Abstract
Corticospinal interactions are considered to play a key role in executing voluntary movements. Nonetheless several different studies have shown directly and indirectly that these interactions take place long before movement starts, when preparation for forthcoming movements dominates. When motor-related parameters are continuously processed in several premotor cortical sites, segmental circuitry is directly exposed to this processing via descending pathways which originate from these sites in parallel to descending fibers that derive from primary motor cortex. Recent studies have highlighted the functional role of these interactions in priming downstream elements for the ensuing motor actions. Time-resolved analysis has further emphasized the dynamic properties of pre-movement preparatory activity.
Introduction
When performing goal-directed movements, information about the direction or distance to the target is often available before the occurrence of the stimulus that initiates movement. Psychophysical studies have demonstrated that prior information is gradually incorporated into the motor command over several hundred milliseconds [1] and that the most obvious behavioral effect of such prior information is to shorten movement reaction time [2-5]. Furthermore, disrupting preparatory activity in dorsal premotor cortex (PMd) causes delayed movement onset [6].
Modulation of neuronal activity preceding movement has been attributed to preparatory processes including the specification of movement direction and amplitude [4,5,7-11]. Various studies have attempted to identify spatially segregated brain regions dedicated to each parameter [5,12-14], but other studies suggest that these parameters are vastly represented in different brain areas and mostly by the same cells [5,11,15]. Regardless of the actual site where these parameters are specified, a full parametric representation of the ensuing movements exists in cortical (and possibly in some subcortical) sites before movement onset. However to date, there is little direct evidence regarding the extent to which spinal circuitry is involved in preparation for actual movement or the specific role that it plays in this process. Specifically, it is unclear whether pre-movement information is accessible to spinal circuitry at all, and if so how (and for what purpose) spinal neurons use this information.
Preparatory activity in downstream elements
Early motor-related activity can be detected in the motor cortex (as well as other motor-related cortical sites) long before movement actually starts [16,17]. It was suggested that this cortical “set-related activity” is responsible for generating the appropriate motor set required for generating movements [18,19]. This concept implies a high-level role of preparatory activity which is not necessarily transmitted to downstream elements. The data nevertheless suggest that preparatory activity may also propagate to downstream motor elements.
Preparation for movement has been shown to involve modulation of spinal reflexes: the monosynaptic H-reflex was found to be suppressed during the preparatory period [20-23], but long-latency reflexes were facilitated [24]. The fact that modulation was also found in the monosynaptic reflex suggests that the source of modulation is likely to be descending inputs which either pre-synaptically affect incoming afferents or modulate the excitability of motoneurons (MNs) [25-27]. Moreover, these reflex studies provide the first indications that both excitatory and inhibitory mechanisms are involved in preparation for movement. Along with the suppression of spinal reflexes, recordings of single motor units revealed a decrease in firing rate and firing variability during the delay period [28] that was not accompanied by changes in the net produced torque. Finally, a large number of studies using transcranial magnetic stimulation (TMS) have shown delay-period modulations in the corticospinal (CS) system. Here the results are less consistent in that both increases and decreases in the evoked motor potentials (MEPs) during the delay period have been observed. In many studies the area under the MEPs decreased during the delay period [29-32] presumably reflecting recruitment of inhibitory cortical and\or spinal circuitry. In other cases, increased CS excitability was found [33]. Some of the discrepancy in the findings may be due to differences in design affecting the structure of the delay period (variability in duration) and the number of pre-specified motor parameters [26,28,31]. When these effects were compensated for, the observed modulations in CS interactions in the TMS data were in fact confined to a specific motor response and were not a mere reflection of some non-specific shift in alertness that preceded the ensuing action [34,35]. Measuring CS synchronization (as opposed to excitability) further revealed a modulation in the strength of gamma-band coherence between cortical activity (measured with MEG) and muscles which was dependent on subjects’ readiness to perform [34,36]. This result suggests that not only the net CS activity is modulated during the delay period but that changes in synchrony among neurons composing the CS system may further modify its excitability without requiring a specific recruitment of cortical and\or spinal interneurons [33].
Attempts at relating the results obtained from reflex analyses and TMS studies to specific neuronal mechanisms have highlighted the complexity of this translation. For example, it was suggested that in TMS, the area of the MEP reflect CS excitability and the silent period that follows the evoked MEP reflects recruitment of intracortical inhibitory interneurons [37]. Yet given the complexity of interactions that might be elicited by TMS pulses it is difficult to derive a clear and distinct description of the neuronal circuitry underlying these modulations.
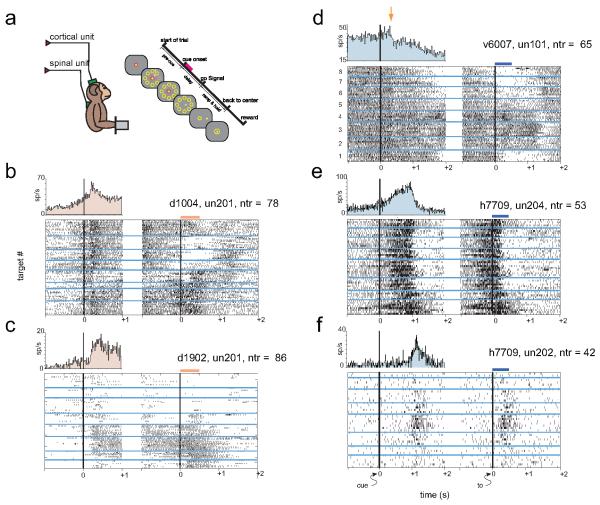
Thus, to further explore the underlying spinal mechanisms that are active during preparation for movement activity of spinal neurons was recorded in primates [38-41] and was shown to be modulated in a manner which includes both inhibitory and excitatory preparatory activity (Fig. 1). The onset time of spinal preparatory activity overlapped the onset of cortical activity, with the inhibitory activity occurring earlier than the excitatory response [39]. Although parametric studies of cortical preparatory activity have revealed a robust and widespread coding of motor parameters relevant to the ensuing movement [5,10,42,43], spinal neurons here often modulated their firing rate in a non-specific way with respect to the upcoming motor action. This finding may limit the implication of spinal preparatory activity and implies that spinal processing is not part of the preparation for specific movements [39].
Figure 1. Examples of cortical and spinal preparatory activity during a 2-dimensional isometric wrist task.
a, monkeys sat in a primate chair in front of a computer screen and controlled an on-screen cursor by generating a 2D isometric torque at the wrist. Task required acquisition of a memorized target out of 8 presented targets in a circular arrangement. Trials were composed of a pre-cue period, a delay period and a “go” signal after which the monkey acquired the cued-target. During task performance neuronal activity was recorded from motor cortex and spinal cord. b-f, examples of cortical (b-c) and spinal (d-f) neurons recording during the task. Each example shows neuronal activity aligned on cue onset (left panel) a torque onset (right panel); the two panels are separated because the duration of the delay period varied. Note also that there was an overlap between the post-cue period and the pre-torque period. Trials were sorted according to target direction (target 1 is the upper-most target and numbering is clockwise). Peri-event time histograms (upper left plot in each example) quantify neuronal activity around cue onset irrespective of target direction. Examples e and f show two spinal neurons recorded simultaneously: one neuron (e) shows robust pre-movement activity and the second neuron (f) shows only torque-related activity. These examples suggest that pre-movement activity is not a mere reflection of subtle pre-movement modulations in torque level.
Population-based analysis of preparatory activity
Often the preparatory activity expressed by single cells is diverse, in that it has complex time-dependent features which vary considerably across cells. In addition, the noisy nature of spiking activity[44] and the limited number of trials available for quantifying single cell responses may further hinder determination of the underlying neuronal processing. An alternative approach to mitigate this cell-to-cell variability is to use a population-based approach where signals that integrate local spiking or synaptic currents are employed to analyze local neuronal processing.
There is evidence that the local field potential (LFP) can capture unique aspects of cortical processing during the delay period. Early studies showed widespread coherent oscillation in the motor cortex which were specifically correlated with motor preparation rather than motor execution [45]. It was further found that LFPs were tuned during the delay period [46]. Motor cortical sites were more likely to exhibit cue-related evoked LFP than motor-related responses [38] if (and only if) the visual cue had motor significance [47,48].
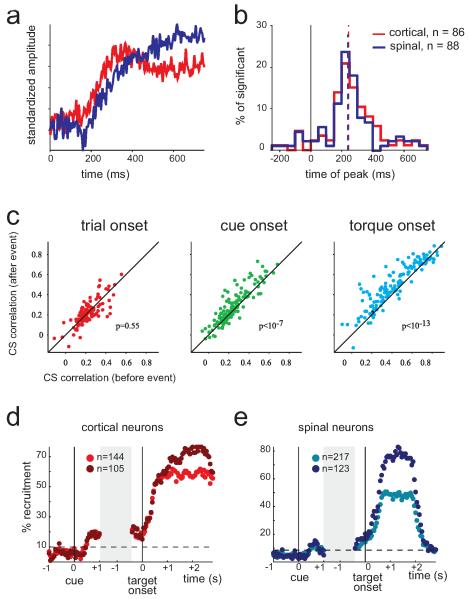
In addition to LFP, a signal commonly used for analyzing activity of neuronal populations is multiunit activity (MUA) which represents the spiking activity of nearby neurons [49,50]. Analyzing the MUA of cortical and spinal sites[38] revealed that both structures expressed early, cue-triggered, evoked responses (EPs - Fig. 2a). Cortical and spinal EPs overlapped in time (Fig. 2b) but differed in structure; namely, the cortical response was positive relative to the pre-cue baseline activity but the spinal response had an initial negative phase which was followed by a positive increase. In this respect, spinal MUA may reflect an integrated image of the single unit responses found among spinal interneurons that included early inhibition in some cells and late excitation in others. A second property that was found using both LFP and MUA signals is the tendency for both cortical and spinal visual EPs to be positively correlated with motor EPs but negatively correlated with reaction time. This finding implies that the extent to which neuronal populations (both upstream and downstream) process early visual cues has direct behavioral consequences (in terms of response timing). Finally, it was found that the visual cue induced an increase in the population-based CS correlation compared to the pre-cue period (Fig 2c). The fact that cue-related EPs in motor cortex and spinal cord were correlated in magnitude but had similar onset times may suggest continuous and reciprocal interactions (i.e., reverberations) in the CS system before and during movements where activity is continuously transmitted between the two sites. The different events and epochs along the trial mostly modify the properties of this reverberation in terms of magnitude and phase properties: after cue onset CS correlations have zero-lag (indicating a co-activation mode) but after torque onset the correlation peak is shifted consistent with the cortical command driving spinal circuitry. This result may further hint at the mechanism underlying the increase in corticomuscular coherence observed during preparation for movement in humans.
Figure 2. Population-based measures of pre-movement delay period activity in motor cortex and spinal cord.
a, we measured the evoked multi-unit activity (MUA) around cue-onset for cortical (red) and spinal (blue) sites recorded from three monkeys during the task described in figure 1a. Single site data were first normalized using pre-cue activity to provide a normalized response (expressed in standard deviation units) and were then averaged across all sites recorded from three different monkeys. Note that about 150 ms after cue onset there was a clear increase in cortical MUA. Spinal MUA at roughly the same time shows a brief decrease followed by a monotonous increase that resembles the cortical response pattern. b, histograms of the peak-time of cue-triggered cortical and spinal responses show overlap indicating a similar time scale for responses at the two sites. Note that as spinal response appeared monotonous with no clear peak we used the peak of the response derivative. c, we first computed the cross-correlation between cortical and spinal evoked MUA potentials before and after each behavioral event. The scatter plots show the peak amplitude of the cross-correlation computed after the event vs. the peak amplitude for the cross-correlation computed before the event. Scatter plots are shown for the following events: trial onset (left), cue onset (middle) and torque onset (right). Structure of trials is shown in figure 1a. Note that although for trial onset (a cue which provided no motor-related information) the event had no effect on peak correlation magnitude, for both cue onset and torque onset CS correlations were stronger after the event compared to the pre-event correlation. d-e, for two monkeys that were trained to perform a one-dimensional wrist task which included only flexion and extension targets we analyzed the fraction of cortical (d ) and spinal (e) neurons that were directionally tuned at different time bins along the trial. For each population of neurons we computed the coding fraction for the entire set of task-related neurons (light red and blue dots) as well as for the subpopulation of neurons that were directionally tuned during the active hold period (dark red and blue dots). The gray block reflects the fact the delay period varied in time on a trial-to-trial basis. Dashed line reflects the threshold above which the fraction of neurons deviated from the expected number based on the pre-cue level and variability. The population recruitment fraction (PRF - fraction of neurons that were significantly tuned to torque direction) varied along the trial and was maximal during hold. Note that cortical PRF exhibited a sustained and robust increase while the spinal PRF at the same period expressed only a transient and smaller peak.
The existence of population-based pre-movement modulation in cortical and spinal activity may suggest that while only a few spinal cells provide consistent information during the delay period regarding the instructed movement, this information could dynamically exist across neurons. When computing the fraction of neurons that express task-related activity in a given time window (Fig. 2d,e) cortical neurons tended to have a consistent representation of direction of ensuing movements during the delay period, but spinal neurons expressed only a transient increase in such a representation. This result is consistent with a previous finding: though many spinal neurons express rate modulation during the delay period [39]spinal representation of movement parameters (before movement onset) is weak.
Dynamic aspects of preparatory activity
It has often been shown that additional information presented during the delay period regarding forthcoming movements greatly modify neuronal processing at this time [12,43,51]. But even when the action is fully predictable during the preparatory period, activity at this time is not necessarily stationary. Several events have been shown to take place during this time, all suggestive that this epoch is used for information processing.
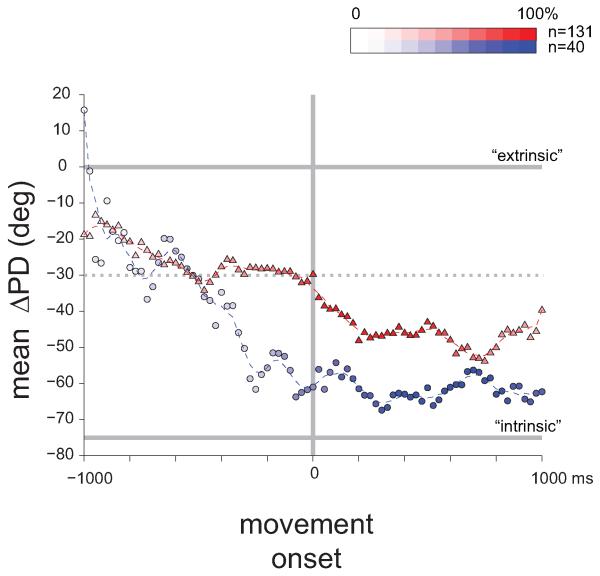
First, the actual time that elapses from cue onset (thus making the go signal more probable) affects the firing of neurons in premotor cortex [52,53] such that the firing of these neurons reflects some subjective time estimation made by the animal. Recently it was shown [38,40,41] that when movement is fully dictated (and unchanged) during the delay period, some parameters slowly change from cue onset until the go signal. For example, the coordinate frame of cortical neurons shifted gradually from a near-external to near internal frame during preparation and through the active ramp period (Fig. 3). This progressive transition is reminiscent of the “mental rotation” exhibited by the population vector when the requested movement is to aim away from the presented cue [54]. In both cases it appears that the cortical circuitry assigns time-dependent weights when integrating multiple sources of information: at cue onset external events dominate but as motor action approaches the weight assigned to internal information (e.g., proprioceptive feedback) increases and thus the coordinate frame rotates towards the muscle-based frame. The mechanism that provides this varying weight at which different inputs are integrated is yet to be determined. Furthermore in this case, it was found that relatively few spinal cells were tuned during the delay period, and for those that were tuned the coordinate frame before movement onset was similar to the one that was used during movement. This indicates again that spinal circuitry does not process motor parameters during preparation for movement despite the fact that spinal neurons modulate their rate during this time.
Figure 3. Dynamic modulation in the coordinate frame during the delay period.
Monkeys were trained to perform the 2-dimensional wrist task with their hand in either a pronation or supination position. The difference between the preferred directions (ΔPD) of tuned neurons in the two hand postures was used to identify the coordinate frame in which these neurons operated as either extrinsic (ΔPD = 0°) or intrinsic (ΔPD ≈ 70°). We found that for cortical neurons (red triangles) the coordinate frame slowly rotated during the trial from a nearly extrinsic frame near cue onset to a nearly intrinsic frame during active hold. Spinal neurons at early stages of the delay period were hardly tuned and the obtained averages were noisy compared to the cortical population. The figure indicates that during the delay period a continuous processing of parametric information takes place especially for cortical neurons.
A possible role for descending control during motor preparation: parallel pathways deliver information and modulation
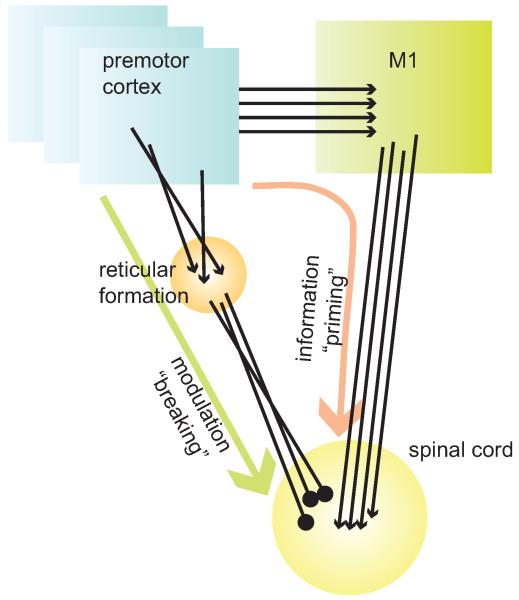
The various methods used to study modulation in the motor system during preparation for movement (in terms of signals, subjects and paradigms) provide a complex picture of the impact of these modulations on downstream elements and the neuronal mechanisms through which this impact is obtained. There is a certain amount of consensus that both excitatory and inhibitory processes take place during this time [30,33,39,55]. It was suggested that these two competing processes act as a “priming and breaking” mechanism [39]: excitation is aimed at priming a specific response, and inhibition acts to prevent this overt excitation from causing a premature movement (a mechanism which was also called “impulse control” [35,56]). A detailed examination of single cell activity of cortical and spinal neurons during this period further revealed some puzzling findings. On one hand, though neurons in premotor and motor cortex contain a representation of the forthcoming movement already at early stages of the delay period, this representation is not transmitted to downstream elements. On the other hand, before movement onset, spinal neurons have a complete parametric representation of the action to be performed. Based on indirect measures, some studies inferred that the source of CS inhibition and excitation is a specific recruitment of cortical inhibitory and excitatory interneurons [30,35,55]. An alternative mechanism which could account for these observations is the existence of parallel pathways from premotor cortex to the spinal cord (Fig. 4). The main pathway that provides motor-related information goes from the premotor to the motor cortex to the spinal cord. Here the termination pattern is consistent, so that directional-torque information is preserved. This suggestion is consistent with previous studies indicating that the premotor cortex exerts most of its excitatory influence upon muscles indirectly via M1 [57-60]. The second pathway goes from the premotor cortex to the reticular formation and from there to the spinal cord [61-64]. The termination pattern of this pathway occurs irrespective of motor parameters and thus specific motor-related parameters are not preserved. The existence of this pathway which can provide a powerful inhibition to spinal circuitry is consistent with previous studies which highlighted the inhibitory impact of premotor cortex on muscle activity [65,66]. In this scenario, premotor activity which dominates during the preparatory period effectively cancels the excitatory command derived from M1 at this period. Yet as soon as movement begins, premotor activity decays [67] and thus the spinal inhibition is lifted, revealing a prepared circuitry in which working elements already exceed the threshold level required for activating muscles. The “priming and breaking” mechanism relies heavily on the existence of a robust PM-reticular-spinal pathway, which was recently shown to affect distal muscles [68]. Clearly, further study is required to directly test the validity of this model during normal motor behavior.
Figure 4. Illustration of possible model to account for the events that take place during preparation for movement in the motor system.
In our model two pathways affect spinal circuitry during preparation for movement. The first delivers information from premotor cortex to motor cortex and subsequently via the corticospinal tract to the spinal cord. The pathway delivers task-related information and its main property is the fact that the convergent pattern of the terminal is organized to preserve the information delivered via this pathway. The second pathway connects premotor cortex with spinal cord indirectly via the reticulospinal pathway. Here, the termination pattern is not organized in a manner that preserves task-related parameters and thus a global, mostly inhibitory signal is transmitted. This pathway serves to modulate spinal circuitry by applying global inhibition and thus prevents premature release of the motor action. This organization is consistent with the fact that when stimulating the premotor cortex, the main excitatory spinal measured by muscle response; the effect is obtained indirectly via M1 (see text for details). In this scheme both excitatory and inhibitory processes that take place during preparation for movement have a supra-spinal origin. Note that the premotor cortex is represented here by multiple boxes to illustrate the fact that several premotor sites may operate in parallel during preparation for movement.
Conclusion
Preparation for movement involves complex and widespread modulation in multiple brain centers which includes both excitatory and inhibitory components. During this time, motor cortex and spinal cord are synchronously active so that both structures express robust cue-related activity. This activity is evident both at the level of spinal single cells and MUA. Delay period activity in motor cortex and spinal cord have similar onset times but differ in two main properties. First, the spinal preparatory activity is both excitatory and inhibitory and second, spinal preparatory activity is only weakly correlated with the parameters of the ensuing movement. To account for these seemingly contradictory findings it is suggested that during preparation for movement, the motor cortex exerts both excitatory (relayed via M1 directly to spinal cord) and inhibitory (relayed indirectly from premotor to spinal cord via reticular formation) impact on downstream elements. In this way movement is preplanned but is not executed prematurely.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ghez C, Favilla M, Ghilardi MF, Gordon J, Bermejo R, Pullman S. Discrete and continuous planning of hand movements and isometric force trajectories. Exp Brain Res. 1997;115:217–233. doi: 10.1007/pl00005692. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum DA. Human movement initiation: specification of arm, direction, and extent. J Exp Psychol Gen. 1980;109:444–474. doi: 10.1037//0096-3445.109.4.444. [DOI] [PubMed] [Google Scholar]
- 3.Bock O, Arnold K. Motor control prior to movement onset: preparatory mechanisms for pointing at visual targets. Exp Brain Res. 1992;90:209–216. doi: 10.1007/BF00229273. [DOI] [PubMed] [Google Scholar]
- 4.Riehle A, MacKay WA, Requin J. Are extent and force independent movement parameters? Preparation- and movement-related neuronal activity in the monkey cortex. Exp Brain Res. 1994;99:56–74. doi: 10.1007/BF00241412. [DOI] [PubMed] [Google Scholar]
- 5.Riehle A, Requin J. Neuronal correlates of the specification of movement direction and force in four cortical areas of the monkey. Behav Brain Res. 1995;70:1–13. doi: 10.1016/0166-4328(94)00180-n. [DOI] [PubMed] [Google Scholar]
- 6.Churchland MM, Shenoy KV. Delay of movement caused by disruption of cortical preparatory activity. J Neurophysiol. 2007;97:348–359. doi: 10.1152/jn.00808.2006. [DOI] [PubMed] [Google Scholar]
- 7.Riehle A, Requin J. The predictive value for performance speed of preparatory changes in neuronal activity of the monkey motor and premotor cortex. Behav Brain Res. 1993;53:35–49. doi: 10.1016/s0166-4328(05)80264-5. [DOI] [PubMed] [Google Scholar]
- 8.Georgopoulos AP, Crutcher MD, Schwartz AB. Cognitive spatial-motor processes. 3. Motor cortical prediction of movement direction during an instructed delay period. Exp Brain Res. 1989;75:183–194. doi: 10.1007/BF00248541. [DOI] [PubMed] [Google Scholar]
- 9.Crammond DJ, Kalaska JF. Neuronal activity in primate parietal cortex area 5 varies with intended movement direction during an instructed-delay period. Exp Brain Res. 1989;76:458–462. doi: 10.1007/BF00247902. [DOI] [PubMed] [Google Scholar]
- 10.Crammond DJ, Kalaska JF. Prior information in motor and premotor cortex: activity during the delay period and effect on pre-movement activity. J Neurophysiol. 2000;84:986–1005. doi: 10.1152/jn.2000.84.2.986. [DOI] [PubMed] [Google Scholar]
- 11.Messier J, Kalaska JF. Covariation of primate dorsal premotor cell activity with direction and amplitude during a memorized-delay reaching task. J Neurophysiol. 2000;84:152–165. doi: 10.1152/jn.2000.84.1.152. [DOI] [PubMed] [Google Scholar]
- 12.Riehle A, Requin J. Monkey primary motor and premotor cortex: single-cell activity related to prior information about direction and extent of an intended movement. J Neurophysiol. 1989;61:534–549. doi: 10.1152/jn.1989.61.3.534. [DOI] [PubMed] [Google Scholar]
- 13.Desmurget M, Grafton ST, Vindras P, Grea H, Turner RS. Basal ganglia network mediates the control of movement amplitude. Exp Brain Res. 2003;153:197–209. doi: 10.1007/s00221-003-1593-3. [DOI] [PubMed] [Google Scholar]
- 14.Desmurget M, Grafton ST, Vindras P, Grea H, Turner RS. The basal ganglia network mediates the planning of movement amplitude. Eur J Neurosci. 2004;19:2871–2880. doi: 10.1111/j.0953-816X.2004.03395.x. [DOI] [PubMed] [Google Scholar]
- 15.Turner RS, Anderson ME. Pallidal discharge related to the kinematics of reaching movements in two dimensions. J Neurophysiol. 1997;77:1051–1074. doi: 10.1152/jn.1997.77.3.1051. [DOI] [PubMed] [Google Scholar]
- 16.Evarts EV, Shinoda Y, Wise SP. Neurophysiological approaches to higher brain functions. John Wiley and Sons; New York: 1984. [Google Scholar]
- 17.Riehle A. Preparation for action: one of the key functions of the motor cortex. In: Riehle A, Vaadia E, editors. Motor cortex in voluntary movements: a distributed system for distributed functions. CRC Press; 2005. pp. 213–240. [Google Scholar]
- 18.Hoshi E, Tanji J. Distinctions between dorsal and ventral premotor areas: anatomical connectivity and functional properties. Curr Opin Neurobiol. 2007;17:234–242. doi: 10.1016/j.conb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Wise SP. The primate premotor cortex: past, present, and preparatory. Annu Rev Neurosci. 1985;8:1–19. doi: 10.1146/annurev.ne.08.030185.000245. [DOI] [PubMed] [Google Scholar]
- 20.Brunia CH, Scheirs JG, Haagh SA. Changes of Achilles tendon reflex amplitudes during a fixed foreperiod of four seconds. Psychophysiology. 1982;19:63–70. doi: 10.1111/j.1469-8986.1982.tb02600.x. [DOI] [PubMed] [Google Scholar]
- 21.Komiyama T, Tanaka R. The differences in human spinal motoneuron excitability during the foreperiod of a motor task. Exp Brain Res. 1990;79:357–364. doi: 10.1007/BF00608245. [DOI] [PubMed] [Google Scholar]
- 22.Bonnet M, Requin J, Stelmach GE. Changes in electromyographic responses to muscle stretch, related to the programming of movement parameters. Electroencephalogr Clin Neurophysiol. 1991;81:135–151. doi: 10.1016/0168-5597(91)90007-k. [DOI] [PubMed] [Google Scholar]
- 23.Hasbroucq T, Kaneko H, Akamatsu M, Possamai CA. The time-course of preparatory spinal and cortico-spinal inhibition: an H-reflex and transcranial magnetic stimulation study in man. Exp Brain Res. 1999;124:33–41. doi: 10.1007/s002210050597. [DOI] [PubMed] [Google Scholar]
- 24.Bonnet M, Requin J. Long loop and spinal reflexes in man during preparation for intended directional hand movements. J Neurosci. 1982;2:90–96. doi: 10.1523/JNEUROSCI.02-01-00090.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonnet M, Requin J, Semjen A. Human reflexology and motor preparation. Exerc Sport Sci Rev. 1981;9:119–157. [PubMed] [Google Scholar]
- 26.Requin J, Brener J, Ring C. Preparation for action. In: Jennings JR, Coles MGH, editors. Handbook of cognitive psychophysiology: central and autonomic nervous system approaches. Johm Wiley and Sons; 1991. pp. 357–448. [Google Scholar]
- 27.Schieppati M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol. 1987;28:345–376. doi: 10.1016/0301-0082(87)90007-4. [DOI] [PubMed] [Google Scholar]
- *28.Duclos Y, Schmied A, Burle B, Burnet H, Rossi-Durand C. Anticipatory changes in human motoneuron discharge patterns during motor preparation. J Physiol. 2008;586:1017–1028. doi: 10.1113/jphysiol.2007.145318. The authors recorded activity of single motor unit in task which included time preparation. Using this paradigm they have managed to obtain a direct measure of motor activity in humans. They found that during the delay period the firing of motor units decreases as well as firing variability. They concluded that during this period inhibition at spinal levels is increased.
- 29.Burle B, Vidal F, Tandonnet C, Hasbroucq T. Physiological evidence for response inhibition in choice reaction time tasks. Brain Cogn. 2004;56:153–164. doi: 10.1016/j.bandc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- **30.Davranche K, Tandonnet C, Burle B, Meynier C, Vidal F, Hasbroucq T. The dual nature of time preparation: neural activation and suppression revealed by transcranial magnetic stimulation of the motor cortex. Eur J Neurosci. 2007;25:3766–3774. doi: 10.1111/j.1460-9568.2007.05588.x. The authors used TMS of M1 in humans which performed a choice reaction time paradigm. By analyzing the MEPs and the following silent period they manage to probe both corticospinal excitability and the recruitment of cortical inhibition. The found that during preparation for movement the corticpspinal system is inhibited and at the same time cortical excitation is increased due to reduced inhibition. The authors concluded that both excitation and inhibition take place during preparation for movement in a mechanism similar to the “priming and breaking” model suggested for motor preparation in primates.
- 31.Hasbroucq T, Osman A, Possamai CA, Burle B, Carron S, Depy D, Latour S, Mouret I. Cortico-spinal inhibition reflects time but not event preparation: neural mechanisms of preparation dissociated by transcranial magnetic stimulation. Acta Psychol (Amst) 1999;101:243–266. doi: 10.1016/s0001-6918(99)00007-4. [DOI] [PubMed] [Google Scholar]
- 32.Touge T, Taylor JL, Rothwell JC. Reduced excitability of the cortico-spinal system during the warning period of a reaction time task. Electroencephalogr Clin Neurophysiol. 1998;109:489–495. doi: 10.1016/s0924-980x(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 33.van Elswijk G, Kleine BU, Overeem S, Stegeman DF. Expectancy induces dynamic modulation of corticospinal excitability. J Cogn Neurosci. 2007;19:121–131. doi: 10.1162/jocn.2007.19.1.121. [DOI] [PubMed] [Google Scholar]
- 34.Mars RB, Bestmann S, Rothwell JC, Haggard P. Effects of motor preparation and spatial attention on corticospinal excitability in a delayed-response paradigm. Exp Brain Res. 2007;182:125–129. doi: 10.1007/s00221-007-1055-4. [DOI] [PubMed] [Google Scholar]
- **35.Duque J, Lew D, Mazzocchio R, Olivier E, Ivry RB. Evidence for two concurrent inhibitory mechanisms during response preparation. J Neurosci. 2010;30:3793–3802. doi: 10.1523/JNEUROSCI.5722-09.2010. The authors have measured motor responses evoked by TMS and H-reflex during reaction time tasks. In this manner they were able to probe modulations in spinal and cortical circuitries. By employing an elegant behavioral paradigm they have managed to dissociate two inhibitory mechanisms which are activated during preparation for movements. The first operate at spinal level for inhibition premature action, and the second operating in supraspinal level as part of response-selection process.
- 36.Schoffelen JM, Oostenveld R, Fries P. Neuronal coherence as a mechanism of effective corticospinal interaction. Science. 2005;308:111–113. doi: 10.1126/science.1107027. [DOI] [PubMed] [Google Scholar]
- 37.Terao Y, Ugawa Y. Basic mechanisms of TMS. J Clin Neurophysiol. 2002;19:322–343. doi: 10.1097/00004691-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Asher I, Zinger N, Yanai Y, Israel Z, Prut Y. Population-Based Corticospinal Interactions in Macaques Are Correlated with Visuomotor Processing. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp095. The authors used population-based signals recorded from the motor cortex and cervical spinal cord of monkeys performing delayed-response paradigm. The authors have shown that in both structures there is a clear cue-related activity and that corticospinal responses were correlated with motor behavior. They further showed an ongoing state of corticospinal synchronization that is modulated both in magnitude and phase along the trial. These results are the first direct evidence for corticospinal interactions obtained in behaving primates performing voluntary movements.
- 39.Prut Y, Fetz EE. Primate spinal interneurons show pre-movement instructed delay activity. Nature. 1999;401:590–594. doi: 10.1038/44145. [DOI] [PubMed] [Google Scholar]
- 40.Yanai Y, Adamit N, Harel R, Israel Z, Prut Y. Connected corticospinal sites show enhanced tuning similarity at the onset of voluntary action. J Neurosci. 2007;27:12349–12357. doi: 10.1523/JNEUROSCI.3127-07.2007. The authors recorded activity of cortical and spinal single neurons during a delayed-response wrist paradigm with two hand postures. They have shown that during preparation for movement there is a gradual shift in the coordinate frame of cortical neurons from near extrinsic to near intrinsic. This is the first study that directly documented the time-resolved tuning properties of cortical and spinal neurons in behaving primates.
- 41.Yanai Y, Adamit N, Israel Z, Harel R, Prut Y. Coordinate transformation is first completed downstream of primary motor cortex. J Neurosci. 2008;28:1728–1732. doi: 10.1523/JNEUROSCI.4662-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexander GE, Crutcher MD. Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol. 1990;64:133–150. doi: 10.1152/jn.1990.64.1.133. [DOI] [PubMed] [Google Scholar]
- 43.Kurata K. Premotor cortex of monkeys: set- and movement-related activity reflecting amplitude and direction of wrist movements. J Neurophysiol. 1993;69:187–200. doi: 10.1152/jn.1993.69.1.187. [DOI] [PubMed] [Google Scholar]
- 44.London M, Roth A, Beeren L, Hausser M, Latham PE. Sensitivity to perturbations in vivo implies high noise and suggests rate coding in cortex. Nature. 466:123–127. doi: 10.1038/nature09086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanes JN, Donoghue JP. Oscillations in local field potentials of the primate motor cortex during voluntary movement. Proc Natl Acad Sci U S A. 1993;90:4470–4474. doi: 10.1073/pnas.90.10.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Leary JG, Hatsopoulos NG. Early visuomotor representations revealed from evoked local field potentials in motor and premotor cortical areas. J Neurophysiol. 2006;96:1492–1506. doi: 10.1152/jn.00106.2006. [DOI] [PubMed] [Google Scholar]
- 47.Boussaoud D, Wise SP. Primate frontal cortex: effects of stimulus and movement. Exp Brain Res. 1993;95:28–40. doi: 10.1007/BF00229651. [DOI] [PubMed] [Google Scholar]
- 48.Boussaoud D, Wise SP. Primate frontal cortex: neuronal activity following attentional versus intentional cues. Exp Brain Res. 1993;95:15–27. doi: 10.1007/BF00229650. [DOI] [PubMed] [Google Scholar]
- 49.Bullock TH. Signals and signs in the nervous system: the dynamic anatomy of electrical activity is probably information-rich. Proc Natl Acad Sci U S A. 1997;94:1–6. doi: 10.1073/pnas.94.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- 51.Rickert J, Riehle A, Aertsen A, Rotter S, Nawrot MP. Dynamic encoding of movement direction in motor cortical neurons. J Neurosci. 2009;29:13870–13882. doi: 10.1523/JNEUROSCI.5441-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Renoult L, Roux S, Riehle A. Time is a rubberband: neuronal activity in monkey motor cortex in relation to time estimation. Eur J Neurosci. 2006;23:3098–3108. doi: 10.1111/j.1460-9568.2006.04824.x. [DOI] [PubMed] [Google Scholar]
- 53.Lucchetti C, Bon L. Time-modulated neuronal activity in the premotor cortex of macaque monkeys. Exp Brain Res. 2001;141:254–260. doi: 10.1007/s002210100818. [DOI] [PubMed] [Google Scholar]
- 54.Georgopoulos AP, Lurito JT, Petrides M, Schwartz AB, Massey JT. Mental rotation of the neuronal population vector. Science. 1989;243:234–236. doi: 10.1126/science.2911737. [DOI] [PubMed] [Google Scholar]
- 55.Sinclair C, Hammond GR. Excitatory and inhibitory processes in primary motor cortex during the foreperiod of a warned reaction time task are unrelated to response expectancy. Exp Brain Res. 2009;194:103–113. doi: 10.1007/s00221-008-1684-2. [DOI] [PubMed] [Google Scholar]
- 56.Duque J, Ivry RB. Role of corticospinal suppression during motor preparation. Cereb Cortex. 2009;19:2013–2024. doi: 10.1093/cercor/bhn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boudrias MH, Lee SP, Svojanovsky S, Cheney PD. Forelimb muscle representations and output properties of motor areas in the mesial wall of rhesus macaques. Cereb Cortex. 2010;20:704–719. doi: 10.1093/cercor/bhp136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boudrias MH, McPherson RL, Frost SB, Cheney PD. Output properties and organization of the forelimb representation of motor areas on the lateral aspect of the hemisphere in rhesus macaques. Cereb Cortex. 2010;20:169–186. doi: 10.1093/cercor/bhp084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *59.Davare M, Montague K, Olivier E, Rothwell JC, Lemon RN. Ventral premotor to primary motor cortical interactions during object-driven grasp in humans. Cortex. 2009;45:1050–1057. doi: 10.1016/j.cortex.2009.02.011. The authors used TMS study in humans to reveal PMv-to-M1 connectivity during preparation for grasp. Using this paradigm they show that the interactions between PMv and M1 are facilitated in an action-specific manner. The premovement processing in PMv is highly relevant for task performance and is relayed to downstream elements via M1.
- *60.Prabhu G, Shimazu H, Cerri G, Brochier T, Spinks RL, Maier MA, Lemon RN. Modulation of primary motor cortex outputs from ventral premotor cortex during visually guided grasp in the macaque monkey. J Physiol. 2009;587:1057–1069. doi: 10.1113/jphysiol.2008.165571. The authors used a chronic stimulation of PMv and M1 and measured the muscle responses. Conditioning M1 stimulation with subthreshold stimulation in PMv enhanced muscle response to M1 test stimulations. These results serve as a further support to data obtained from human studies by the same authors showing that PMv can facilitate the corticospinal output from M1 in a response-specific manner.
- 61.Borra E, Belmalih A, Gerbella M, Rozzi S, Luppino G. Projections of the hand field of the macaque ventral premotor area F5 to the brainstem and spinal cord. J Comp Neurol. 2010;518:2570–2591. doi: 10.1002/cne.22353. [DOI] [PubMed] [Google Scholar]
- 62.Keizer K, Kuypers HG. Distribution of corticospinal neurons with collaterals to the lower brain stem reticular formation in monkey (Macaca fascicularis) Exp Brain Res. 1989;74:311–318. doi: 10.1007/BF00248864. [DOI] [PubMed] [Google Scholar]
- 63.Kuypers HGJM. The anatmomical organization of the descending pathways and their contribution to motor control especially in primates. In: Desmedt JE, editor. New developments in EMG and clinical neurophysiology. Vol. 3. Karger; 1973. pp. 38–68. [Google Scholar]
- 64.Kuypers HG. Anatomy of descending pathways. In: Brooks VB, editor. Motor control, part 1. II. American Physiological Society; 1981. Handbook of Physiology. [Google Scholar]
- 65.Moll L, Kuypers HG. Premotor cortical ablations in monkeys: contralateral changes in visually guided reaching behavior. Science. 1977;198:317–319. doi: 10.1126/science.410103. [DOI] [PubMed] [Google Scholar]
- 66.Sawaguchi T, Yamane I, Kubota K. Application of the GABA antagonist bicuculline to the premotor cortex reduces the ability to withhold reaching movements by well-trained monkeys in visually guided reaching task. J Neurophysiol. 1996;75:2150–2156. doi: 10.1152/jn.1996.75.5.2150. [DOI] [PubMed] [Google Scholar]
- 67.Weinrich M, Wise SP. The premotor cortex of the monkey. J Neurosci. 1982;2:1329–1345. doi: 10.1523/JNEUROSCI.02-09-01329.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *68.Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci. 2009;29:4993–4999. doi: 10.1523/JNEUROSCI.3720-08.2009. The authors used intracelleular recording in anaesthetized monkeys to identify the synaptic connection between reticulospinal neurons and upper limb motoneurons. The found robust mono- and disynaptic connections (including monosynaptic excitations) with motonueonrs that innervates distal hand muscles. These findings suggest a role for the reticulospinal system in controlling upper limb muscles in parallel to the corticospinal system.