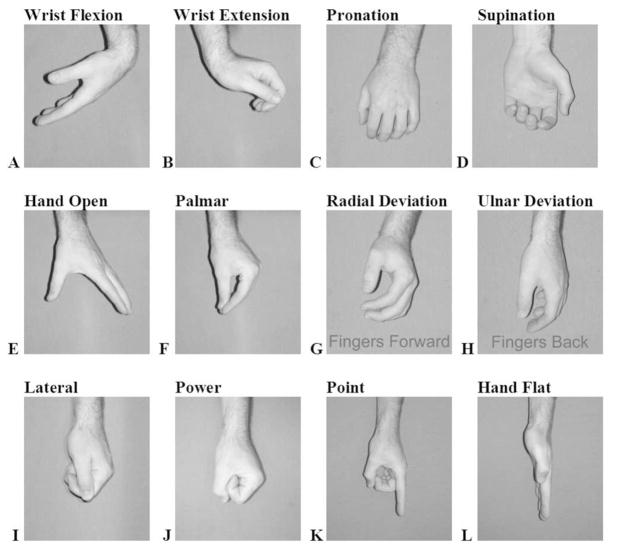
A preliminary power analysis conducted on pilot data found that 11 subjects would be required to obtain sufficient statistical power for the comparison of the different electrode conditions [55]. Each of the 11 subjects performed a series of trials in which they alternated between relaxing and performing 1 of 12 movements of the hand and wrist. In each of the trials, the users were cued to contract or relax via audio cues that were spaced by approximately 4 s. Four contractions were contained in each trial and four trails were collected for each movement. To increase the robustness of the estimated classification accuracies, all of the reported accuracies were calculated using a fourfold cross-validation technique in which the accuracies were calculated four times for each condition and then averaged. The four accuracies were calculated by rotating the three trials from each movement class used to train the classifier and the remaining trail from each class used to calculate the classification accuracy. The 12 movements investigated were as follows: wrist flexion, wrist extension, pronation, supination, hand open, palmar grasp, radial deviation, ulnar deviation, lateral grasp, power grasp, and point and hand flat hand postures (Fig. 3). The order in which the movements were performed was randomized for each subject.
Fig. 3.
Photographs of the 12 movement classes that were investigated in this study. (A) Wrist flexion. (B) Wrist extension. (C) Pronation. (D) Supination. (E) Hand open. (F) Palmar prehension. (G) Radial deviation. (H) Ulnar deviation. (I) Lateral prehension. (J) Power prehension. (K) “Point.” (L) “Hand flat” hand postures.
The users were first shown a photograph of the final hand posture and the desired movement was demonstrated. The user’s arm hung in a relaxed position at their side with their fingers pointed toward the floor. After performing a contraction, the users were instructed to relax and allow gravity to return them to a neutral position instead of actively returning to neutral. The users were instructed to keep their fingers relaxed when they performed movements of the wrist and were also instructed to produce a comfortable amount of force after achieving the different hand grasp patterns.
D. EMG Data Processing
The EMG data were preprocessed by filtering, decimating, and extracting signal features from the raw data. The cutoff frequencies of the EMG preamplifier filters were set to 1500 Hz and the EMG data were initially collected at 3000 Hz. Matlab (Mathworks, Natick, MA) software was then used to apply a fourth-order Butterworth bandpass filter (30–1000 Hz) and then down-sample the data to 1000 Hz.
As mentioned previously, prior work has shown success in increasing classification accuracies by extracting additional signal features from the EMG signal. The group at the University of New Brunswick (UNB) has extensively explored the use of signal features for multifunctional prosthesis control. Features that have been investigated by this group include TD features, several forms of wavelet analysis, Fourier transforms, and AR coefficients. The group’s most recent work found that when used in isolation, AR features produced the best classification accuracies [42]. In a previous work, it was reported that combining additionally, Hudgins et al.’s [28] TD features with the AR features produced even higher classification accuracies than either feature set by itself [8]. Thus, TD features and AR coefficients were the features that were chosen for this study. Additionally, the rms of the EMG signal was calculated for each data window to extract basic amplitude information.
E. Classifier
Classification accuracies are dependent upon a number of different variables, several of which are associated with the steps shown in Fig. 1. Classification accuracy can be affected by the length of the data analysis windows, EMG signal features, classifier, and number of majority votes as well as the composition of the training data set. Many different combinations of these variables have been examined by previous researchers. However, due to differences in these studies with regard to the number and placement of electrodes, number and type of output classes, training data set compositions, as well as classifiers and features used, there is little consensus on the features, classifiers, etc., that produce the best classification accuracies. Farrell [55] examined a number of conditions in an attempt to optimize each of these variables for the data collected in this study before comparing the effects of electrode targeting, the use of surface versus intramuscular electrodes, and the effects of varying the number of channels or output classes.
Farrell examined different combinations of analysis window lengths and majority votes, the use of linear discriminant analysis and multinomial logistic-regressive-based classifiers, the use of rms, TD, and AR feature sets, and the effect of including transient data into the training data sets. The following was found.
160 ms analysis windows shifted in 10 ms increments with no majority voting providing the best combination of computational efficiency and classification accuracy when compared to smaller analysis window lengths that employed majority voting.
Fourth-order AR models provided the best combination of computational efficiency and classification accuracy.
Feature sets utilizing all signal features produced the highest accuracies.
Linear discriminant classifiers produce statistically similar accuracies to multinomial logistic regression classifiers at a much lower computational cost.
Including the onset transient of each contraction in the training data improved classification accuracies when compared to training matrices that included only steady-state data.
Therefore, the classification accuracies that are reported in this paper were obtained using a linear discriminant analysis (LDA) classifier with 160 ms analysis windows and no majority voting, all features (rms, TD, and fourth-order AR coefficients) and training data sets that included the onset transient.
F. Analysis
As mentioned previously, it appears as though most researchers either targeted or did not target their electrodes based upon whether or not additional signal features were used. Therefore, classification accuracies were calculated for feature sets that both included and excluded additional signal features. A two-factor, two-level (targeted/untargeted and surface/intramuscular) repeated-measures analysis of variance (ANOVA) was conducted on each data set. All results from ANOVA analyses conducted in this paper underwent Bonferroni correction for multiple comparisons. Additionally, all data sets underwent Mauchly’s test of sphericity and, if the data did not show equal variances, a Greenhouse–Geisser correction was used [56]. In addition to comparing the classification accuracies obtained from the full complement of eight electrodes classifying all 12 output classes, subsets of these two variables were also investigated.
1) Class Subsets
Twelve different movement classes were chosen to make the classification problem difficult in an attempt to highlight the potential differences between the electrode conditions. However, current commercially available devices are not capable of producing the full set of 12 movements. Six degrees of freedom can be implemented in a transradial prosthesis using commercially available hardware: hand open/close (many options), pronation/supination (Otto Bock wrist rotator, Otto Bock Healthcare, Duderstadt, Germany) and wrist flexion/extension (Kesheng Prostheses Company, Ltd., Shanghai, China). Therefore, to examine the potential differences in the electrode conditions for more currently clinically relevant problems, classification accuracies were calculated for smaller numbers of output classes. Two types of class subsets were investigated: the first subsets were chosen based on clinical criteria while the other subsets were chosen at random. A two-factor (electrode type and number of classes) repeated-measures ANOVA was conducted on the resulting classification accuracies.
The accuracy for the clinically relevant two-class classifier was calculated using the hand open and palmar prehension classes to mimic the results that would be observed for a typical single-degree-of-freedom transradial prosthesis. The four-class condition used the hand open and palmar prehension classes in combination with pronation and supination, as these four movements are the most frequently implemented in transradial prostheses with two degrees of freedom. The six-class condition examined all of the commercially available movements described in the previous paragraph. Finally, the eight-class condition examined the hand open and palmar prehension classes along with all of the wrist movements (pronation/supination, wrist flexion/extension, and radial/ulnar deviation).
The classification accuracies for the random combinations of classes were obtained from 12 random combinations of two-, four-, six-, eight-, and ten-class subsets. In addition to the repeated measures ANOVA analysis described earlier, a linear mixed effects model was constructed on the random subset accuracies to provide an estimate of the effect of adding additional classes to the classification problem as well as examining the effects of the different electrode conditions and different numbers of output classes.
2) Channel Subsets
Eight channels of data were collected for each of the four electrode conditions. However, if similar classification accuracies could be obtained using a smaller number of channels, then fewer sensors would need to be implanted into the forearm or fewer surface electrodes would have to be laminated into the socket. Therefore, the effect of reducing the number of channels on classification accuracy was also investigated.
The channel subsets were chosen using a forward addition approach, which is a computationally efficient means of finding subsets of channels that produce high classification accuracies across the subject population. All eight channels were tested individually and the single channel that produced the best classification accuracy across all subjects was chosen for the one-channel condition. Classification accuracies were then calculated for each of the remaining seven channels paired with the best single channel. Then, the remaining six channels were each paired with the prevailing two-channel subset, and so on. A two-factor (electrode condition/number of channels) repeated-measures ANOVA was conducted on the resulting classification accuracies to examine the effects of reducing the number of channels for each electrode condition.
III. Results
A. EMG Amplitude Only With All Channels and All Classes
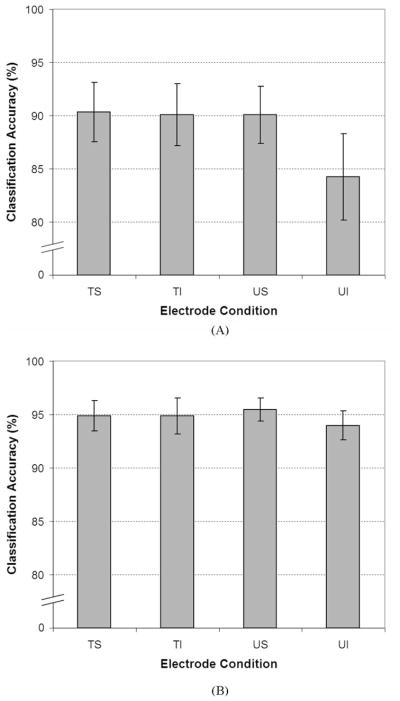
The average classification accuracies for the four electrode conditions using only EMG amplitude features to classify all 12 movement classes using all eight channels for each condition are shown in Fig. 4(A). Two ANOVA analyses, which will be described in greater detail, found that the TS, TI, and US electrode conditions produced similar classification accuracies while the accuracies resulting from the UI electrodes were significantly lower.
Fig. 4.
(A) Average classification accuracies for the four electrode conditions using only amplitude features with 12 movement classes. ANOVA analyses reported no statistical differences between three of the conditions: TS, TI, and US. However, statistical differences were found between these three conditions and the UI condition. (Note: Nonzero y-axis for clarity.) (B) Average classification accuracies for the four electrode conditions using all available signal features with 12 movement classes and all eight electrodes for each condition. A repeated-measures ANOVA reported no statistical differences between the four conditions. Electrode conditions—TS: targeted surface; TI: targeted intramuscular; US: untargeted surface; UI: untargeted intramuscular. (Note: Nonzero y-axis for clarity.)
Initially, a two-factor, two-level (targeted/untargeted and surface/intramuscular) repeated-measures ANOVA was conducted. The ANOVA found statistical differences between the targeted and untargeted electrode conditions (p < 0.0005), surface and intramuscular electrode conditions (p = 0.003), and the interaction between the two (p = 0.017). The post hoc test results from the ANOVA analysis and the data in Fig. 4(A) appeared to indicate that the TS, TI, and US conditions were statistically similar while the UI condition was statistically different from these three. However, the two-factor repeated-measures ANOVA does not allow for a direct comparison of the TS and UI conditions or the US and TI conditions.
Therefore, a one-factor, four-level (one for each of the electrode conditions), repeated-measures ANOVA was performed to verify these assumptions. As expected, the ANOVA found a statistical difference between the electrode conditions (p < 0.0005). Bonferroni-corrected post hoc pair-wise analysis showed no difference between the TS, TI, and US conditions (p = 1.000 for all comparisons) and that the UI condition differed from the other three: TS (p = 0.005), TI (p = 0.011), and US (p = 0.013).
B. All Signal Features With All Channels and All Classes
The average classification accuracies for the four electrode conditions using TD and AR features in addition to the signal amplitude to classify all 12 movements using all eight channels for each condition are shown in Fig. 4(B). Comparing Fig. 4(B) to Fig. 4(A) highlights the increases in classification accuracy created by including the additional signal features (~90% to ~95%).
No statistical differences were detected between the electrode conditions when additional signal features were used. The repeated-measures ANOVA found that electrode targeting (p = 0.636) and using surface versus intramuscular electrodes (p = 0.053) did not produce statistically significant differences in classification accuracy. While the effect of using surface versus intramuscular electrodes may be considered “marginally significant,” it is greater than the preestablished a threshold of 0.05.
C. Effect of Output Class Number
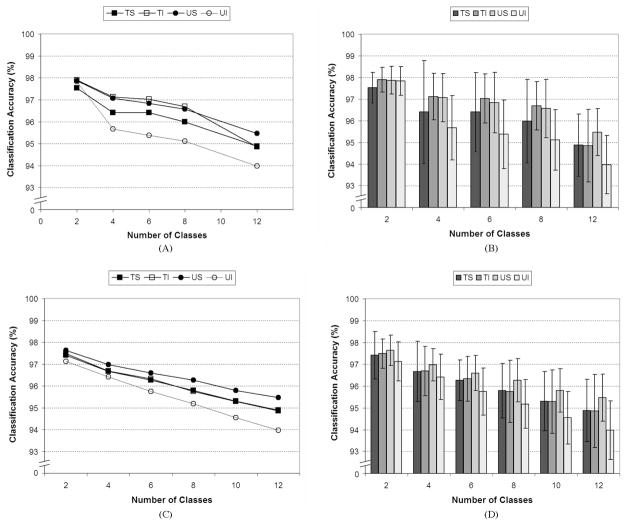
The classification accuracies for the “clinically relevant” subsets of output classes (described in Section II-F1) for each of the four electrode conditions using all available signal features and all eight channels for each condition are shown in Fig. 5(A) and (B). Fig. 5(A) shows the relationship between the average classification accuracy and the number of output classes while Fig. 5(B) contains the mean and standard deviation for each condition. A nearly monotonic trend of decreasing classification accuracy with increasing output class number was observed.
Fig. 5.
(A) and (B). Average classification accuracies for either 2 (hand open and palmar prehension), 4 (pronation, supination, hand open, and palmar prehension), 6 (wrist flexion, wrist extension, pronation, supination, hand open, and palmar prehension), 8 (ulnar deviation, radial deviation, wrist flexion, wrist extension, pronation, supination, hand open, and palmar prehension), or 12 [shown in Fig. 4(B)] output classes using all eight EMG channels for each condition. (A) Relationship between the average classification accuracy and the number of output classes. (B) Mean and standard deviation information for each data set. [Note: Nonzero y-axes for clarity and y-axes are zoomed in from those shown in Fig. 4(A) and Fig. 4(B)]. (C) and (D). Average classification accuracies for random combinations of either two, four, six, eight, or ten output classes using all eight EMG channels for each condition. Twelve random combinations of output movements were selected for each condition with less than 12 output classes. The 12-class conditions used all of the available classes. (C) Relationship between the average classification accuracy and the number of output classes. (D) Mean and standard deviation information for each data set. Electrode conditions—TS: targeted surface; TI; targeted intramuscular; US: untargeted surface; UI: untargeted intramuscular. [Note: Nonzero y-axis for clarity and y-axes are zoomed in from those shown in Fig. 4(A) and Fig. 4(B)].
The repeated-measures ANOVA results showed statistical differences between the different numbers of output classes (p < 0.0005). However, post hoc analyses from the ANOVA found no statistical differences between the different electrode conditions (p > 0.08 for all comparisons).
When the class subsets were chosen based upon clinical criteria, a nonlinear relationship results [Fig. 5(A)]. However, when the classes were selected at random, a more linear relationship was observed [Fig. 5(C)]. A repeated-measures ANOVA was also conducted on the data in Fig. 5(C) and (D). As with the previous analysis, there were no statistical differences between the electrode conditions (p = 0.12) but a difference was found between the different numbers of classes (p < 0.0005). Post hoc analyses found differences between each number of output classes for every electrode condition (p < 0.01 for all comparisons).
Additionally, a linear mixed effects model was constructed on the data shown in Fig. 5(C) and (D) with both fixed and random (to account for intersubject differences) effects for the intercept, the electrode condition, and the number of output classes. This model yielded the same statistical conclusions as the repeated-measures ANOVA by finding that there was no difference between the electrode conditions, but there were differences between the different numbers of output classes. Additionally, the model was able to provide an estimate of the decrease in accuracy that would be expected if the classifier was required to classify an additional movement class. The co-efficient of the fixed parameter associated with the number of output classes indicated that a decrease of 0.26% in classification accuracy was observed for each additional movement class that was included in the classifier.
D. Effect of Output Class Number
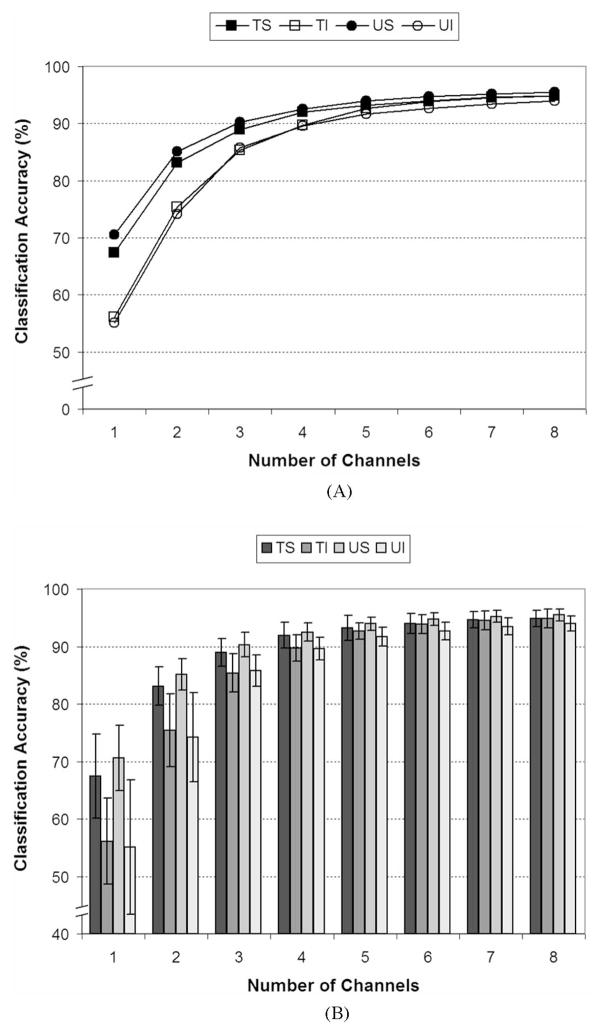
The average classification accuracy results for the reduced subsets of channels obtained using the methods described in Section II-F2 are shown in Fig. 6. Fig. 6 shows a trend of increasing classification accuracy with an increasing number of channels. While large increases in classification accuracy were observed as the first few channels were included, there were diminishing returns with each additional channel. The reduction was especially apparent after approximately four channels were included.
Fig. 6.
Average classification accuracies for the four electrode conditions for all 12 output classes as a function of the number of channels used for classification. (A) Relationship between the average classification accuracy and the number of channels used. (B) Mean and standard deviation information for each data set. The open points in (A) represent the intramuscular conditions while the filled points represent the surface conditions. TS: targeted urface; TI: targeted intramuscular; US: untargeted surface; UI: untargeted intramuscular. (Note: Nonzero y-axis for clarity.)
The order/rank of the channels that were selected using the forward addition process was as follows.
Targeted surface: FDS, ECR, ECU, FCU, EDC, SUP, PT, FCR.
Targeted intramuscular: EDC, FDS, ECU, ECR, PT, SUP, FCU, FCR.
Untargeted surface: 7, 3, 1, 6, 2, 8, 5, 4.
Untargeted intramuscular: 1, 3, 7, 2, 5, 4, 8, 6.
Refer to Fig. 2(B) for the relative anatomical positions of the different muscles.
The repeated-measures ANOVA analysis found that the number of channels (p < 0.0005), electrode condition (p <0.0005), and the interaction term (p < 0.0005) were all found to be statistically significant. In almost all cases, post hoc testing showed that reducing the number of channels by one caused a statistically significant drop in classification accuracy.
Fig. 6 shows that the intramuscular EMG conditions appear to be more affected by the loss of channels than the surface recordings [compare open and filled points in Fig. 6(A)]. No statistical differences were detected between the electrode conditions when eight channels were used and post hoc pair-wise analysis showed that this lack of difference was generally true when five to seven electrodes were used. For the one through three channel conditions, differences were generally detected between each of the surface and intramuscular conditions but not within the surface or intramuscular conditions, i.e., TS/US were different from both TI/UI but were not different from one another and vice versa.
IV. Discussion
A. Conditions Using All Channels and All Output Classes
Feature sets using either EMG amplitude or TD and AR features in combination with EMG amplitude were investigated. Classifiers using only the amplitude of the EMG signal will be discussed first. However, as it has been shown that the classification accuracies can be substantially increased for all conditions by extracting additional signal features and the relative cost of doing so is minimal (calculations can be relatively easily performed by digital signal processors), the results from the data using all available features will be used to address the primary goals of this paper.
1) Classifiers Using Only EMG Signal Amplitude
The statistical analysis performed on the data shown in Fig. 4(A) indicated that the TS, TI, and US electrodes produced similar classification accuracies while the accuracies from the UI electrodes were significantly lower. These results suggest that if additional signal features are not going to be used with data from all eight channels, it is necessary to make sure that the implanted sensors are targeted to muscles that are involved in the movements being controlled. In addition, it does not appear necessary to target the surface electrodes to specific muscles, which simplifies socket fabrication.
The authors hypothesize that when amplitude information was available from most of the residual musculature involved in the movements being controlled, the classifiers tended to show higher classification accuracies. Attaining amplitude information from most of the residual musculature could be done by either using surface electrodes with a large pickup area (TS, US) or targeting the intramuscular electrodes to specific muscles (TI). When the intramuscular electrodes were not targeted to specific muscles (UI), a smaller amount of information was available to the classifier because the electrodes were unable to detect any activity from some muscles (e.g., pronator teres) that were heavily involved in some of the movements. This lack of information likely resulted in lower classification accuracies. Comparing Fig. 4(A) and (B) shows that including signal features in addition to amplitude metrics substantially increased the classification accuracies for all electrode conditions. This increase in accuracy is likely a result of increased information made available to the classifier by the features.
The literature review found that most of those groups that did not use additional signal features targeted their electrodes to specific muscles. Additionally, each of these previous attempts used surface EMG electrodes. The data presented in this paper show that targeting electrodes to specific muscles does not increase classification accuracy when surface electrodes were employed. An additional analysis shows this to be true regardless of the number of electrodes used [55]. These data appear to indicate that it was unnecessary for previous researchers that used only EMG amplitude to target their electrodes to specific muscles and that similar results would have been obtained with a symmetric electrode orientation.
2) Classifiers Using Additional Signal Features
The two main goals of these experiments were to compare the use of surface and intramuscular electrodes as well as targeted and untargeted recordings. Section III-B showed that when additional features are extracted from the EMG signals, there were no statistical differences between the electrode conditions. Two conclusions that can be drawn from these results are that similar classification accuracies can be obtained when using 1) surface or intramuscular electrodes and 2) targeted or untargeted electrodes.
From the point of view of electrode targeting, this finding has application to both surface and intramuscular electrodes. The fact that targeting surface channels does not increase classification accuracy means that the socket does not need to be fabricated such that the electrodes are placed over specific muscles bellies; a requirement that increases the difficulty of socket fabrication. Instead, socket fabrication can be simplified by arranging the electrodes in a symmetric array around the circumference of the forearm.
The lack of improvement seen by targeting the intramuscular electrodes is also noteworthy. As stated earlier, targeting the implants to specific muscles is not trivial and may require additional equipment/procedures (e.g., ultrasound guidance) to accomplish. Since targeting the intramuscular electrodes to specific muscles did not improve classification accuracy, these more complicated medical procedures may not be necessary if a pattern-recognition-based control scheme is implemented. However, these results need to be used with caution as long-term studies have yet to be conducted on chronically implanted myoelectric sensors. These long-term studies may determine that the electrodes do need to be targeted to specific muscles for clinical reasons not related to maximizing classification accuracy, such as the prevention of sensor migration.
Both surface and intramuscular electrodes were thought to have their advantages for pattern-recognition-based prosthesis control. Surface electrodes have a large pickup area that allows for information to be recorded from a larger volume of muscle whereas intramuscular electrodes provide the ability to record focally and obtain independent recordings from the deep muscles of the forearm. However, similar classification accuracies were obtained for all electrode conditions.
The similarity in accuracies shows that intramuscular electrodes can perform as well as surface electrodes. Therefore, the choice of using implanted versus surface electrodes should be made, not based upon classification accuracy, but based upon other clinical factors. Clinical factors include comfort, cost, consistent electrode contact (i.e., surface electrode “liftoff”), invasiveness, signal consistency with donning and doffing, signal robustness, and skin impedance changes. While work is underway to create classifiers that are able to adjust to erroneous signals due to electrode liftoff, changes in skin impedance, or variable electrode locations with donning and doffing, intramuscular electrodes would likely address these problems.
From Fig. 4(B), it is evident that good performance is achieved with all electrode conditions when additional signal features are used. From the point of view of strictly maximizing the classification accuracy, the US electrodes provide similar classification accuracies to the other three conditions and do so with the lowest cost, invasiveness, and difficulty in socket fabrication. However, good clinical performance is based on more than having a high accuracy in a laboratory setting. Two major clinical problems with surface electrodes for pattern-recognition-based control, i.e., liftoff and repeatability/consistency, were not considered in this study and may be addressed with intramuscular electrodes. Therefore, it needs to be stressed that the electrodes should be selected based upon these other clinical criteria as all of the electrodes produced similar classification accuracies in the laboratory.
B. Output Class Subsets
As expected, classifiers attempting to differentiate between smaller numbers of output classes tended to have higher classification accuracies for each electrode condition. If the feature space is less densely populated by different classes, it makes intuitive sense that the classifier is able to more accurately separate the different movements.
Twelve output classes were used to make the classification problem difficult in an attempt to tease out differences between the electrode conditions. However, the analyses performed on the class subsets chosen using clinical criteria or random methods showed a consistent lack of statistical difference between the electrode conditions regardless of the number of output classes. These results provide further evidence that similar accuracies can be obtained using either surface or intramuscular as well as targeted or untargeted electrodes when pattern recognition is used to control gross movements of the wrist and hand. Therefore, the conclusions from the previous section also hold for prostheses that utilize commercially available componentry.
When the output classes were chosen based upon clinical criteria, the relationship between the number of output classes and the classification accuracy [Fig. 5(A)] appears to be nonlinear with steeper drops in accuracy occurring between the two- and four-class conditions as well as between the 8- and 12-class conditions. The increase in the slope from the 8- to 12-class conditions (when compared to slopes from the four- to six- and six- to eight-class conditions) is likely a result of the fact that the four added classes were all hand grasp patterns (point, lateral, hand flat, and power). The proximal location of the electrodes does not allow for the detection of activity from many of the muscles needed to produce these motions, and therefore, a larger percentage drop in classification accuracy would be expected as hand motions are added to the classifier.
When the subsets of classes were chosen in a random manner, a more consistent linear relationship was observed [Fig. 5(C)]. Analyzing the data for the two-class condition is beneficial in that it represents a baseline error (2.6%). Even when only attempting to differentiate between two classes, a small number of errors are produced as a result of increased tonic activity from pain associated with the intramuscular electrodes, the subjects not responding at precisely the same time to the movement cues, etc. Any error in addition to this baseline level can be assumed to be a result of misclassifications caused by the increase in the number of output classes.
By examining random combinations of classes, an estimate of the expected decrease in classification accuracy with additional output classes can be determined. The linear mixed effects model provides a rule of thumb that each additional output class that is included into the classification problem will cause the classification accuracy to drop by 0.26%. The linear relationship that was observed for the randomly selected classes may become nonlinear if more classes were added, but the relationship holds when a maximum of 12 classes are examined.
Another observation from Fig. 5(C) is that the difference between the electrode conditions appears to grow with an increasing number of output classes. While no differences were detected with 12 output classes, if the trend of increasing separation between the electrode conditions continues, it is likely that a difference may have been detected if a larger number of output classes were tested.
Finally, average classification accuracies for the six output classes that represent movements that can be implemented through commercially available products range between 95% and 97%. These results, in combination with previously conducted studies, indicate that while there may be clinical issues that will need to be addressed before a commercial system can be successfully implemented, there appears to be substantial potential for the clinical success of myoelectrically controlled multifunctional transradial prostheses with at least three degrees of freedom.
C. Channel Subsets
Fig. 6 shows a monotonic increase in classification accuracy as the number of channels is increased. The increase in accuracy was expected as a larger number of channels provide the classifier with more information about the muscular activity of the forearm. Diminishing returns were observed with larger numbers of channels and the “knee” in the curve occurred at approximately three to four channels. Davidge et al. [57] and Hargrove et al. [42] showed a similar relationship in the improvement in classification accuracy with increasing numbers of channels in studies that used US electrodes. Davidge et al. and Hargrove et al. also found substantially diminishing returns above approximately four channels.
The ANOVA results generally showed a statistically significant decrease in classification accuracy with each incremental decrease in the number of channels. While this decrease in performance was statistically significant, the changes in accuracy were relatively small for the conditions with larger numbers of channels. For example, decreasing the number of channels from seven to six (which was statistically significant for all electrode conditions) produced an average decrease of only 0.64% across the four electrode conditions. A small but consistent decrease across subjects will show statistical significance when repeated measures approaches are used. While these differences were statistically significant, the authors hypothesize that this small decrease in accuracy would have little impact on the clinical performance of the device. Regardless, using at least four electrodes appears prudent as larger decreases in classification accuracy are observed when fewer than four channels were used.
Much like the results shown in Section III-B, the ANOVA results for the channel subset data showed that there was generally no difference between most combinations of electrode conditions when as few as five electrodes were used. However, the intramuscular electrodes showed consistently lower classification accuracies than the surface electrodes when three or fewer channels were used. These results indicate that surface channels are preferable if small numbers of channels are used for pattern-recognition-based control.
The relatively poor performance of the intramuscular electrodes with small channel numbers is likely a result of the fact that closely spaced (2–3 mm) intramuscular electrodes were used. These closely spaced electrodes produce more focal recordings [58], [59] with a smaller pickup area. The intramuscular electrodes are likely only measuring EMG from the muscle in which they are implanted with the possibility of measuring small amounts activity from muscles that are immediately adjacent to the targeted muscle. When many electrodes are used, the intramuscular recordings are able to acquire data from many individual muscles, and thus, most of the forearm musculature’s activity is captured. However, when an intramuscular channel is removed, the classifier likely no longer receives information from that muscle and will suffer accordingly. The superior performance of the surface electrodes with smaller numbers of channels is likely a result of their larger pickup area, which allows them to acquire activity from muscles that are relatively distant to the recording site. Therefore, even when a surface channel is removed, adjacent surface channels are still able to detect some activity from the muscles that were the primary contributors to the EMG measured on the removed channel.
Several observations can be made with regard to the channels that were selected using the forward addition approach. FDS, ECR, and ECU were all contained in the best four channels for both of the targeted electrode conditions. Likewise, PT, SUP, and FCR were all contained in the four least important channels for both targeted conditions. Similar correlations were observed with the untargeted conditions with channels 1, 3, and 7 being the best three channels and channels 4, 5, and 8 being added later for both conditions. It is not surprising that the channels that gave the best accuracies for small subsets (1, 3, and 7) were spaced relatively far from one another. When incrementally adding new channels, classification accuracy will be maximized by adding channels that provide the most new information to the classifier. The channels that will possess the most new information are likely not going to be immediately adjacent to channels that are currently being used but instead will be located at some relatively distant section of the forearm. Finally, it was found that the least useful channel for the untargeted electrodes was channel 4, which Fig. 2(B) shows was usually placed over the brachioradialis. This makes intuitive sense as elbow flexion, which is the primary movement produced by brachioradialis, was not included in the movement classes used in this study.
D. General Observations
Pain was experienced by almost all subjects for at least a few of the 12 movements. The pain appeared to increase tonic muscle activity and likely caused increased cocontraction. In turn, these increases in muscle activity may have caused the classification accuracies reported here to be lower than those that would be observed if no fine wire electrodes were used. However, this problem was addressed by collecting data for both the surface and intramuscular conditions when fine wires were present in the forearm. Collecting both surface and intramuscular activity simultaneously required the classifiers for both electrode conditions to have to cope with the increased tonic activity and cocontraction.
The initial impetus for the experiments comparing the surface and intramuscular recordings was the development of the implantable myoelectric sensor (IMES) [60]–[62]. IMESs are small, hermetically sealed, implantable devices that were designed to acquire focal EMG recordings to control a prosthesis via independent site control (one EMG site for each degree of freedom) or forward dynamic models. The focal recordings from the IMES were not initially intended to be used with pattern recognition systems and, as shown in the previous section, intramuscular recordings may not be advantageous for pattern-recognition-based control if a small number of channels are used. However, these devices are expected to possess certain clinical advantages (e.g., produce consistent recording sites) over surface electrodes that may allow them to be particularly useful with pattern recognition systems and it was encouraging to see that intramuscular recordings can perform as well as surface recordings in these systems.
The IMES use a transcutaneous magnetic link to both provide power to the implants and telemeter out the recorded EMG data. The capsules are approximately 13 mm in length and have an electrode at either end. While the goal was to try to mimic IMES recordings, it was determined that it was not reasonable to attempt to obtain the electrode spacing of the IMES as it would require two successful needle sticks for each muscle. The present protocol required only one successful insertion per muscle as two wire electrodes were contained in each needle. Using two wires per needle made the study more tractable but also meant that the spacing of the IMES sensors was no longer being reproduced. Using larger electrode spacing would increase the pickup area of the intramuscular electrodes and may increase their classification accuracies by allowing activity from neighboring muscles to be detected by the implants. However, Lowery et al. [63] performed simulations to show that the pickup area of the IMES sensors was relatively small. While small amounts of activity could be detected from neighboring muscles, if the implants were located in the center of the muscle belly and aligned along the length of the muscle, a vast majority of the activity measured by the IMES would originate from the muscle in which it was implanted. Therefore, while the focal recordings from the closely spaced electrodes used in these experiments did not exactly recreate those signals that would be measured by the IMES, they provided a reasonable estimate of these recordings.
The classification accuracies reported in this paper may differ from those that would be observed during clinical use. For example, these experiments did not account for variable electrode positions as the user dons and doffs the prosthesis, motion artifacts, surface electrodes “lifting off,” skin impedance changes throughout the day, other motions of the arm being conducted (i.e., flexion/extension of the elbow) while the hand/wrist are being controlled, etc. These other sources of noise, etc., may have different effects on the relative performance of each electrode condition (i.e., the intramuscular electrodes would not have the problem of ‘lifting off’), which could affect the relative advantages/disadvantages of each electrode type. However, these experiments successfully provided a comparison of the different electrodes in a laboratory setting and concluded that further investigation into the clinical advantages of the different electrode conditions is warranted.
Inconsistent electrode location, as well as skin impedance changes and electrode liftoff can be particularly problematic in a pattern-recognition-based system. These problems associated with surface electrodes cause changes in the EMG that will cause the classifier to be operated with signals that are different from those that were used to train it. For example, Hargrove et al. [64] showed substantial decreases in accuracy with 1 cm shifts in the electrode position. The fact that intramuscular electrodes can produce classification accuracies that are as high as those produced by surface electrodes and have the potential ability to address the major problems associated with surface electrodes (e.g., liftoff, consistent placement, skin impedance changes, etc.) indicates that these devices may produce a better clinically functioning prosthesis. However, further investigation is necessary to verify that intramuscular electrodes will indeed address these problems.
V. Conclusion
The primary goals of this paper were to compare the pattern recognition classification accuracies produced by both targeted and untargeted as well as surface and intramuscular electrodes. No previous work had compared the use of targeted and untargeted electrodes and only a single comparison of surface and intramuscular electrodes had been conducted. This study found that when additional signal features are extracted from the EMG, there was no difference between the electrode conditions. Both targeted and untargeted as well as surface and intramuscular electrodes produced similar classification accuracies. The similarity between the electrode conditions was also seen for several subsets of the 12 output classes when the full complement of electrodes was used. Differences between the electrode conditions for classifiers utilizing additional signal features were only observed when small numbers of electrodes (≤3) were employed. In these cases, the surface EMG recordings produced higher classification accuracies. Classification accuracies were shown to increase with increasing numbers of channels but diminishing returns were observed for all electrode conditions when more than four electrodes were used. Finally, when no additional signal features were used, the UI electrodes produced statistically lower classification accuracies than the other three conditions.
From the point of view of strictly increasing classification accuracy in a laboratory setting, the US electrodes perform as well as the other three conditions and do so with the lowest cost, invasiveness, and difficulty in socket fabrication. However, good performance in the clinical realm is dependent upon more than producing a high classification accuracy in a laboratory setting. Several clinical issues that are known to profoundly affect pattern-recognition-based control, such as electrode liftoff and signal repeatability/consistency, were not considered in this study. Therefore, the similarity of the classification accuracies produced by both the surface and intramuscular electrodes in the laboratory indicate that electrodes should be chosen based upon clinical issues such as signal robustness as opposed to which electrodes produce the highest classification accuracies.
Acknowledgments
This work was supported in part by the National Institutes of Health (NIBIB/NICHD) under Grant 1 R01 EB01672-01, in part by the Department of Veterans Affairs, Rehabilitation Research and Development Service and is administered through the Jesse Brown Veterans Affairs Medical Center, Chicago, IL, and in part by the National Institute of Disability and Rehabilitation Research (NIDRR) of the Department of Education under Grant H133E030030.
The authors would like to acknowledge A. Ajiboye, Ph.D., R. Bogey, D.O., and T. Kuiken, M.D./Ph.D., for their assistance in the development and implementation of the fine wire protocol. They would also like to acknowledge D. Kamper, Ph.D., S. Koehler, M.S., and L. Welty, Ph.D., for their assistance with the statistical analyses contained in this paper. Additionally, the authors would like to recognize the Coleman Foundation for the use of the Coleman Foundation Computing Center. The opinions contained in this paper are those of the grantee and do not necessarily reflect those of the Department of Education.
Biographies

Todd R. Farrell (M′02) was born in Albany, NY. He received the Bachelor’s degree from the Catholic University of America, Washington, DC, in 2000, and the M.S. and Ph.D. degrees from the Northwestern University, Evanston, IL, in 2003 and 2007, respectively, all in biomedical engineering.
He is currently with Liberating Technologies, Holliston, MA. His current research interests include the area of multifunctional prosthesis control, specifically the use of intramuscular EMG, as well as the effect of delay and controller accuracy on prosthesis performance.

Richard F. ff. Weir (M′91) was born in Dublin, Ireland. He received the B.A. degree in mathematics and the B.A.I. degree in microelectronics and electrical engineering from Trinity College, University of Dublin, Dublin, both in 1983, and the M.S. and Ph.D. degrees in biomedical engineering from the Northwestern University, Evanston, IL, in 1989 and 1995, respectively.
He was a Control Engineer in U.K. He is currently the Director of the Biomechatronics Development Laboratory, Rehabilitation Institute of Chicago, Chicago, IL, where he is a Research Scientist at Jesse Brown VA Medical Center. He is also a Research Assistant Professor in the Departments of Physical Medicine and Rehabilitation and Biomedical Engineering, Northwestern University. His current research interests include the area of neural control, biomechatronics and rehabilitation, specifically arm/hand systems, manipulators, robotics, and their associated control. The current focus of this work is the development of a multichannel/multifunction prosthetic hand/arm controller system based on implantable myoelectric sensors (IMES), the development of an externally powered partial hand prostheses, the development of a multifunction externally powered prosthetic hand, as well as an exploration of the use of series elastic actuators for use in prosthetic devices.
Contributor Information
Todd R. Farrell, The Department of Biomedical Engineering, McCormick School of Engineering and Applied Science, Northwestern University, Evanston, IL 60208 USA, and also with the Biomechatronics Development Laboratory of the Rehabilitation Institute of Chicago, Chicago, IL 60611 USA.
Richard F. ff. Weir, Email: rweir@northwestern.edu, The Biomechatronics Development Laboratory, Rehabilitation Institute of Chicago, Chicago, IL 60208 USA. He is also with Jesse Brown VA Medical Center, Department of Physical Medicine and Rehabilitation, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611 USA, and also with the Department of Biomedical Engineering, McCormick School of Engineering and Applied Science, Northwestern University Chicago, IL 60611 USA.
References
- 1.Graupe D, Magnussen J, Beex AA. Microprocessor system for multifunctional control of upper-limb prostheses via myoelectric signal identification. IEEE Trans Autom Control. 1978 Aug;AC-23(4):538–544. [Google Scholar]
- 2.Lee SP, Kim JS, Park SH. An enhanced feature extraction algorithm for EMG pattern classification. IEEE Trans Rehabil Eng. 1996 Dec;4(4):439–443. doi: 10.1109/86.547948. [DOI] [PubMed] [Google Scholar]
- 3.Park SH, Lee SP. EMG pattern recognition based on artificial intelligence techniques. IEEE Trans Rehabil Eng. 1998 Dec;6(4):400–405. doi: 10.1109/86.736154. [DOI] [PubMed] [Google Scholar]
- 4.Ajiboye AB, Weir RF. A heuristic fuzzy logic approach to EMG pattern recognition for multifunctional prosthesis control. IEEE Trans Neural Syst Rehabil Eng. 2005 Sep;13(3):280–291. doi: 10.1109/TNSRE.2005.847357. [DOI] [PubMed] [Google Scholar]
- 5.Chan FH, Yang YS, Lam FK, Zhang YT, Parker PA. Fuzzy EMG classification for prosthesis control. IEEE Trans Rehabil Eng. 2000 Sep;8(3):305–311. doi: 10.1109/86.867872. [DOI] [PubMed] [Google Scholar]
- 6.Micera S, Sabatini AM, Dario P. On automatic identification of upper-limb movements using small-sized training sets of EMG signals. Med Eng Phys. 2000 Oct;22:527–533. doi: 10.1016/s1350-4533(00)00069-2. [DOI] [PubMed] [Google Scholar]
- 7.Saridis GN, Stephanou HE. Fuzzy decision-making in prosthetic devices. In: Gupta MM, editor. Fuzzy Automata and Decision Processes. New York: North Holland; 1977. pp. 387–402. [Google Scholar]
- 8.Huang Y, Englehart KB, Hudgins B, Chan AD. A Gaussian mixture model based classification scheme for myoelectric control of powered upper limb prostheses. IEEE Trans Biomed Eng. 2005 Nov;52(11):1801–1811. doi: 10.1109/TBME.2005.856295. [DOI] [PubMed] [Google Scholar]
- 9.Chan AD, Englehart KB. Continuous myoelectric control for powered prostheses using hidden Markov models. IEEE Trans Biomed Eng. 2005 Jan;52(1):121–124. doi: 10.1109/TBME.2004.836492. [DOI] [PubMed] [Google Scholar]
- 10.Englehart K, Hudgins B, Chan A. Continuous multifunction myoelectric control using pattern recognition. Technol Disabil. 2003;15:95–103. [Google Scholar]
- 11.Englehart K, Hudgins B, Parker PA. A wavelet-based continuous classification scheme for multifunction myoelectric control. IEEE Trans Biomed Eng. 2001 Mar;48(3):302–311. doi: 10.1109/10.914793. [DOI] [PubMed] [Google Scholar]
- 12.Englehart K, Hudgins B, Parker PA, Stevenson M. Classification of the myoelectric signal using time-frequency based representations. Med Eng Phys. 1999 Jul./Sep;21:431–438. doi: 10.1016/s1350-4533(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 13.Herberts P, Almstrom C, Caine K. Clinical application study of multifunctional prosthetic hands. J Bone Joint Surg Br. 1978 Nov;60-B:552–560. doi: 10.1302/0301-620X.60B4.711808. [DOI] [PubMed] [Google Scholar]
- 14.Herberts P, Almstrom C, Kadefors R, Lawrence PD. Hand prosthesis control via myoelectric patterns. Acta Orthop Scan. 1973;44:389–409. doi: 10.3109/17453677308989075. [DOI] [PubMed] [Google Scholar]
- 15.Saridis GN, Gootee TP. EMG pattern-analysis and classification for a prosthetic arm. IEEE Trans Biomed Eng. 1982 Jun;BE-29(6):403–412. doi: 10.1109/TBME.1982.324954. [DOI] [PubMed] [Google Scholar]
- 16.Yamada M, Niwa N, Uchiyama A. Evaluation of a multifunctional hand prosthesis system using EMG controlled animation. IEEE Trans Biomed Eng. 1983 Nov;BE-30(11):759–763. doi: 10.1109/tbme.1983.325192. [DOI] [PubMed] [Google Scholar]
- 17.Hu X, Nenov V. Multivariate AR modeling of electromyography for the classification of upper arm movements. Clin Neurophysiol. 2004 Jun;115:1276–1287. doi: 10.1016/j.clinph.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 18.Lyman J, Freedy A, Solomonow M. System integration of pattern-recognition, adaptive aided, upper limb prostheses. Mech Mach Theory. 1977;12:503–514. [Google Scholar]
- 19.Doerschuk PC, Gustafson DE, Willsky AS. Upper extremity limb function discrimination using EMG signal analysis. IEEE Trans Biomed Eng. 1983 Jan;BE-30(1):18–29. doi: 10.1109/tbme.1983.325162. [DOI] [PubMed] [Google Scholar]
- 20.Dening DC, Gray FG, Haralick RM. Prosthesis control using a nearest neighbor electromyographic pattern classifier. IEEE Trans Biomed Eng. 1983 Jun;BE-30(6):356–360. doi: 10.1109/tbme.1983.325138. [DOI] [PubMed] [Google Scholar]
- 21.Peleg D, Braiman E, Yom-Tov E, Inbar GF. Classification of finger activation for use in a robotic prosthesis arm. IEEE Trans Neural Syst Rehabil Eng. 2002 Dec;10(4):290–293. doi: 10.1109/TNSRE.2002.806831. [DOI] [PubMed] [Google Scholar]
- 22.Al-Assaf Y, Al-Nashash H. Surface myoelectric signal classification for prostheses control. J Med Eng Technol. 2005 Sep./Oct;29:203–207. doi: 10.1080/03091900412331289906. [DOI] [PubMed] [Google Scholar]
- 23.Bu N, Fukuda O, Tsuji T. EMG-based motion discrimination using a novel recurrent neural network. J Intell Inf Syst. 2003;21:113–126. [Google Scholar]
- 24.Chu JU, Moon I, Mun MS. A real-time EMG pattern recognition system based on linear-nonlinear feature projection for a multifunction myoelectric hand. IEEE Trans Biomed Eng. 2006 Nov;53(11):2232–2239. doi: 10.1109/TBME.2006.883695. [DOI] [PubMed] [Google Scholar]
- 25.Farry KA, Walker ID, Baraniuk RG. Myoelectric teleoperation of a complex robotic hand. IEEE Trans Robot Autom. 1996 Oct;12(5):775–788. [Google Scholar]
- 26.Fukuda O, Tsuji T, Kaneko M, Otsuka A. A human-assisting manipulator teleoperated by EMG signals and arm motions. IEEE Trans Robot Autom. 2003 Apr;19(2):210–222. [Google Scholar]
- 27.Gallant PJ, Morin EL, Peppard LE. Feature-based classification of myoelectric signals using artificial neural networks. Med Biol Eng Comput. 1998 Jul;36:485–489. doi: 10.1007/BF02523219. [DOI] [PubMed] [Google Scholar]
- 28.Hudgins B, Parker P, Scott RN. A new strategy for multifunction myoelectric control. IEEE Trans Biomed Eng. 1993 Jan;40(1):82–94. doi: 10.1109/10.204774. [DOI] [PubMed] [Google Scholar]
- 29.Karlik B, Tokhi MO, Alci M. A fuzzy clustering neural network architecture for multifunction upper-limb prosthesis. IEEE Trans Biomed Eng. 2003 Nov;50(11):1255–1261. doi: 10.1109/TBME.2003.818469. [DOI] [PubMed] [Google Scholar]
- 30.Kelly MF, Parker PA, Scott RN. The application of neural networks to myoelectric signal analysis: A preliminary study. IEEE Trans Biomed Eng. 1990 Mar;37(3):221–230. doi: 10.1109/10.52324. [DOI] [PubMed] [Google Scholar]
- 31.Koike Y, Kawato M. Estimation of dynamic joint torques and trajectory formation from surface electromyography signals using a neural network model. Biol Cybern. 1995;73:291–300. doi: 10.1007/BF00199465. [DOI] [PubMed] [Google Scholar]
- 32.Kumar DK, Ma N, Burton P. Classification of dynamic multi-channel electromyography by neural network. Electromyogr Clin Neurophysiol. 2001 Oct./Nov;41:401–408. [PubMed] [Google Scholar]
- 33.Kuruganti U, Hudgins B, Scott RN. Two-channel enhancement of a multifunction control system. IEEE Trans Biomed Eng. 1995 Jan;42(1):109–111. doi: 10.1109/10.362912. [DOI] [PubMed] [Google Scholar]
- 34.Light CM, Chappell PH, Hudgins B, Englehart K. Intelligent multifunction myoelectric control of hand prostheses. J Med Eng Technol. 2002 Jul./Aug;26:139–146. doi: 10.1080/03091900210142459. [DOI] [PubMed] [Google Scholar]
- 35.Nishikawa D, Yu WW, Yokoi H, Kakazu Y. On-line supervising mechanism for learning data in surface electromyogram motion classifiers. Syst Comput Jpn. 2002;33:1–11. [Google Scholar]
- 36.Sebelius F, Eriksson L, Balkenius C, Laurell T. Myoelectric control of a computer animated hand: A new concept based on the combined use of a tree-structured artificial neural network and a data glove. J Med Eng Technol. 2006 Jan./Feb;30:2–10. doi: 10.1080/03091900512331332546. [DOI] [PubMed] [Google Scholar]
- 37.Sebelius F, Eriksson L, Holmberg H, Levinsson A, Lundborg G, Danielsen N, Schouenborg J, Balkenius C, Laurell T, Montelius L. Classification of motor commands using a modified self-organising feature map. Med Eng Phys. 2005 Jun;27:403–413. doi: 10.1016/j.medengphy.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Soares A, Andrade A, Lamounier E, Carrijo R. The development of a virtual myoelectric prosthesis controlled by an EMG pattern recognition system based on neural networks. J Intell Inf Syst. 2003 Sep;21:127–141. [Google Scholar]
- 39.Patterson PE, Anderson M. The use of self organizing maps to evaluate myoelectric signals. Biomed Sci Instrum. 1999;35:147–152. [PubMed] [Google Scholar]
- 40.Stein RB, Charles D, Walley M. Bioelectric control of powered limbs for amputees. Adv Neurol. 1983;39:1093–1108. [PubMed] [Google Scholar]
- 41.Tucker F, Peteleski N. Microelectronic telemetry implant for myoelectric control of a powered prosthesis. Can Electr Eng J. 1977;2:3–7. [Google Scholar]
- 42.Hargrove LJ, Englehart K, Hudgins B. A comparison of surface and intramuscular myoelectric signal classification. IEEE Trans Biomed Eng. 2007 May;54(5):847–853. doi: 10.1109/TBME.2006.889192. [DOI] [PubMed] [Google Scholar]
- 43.Santa-Cruz MC, Riso RR, Lange B, Sepulveda F. Natural control of key grip and precision grip movements for a myoelectric prosthesis. Proc. Myoelectric Controls Symp.—MEC; 1999; Fredericton, NB, Canada. pp. 106–112. [Google Scholar]
- 44.Santa-Cruz MC, Riso RR, Sepulveda F. Evaluation of neural network parameters towards enhanced recognition of naturally evoked EMG for prosthetic hand grasp control. Proc. Congr. Int. FES Soc.; Aalborg, Denmark. 2000. pp. 436–439. [Google Scholar]
- 45.Santa-Cruz MC, Riso RR, Sepulveda F, Lange B. Natural control of wrist movements for myoelectric prostheses. Proc. 21st Annu. Conf. IEEE Eng. Med. Biol. Soc. Fall Meeting Biomed. Eng. Soc.; Atlanta, GA. 1999. p. 642. [Google Scholar]
- 46.Almstrom C, Herberts P, Korner L. Experience with Swedish multifunctional prosthetic hands controlled by pattern recognition of multiple myoelectric signals. Int Orthop. 1981;5:15–21. doi: 10.1007/BF00286094. [DOI] [PubMed] [Google Scholar]
- 47.Sebelius FC, Rosen BN, Lundborg GN. Refined myoelectric control in below-elbow amputees using artificial neural networks and a data glove. J Hand Surg Amer. 2005 Jul;30:780–789. doi: 10.1016/j.jhsa.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Tsuji T, Fukuda O, Kaneko M, Ito K. Pattern classification of time-series EMC signals using neural networks. Int J Adapt Control. 2000 Dec;14:829–848. [Google Scholar]
- 49.Englehart K, Hudgins B. A robust, real-time control scheme for multifunction myoelectric control. IEEE Trans Biomed Eng. 2003 Jul;50(7):848–854. doi: 10.1109/TBME.2003.813539. [DOI] [PubMed] [Google Scholar]
- 50.Graupe D, Salahi J, Kohn KH. Multifunctional prosthesis and orthosis control via microcomputer identification of temporal pattern differences in single-site myoelectric signals. J Biomed Eng. 1982 Jan;4:17–22. doi: 10.1016/0141-5425(82)90021-8. [DOI] [PubMed] [Google Scholar]
- 51.Graupe D, Salahi J, Zhang DS. Stochastic analysis of myoelectric temporal signatures for multifunctional single-site activation of prostheses and orthoses. J Biomed Eng. 1985 Jan;7:18–29. doi: 10.1016/0141-5425(85)90004-4. [DOI] [PubMed] [Google Scholar]
- 52.Nishikawa D, Yu WW, Yokoi H, Kakazu Y. On-line learning method for EMG prosthetic hand control. Electron Commun Jpn III—Fundam Electron Sci. 2001;84:35–46. [Google Scholar]
- 53.Latwesen A, Patterson PE. Identification of lower arm motions using the EMG signals of shoulder muscles. Med Eng Phys. 1994 Mar;16:113–121. doi: 10.1016/1350-4533(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 54.Micera S, Sabatini AM, Dario P, Rossi B. A hybrid approach to EMG pattern analysis for classification of arm movements using statistical and fuzzy techniques. Med Eng Phys. 1999 Jun;21:303–311. doi: 10.1016/s1350-4533(99)00055-7. [DOI] [PubMed] [Google Scholar]
- 55.Farrell T. PhD dissertation. Dept. Biomed. Eng., Northwestern Univ; Evanston, IL: 2007. Multifunctional prosthesis control: The effects of targeting surface and intramuscular electrodes on classification accuracy and the effect of controller delay on prosthesis performance. [Google Scholar]
- 56.Field A. Discovering Statistics Using SPSS. 2. London, U.K: Sage; 2005. [Google Scholar]
- 57.Davidge KA, Buerkle VR, Englehart K, Parker P. Multi-functional myoelectric control using a linear array. Proc. 15th Congr. Int. Soc. Electrophysiol. Kinesiol.; Boston, MA. 2004. p. 59. [Google Scholar]
- 58.Giroux B, Laontagne M. Comparison between surface electrodes and intramuscular wire electrodes in isometric and dynamic conditions. Electromyogr Clin Neur. 1990;30:397–405. [PubMed] [Google Scholar]
- 59.Perry J, Easterday CS, Antonelli DJ. Surface versus intramuscular electrodes for electromyography of superficial and deep muscles. Phys Ther. 1977;61:7–15. doi: 10.1093/ptj/61.1.7. [DOI] [PubMed] [Google Scholar]
- 60.Weir RF, Troyk PR, DeMichele G, Kerns D. Technical details of the implantable myoelectric sensor (IMES) system for multifunction prosthesis control. Proc. 27th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc.; 2005. pp. 7337–7340. [DOI] [PubMed] [Google Scholar]
- 61.Weir RF, Troyk PR, DeMichele G, Kuiken TA. Implantable myoelectric sensors (IMES) for upper-extremity prosthesis control—Preliminary work. Proc. 25th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc.; 2003. pp. 1562–1565. [Google Scholar]
- 62.Weir RFff, Troyk PR, DeMichele GA, Kerns DA, Schorsch JF, Maas H. Implantable myoElectric sensors (IMES) for prosthesis control: Development and testing. IEEE Trans Biomed Eng. to be published. [Google Scholar]
- 63.Lowery MM, Weir RF, Kuiken TA. Simulation of intramuscular EMG signals detected using implantable myoelectric sensors (IMES) IEEE Trans Biomed Eng. 2006 Oct;53(10):1926–1933. doi: 10.1109/TBME.2006.881774. [DOI] [PubMed] [Google Scholar]
- 64.Hargrove L, Hudgins B, Englehart K, Leckey R. A comparison of surface and internally measured myoelectric signals for use in prosthetic control. Proc. Myoelectric Controls Symp.—MEC; 2005; Fredericton, NB, Canada. pp. 102–106. [Google Scholar]