Abstract
This pilot case-control study retrospectively assessed between-group differences in subjective opioid effects in patients treated for the first time with opioids for chronic pain. Cases were individuals in an inpatient substance abuse treatment center for primary prescription opioid addiction whose initial exposure to prescription opioids was reported for chronic pain. Controls had not developed prescription opioid addiction as measured in part by close monitoring on long-term opioid therapy at a pain management center. Twenty subjects in each group completed a battery of measures to capture data related to the individual’s first exposure to prescription opioids. The Morphine Benzedrine Group subscale of an adapted 49-item Addiction Center Research Inventory (ARCI), designed to measure euphoria and other drug effects, showed an average score of 8.70 (+/− 4.18) in cases vs. 2.55 (+/− 3.36) in controls (p<0.001), indicating a significantly greater “euphoric” effect of opioids in the cases compared to the controls. Differences in the subjective response to opioids suggest that: (1) a subgroup of patients does develop euphoria when taking opioids for pain, which may be a risk factor for eventual development of prescription opioid addiction; and (2) subjective effects predictive of eventual addiction may include stimulation and other experiences not typically associated with opioids.
Keywords: chronic pain, drug addiction, euphoria, prescription opioids, subjective effects
Introduction
Opioid analgesics remain a controversial option for long term management of chronic pain. They are recognized as being safe and effective in cancer and acute pain. Long term use in chronic non-cancer pain is less well accepted, in part because of the risk of development of abuse and addiction to these drugs. Many patients have inadequate access to opioids and therefore must live with the costly and debilitating condition of intractable pain (Green, Todd, Lebovits, & Francis, 2006). Conversely, inappropriate access to opioids increases the risk of addiction, which can be similarly costly and debilitating (Birnbaum et al., 2006). Thus, clinicians are faced with a dilemma. They wish to minimize risk of addiction while providing appropriate analgesia for pain relief. A strategy that could improve decision-making, with regard to prescribing opioid analgesics, involves improving physicians’ ability to reliably determine patients’ risk for opioid addiction (Butler, Budman, Fernandez, & Jamison, 2004). If addiction risk could be reliably determined (through questionnaires, genetic mapping or other tests), then patients could be matched to an appropriate treatment, including both pharmacologic approaches and treatment setting, whether or not opioids are prescribed (Gourlay, Heit, & Almarhezi, 2005). Definition of the risk phenotype through genetic predictors would provide an objective measure of addiction susceptibility (Kreek, Nielsen, Butelman, LaForge, 2005). Less objective methods for determining risk have included the development of such tools such as Screener and Opioid Assessment for Patients in Pain, SOAPP (Butler et al., 2004) and the Opioid Risk Tool, ORT questionnaire (Webster & Webster, 2005), that rely to a large degree on the patient history of earlier or current drug abuse.
In this report we describe differences in the subjective effects of opioids when given for chronic pain, comparing patients who ultimately developed addiction to prescription opioids, to patients who did not develop addiction under close monitoring over a period of years. . We also sought to explore the relationship between the experience of euphoria in pain patients initiating opiate treatment and subsequent addiction. The results of this analysis are part of a larger study to develop and assess the feasibility of a battery of measures of potential risks for prescription opioid abuse and addiction.
Materials and Methods
Institutional review board approval was granted and informed consent was obtained from all subjects. A sample of 20 subjects per group was sought without sample size justification. Cases were selected from a convenience sample of opioid analgesic addicted patients admitted to the Alcohol and Drug Abuse Treatment Program at McLean Hospital (Belmont, MA) for the primary treatment of prescription opioid addiction. Controls were selected from a convenience sample of patients receiving opioid maintenance therapy for chronic pain at a Connecticut-based pain treatment facility. Inclusion criteria for cases were: (a) at least 18 years of age, (b) able to give informed consent, (c) had pain for a period of at least three months which preceded their first opioid exposure, (d) treated for chronic pain with prescription opioid analgesics for at least three months, (e) first exposure to prescription opioids was for the treatment of their chronic pain, and (f) subsequently admitted for treatment of primary prescription opioid addiction. A previous history of a substance use disorder did not exclude subjects from either group. Inclusion criteria for controls were the same except that the patients had not developed prescription opioid addiction in the context of long-term opioid therapy for pain under close monitoring. This was determined through repeated negative urine toxicology screens administered at the initial visit, twice yearly on scheduled dates, and at random unannounced times throughout the year. The treating physician assessed patient risk using the ORT at initial visit and followed up monthly indicating the absence of any problematic opioid-related behavior. Subjects were informed that the purpose of the study was to try to understand the experiences of patients being treated with prescription opioids to improve treatment services and their responses would be kept confidential. All subjects completed a battery of self-report assessments which were mailed to them. The battery consisted of thirteen assessments measuring; personal and family drug use history, routes of administration, social functioning, trauma exposure, adverse life events, craving, pain intensity, somatic symptom severity, and psychological co-morbidity.
Compensation for completion was a $75 gift certificate to local retail stores. This report describes the results of the Martin 49-item ARCI modified to 46 items (Martin, Sloan, Sapira, & Jasinski, 1971). Martin’s original ARCI 49-item short form measures euphoria and other drug effects at the time of exposure, and asks participants to respond “true” or “false” to a series of statements about their experiences after drug administration. We adapted this instrument by modifying the instructions to ask subjects to, “Think about the way you felt when you were first taking prescription opioids for your chronic pain.” We removed three questions, two because they could not be adapted to our retrospective recall paradigm (“Answering these questions was very easy today” and “It seems I'm spending longer than I should on each of these questions”), and an additional question which, if modified to reflect a recalled experience would have created a redundant item: “I would be happy all the time if I felt as I do now.”
Analysis was conducted using SPSS version 14 (SPSS, 2005). We constructed the five subscale scores using the algorithm of Martin et al. (1971) comparing the means using t-tests. These subscales consisted of the Pentobarbital, Chlorpromazine, Alcohol Group (PCAG), the Morphine Benzedrine Group (MBG), the Lysergic Acid Diethylamide Group (LSD), the Benzedrine Group (BG), and the Amphetamine Group (A). The three items, noted above, that we removed from the original Martin ARCI were not included in the scoring. Two-sided Fisher’s exact tests were used to evaluate differences between groups on each item on our modified ARCI with a difference of interest defined by a p-value of ≤0.05, highlighting items from the MBG subscale or that were identified by Hill et al. (Hill, Haertzen, Wolbach, & Miner, 1963) to be significant for morphine.
Results
Thirty-five cases and 27 controls were recruited and 20 cases and 20 controls completed the battery of instruments (Table 1). The evaluable data set for this report was defined as subjects who completed the ARCI. Twenty-two of the recruited participants did not return the battery of assessments. Gender was approximately evenly distributed in each group (cases 10 male, 10 emale; controls 11 male, 9 female). Ages ranged from 19 to 70 years with a mean age of 44. The average length of exposure to prescription opioids was shorter for cases than controls (cases 7.06 years, range= 1–28, controls 12.28 years, range= 1–31). Cases also on average had a shorter elapsed time between opiate initiation and participation in this study. (cases 8.05 years, range= 1– 28, controls 12 years, range= 1–31). A greater number of controls reported having sought treatment for a non-substance related emotional or psychiatric disorder (cases 80%, controls 45%, p= .02).
Table 1.
Demographics
| Variable | N | % | Cases | % | Controls | % |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 21 | 52.5 | 10 | 50 | 11 | 55 |
| Female | 19 | 47.5 | 10 | 50 | 9 | 45 |
| Age | ||||||
| mean | 44 | 37 | 50 | |||
| Sd | 11.07 | 9.73 | 8.24 | |||
| range | 19–70 | 19–56 | 37–70 | |||
| Educational Level | ||||||
| Did not complete high school | 1 | 2.5 | 0 | 0 | 1 | 5 |
| Graduated high school or received a G.E.D. | 8 | 20 | 4 | 20 | 4 | 20 |
| Some College | 20 | 50 | 11 | 55 | 9 | 45 |
| 4 years of college or a bachelor’s degree | 8 | 20 | 3 | 15 | 5 | 25 |
| Some Graduate education or a Master’s degree | 6 | 15 | 4 | 20 | 2 | 10 |
| Marital Status | ||||||
| Married or living with partner | 30 | 75 | 12 | 60 | 18 | 90 |
| Separated, Divorced, Widowed | 2 | 5 | 2 | 10 | 0 | 0 |
| Never married and not living with a partner | 8 | 20 | 6 | 30 | 2 | 10 |
| Employment | ||||||
| Full time | 13 | 32.5 | 5 | 25 | 8 | 40 |
| Part time (<35 hrs/wk) | 7 | 17.5 | 4 | 20 | 3 | 15 |
| Student (full time) | 2 | 5 | 2 | 10 | 0 | 0 |
| Unemployed | 5 | 12.5 | 1 | 5 | 4 | 20 |
| On disability | 13 | 32.5 | 8 | 40 | 5 | 25 |
| Income | ||||||
| Less than $19,999 | 6 | 15 | 3 | 15 | 3 | 15 |
| $20,0000 – $49,999 | 15 | 37.5 | 9 | 45 | 6 | 30 |
| $50,000 – $99,999 | 14 | 35 | 5 | 25 | 9 | 45 |
| $100,000 or more | 5 | 12.5 | 3 | 15 | 2 | 10 |
As indicated in Table 2 the majority of cases and controls had a history of drug use prior to or concurrent with the initiation of prescription opioids for pain. A greater number of cases initiated the use of heroin concurrently with prescription opioids than controls (cases 50%, controls 5%, p= .018). Across all drug classes 89% of cases and 75% of controls had used at least one drug prior to exposure to prescription opioids. These data do not differentiate between sedative drug use and abuse.
Table 2.
Timing of other drug use compared to initiation of prescription opioids for pain
| Drug Classification | Prior to Opioid Rx (Lifetime) n (%) |
The Same Year as Initiation of Opioid Rx n (%) |
> 1 year after Initiation of Opiate Rx n (%) |
|||
|---|---|---|---|---|---|---|
| Case | Control | Case | Control | Case | Control | |
| PCP | 7 (35) | 1 (5) | 0 | 0 | 0 | 0 |
| Cocaine | 10 (50) | 6 (30) | 0 | 0 | 3 (15) | 1 (5) |
| Heroin | 0 | 1 (5) | 10 (50) | 1 (5) | 2 (10) | 0 |
| Sedatives | 7 (35) | 7 (35) | 3 (15) | 2 (10) | 4 (20) | 2 (10) |
| Marijuana | 15 (75) | 14 (70) | 1 (5) | 0 | 0 | 0 |
Note: The values in Table 2 were derived by comparing the year subjects initiated a specific drug to the year prescription opioids were initiated. Columns are mutually exclusive.
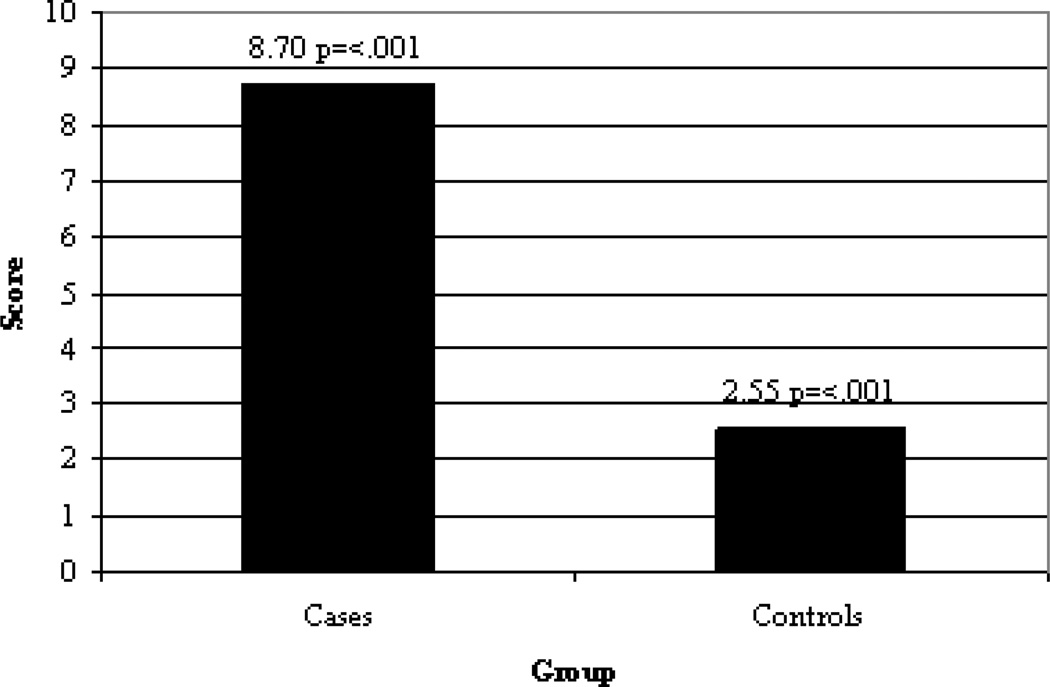
The mean score on the MBG subscale of the ARCI was for cases 8.70 +/− 4.18 and for controls 2.55 +/− 1.51 (p= <0.001), indicating that cases recalled significantly greater euphoric effects from opioids at the time of initial exposure for the treatment of pain than did controls (Figure 1). The mean scores for cases also was higher than for controls on each of the non-opioid-related ARCI subscales, the greatest difference being for the Amphetamine Group (cases 5.20 +/− 2.67, controls 1.80 +/− 1.51, p= <0.001) (Table 3). Between cases who did and did not report using heroin the means MBG score was similar (heroin using cases 9 +/− 4.19, non-heroin using cases 8 +/− 4.31).
Figure 1.
Mean score of the modified 46-Item ARCI MBG subscale between cases and controls
Table 3.
ARCI subscale T-Tests
| Scale | Group | Mean | sd | p-value |
|---|---|---|---|---|
| MBG | <.001 | |||
| Case | 8.70 | 4.181 | ||
| Control | 2.55 | 3.364 | ||
| PCAG | .094 | |||
| Case | 7.45 | 3.967 | ||
| Control | 5.50 | 3.171 | ||
| A | <.001 | |||
| Case | 5.20 | 2.668 | ||
| Control | 1.80 | 1.508 | ||
| LSD | <.001 | |||
| Case | 7.00 | 2.902 | ||
| Control | 4.00 | 1.487 | ||
| BG | .089 | |||
| Case | 5.40 | 2.137 | ||
| Control | 4.30 | 1.838 | ||
PCAG (Pentobarbital, Chlorpromazine, Alcohol Group)
G (Morphine Benzedrine Group)
LSD (Lysergic Acid Diethylamide Group)
BG (Benzedrine Group)
A (Amphetamine Group)
A separation between the cases and controls on a number of individual items from the modified ARCI was also revealed in the analysis. The greatest difference was within items on the MBG subscale, such as “I feared I would lose the contentment I had then” (cases 80%, controls 10%, p= <.001); “I would be happy all the time if I felt as I felt then” (cases 75%, controls 5%, p=<.001); and “I felt as if something pleasant had just happened to me” (cases 78.9%, controls 10.5%, p= <.001). However, many items outside of the MBG subscale also showed a significant difference between case and control responses, such as “A thrill had gone through me” (cases 85%, controls 10%, p= <.001); and “Some parts of my body were tingling” (cases 80%, controls 15.8%, p= <.001). Of the 33 items on the modified ARCI that were identified by Hill et al. (1963) as significant or marginally significant for morphine, 14 were identified as significant in our sample. Only one item, “People might have said that I was a little dull” (cases 70%, controls 10%, p= <.001) was significant in our sample but not identified as significant for morphine by Hill et al. (1963) (Table 4).
Table 4.
Between-group differences by individual item on the 46-item modified ARCI.
| Item | Subscale | % Cases | % Controls | p-value** |
|---|---|---|---|---|
| 1. My speech was sluned. + | P | 50 | 10 | .014 |
| 2. I was not as active as usual. + | P | 75 | 45 | .105 |
| 3. I had a feeling of just dragging along rather than coasting. | P | 50 | 15 | .041 |
| 4. I felt sluggish. | P | 60 | 40 | .343 |
| 5. My head felt heavy. | P | 30 | 20 | .716 |
| 6. I felt like avoiding people although I usually did not feel this way. | P | 60 | 10 | .002 |
| 7. I felt dizzy | P | 30 | 15 | .451 |
| 8. It seemed harder than usual to move around. | P | 40 | 5 | .020 |
| 9. I was moody. | P | 75 | 30 | .010 |
| 10. People might have said that I was a little dull. | P/B | 70 | 10 | <.001 |
| 11. I felt drowsy. | P/L | 70 | 50 | .333 |
| 12. I was full of energy | P | 55 | 30 | .2 |
| 13. I said things in the easiest possible way. | M | 50 | 25 | .191 |
| 14. Things around me seemed more pleasing than usual. | M | 75 | 25 | .004 |
| 15. I had a pleasant feeling in my stomach. * | M | 47.4 | 30 | .333 |
| 16. I feared I would lose the contentment that I had then. | M | 80 | 10 | <.001 |
| 17. I felt in complete harmony with the world and those about me. | M | 60 | 25 | .054 |
| 18. I could completely appreciate what others were saying when I was in that mood. * | M | 55 | 15.8 | .019 |
| 19. I would be happy all the firm if I felt as I felt then. | M | 75 | 5 | <.001 |
| 20. I felt so good that I knew other people could tell it. | M | 55 | 5 | .001 |
| 21. I felt as if something pleasant had just happened to me. * | M | 78.9 | 10.5 | <.001 |
| 22. I felt more clear headed than dreamy. | M/B/P | 55 | 35 | .341 |
| 23. I felt as if I would be more popular with people. | M/A | 30 | 5 | .091 |
| 24. I felt a very pleasant emptiness. | M/B | 35 | 5 | .044 |
| 25. My thoughts came more easily than usual. | M/A/B | 55 | 5 | .001 |
| 26. I felt less discouraged than usual. | M/A | 75 | 25 | .004 |
| 27. I was in the mood to talk about the feelings I had. | M/B | 50 | 30 | .333 |
| 28. I felt more excited than dreamy. + | A/P | 68.4 | 25 | .010 |
| 29. My memory seemed sharper to me than usual. | A/B | 50 | 15 | .041 |
| 30. I felt as if I could write for hours. | A/B | 40 | 5 | .020 |
| 31. I felt very patient. | A/L | 25 | 40 | .501 |
| 32. Some parts of my body were tingling. * | A/B/L | 80 | 15.8 | <.001 |
| 33. I had a weird feeling. | A/L | 65 | 40 | .205 |
| 34. My movements seemed faster than usual. | B | 40 | 5 | .020 |
| 35. I had better control over myself than usual. | B | 45 | 25 | .320 |
| 36. My movements seemed slower than usual. | B | 35 | 40 | 1.00 |
| 37. I found it hard to keep my mind on a task or job.* | B | 57.9 | 30 | .111 |
| 38. I didn't feel like reading anything then. | L | 60 | 30 | .111 |
| 39. My hands felt clumsy. | L | 20 | 5 | .342 |
| 40. I noticed my hand shook when I tried to write. | L | 35 | 5 | .044 |
| 41. I had a disturbance in my stomach. | L | 50 | 15 | .041 |
| 42. I felt an increasing awareness of bodily sensations. | L | 55 | 30 | .2 |
| 43. I felt anxious and upset. | L | 40 | 10 | .065 |
| 44. I had unusual weakness of my muscles.+ | L | 40 | 0 | .003 |
| 45. A thrill had gone through me one or more times. | L/P | 85 | 10 | <.001 |
| 46. My movements were free, relaxed, and pleasurable.* | L | 73.7 | 40 | .054 |
(P) - Pentobarbital, Chlorpromazine, Alcohol Group, (M) - Morphine Benzedrine Group, (L) - Lysergic Acid Diethylamide Group, (B) - Benzedrine Group, (A) - Amphetamine Group
Italicized items were identified as significant for morphine by Hill [12]
one or more individuals did not respond
item identified as marginally significant for morphine by Hill [12]
2- sided Fisher’s exact test.
Discussion
The results suggest that there is a difference in the subjective experience of opioid exposure for the treatment of pain recalled by individuals who ultimately developed addiction to prescription opioids compared to individuals who ultimately did not develop addiction despite long-term exposure. Our study is unique in that respondents were asked to focus on their initial experience with opioids for the treatment of pain. Moreover, our study uniquely compares prescription opioid addicts to individuals who did not develop addiction despite long-term exposure, as opposed to many studies which compare addicts to non-addicts without considering exposure. Our data have several implications.
First, the subjective experience, particularly if captured with a validated questionnaire designed to evaluate this experience, may have utility as a risk predictor, which would have potentially important clinical implications. This was demonstrated by the robustly different scores between cases and controls on the MBG subscale. Development and validation of future measures may be useful in more accurately capturing these subjective experiences.
Second, a careful characterization of the phenotype of the patient at risk for prescription opioid addiction, as distinct from the patient not at risk, would be useful for genetic studies aiming to identify genetic risk (or protective) factors for prescription opioid addiction in patients with pain. Third, we found that differences in the subjective experience of opioid exposure between those who safely use opioids and those who became addicted may be marked by experiences not typically associated with intended opioid effects, as indicated by the significance of items and subscales not in the MBG subscale. More specifically, our data suggest that individuals at risk for prescription opioid abuse experience a more stimulating or euphoric effect of opioids than do those not at such risk. The patient’s state or trait (genetics, environment, psychological, clinical, etc.) may predispose them to becoming addicted, and their subjective experience may be a surrogate marker for this predisposition. Further efforts to develop predictive scales may therefore need to be open to the possibility that the predictive subjective effects may go beyond those traditionally associated with opioids.
Our study has a number of obvious limitations. We used convenience samples of both cases and controls and a small sample size. Our cases and controls differed not only on their addiction status, but on a number of other variables, most prominently the point of recruitment: individuals in an urban addiction treatment center can be expected to differ from those in a community pain management center in many ways. In addition, we did not attempt to determine if one opioid was a greater risk for the development of addiction, or if it is a class effect. A high percentage of both cases and controls used illicit drugs prior to prescription opioid initiation. This may have influenced the participant’s account of their first exposure to prescription opioids. The differences in psychopathology reported by cases and controls may account for some differences in subjective opioid effects. It is also possible some cases did not truthfully report heroin use prior to opioid initiation. Finally, our questionnaire asked subjects to recall experiences that had taken place long in the past. While our subjects appeared quite able to do so, a variety of recall biases could have influenced their reports.
Conclusions
Our data suggest that patients at risk for developing addiction to opioids prescribed for the treatment of pain may differ from patients not at such risk in their subjective reaction to opioid exposure. Our design compared individuals exposed to opioids who developed addiction to individuals exposed to opioids who did not develop addiction, none of whom had pre-exposure opioid addiction problems. We believe that these results bear further investigation. A larger study would allow modeling approaches to identify interaction effects and protective factors. Just as the subjective experience of initial opioid exposure may be predictive of subsequent addiction, other factors present at the time of initial exposure, such as additional subjective experiences, psychological trait or state, social background or status at the time, may also increase or decrease risk. It is likely that the interaction of intrinsic risk and protective factors together with extrinsic environmental factors ultimately determines outcome.
Acknowledgements
This study was supported by the Tufts Health Care Institute Program in Opioid Risk Management, by Inflexxion, Inc, by Analgesic Research (NK), and grants K02 DA00326 (RDW) and U10 DA15831 (RDW, JSP) from the National Institute on Drug Abuse.
References
- Birnbaum HG, White AG, Reynolds JL, Greenberg PE, Zhang M, Vallow S, Schein JR, Katz NP. Estimated costs of prescription opioid analgesic abuse in the United States in 2001: a societal perspective. Clinical Journal of Pain. 2006;22(8):667–676. doi: 10.1097/01.ajp.0000210915.80417.cf. [DOI] [PubMed] [Google Scholar]
- Butler SF, Budman SH, Fernandez K, Jamison RN. Validation of a screener and opioid assessment measure for patients with chronic pain. Pain. 2004;112:65–75. doi: 10.1016/j.pain.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Gourlay D, Heit H, Almarhezi A. Universal precautions in pain medicine: A rational approach to the treatment of chronic pain. Pain Medicine. 2005;6(2):107–112. doi: 10.1111/j.1526-4637.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- Green C, Todd KH, Lebovits A, Francis M. Disparities in pain: ethical issues. Pain Medicine. 2006;7(6):530–533. doi: 10.1111/j.1526-4637.2006.00244.x. [DOI] [PubMed] [Google Scholar]
- Hill HE, Haertzen CA, Wolbach AB, Miner EJ. The Addiction Research Inventory: Standardization of scales which evaluate subjective effects of morphine, amphetamine, pentobarbital, alcohol, LSD-25, pyrahexyl, and chlorpromazine. Psychopharmacologia. 1963;4:184–205. doi: 10.1007/BF02584089. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nature Neuroscience. 2005;8(11):1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan BS, Sapira JD, Jasinski DR. Physiologic, subjective and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine and methylphenidate in man. Clinical Pharmacology and Therapeutics. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- SPSS Inc. SPSS Version 14 [computer software] Chicago: Illinois: 2005. [Google Scholar]
- Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: Preliminary validation of the Opioid Risk Tool. Pain Medicine. 2005;6(6):432–442. doi: 10.1111/j.1526-4637.2005.00072.x. [DOI] [PubMed] [Google Scholar]