This cost-effectiveness study shows that sputum smear microscopy is the most cost-effective test for active tuberculosis (TB) in India, and liquid culture plus microscopy is more cost-effective for TB diagnosis than serological tests.
Abstract
Background
Undiagnosed and misdiagnosed tuberculosis (TB) drives the epidemic in India. Serological (antibody detection) TB tests are not recommended by any agency, but widely used in many countries, including the Indian private sector. The cost and impact of using serology compared with other diagnostic techniques is unknown.
Methods and Findings
Taking a patient cohort conservatively equal to the annual number of serological tests done in India (1.5 million adults suspected of having active TB), we used decision analysis to estimate costs and effectiveness of sputum smear microscopy (US$3.62 for two smears), microscopy plus automated liquid culture (mycobacterium growth indicator tube [MGIT], US$20/test), and serological testing (anda-tb ELISA, US$20/test). Data on test accuracy and costs were obtained from published literature. We adopted the perspective of the Indian TB control sector and an analysis frame of 1 year. Our primary outcome was the incremental cost per disability-adjusted life year (DALY) averted. We performed one-way sensitivity analysis on all model parameters, with multiway sensitivity analysis on variables to which the model was most sensitive.
If used instead of sputum microscopy, serology generated an estimated 14,000 more TB diagnoses, but also 121,000 more false-positive diagnoses, 102,000 fewer DALYs averted, and 32,000 more secondary TB cases than microscopy, at approximately four times the incremental cost (US$47.5 million versus US$11.9 million). When added to high-quality sputum smears, MGIT culture was estimated to avert 130,000 incremental DALYs at an incremental cost of US$213 per DALY averted. Serology was dominated by (i.e., more costly and less effective than) MGIT culture and remained less economically favorable than sputum smear or TB culture in one-way and multiway sensitivity analyses.
Conclusions
In India, sputum smear microscopy remains the most cost-effective diagnostic test available for active TB; efforts to increase access to quality-assured microscopy should take priority. In areas where high-quality microscopy exists and resources are sufficient, MGIT culture is more cost-effective than serology as an additional diagnostic test for TB. These data informed a recently published World Health Organization policy statement against serological tests.
Please see later in the article for the Editors' Summary
Editors' Summary
Background
Every year, about 2 million people develop tuberculosis in India—a fifth of the global incidence of this highly contagious bacterial infection. Mycobacterium tuberculosis, the bacterium that causes tuberculosis, is spread in airborne droplets when people with the disease cough or sneeze and usually infects the lungs although it can also infect other organs. The characteristic symptoms of tuberculosis are a persistent cough, weight loss, and night sweats. Diagnostic tests for tuberculosis include sputum smear microscopy (microscopic analysis of mucus brought up from the lungs by coughing), culture (growth) of M. tuberculosis from sputum samples in liquid media (using, for example, a commercial product called the mycobacteria growth indicator tube or MGIT), and nucleic acid amplification tests (which detect the bacterium's genome in patient samples) such as the Xpert MTB/RIF system. Tuberculosis can usually be cured by taking several powerful antibiotics daily for at least 6 months.
Why Was This Study Done?
In India, as elsewhere, undiagnosed and misdiagnosed tuberculosis drives the tuberculosis epidemic by increasing the transmission of M. tuberculosis. Unfortunately, sputum smear microscopy, the current mainstay of tuberculosis diagnosis worldwide, detects only half of tuberculosis cases, mycobacterial culture can take weeks to provide a diagnosis, and rapid techniques such as nucleic acid amplification require infrastructure that is often not available in developing countries. Consequently, in India and other developing countries, serological tests are widely used for the diagnosis of tuberculosis. Serological tests detect antibodies against M. tuberculosis in the blood (antibodies are proteins made by the immune system in response to infections). Serological tests are fast and simple to perform, but they are not recommended for clinical use, and the available evidence suggests that they do not diagnose tuberculosis accurately. Even so, and in the absence of information about the cost and impact (cost-effectiveness) of serological testing, about 1.5 million serological tests for tuberculosis are conducted every year in India at a cost of more than US$15 million. Here, the researchers analyze the cost-effectiveness of serological tests compared to other diagnostic tests from the perspective of tuberculosis control in India.
What Did the Researchers Do and Find?
The researchers used “decision analysis” to estimate the cost-effectiveness of sputum smear microscopy, microscopy plus liquid culture using the MGIT system, and serological testing using the widely used anda-tb ELISA commercial test in a hypothetical group of 1.5 million people suspected of having tuberculosis. Decision analysis formally assesses the decision-making process by using models that evaluate outcomes under different scenarios. By feeding data on the costs and accuracy of different diagnostic tests into their decision-analysis model, the researchers estimate that, over a year, serology would generate 14,000 more tuberculosis diagnoses than sputum microscopy. However, it would also generate 121,000 more false-positive diagnoses and 32,000 more tuberculosis transmissions to other people (secondary transmissions), and avert 102,000 fewer disability-adjusted life years (DALYs; a DALY is a year of healthy life lost because of premature death or disability) at four times the incremental cost of sputum microscopy. MGIT culture added to sputum smear microscopy would avert 130,000 DALYs at an incremental cost of US$213 per DALY averted. Finally, sensitivity analyses (reruns of the decision-analysis model using different values for test costs and accuracy) identified no scenario in which serology was either less costly or more effective than sputum smear microscopy alone or in which serology plus sputum microscopy was more cost-effective than MGIT culture plus sputum microscopy.
What Do These Findings Mean?
These findings identify sputum smear microscopy as the most cost-effective existing diagnostic test for tuberculosis in India. Moreover, they suggest that in areas where high-quality microscopy is available, resources are sufficient, and infrastructure to effectively use culture exists, the addition of MGIT culture to sputum smear microscopy would be more cost-effective than the addition of serology. Importantly, these findings suggest that, if used as an initial test for tuberculosis in India, serology would result in more DALYs, more secondary infections, and more false-positive diagnoses than sputum smear microscopy while increasing per-patient costs to the Indian tuberculosis control sector. Given these findings and the results of a recent updated systematic review on the accuracy of serological tests, the World Health Organization's Strategic and Technical Advisory Group for Tuberculosis recently advised against the use of currently available serological tests for the diagnosis of tuberculosis. The WHO negative policy against serological tests must now be implemented in India.
Additional Information
Please access these Web sites via the online version of this summary at http://dx.doi.org/10.1371/journal.pmed.1001074.
Details of the recent systematic review of serological tests for tuberculosis diagnosis are available in a PLoS Medicine Research Article by Steingart et al.
The World Health Organization provides information on all aspects of tuberculosis, including tuberculosis diagnostics and the Stop TB Partnership (some information is in several languages); its Strategic and Technical Advisory Group for Tuberculosis recommendations on tuberculosis diagnosis are available
The Evidence-based TB Diagnosis Web site by the Stop TB Partnership's New Diagnostics Working Group provides evidence syntheses on various TB tests, along with guidelines, resources, and training materials
The US Centers for Disease Control and Prevention has information about tuberculosis, including information on the diagnosis of tuberculosis disease
The US National Institute of Allergy and Infectious Diseases also has information on all aspects of tuberculosis
MedlinePlus has links to further information about tuberculosis (in English and Spanish)
Introduction
Improved diagnostic testing represents a key component of tuberculosis (TB) control [1]–[3], and inadequate case detection is a major hurdle for effective control of the TB epidemic. Sputum smear microscopy, the current backbone of TB diagnosis worldwide, misses 50% of all cases and is often performed under suboptimal laboratory conditions [4]–[6]. Thus, if targets for TB control are to be achieved, newer diagnostic tools for active TB must be used. On the basis of available evidence [7],[8], the World Health Organization (WHO) currently recommends several technologies for TB diagnosis [9], including culture of Mycobacterium tuberculosis using commercial liquid media (e.g., mycobacteria growth indicator tube [MGIT], BD Diagnostics) or noncommercial methods (e.g., microscopic observation drug susceptibility [MODS]), and more recently, rapid molecular testing with the Xpert MTB/RIF system (Cepheid, Inc.) [10],[11]. However, TB culture often takes weeks to obtain results, and all currently recommended diagnostic tests (other than microscopy) require infrastructure currently unavailable in many settings [7],[8],[12]. Although promising, technologies such as MODS and Xpert MTB/RIF are yet to be scaled up in India. Before investing in additional infrastructure, it is important to assess whether other, more immediately accessible, TB diagnostic tests might be cost-effective.
Serological tests for active TB are based on detection of antibodies elicited by antigens of Mycobacterium tuberculosis that are recognized by the humoral immune system. Many serological tests utilize an ELISA format, while several others are available as rapid point-of-care tests (e.g. immunochromatographic, lateral flow assays). Thus, serological tests are attractive because they are faster and simpler to perform than most sputum-based methods [13]. However, as demonstrated in a recent updated systematic review [14], existing evidence supporting commercial serological tests is inconsistent and of low quality. Although no international guideline recommends their use, serological tests for active TB are readily available and widely used in parts of the developing world, including India and at least 16 of 21 other high-burden countries [15]. Thus, while unlikely to be as accurate as TB culture, serological tests might still be preferred over sputum smear microscopy, and serology might also be economically attractive relative to culture and molecular tests given fewer infrastructure requirements, faster turnaround time, and higher volume of usage.
The economic implications of serological testing for TB are substantial. In India, at least 13 different TB serological kits are on the market (Table 1), and an estimated 1.5 million serological tests for active TB are performed every year (primarily in the private medical sector), at a cost of over US$15 million for testing alone [15],[16]. To better understand the economic and epidemiological consequences of serological testing for active TB in India, we analyzed costs and effectiveness from the perspective of the combined public and private TB control sector of the health care system.
Table 1. Serological assays for tuberculosis on the Indian market.
| Company | Kit | Assay Technique | Sensitivity and Specificity from Package Insert | URL |
| Anda Biologicals, Strasbourg, France | anda TB-ELISA | ELISA | Not listed, refers to publications | http://www.andabiologicals.com |
| Omega Diagnostics, Alva, Scotland | Pathozyme TB Complex Plus | ELISA | 37% and 100% | http://www.omegadiagnostics.com |
| Tulip Group, Goa | Qualisa TB | ELISA | 100% and 99% | http://www.tulipgroup.com |
| Tulip Group, Goa | Serocheck-MTB | Rapida | 100% and 100% | http://www.tulipgroup.com |
| Span Diagnostics, Surat | TB Spot Ver 2.0 | Rapida | 80% and 99% | http://www.span.co.in |
| Bhat Biotech, Bangalore | Bhat Bioscan TB card | Rapida | 83% and 99% | http://www.bhatbiotech.com/ |
| Span Diagnostics, Surat | Mycowell | ELISA | “Superior sensitivity and specificity” | http://www.span.co.in |
| J Mitra, New Delhi | TB IgG, IgM, IgA Elisa | ELISA | 80% and 97% | http://www.jmitra.co.in |
| JB Trop Dis Res Centre, Sevagram | SEVA TB ELISA | ELISA | 97% and 99% | http://www.jbtdrc.org/SEVA_TB.pdf |
| S.D. Bio Standard Diagnostic India | SD BIOLINE Rapid TB | Rapida | 98% and 99% | http://sdbiostandard.tradeindia.com/ |
| Bisen Biotech, Gwalior | TB SCREEN TEST | Rapida | 94% and 98% | http://www.bisenbiotechindia.com |
| Lab-care Diagnostics Pvt Ltd, Sarigam | Accucare Rapid TB test | Rapida | >80% sensitivity and specificity | http://www.labcarediagnostics.com/RapidTest_sub.html |
| Tashima Inc, Bangalore | TB IgG/IgM 3 Line Rapid test | Rapida | 93% and 100% | http://www.tashima.net |
Rapid chromatographic immunoassay.
Methods
We constructed a decision-analytic model to estimate the costs and effectiveness of serological testing for active TB. The basic model structure (Figure 1) was adapted from a previously described cost-effectiveness analysis of novel TB diagnostics [17]. This static model evaluates outcomes (including secondary TB transmission) in a cohort of TB suspects. We took as our study population a hypothetical cohort of 1.5 million adult TB suspects in India, presenting for diagnosis in settings with access to serological testing. Thus, this cohort provides a conservative estimate of costs and outcomes among all Indian patients receiving serological testing for active TB in a given year. These patients were assumed to have TB prevalence, HIV prevalence, and access to antiretroviral therapy representative of adults with suspected TB in India as a whole [18],[19].
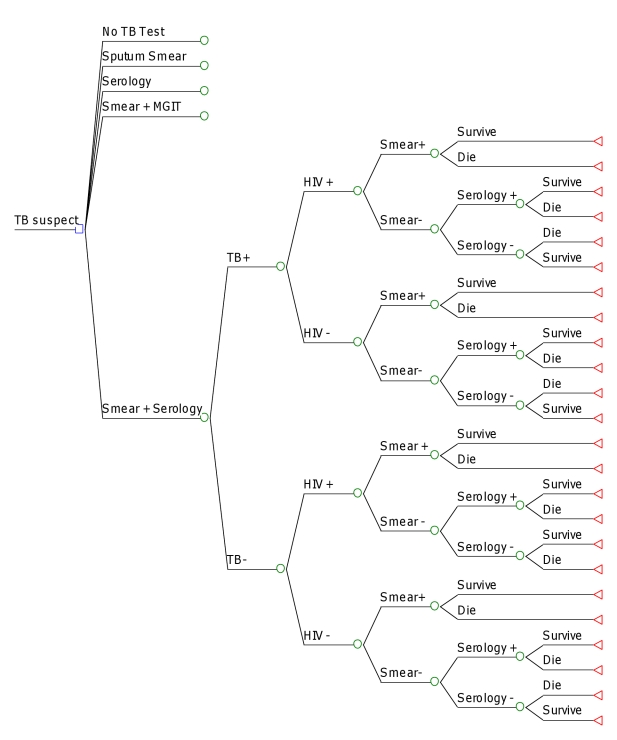
Figure 1. Study decision tree.
Depicted is a simplified version of the analytic framework for this decision analysis. The square represents a decision node, circles chance nodes, and triangles terminal nodes. The branch of the decision node (sputum smear + serology) is compared to similar branches corresponding to scenarios of no TB-specific diagnosis, sputum smear only, and sputum smear plus TB culture (commercial liquid media), as described in the text. Probabilities, costs, and DALYs are calculated at each terminal node according to the parameters described in Table 2. ARV, antiretroviral therapy; +, positive; −, negative.
To evaluate various diagnostic strategies, we conducted a two-stage analysis. In the first stage, we sought to identify the best initial test for TB. In the second stage, we sought to evaluate the optimal diagnostic strategy for improved TB diagnosis in settings where sputum smear microscopy is routinely performed. The baseline scenario for the first analysis assumed that the study population receives the combination of tests (e.g., chest X-ray [CXR]) and clinical strategies (e.g., antibiotic trials) that is typical in India, with the exception of using no TB-specific microbiological test (including sputum smear microscopy, culture, or serology). We assumed that the country-wide smear-negative TB case detection rate in India approximates the ability of clinicians to successfully diagnose TB in this situation. To evaluate the most appropriate initial test for TB in this situation, we modeled the addition to the baseline scenario of sputum smear microscopy versus serological testing using anda-tb ELISA (Anda Biologicals; the most widely studied and used serological test in India) [14]. In the second analytic stage, we assumed that sputum smear microscopy was performed as an initial test, followed by either anda-tb ELISA or automated liquid culture on smear-negative specimens. Our primary outcome for all analyses was the incremental cost-effectiveness ratio (ICER) relative to the reference scenario for each analytic stage.
Serology is primarily available in India through stand-alone and large network (chain) private laboratories which also offer TB cultures. Thus, we assumed that the population had existing access to serology (at market prices), and that similar access to TB culture could be established by constructing an appropriate laboratory facility; there are currently 27 reference laboratories capable of culture and drug-susceptibility testing, accredited by the Indian Revised National TB Control Programme (RNTCP). We assumed that sputum smear and serology could be performed by send-out testing to a public or private laboratory with a 1-wk turnaround time, and that TB culture would incur additional delays of 8 wk for specimen incubation, reporting of results, and initiation of treatment [20]. These assumptions were tested in sensitivity analyses.
Regarding costs, we conservatively estimated that anda-TB would cost US$20 per person with suspected active TB on the basis of data from private labs in India [15],[16], although the total cost per patient often exceeds US$40 (because three antibodies—IgA, IgG, and IgM—are often requested). We assumed that the cost per TB culture was US$20, approximately equal to the cost of automated MGIT culture, including the costs of laboratory construction and overheads, when run at half-maximum throughput in Zambia [21]. Patients diagnosed with TB were assumed to be treated according to RNTCP national standards.
Accuracy for each test was estimated from published literature, including meta-analyses where available [14],[22],[23]. For TB serology, we used the sensitivity and specificity of anda-TB, the most widely studied test according to a recently updated meta-analysis [14]. Since anda-TB is likely to outperform more poorly studied in-house serological tests and less accurate rapid test formats [14], and laboratory accuracy is likely to exceed that in the field, our analysis likely overestimates the accuracy of serology as actually performed in practice. A full listing of estimated parameter values is given in Table 2.
Table 2. Parameter estimates used in the model.
| Parameter | Base Value | Range for Sensitivity Analysis | Reference |
| TB dynamicsa | |||
| Probability of death, untreated smear-positive TB | 0.70 | 0.5–0.95 | [4] |
| Probability of death, untreated smear-negative TB | 0.20 | 0.15–0.25 | [4] |
| Secondary TB infections per year, smear-positive TB | 10 | 8–12 | [24] |
| Relative infectiousness of smear-negative TB | 0.22 | 0.16–0.28 | [37] |
| Fraction of new TB cases that are smear-positive | 0.53 | 0.4–0.66 | [4] |
| Characteristics of TB diagnosis | |||
| Prevalence of active TB among persons with suspected active TB | 0.14 | 0.11–0.18 | [19] |
| Sensitivity of clinician diagnosisb | 0.53 | 0.40–0.67 | [26] |
| Sensitivity for smear-positive TB, serology (anda-TB) | 0.76 | 0.63–0.87 | [14] |
| Sensitivity for smear-negative TB | |||
| MGIT TB culture (single specimen) | 0.73 | 0.55–0.91 | [23] |
| Serology | 0.59 | 0.40–0.85 | [14] |
| Specificity for active TB | |||
| Clinician diagnosisb | 0.94 | 0.75–1.0 | [3] |
| Sputum smear microscopy (two smears) | 0.97 | 0.9–1.0 | [6] |
| MGIT TB culture | 0.99 | 0.95–1.0 | [23] |
| Serology | 0.87 | 0.74–0.98 | [14] c |
| Time to TB diagnosis | |||
| MGIT TB culture | 8 wk | 1–4 mo | [20] |
| Sputum smear, serology | 1 wk | 3–14 d | [38],[39] |
| Loss to follow-up | |||
| MGIT TB culture | 0.25 | 0.19–0.31 | Estimated |
| Sputum smear, serology | 0.15 | 0.11–0.19 | [40],[41] |
| Characteristics of TB treatment | |||
| Proportion of treated TB patients who die | 0.045 | 0.033–0.056 | [26] |
| Proportion of treated HIV/TB patients who die | 0.090 | 0.068–0.114 | [26] |
| Proportion of treated TB patients infectious at 1 y | 0.045 | 0.033–0.056 | [26] |
| HIV/TB | |||
| HIV prevalence, general population | 0.3% | 0.225%–0.4% | [18] |
| HIV prevalence, patients with TB | 5.3% | 4.0%–6.6% | [26] |
| Proportion of HIV-infected patients with ART access | 0.10 | 0.075–0.125 | [18] |
| Costs and effectiveness, US$ | |||
| Unit cost, independent laboratory | |||
| Sputum smear microscopy (two smears) | US$3.62 | US$1–US$5 | [19] |
| TB culture (MGIT) | US$20 | US$10–US$30 | [21] |
| Serology | US$20 | US$10–US$30 | [19] |
| Mean cost of treating one case of TB | US$82.40 | US$60–US$100 | [26] |
| DALY weightsa | |||
| Active TB | 0.264 | 0.198–0.330 | [42] |
| TB treatment | 0.132 | 0.099–0.165 | Estimated |
| Life expectancy after TB cure (y) | 40 | 30–50 | [43],[44] |
Parameter values for HIV-positive patients are excluded from this table owing to low HIV prevalence but were incorporated into the model and can be found in reference [17].
In the absence of any TB-specific microbiological test.
Excludes studies not performed in developing countries.
ART, antiretroviral therapy.
We assumed that all incremental costs and effects of TB treatment occur during the first year after presentation with symptoms of active TB (i.e., that any patient who would be successfully diagnosed with TB would be diagnosed within 1 y of presentation). Patients with active TB at the end of this year are assumed to remain infectious for an additional year [24], acknowledging that the period of infectivity depends on numerous variables including HIV status, age, gender, and type (e.g., cavitary nature) of disease. Outcomes (e.g., death) occurring within that year are discounted over the cohort's lifetime, which was the analytic time horizon. We lack reliable data on the costs of hospitalization for TB, lost wages due to TB, or treatment for other related conditions (e.g., bacterial pneumonia). Thus, we adopted the perspective of the Indian TB control sector, including both the public RNTCP and those elements of the private health care sector devoted to diagnosis and treatment of TB. Our primary outcome was the incremental cost per disability-adjusted life year (DALY) averted. All costs were converted to US dollars using historical exchange rates and inflated to the year 2010 using the medical care component of the US Consumer Price Index [25]. We discounted all future costs and effects (DALYs and secondary TB transmissions) by 3% per year, with sensitivity analysis for 0% and 7%.
One-way sensitivity analysis was performed on all parameters across ranges shown in Table 2, with further two-way sensitivity analysis on those variables to which the model was most sensitive. In the absence of data to suggest a reasonable parameter range, we varied each parameter over +/−25% of its baseline value. Since published estimates of serological test accuracy likely overestimate their actual accuracy in the field, we also evaluated an alternative scenario using the lower bounds of sensitivity and specificity for serological testing from a recent meta-analysis [14]. Analyses were performed using TreeAge Pro 2009 (TreeAge Software, Inc.).
Results
The hypothetical study population of 1.5 million adults with suspected active TB included an estimated 214,000 cases of active TB, or 16% of India's annual burden of incident TB [26]. We estimated that, in the absence of TB-specific microbiological testing (i.e., in the baseline scenario), clinicians would diagnose 114,000 (53%) of these patients with TB on the basis of clinical judgment and nonmicrobiological tests (e.g., chest X-ray [CXR], antibiotic therapeutic trials) alone. Addition of sputum smear microscopy to this scenario of no TB-specific testing resulted in diagnosis of an estimated 44,000 more cases of TB, or 44% of remaining undiagnosed cases (Table 3). Replacing sputum smear with serological testing resulted in an estimated 14,000 more diagnosed cases of TB than did sputum smear, but also resulted in 121,000 additional false-positive diagnoses relative to microscopy. Because the cases detected by sputum smear are assumed to be more infectious than those detected by serology, smear was estimated to avert 102,000 more DALYs and 32,000 more secondary cases than did serology, at approximately one-fourth the incremental cost. If performed only on smear-negative specimens, TB culture was both cheaper and more effective (i.e., dominant) compared with serological testing (Table 3). For each additional smear-negative TB case diagnosed by serology, more than six additional false positives were inappropriately diagnosed.
Table 3. Cost-effectiveness of diagnostic strategies for 1.5 million persons with suspected active TB in India.
| Diagnostic Test | Cost (US$) | Additional TB Cases Treated | Additional False-Positive Cases Treated | Secondary Cases Averted | DALYs Averted | Incremental DALYs Averted | Incremental Cost per DALY Averted (US$) |
| Performed alone, relative to no microbiological testing | |||||||
| Sputum smear microscopy | 11.9 million | 44,000 | 36,000 | 443,000 | 623,000 | 623,000 | 19 |
| anda-TB serology | 47.5 million | 58,000 | 157,000 | 411,000 | 520,000 | (Dominated) | (Dominated) |
| Performed on smear-negative specimens only, relative to sputum smear alone | |||||||
| MGIT culture | 27.6 million | 26,000 | 12,000 | 112,000 | 130,000 | 130,000 | 213 |
| anda-TB serology | 39.0 million | 24,000 | 152,000 | 112,000 | 110,000 | (Dominated) | (Dominated) |
When we conducted one-way sensitivity analysis of all variables across the ranges specified in Table 2, no scenario was identified in which serology was either less costly or more effective than sputum smear microscopy alone, nor serology plus sputum smear more cost-effective than MGIT culture plus sputum smear. Reducing the time to culture-based diagnosis from 8 wk (with 25% loss to follow-up) to 2 wk (with 15% loss to follow-up, similar to serology) reduced the incremental cost-effectiveness of culture plus smear, relative to smear alone, from US$213 to US$188.
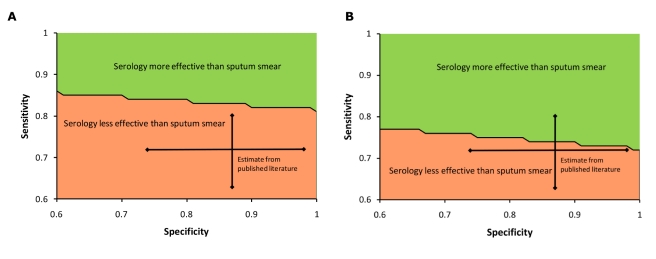
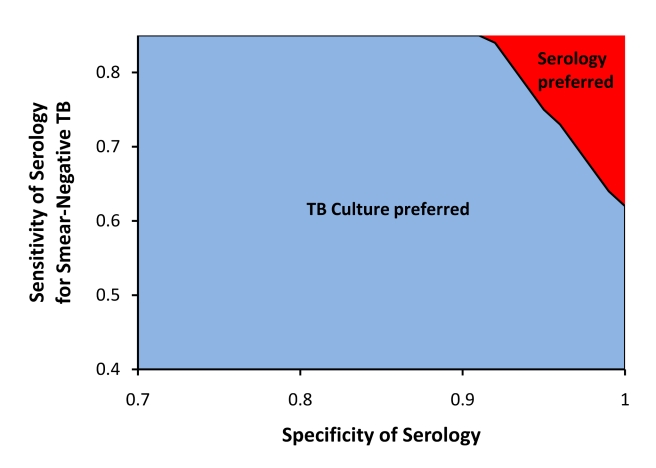
The incremental cost-effectiveness of serology was most sensitive to the sensitivity and specificity of the serological test. By contrast, the influence of specificity on the cost-effectiveness of sputum smear was small (incremental cost per DALY averted of US$19 versus US$14 for sputum smear specificity of 97% versus 100%). Thus, comparing serology alone to sputum smear, we performed two-way sensitivity analysis around sensitivity and specificity of serology, plus three-way sensitivity analysis sequentially incorporating the price of serology and proportion of TB cases presenting with smear-positive TB. For serological testing to achieve equivalent effectiveness (in terms of DALYs averted) to sputum smear microscopy, sensitivity for both smear-positive and smear-negative TB would be required to exceed 81%–86%, depending on specificity (Figure 2A). These thresholds are less stringent in settings where a lower proportion of TB patients present with smear-positive disease (Figure 2B). Because of the excess cost of treating false positives, serology (with any sensitivity) would need to achieve a specificity of >0.92 before a test, even with a price of US$0.01, could be less costly to the TB control sector than sputum smear. When we evaluated the lower bounds of published values for the specificity (0.74) and smear-positive sensitivity (0.63) of serological testing to compensate for possible publication bias, serology was estimated to avert 171,000 fewer DALYs and cost US$48.1 million more than sputum smear microscopy alone. When added to sputum smear microscopy, serology would need to achieve exceptional sensitivity and specificity (e.g., 70% sensitivity and 97% specificity for smear-negative TB) to achieve more favorable cost-effectiveness ratios than TB culture (Figure 3).
Figure 2. Three-way sensitivity analysis: sensitivity and specificity of serology for active TB.
Specificity and sensitivity (for smear-positive and smear-negative TB) would be required to achieve values above the primary line for serology to be more effective than sputum smear microscopy alone. The vertical and horizontal lines denote the 95% credible intervals of estimated accuracy from published studies of anda-TB [14], the most widely used serological test in India. (A) compares serology alone against sputum smear microscopy alone in a setting where 53% of TB patients are smear-positive (base case scenario), while (B) considers a scenario in which only 40% of TB patients are smear-positive.
Figure 3. Two-way sensitivity analysis: serology versus culture for diagnosis of drug-sensitive TB.
For the incremental cost-effectiveness ratio (ICER) to be more favorable for serology than for TB culture using commercial liquid media (MGIT) in the base-case scenario (73% sensitivity and 99% specificity), the sensitivity and specificity of serology for smear-negative TB must achieve values above the primary line. The estimated sensitivity and specificity of serology in the base case are 59% and 87%, respectively [14].
Discussion
This study, in a hypothetical cohort of 1.5 million adult Indian persons with suspected active TB, suggests that sputum smear microscopy remains the most cost-effective initial diagnostic test for active TB, across a wide range of plausible parameter estimates. In areas where quality-assured microscopy is already available and resources are sufficient, liquid TB culture is a more cost-effective addition than serology. As an addition to sputum smear plus clinical judgment, serology detects more than six false positives for every new smear-negative TB case appropriately diagnosed, and from the economic perspective of the Indian TB control sector, is less favorable than (i.e., dominated by) other diagnostic options.
This study highlights the substantial adverse economic consequences of serological TB testing in India. Although most serological testing in India is performed in the private sector, patients diagnosed with TB (including false positives) are often sent for treatment in public facilities [27]. The scale-up of the RNTCP is estimated to have added over US$88 billion in economic benefit to India over the last 10 y through country-wide implementation of directly observed therapy, short-course (DOTS) services, including sputum smear microscopy [28]. Given the scale of the RNTCP's activities and its limited budget, any diagnostic strategy demanding additional resources may divert those resources from further improvement of sputum smear microscopy or engagement of the private sector to provide broader access to microscopy or other new WHO-endorsed diagnostics. If serology is used as a replacement for sputum smear microscopy in one-third of the estimated 1.5 million patients receiving serologic TB diagnosis annually in India [16], we estimate that the Indian TB control sector would lose an estimated US$11.9 million (approximately one-sixth the annual budget of the RNTCP) [16], generate 11,000 additional secondary cases of TB (nearly 1% of all incident TB cases in India) [26], treat 40,000 false-positive patients unnecessarily, and cause 34,000 DALYs.
Although we designed this analysis primarily as an evaluation of serological testing, our secondary results regarding TB culture suggest that, from the perspective of the Indian TB control sector, MGIT would be cost-effective, assuming that systems could be put in place to translate culture results consistently into treatment decisions [20]. While there is no universal standard for cost-effectiveness of interventions, those whose cost per DALY averted is less than a country's per-capita gross domestic product (GDP, US$3,100 in India [29]) are considered by the Commission for Macroeconomics and Health and WHO to be “very cost-effective” [30]. Sputum smear microscopy is estimated to cost US$19 per DALY averted, and addition of TB culture to sputum smear US$213 per DALY averted, speaking to the importance of efforts to increase India's TB control budget and to deploy TB diagnostics in settings where current diagnostic standards are poor [17]. Indeed, the RNTCP will require substantially higher resources to scale-up improved and new TB diagnostics in India, as it begins a new phase (2012–2017).
One important, and often overlooked, consideration in cost-effectiveness analyses of TB diagnostics is the role of false-positive results [31]. Most prior cost-effectiveness analyses of TB diagnostics (e.g., [3],[17]) have focused on the importance of high sensitivity in controlling the TB epidemic, assessing small costs (e.g., the cost of first-line TB drugs) to patients incorrectly diagnosed with TB. However, in a setting where a diagnostic test could result in over six times as many incremental false-positive as true-positive diagnoses (Table 3), other considerations including loss of faith in the health care system, ethical concerns of physicians, and adverse effects of TB therapy may be increasingly relevant. Furthermore, we assumed a setting where one in seven persons with suspected TB has active disease; where this ratio is lower, low-specificity diagnostics will perform even more poorly. Thus, our analysis may be biased against those diagnostics (i.e., smear and TB culture) with highest specificity.
A key limitation to this study is its reliance on published data for parameter estimates, many of which are subject to substantial variability, as well as the lack of direct data on the full costs (including purchase of equipment, laboratory scale-up, and training of staff) of culture. Nevertheless, we were unable to generate a model in which serology was more effective than sputum smear or more cost-effective than TB culture despite variation of each individual parameter by at least 25% of its baseline value. More importantly, published data on the sensitivity and specificity of serological testing are limited. For example, a total of 686 patients (105 from developing countries) have been reported in the published literature for anda-TB: the most widely studied serological test, available since 1990 [14]. This number compares with 1,730 patients reported in the first publication of Xpert MTB/RIF, a novel molecular test for TB [10], and over 14,000 specimens evaluated by MGIT [23]. Since published estimates likely overestimate the true performance of novel diagnostic tests, and in-house ELISA and rapid tests perform worse than commercial ELISA-based assays [14],[32], our analysis based on published estimates of a commercial ELISA may overestimate the effectiveness and cost-effectiveness of serology as implemented in actual practice.
We also acknowledge other limitations to our approach. First, we were unable to adopt a societal perspective, owing to the lack of reliable data on the societal costs of TB diagnosis and treatment in India. Thus, while internally consistent, our analysis should not be used to compare the cost-effectiveness of improved TB diagnostics to interventions in other areas of the health care sector. Our estimate of the cost-effectiveness of sputum smear microscopy plus TB treatment (US$19 per DALY averted) is nonetheless similar to that of the World Bank (US$7–US$11 in 2010 dollars for a low-income country) [33]. Second, since our primary focus was on serology, we limited our analysis to drug-sensitive TB and did not conduct a full costing study for culture. Third, we assume a population (urban and peri-urban) with access to a laboratory capable of performing serology. This population is not representative of the entire Indian population, and results from our model thus should not be generalized to all Indian persons with suspected active TB. Furthermore, the cost of establishing such laboratory services may not be entirely reflected in the market prices for these tests; thus, our model may underestimate the cost of diagnostic testing in areas where laboratory infrastructure is not preexisting. Finally, although we estimated secondary transmissions from our primary cohort, our model has a static 1-y analysis frame and does not account for changes over time including repeated rounds of TB transmission. We may therefore underestimate the long-term cost-effectiveness of improved TB diagnosis [34].
These data were presented to a WHO Expert Group that met in July 2010 to review the evidence on TB serological assays. This Expert Group considered the updated meta-analysis on commercial serological tests [14], as well as data from the present analysis demonstrating that serology is not cost-effective. Based on the Expert Group's recommendation, in July 2011, the WHO published a policy statement on commercial serodiagnostic tests for diagnosis of TB. The policy states that Commercial serological tests provide inconsistent and imprecise estimates of sensitivity and specificity. There is no evidence that existing commercial serological assays improve patient-important outcomes, and high proportions of false-positive and false-negative results adversely impact patient safety. Overall data quality was graded as very low, with harms/risks far outweighing any potential benefits (strong recommendation). It is therefore recommended that these tests should not be used in individuals suspected of active pulmonary or extra-pulmonary TB, irrespective of their HIV status. The WHO policy strongly encourages targeted further research to identify new/alternative point-ofcare tests for TB diagnosis and/or serological tests with improved accuracy [34],[35,45].
In conclusion, this cost-effectiveness analysis suggests that, as an initial test for active TB among adults in India, serology results in more DALYs, secondary infections, and false-positive diagnoses than sputum smear microscopy, while increasing per-patient costs to the Indian TB control sector. In areas where high-quality sputum microscopy is available, adding automated liquid culture is more effective and less costly than adding serology. These data have been considered by the WHO in recommending against serological testing for active TB, and will need to be considered by Indian regulatory and governmental agencies that need to implement the WHO recommendations.
Acknowledgments
We are grateful to David Bishai for his contributions to the original cost-effectiveness model.
Abbreviations
- DALY
disability-adjusted life year
- MGIT
mycobacteria growth indicator tube
- RNTCP
Revised National TB Control Programme
- TB
tuberculosis
- WHO
World Health Organization
Footnotes
None of the authors have any financial/industry conflicts to declare. All authors participated in the WHO Expert Group meeting on TB serological tests held in July 2010. KRS and MP have contributed to published and updated meta-analyses on TB serological tests. The updated meta-analysis on TB serological tests was commissioned by WHO with funding provided by USAID through a grant administered by the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR). KRS and MP are affiliated with the Stop TB Partnership's New Diagnostics Working Group. KRS serves as co-chair of the Evidence Synthesis subgroup of Stop TB Partnership's New Diagnostics Working Group, while MP serves as Co-chair of the Working Group. MP also serves as a consultant to the Bill & Melinda Gates Foundation (BMGF). BMGF had no involvement in this manuscript. MP serves on the editorial boards of PLoS Medicine and PLoS One.
This work was funded by the Stop TB Partnership's New Diagnostics Working Group, via the subgroup on Evidence Synthesis. MP is a recipient of support from the Canadian Institutes of Health Research (CIHR). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Small PM, Pai M. Tuberculosis diagnosis--time for a game change. N Engl J Med. 2010;363:1070–1071. doi: 10.1056/NEJMe1008496. [DOI] [PubMed] [Google Scholar]
- 2.Perkins MD, Cunningham J. Facing the crisis: improving the diagnosis of tuberculosis in the HIV era. J Infect Dis. 2007;196(Suppl 1):S15–S27. doi: 10.1086/518656. [DOI] [PubMed] [Google Scholar]
- 3.Keeler E, Perkins MD, Small P, Hanson C, Reed S, et al. Reducing the global burden of tuberculosis: the contribution of improved diagnostics. Nature. 2006;444(Suppl 1):49–57. doi: 10.1038/nature05446. [DOI] [PubMed] [Google Scholar]
- 4.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 5.Gilpin C, Kim SJ, Lumb R, Rieder HL Van Deun A; Working Group on Sputum Smear Microscopy. Critical appraisal of current recommendations and practices for tuberculosis sputum smear microscopy. Int J Tuberc Lung Dis. 2007;11:946–952. [PubMed] [Google Scholar]
- 6.Steingart KR, Ng V, Henry M, Hopewell PC, Ramsay A, et al. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: A systematic review. Lancet Infect Dis. 2006;6:664–674. doi: 10.1016/S1473-3099(06)70602-8. [DOI] [PubMed] [Google Scholar]
- 7.Pai M, Ramsay A, O’Brien R. Evidence-based tuberculosis diagnosis. PLoS Med. 2008;5:e156. doi: 10.1371/journal.pmed.0050156. doi: 10.1371/journal.pmed.0050156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pai M, Minion J, Sohn H, Zwerling A, Perkins MD. Novel and improved technologies for tuberculosis diagnosis: progress and challenges. Clin Chest Med. 2009;30:701–716. doi: 10.1016/j.ccm.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. 2010. Framework for implementing new tuberculosis diagnostics. Available: http://www.who.int/tb/laboratory/whopolicyframework_july10_revnov10.pdf. Accessed 13 March 2011.
- 10.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. 2010. Available: http://whqlibdoc.who.int/publications/2011/9789241501545_eng.pdf. Accessed 13 July 2011.
- 12.Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–2049. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 13.Perkins MD, Roscigno G, Zumla A. Progress towards improved tuberculosis diagnostics for developing countries. Lancet. 2006;367:942–943. doi: 10.1016/S0140-6736(06)68386-4. [DOI] [PubMed] [Google Scholar]
- 14.Steingart KR, Flores LL, Dendukuri N, Schiller I, Laal S, et al. Commercial serological tests for the diagnosis of tuberculosis: an updated systematic review and meta-analysis. PLoS Med. 2011;8:e1062. doi: 10.1371/journal.pmed.1001062. doi: 10.1371/journal.pmed.1001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grenier J, Pinto L, Nair D, Steingart KR, Dowdy D, et al. Eur Respir J.; 2011. Widespread use of serological tests for tuberculosis: data from 22 high-burden countries. In press. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Geneva: WHO; 2010. Expert group meeting report on commercial serodiagnostic tests for diagnosis of tuberculosis. [PubMed] [Google Scholar]
- 17.Dowdy DW, O’Brien MA, Bishai D. Cost-effectiveness of novel diagnostic tools for the diagnosis of tuberculosis. Int J Tuberc Lung Dis. 2008;12:1021–1029. [PubMed] [Google Scholar]
- 18.Joint United Nations Programme on HIV/AIDS (UNAIDS) AIDS epidemic update 2009. Geneva: UNAIDS/WHO. Report UNAIDS/09.36E/ 2009;JC1700E [Google Scholar]
- 19.Special Programme for Research and Training in Tropical Diseases. Geneva: WHO; 2008. Diagnostics for tuberculosis: global demand and market potential. p. 203 p. [Google Scholar]
- 20.Dowdy DW, Lourenco MC, Cavalcante SC, Saraceni V, King B, et al. Impact and cost-effectiveness of culture for diagnosis of tuberculosis in HIV-infected Brazilian adults. PLos ONE. 2008;3:e4057. doi: 10.1371/journal.pone.0004057. doi: 10.1371/journal.pone.0004057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller DH, Mwenge L, Muyoyeta M, Muvwimi MW, Tembwe R, et al. Costs and cost-effectiveness of tuberculosis cultures using solid and liquid media in a developing country. Int J Tuberc Lung Dis. 2008;12:1196–1202. [PubMed] [Google Scholar]
- 22.Steingart KR, Henry M, Laal S, Hopewell PC, Ramsay A, et al. Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med. 2007;4:e202. doi: 10.1371/journal.pmed.0040202. doi: 10.1371/journal.pmed.0040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cruciani M, Scarparo C, Malena M, Bosco O, Serpelloni G, et al. Meta-analysis of BACTEC MGIT 960 and BACTEC 460 TB, with or without solid media, for detection of mycobacteria. J Clin Microbiol. 2004;42:2321–2325. doi: 10.1128/JCM.42.5.2321-2325.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Styblo K. The Hague, The Netherlands: Royal Netherlands Tuberculosis Association (KNCV); 1991. Epidemiology of tuberculosis. p. 136 p. [Google Scholar]
- 25.United States Bureau of Labor Statistics. 2010. Consumer price index: all urban consumers. Washington (D.C.): Bureau of Labor Statistics. Available: http://data.bls.gov/cgi-bin/surveymost?cu. Accessed 23 October 2010.
- 26.World Health Organization. Report WHO/HTM/TB/2009.411. Geneva: WHO; 2009. Global tuberculosis control – epidemiology, strategy, financing. p. 78 p. [Google Scholar]
- 27.Pantoja A, Floyd K, Unnikrishnan KP, Jitendra R, Padma MR, et al. Economic evaluation of public-private mix for tuberculosis care and control, India. Part I. Socio-economic profile and costs among tuberculosis patients. Int J Tuberc Lung Dis. 2009;13:698–704. [PubMed] [Google Scholar]
- 28.Goodchild M, Sahu S, Wares F, Dewan P, Shukla RS, et al. A cost-benefit analysis of scaling up tuberculosis control in India. Int J Tuberc Lung Dis. 2011;15:358–362. [PubMed] [Google Scholar]
- 29.Central Intelligence Agency. Washington (D.C.): Central Intelligence Agency; 2009. The world factbook. Available: https://www.cia.gov/library/publications/the-world-factbook. Accessed 23 October 2010. [Google Scholar]
- 30.Commission on Macroeconomics and Health. Geneva: WHO; 2001. Macroeconomics and health: investing in health for economic development. p. 200 p. [Google Scholar]
- 31.Dowdy DW, Cattamanchi A, Steingart KR, Pai M. Is scale-up worth it? Challenges in economic analysis of diagnostic tests for tuberculosis. PLoS Med. 2011;8:e1001063. doi: 10.1371/journal.pmed.1001063. doi: 10.1371/journal.pmed.1001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization on behalf of the Special Programme for Research and Training in Tropical Diseases. Geneva: World Health Organization; 2008. Laboratory-based evaluation of 19 commercially available rapid diagnostic tests for tuberculosis. p. 70 p. [Google Scholar]
- 33.World Bank. Oxford: Oxford University Press; 1993. World development report 1993: investing in health. p. 329 p. [Google Scholar]
- 34.Dowdy DW, Chaisson RE, Moulton LH, Dorman SE. The potential impact of enhanced diagnostic techniques for tuberculosis driven by HIV: a mathematical model. AIDS. 2006;20:751–762. doi: 10.1097/01.aids.0000216376.07185.cc. [DOI] [PubMed] [Google Scholar]
- 35.Morris K. WHO recommends against inaccurate tuberculosis tests. Lancet. 2011;377:113–114. doi: 10.1016/s0140-6736(11)60005-6. [DOI] [PubMed] [Google Scholar]
- 36.Strategic and Technical Advisory Group for Tuberculosis (STAG-TB) 2010. Report of the tenth meeting. Available: http://www.who.int/tb/advisory_bodies/stag_tb_report_2010.pdf. Accessed 13 March 2011.
- 37.Behr MA, Warren SA, Salamon H, Hopewell PC, Ponce de Leon A, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353:444–449. doi: 10.1016/s0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 38.Pronyk RM, Makhubele MB, Hargreaves JR, Tollman SM, Hausler HP. Assessing health seeking behaviour among tuberculosis patients in rural South Africa. Int J Tuberc Lung Dis. 2001;5:619–627. [PubMed] [Google Scholar]
- 39.Salaniponi FM, Harries AD, Banda HT, Kang'ombe C, Mphasa N, et al. Care-seeking behaviour and diagnostic processes in patients with smear-positive pulmonary tuberculosis in Malawi. Int J Tuberc Lung Dis. 2000;4:327–332. [PubMed] [Google Scholar]
- 40.Squire SB, Belaye AK, Kashoti A, Salaniponi FM, Mundy CJ, et al. ‘Lost’ smear-positive pulmonary tuberculosis cases: where are they and why did we lose them? Int J Tuberc Lung Dis. 2005;9:25–31. [PubMed] [Google Scholar]
- 41.Creek TL, Lockman S, Kenyon TA, Makhoa M, Chimidza N, et al. Completeness and timeliness of treatment initiation after laboratory diagnosis of tuberculosis in Gaborone, Botswana. Int J Tuberc Lung Dis. 2000;4:956–961. [PubMed] [Google Scholar]
- 42.Murray CL, Lopez AD. Boston, Massachusetts: Harvard School of Public Health; 1996. The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020.1022 [Google Scholar]
- 43.Ministry of Health and Family Welfare. TB India 2010: RNTCP Status Report. New Delhi: Central TB Division, Directorate General of Health Services (India). 2010. Available: http://www.tbcindia.org/pdfs/TB%20India%202010.pdf. Accessed 23 October 2010.
- 44. Institute of Actuaries of India. Published Mortality Tables. Available: http://www.actuariesindia.org/Publication%20and%20Library%20Facility/mortality%20tables.htm. Accessed 23 October 2010.
- 45.World Health Organization. http://www.who.int/tb/laboratory/policy_statement/en. Accessed: 12 July 2011.