Abstract
Epileptic seizures cause dynamic, reversible changes in brain function and are often associated with loss of consciousness. Of all seizure types, absence seizures lead to the most selective deficits in consciousness, with relatively little motor or other manifestations. Impaired consciousness in absence seizures is not monolithic, but varies in severity between patients and even between episodes in the same patient. In addition, some aspects of consciousness may be more severely involved than other aspects. The mechanisms for this variability are not known. Here we review the literature on human absence seizures and discuss a hypothesis for why effects on consciousness may be variable. Based on behavioral studies, electrophysiology, and recent neuroimaging and molecular investigations, we propose absence seizures impair focal, not generalized brain functions. Imapired consciousness in absence seizures may be caused by focal disruption of information processing in specific corticothalamic networks, while other networks are spared. Deficits in selective and varying cognitive functions may lead to impairment in different aspects of consciousness. Further investigations of the relationship between behavior and altered network function in absence seizures may improve our understanding of both normal and impaired consciousness.
Introduction
What is the relationship between brain activity and conscious thought? In science, difficult questions of this kind often yield to investigation, provided a good model system is available. One approach is to determine relationships between brain function and consciousness in situations where both vary. Examples include the investigation of sleep, anesthesia, brain lesions, evolution, development, and epilepsy. Epilepsy is an attractive model system for studying consciousness because epileptic seizures can cause selective, dynamic, and rapidly reversible changes in consciousness associated with altered function in specific brain networks. In this review, we will first introduce the major seizure types that cause impaired consciousness, including absence seizures. We next discuss electrophysiology and behavioral studies of impaired consciousness in absence seizures. Finally, we will briefly discuss recent neuroimaging and molecular studies suggesting that selective corticothalamic network involvement in absence seizures may, ultimately, explain the specific cognitive impairments that cause loss of consciousness.
Epilepsy models for studying impaired consciousness
Epileptic seizures are usually classified as either partial, involving focal brain regions, or generalized, involving widespread regions of the brain bilaterally (ILAE, 1981). Partial seizures associated with impaired consciousness are referred to as complex partial seizures, while those that spare consciousness are called simple partial seizures. Complex partial seizures arise most commonly from the temporal lobe. They typically begin with an unusual abdominal sensation or premonition, followed by staring, unresponsiveness, and simple repetitive movements of the mouth and limbs lasting 1–2 min, followed by amnesia for the episode. We and others have recently discussed a possible mechanism for impaired consciousness in temporal lobe complex partial seizures, in which network interactions with the upper brainstem and medial thalamus inhibit function of the bilateral fronto-parietal association cortex (Menzel et al., 1998; Lee et al., 2002; Norden and Blumenfeld, 2002; Blumenfeld and Taylor, 2003; Van Paesschen et al., 2003; Blumenfeld et al., 2004a, b).
Generalized seizures should invariably cause loss of consciousness if they truly involve the whole brain. Interestingly, however, this is not the case. Consciousness is spared in several types of generalized seizures (Gokygit and Caliskan, 1995; Bell et al., 1997; Vuilleumier et al., 2000). Moreover, recent work suggests that the so-called “generalized” seizures preferentially involve certain cortical–subcortical networks, while sparing others (Blumenfeld, 2003; McNally and Blumenfeld, 2004; McNally et al., 2004a, b; Nersesyan et al., 2004a, b). An important and common form of generalized seizure is the grand mal, or generalized tonic-clonic seizure. These typically consist of a dramatic rigid stiffening of the limbs for approximately 10 s, followed by synchronous contractions of the extremities for 1–2 min, with unresponsiveness throughout, and profound lethargy and confusion for a variable period after the episode ends. We recently proposed that the severe impairment in consciousness during and following generalized tonic-clonic seizures is caused by intense, abnormally increased neuronal activity in focal regions of the fronto-parietal association cortex and their related subcortical networks (Fig. 1) (Blumenfeld et al., 2003a, b; Blumenfeld and Taylor, 2003; McNally and Blumenfeld, 2004). Thus, abnormal decreased activity in bilateral fron-to-parietal networks may impair consciousness in complex partial seizures, while abnormal increased activity in these same networks may impair consciousness in generalized tonic-clonic seizures.
Fig. 1.
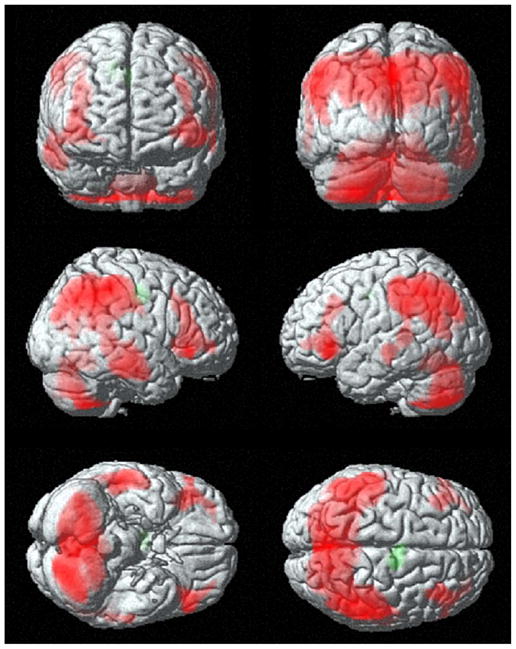
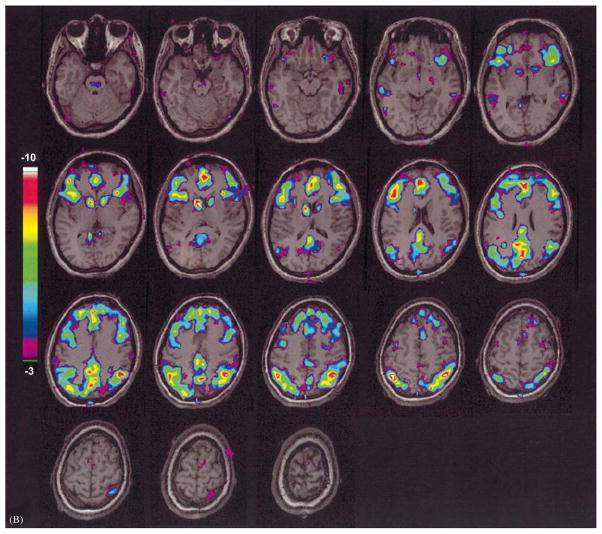
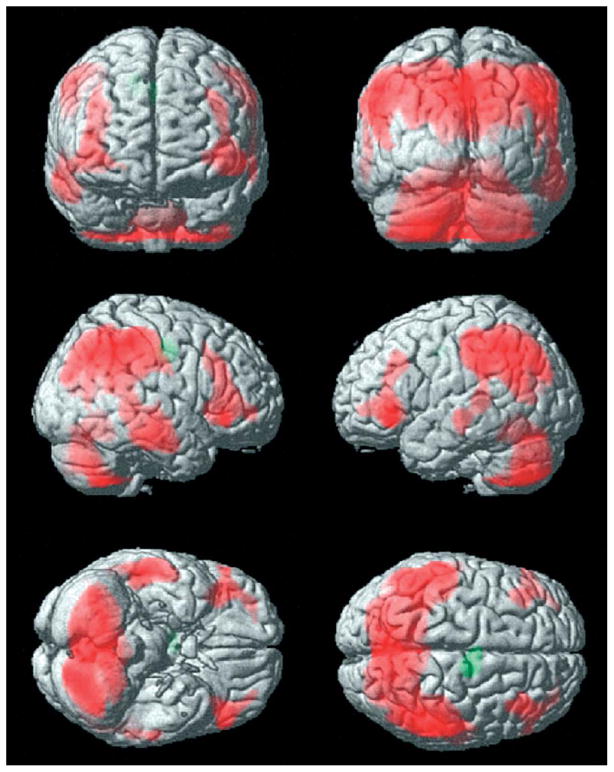
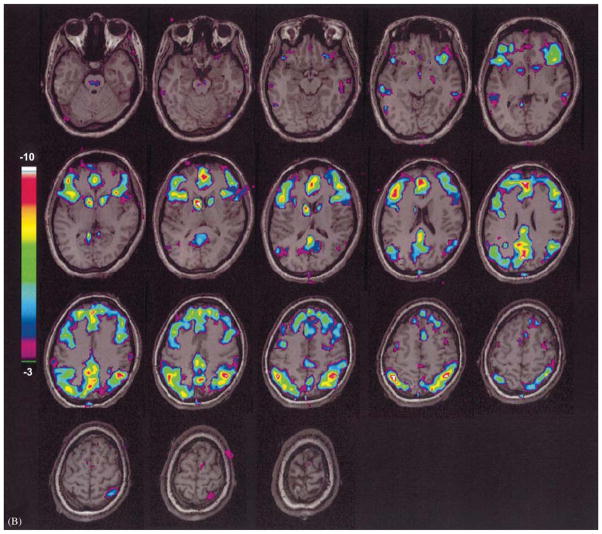
Fronto-parietal cortical involvement in generalized tonic-clonic seizures. Generalized tonic-clonic seizures were induced by electroconvulsive therapy for treatment of refractory depression (American Psychiatric Association, 2001) and cerebral blood flow (CBF) was imaged by single photon emission computed tomography (SPECT). Red represents relative increases in ictal compared to interictal CBF on SPECT scans, and green represents decreases. Despite the clinically generalized convulsions, focal relative signal increases are present in higher-order frontal and parietal association cortex, while many other brain regions are relatively spared. Ictal versus interictal SPECT images were analyzed with statistical parametric mapping. Reproduced with permission from Blumenfeld et al. (2003b). See Plate 20.1 in Colour Plate Section.
The most “pure” example of impaired consciousness in epilepsy is absence seizures. Like generalized tonic-clonic seizures, absence seizures (also called petit mal) are classified as generalized. However, in absence seizures the impaired consciousness is accompanied by relatively few additional motor or other complicating phenomena (like those seen in tonic-clonic and complex partial seizures).
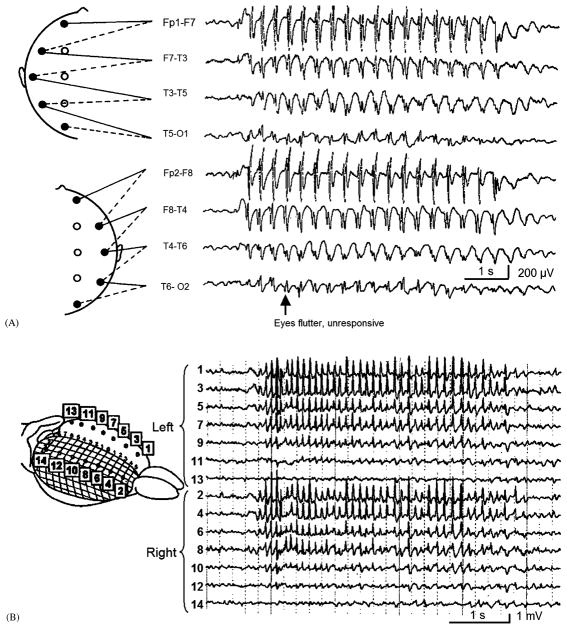
Typical absence seizures consist of brief episodes of staring and unresponsiveness, often accompanied by mild eyelid fluttering or myoclonic jerks. Duration is usually less than 10 s, and no obvious deficits are present after the seizure concludes, apart from the “missed time” during the seizure. Absence seizures are most common in childhood, and can occur up to hundreds of times per day, leading to impaired school performance. Electroencephalogram (EEG) recordings during typical absence seizures reveal large amplitude bilateral 3–4 Hz spike-wave discharges (Fig. 2). In atypical forms of absence seizures, and in absence status epilepticus, consciousness may be preserved, and the EEG usually reveals a slower or more irregular spike-wave pattern. In this review we will focus on typical absence seizures, and on the impaired consciousness accompanying these episodes. It should be noted, however, that typical and atypical absence are not always clearly distinct categories, and in fact lie along the same diagnostic spectrum (Holmes et al., 1987).
Fig. 2.
Typical spike-wave EEG discharges during absence seizures have a focal frontal predominance. (A) EEG recording from a 7-year-old girl during a typical absence seizure reveals bilateral synchronous 3–4 Hz spike-wave discharges, with an anterior to posterior amplitude gradient. (Inset of electrode positions modified with permission from Fisch, B.J. (1991) Spehlmann’s EEG Primer. Elsevier, Amsterdam. EEG recording modified with permission from Daly, D.D. and Pedley, T.A. (1990) Current Practice of Clinical Electroencephalography. Raven Press, New York.) (B) Electrocorticography from the surface of the WAG/Rij rat cortex during spike-and-wave seizures reveals intense involvement of the anterior cortex and relative sparing of the occipital lobes. (Reprinted with permission from Meeren et al., 2002. Copyright 2002, Society for Neuroscience.)
Numerous human and animal studies have suggested that absence seizures are generated through abnormal network oscillations involving both the cortex and thalamus (Williams, 1953; Avoli et al., 1990; Blumenfeld and McCormick, 2000; Kostopoulos, 2001; McCormick and Contreras, 2001; Blumenfeld, 2002; Crunelli and Leresche, 2002; Blumenfeld 2003). Despite much work, it is still not known with certainty whether absence seizures are generated through an overall increase or decrease in neuronal activity in cortical and subcortical circuits (Engel et al., 1985; Salek-Haddadi et al., 2003; Aghakhani et al., 2003; Nersesyan et al., 2004a, b). In addition, even in typical absence seizures there is substantial variability in the degree of impaired consciousness from one patient to another, and even from one episode to the next in the same patient. The cause of this variability is not known.
To understand the fundamental mechanisms for impaired consciousness in absence seizures it will be necessary to obtain a more detailed understanding of both the physiological changes in the brain and the specific cognitive deficits that occur during these events. As we will discuss, prior studies have begun to investigate the anatomy and physiology of absence seizures, and altered behavior during these events, but little work has been done to directly relate impaired brain function to behavior. For example, can the variability in impaired consciousness during absence seizures be explained by variability in brain dysfunction during these episodes? Are there specific anatomical regions of the brain that are involved or spared when patients do or do not show impaired responsiveness during absence seizures? Is the impaired brain function caused by abnormally increased activity, abnormally decreased activity, or both? What are the molecular and circuit mechanisms causing specific regions but not others to be involved during seizures? We will now review prior work on the physiology and behavior of typical human absence seizures, and highlight areas that need to be addressed through further investigations. We mention atypical absence, and animal models only briefly, as these are reviewed in detail elsewhere (Holmes et al., 1987; Avoli et al., 1990; Yaqub, 1993; Crunelli and Leresche, 2002; Coenen and Van Luijtelaar, 2003).
Electrophysiology
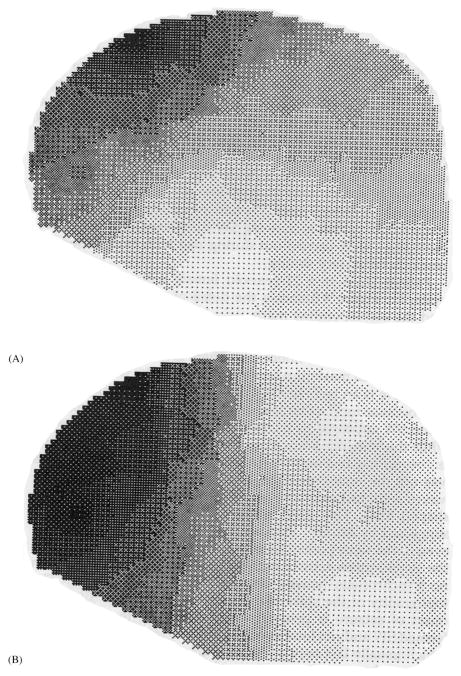
The characteristic 3–4 Hz spike-wave discharge of absence seizures was first described by Gibbs and colleagues in 1935 (Gibbs et al., 1935), just 6 years after Berger (1929) developed human EEG. Since then, countless recordings have been made of these discharges, which are usually bilaterally symmetric, but with an occasional left or right amplitude preponderance (Ebersole and Pedley, 2003). Although considered a form of generalized epilepsy, it has long been appreciated that the amplitude of spike-wave discharges on scalp EEG is not uniform in all electrodes. In most cases, there is a clear bifrontal amplitude maximum, greatest in the midline (Figs. 2A and 3) (Weir, 1965; Rodin and Ancheta, 1987; Coppola, 1988; Holmes et al., 2004). The focal electrographic distribution of spike-wave discharge is also supported by recent studies in rodent models of absence seizures (Vergnes et al., 1990; Meeren et al., 2002; Nersesyan et al., 2004a, b). In agreement with human scalp EEG recordings, invasive recordings from rodent models show bilateral spike-wave discharges of maximal amplitude in the anterior regions, sparing the occipital cortex (Fig. 2B). Thalamic nuclei corresponding to the anterior cortical regions are intensely involved, while the thalamic lateral geniculate nuclei (with connections to the occipital cortex), are spared (Nersesyan et al., 2004b). Kostopoulos and others (Kostopoulos, 2001; Blumenfeld and Taylor, 2003) have proposed that focal involvement of specific thalamocortical sectors (e.g. in the frontal cortex), during absence seizures may cause selective deficits during absence seizures, affecting some cognitive modalities, and sparing others. To more fully investigate this hypothesis in humans, noninvasive methods with higher spatial resolution than scalp EEG, such as neuroimaging, will be needed. However, it is first necessary to understand in as much detail as possible the specific cognitive deficits caused by absence seizures.
Fig. 3.
Topographic mapping showing frontal predominance of spike-wave discharges in absence. Instantaneous EEG amplitude maps were generated from recordings during a generalized spike-wave complex in a patient with typical absence seizures (petit mal). (A) Amplitude map at the peak of the spike. (B) Amplitude map at the peak of the wave. Increased gray scale indicates greater negativity relative to the reference. Reproduced with permission from Coppola (1988).
Behavior
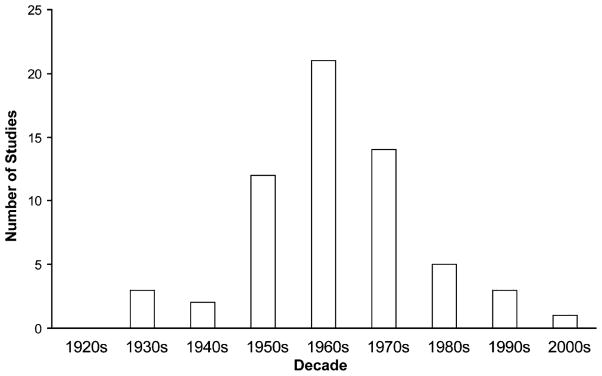
There has been an interesting historical trend in behavioral studies of impaired consciousness during absence seizures (Fig. 4). These studies, initiated by Schwab and Jung in the late 1930s (Jung, 1939; Schwab, 1939), reached a peak in the 1960s, and then dropped off precipitously, with hardly any published investigations in the last two decades. The rise in behavioral studies of absence seizures can easily be explained by the development of EEG (Berger, 1929), improved quantitative testing methods, and increasing interest in experimental psychology. However, the cause for the decline is more elusive, especially given the explosion of interest in cognitive neuroscience and consciousness in recent years. One possible explanation is the development of effective anti-absence medications such as ethosuximide in 1960 and valproate in 1978, leading to a smaller patient pool with uncontrolled episodes to study. However, studies should still be possible using patients with newly diagnosed absence, or patients who are unable to tolerate therapeutic doses of these medications. Another, more intriguing possibility is that studies on impaired consciousness in absence stopped because most questions were answered within the technical limitations of the times. In other words, once the major behavioral features of absence seizures were described, and their relationship to EEG discharges measured, there was little novel work left to be done until better methods for studying brain function were developed. As we discuss in the next section, it is our hope that this “forgotten science” will be reinvigorated now that modern functional neuroimaging and other methods are available.
Fig. 4.
Historical trends in behavioral studies of impaired consciousness in absence seizures. Number of studies on impaired consciousness during absence seizures were tabulated for each decade, peaking in the 1960s, and then showing a sharp drop-off, with few studies in recent years. References were found using medline search or based on citation in other articles, and then verified by reviewing content directly. Only original articles that investigated behavioral impairments during clinical absence seizures or subclinical generalized 3–4 Hz spike-wave discharges have been included.
Meanwhile, there remains much to be learned from the first wave of behavioral studies of absence seizures. These investigations utilized a variety of techniques to assess patients’ cognitive function during absence seizures. Because the literature in this field is finite, an effort has been made to include all or nearly all pertinent studies in this review. Some of the studies have significant methodological limitations, including heterogeneous patient types, limited sample size, and descriptive rather than quantitative analysis. Nevertheless, the majority provide useful data, allowing some preliminary conclusions to be drawn, which may spark future research. We will now review three common themes in these studies: (1) Which cognitive functions are involved and which preserved during absence seizures? (2) What is the timing of deficits relative to spike-wave discharges on EEG? (3) Is the variability in impaired consciousness from one episode to the next related to any properties of the EEG?
Which cognitive functions are impaired during absence?
A variety of different testing paradigms have been used to study impaired consciousness during absence seizures (Table 1). In fact, the different tests used illustrate that several cognitive functions may contribute to consciousness. There has been considerable dispute in the absence literature, whether impairment of some cognitive functions with sparing of others, qualifies as impaired consciousness. Does a patient who appears normal and is able to talk, but has transient slowing of reaction times during spike-wave episodes, have impaired consciousness? On the other extreme, can a patient who is completely unresponsive and has no recall afterwards of episodes be considered unconscious, or is the patient simply immobile, mute, and amnestic due to selective motor, verbal, and memory impairment (Gloor, 1986)? Ultimately, to answer questions of this kind, a more specific, mechanistic definition of consciousness is needed, along with a better understanding of the underlying physiological processes. Until this is achieved, and perhaps as a means of achieving such a definition, it is useful to study each of the specific cognitive processes that may contribute to loss of consciousness.
Table 1.
Tests used to asses consciousness during absence seizuresa
Tests are arranged in approximate order of impairment during absence seizures. Thus, tests most spared during absence seizures are listed towards the top, while those most impaired by absence seizures are listed towards the bottom.
Testing paradigms during absence seizures have varied from simple repetitive motor tasks to higher order decision making (Table 1). Some functions are more severely impaired than others during absence seizures. The tests in Table 1 are listed in approximate order of impairment by absence seizures. For example, several investigators reported that simple repetitive motor tasks such as finger tapping are more often conserved than more demanding tasks, such as counting aloud, and impairment is even more common with tasks requiring a response to verbal questions (Schwab, 1939; Cornil et al., 1951; Courtois et al., 1953; Gastaut, 1954; Goldie and Green, 1961; Davidoff and Johnson, 1964). Decision making based on sensory input, and complex motor performance were more severely affected than simpler tests (Guey et al., 1965; Mirsky and Buren, 1965). Furthermore, impaired memory of items presented during seizures depended on task difficulty (Jus and Jus, 1962; Mirsky and Buren, 1965; Geller and Geller, 1970). Interestingly, while verbal responses were very often interrupted during seizures, some patients recalled questions or commands given during a seizure, and responded appropriately after the seizure ended (Courtois et al., 1953; Kooi and Hovey, 1957; Boudin et al., 1958). In addition, while some patients were unable to respond verbally, they were able to turn their gaze toward the examiner (Goldie and Green, 1961). Some investigators also found that absence seizures could be terminated by external stimulation, with loud or painful stimuli being most effective (Jung, 1939, 1954; Boudin et al., 1958), and seizure frequency was diminished by tasks requiring active mental attention (Lennox et al., 1936; Gastaut, 1954; Kooi and Hovey, 1957; Davidoff and Johnson, 1964; Bureau et al., 1968). Another interesting observation was that even “subclinical” (also called “larval” or “phantom”) spike-wave discharges, with no obvious clinical impairment, were often associated with more subtle deficits in reaction time or continuous motor performance (Schwab, 1939; Kooi and Hovey, 1957; Yeager and Guerrant, 1957; Tuvo, 1958; Grisell et al., 1964; Guey et al., 1965).
In summary, these findings suggest that absence seizures can independently affect some cognitive functions that may be important for consciousness, while sparing certain others. The mechanism for this remains unknown, but it can be hypothesized that selective cognitive deficits may be related to selective impairment of specific brain networks during seizures, while others are spared.
What is the timing of impairments during absence?
On the basis of verbal response to questions and motor tests, it was observed that patients tend to have little impairment in the initial 1–3 s and final 3–5 s of absence seizures, but enter a “trough” of impaired consciousness in between (Shimazono et al., 1953; Goldie and Green, 1961; Mirsky and Buren, 1965; Goode et al., 1970). On the other hand, some investigators reported on the basis of vigilance or reaction time tasks that impairment coincides with electrographic seizure onset (Browne et al., 1974), or may even occur a few seconds before the onsets (Mirsky and Buren, 1965). In addition, although impairment of vigilance (continuous performance task) was reported to end about 5 s before the end of seizures, impaired simple tapping and sometimes responses to verbal questions have been reported to be impaired for several seconds after the end of the electrographic seizure (Goldie and Green, 1961; Mirsky and Buren, 1965). The different timing effects in these studies may again reflect the different tasks and methods used. Thus, some cognitive functions may be more severely affected during certain phases of seizures, while others are left relatively unharmed. This, once again, supports the notion that absence seizures dynamically disrupt specific functional brain networks, while sparing others.
There has also been some variability in reports of the timing of amnesia during absence seizures. Thus, some investigators reported no retrograde amnesia for stimuli presented prior to seizure onset (Courtois et al., 1953), while others reported retrograde amnesia extending up to 14 s before seizure onset, with gradual shrinking of the retrograde amnesia after the seizure ended (Jus and Jus, 1962; Mirsky and Buren, 1965; Geller and Geller, 1970).
Are EEG duration, amplitude, or any other features related to degree of impairment?
One important common theme in all these studies is that impaired responsiveness is not a monolithic feature of absence seizures, but rather highly variable between patients, and even from one episode to the next within a single patient. The mechanism for this variability is of great potential importance for understanding the fundamental mechanisms of impaired consciousness in absence seizures. Efforts have been made to relate certain EEG features to the degree of impairment. This subject has also been a matter of some controversy among investigators, most likely because of varying testing methods and cognitive tasks that were employed.
Longer seizure durations, especially those lasting more than 3–4 s are reported to cause more severe impairments in simple reaction time, continuous performance task, memory testing, and continuous motor performance (Schwab, 1939; Guey et al., 1965; Mirsky and Buren, 1965; Goode et al., 1970). Some impairment can be seen on careful reaction time testing even with brief spike-wave discharges (Browne et al., 1974). However, the impairment in this case is more obvious and lasts longer with longer duration discharges. Amplitude (Courtois et al., 1953) and “generalization” of discharges were associated with worse performance on reaction time and other tasks (Sellden, 1971; Porter and Penry, 1973; Browne et al., 1974). Mirsky and Van Buren conducted a detailed investigation of EEG characteristics, and found that spike-wave duration, amplitude, rhythmicity, and fronto-central distribution were all associated with more severe impaired performance on vigilance and memory testing (Mirsky and Buren, 1965). A recent small case series suggested different EEG spike-wave distributions during impaired or preserved consciousness (Vuilleumier et al., 2000). Other investigators, however, found no association between EEG duration (Browne et al., 1974), or any other EEG features with impaired consciousness (Gastaut, 1954).
The majority of these studies suggest that an association exists between certain EEG features, and impaired performance on cognitive tasks contributing to impaired consciousness in absence. However, the precise anatomical and physiological source of this variability in EEG and behavioral impairment will require further investigation with more advanced methods.
Neuroimaging and molecular mapping
Human imaging studies during absence seizures have been highly controversial. Some studies have shown global increases in cerebral metabolism or blood flow (Engel et al., 1982, 1985; Theodore et al., 1985; Prevett et al., 1995; Diehl et al., 1998; Yeni et al., 2000), while others have shown no change, focal or generalized increases or decreases (Theodore et al., 1985; Ochs et al., 1987; Park et al., 1994; Ferrie et al., 1996; Diehl et al., 1998; Salek-Haddadi et al., 2003; Aghakhani et al., 2003; Archer et al., 2003). Some of these variabilities may reflect technical limitations, such as limited temporal resolution of fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) or Tc99 m single photon emission computed tomography (SPECT) imaging relative to absence seizure duration. In addition, the variability may result from heterogeneous patient populations, and the inclusion in these studies of pediatric and adult patients along with variable numbers of patients with atypical absence seizures.
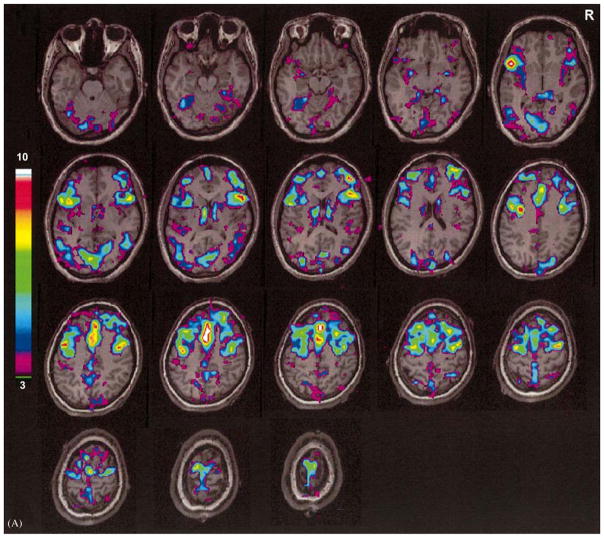
Because absence episodes are relatively brief, it is crucial to simultaneously record EEG with neuroimaging so that images acquired during seizures and baseline can be identified and analyzed separately. In addition, an imaging modality with sufficient temporal resolution to capture individual absence seizures should be used. Functional magnetic resonance imaging (fMRI) fulfils both of these criteria, and several recent studies have begun to show interesting results with this method (Aghakhani et al., 2003; Archer et al., 2003; Salek-Haddadi et al., 2003). Interestingly, fMRI changes during absence seizures include both increases and decreases in signal, and these changes are heterogeneous, not involving the entire brain in a uniform fashion (Fig. 5). These findings agree with behavioral and EEG studies suggesting that absence seizures may selectively involve certain networks, while sparing others. Additional neuroimaging studies with improved spatial and temporal resolution will be needed to investigate the possible role of focal brain dysfunction in loss of consciousness during absence seizures. In addition, fMRI studies are limited because the signals measured are only indirectly related to neuronal activity. Further work will be needed to fully interpret the relationship of fMRI signal increases and decreases during absence seizures with the underlying changes in neuronal activity (Smith et al., 2002; Nersesyan et al., 2004b).
Fig. 5.
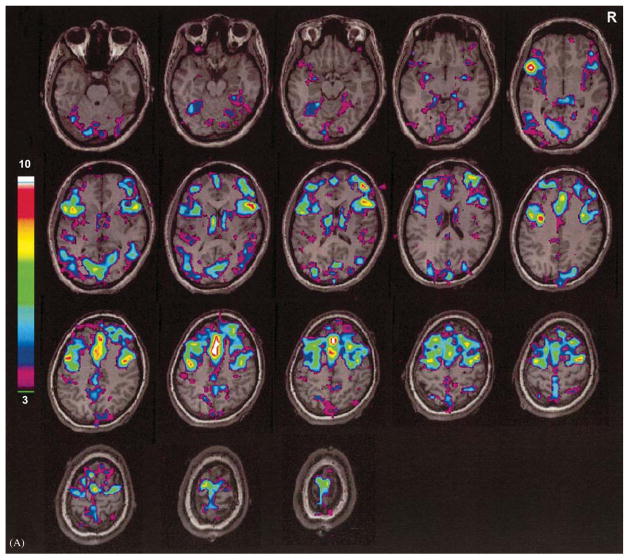
fMRI during human absence seizures. 18-year-old patient with onset of absence seizures at age 8. EEG and fMRI were recorded simultaneously. (A) fMRI activation. (B) fMRI deactivation. Focal regions of increased and decreased signal are seen bilaterally. Color bars show t scores. Reproduced with permission from Aghakhani et al. (2003). See Plate 20.5 in Colour Plate Section.
Studies in animal models may also shed light on mechanisms of spatial heterogeneity in spike-wave seizures (Blumenfeld, 2003). Recent studies on rodent models on absence seizures have demonstrated that, as in humans, seizure discharges often have an anterior predominance, with the posterior brain regions relatively spared (Vergnes et al., 1990; Meeren et al., 2002; Klein et al., 2004; Nersesyan et al., 2004a, b) (Fig. 2B). These investigations have included electrophysiologic, fMRI, and Doppler flow measurements, all implying that spike-wave seizures that appear fairly generalized on scalp EEG recordings, in fact, may intensely involve some corticothalamic networks, while sparing others.
Ultimately, it will be crucial to identify the underlying mechanisms that cause certain corticothalamic networks to be intensely involved in spike-wave seizures, while others remain functional. Many factors can contribute to altered excitability and synchronous firing of corticothalamic networks, and only a few candidate molecules and mechanisms have begun to be explored (Crunelli and Leresche, 2002). It was recently found in a rodent model of absence seizures that enhanced excitability in focal cortical regions may be related to altered expression of voltage gated sodium channels (Klein et al., 2004) or to reduced function of hyperpolarization-activated cation channels (Ih) (Strauss et al., 2004). If the specific genes and proteins can be identified, which generate absence seizures in selective brain regions, but not others, it may ultimately be possible to map networks predisposed to seizure discharges using molecular techniques. Furthermore, identification of these molecular changes will allow targeted therapies aimed at reducing abnormal excitability in these networks, and to prevent absence seizures and the accompanying loss of consciousness.
Conclusions
Loss of consciousness during absence seizures may serve as a good model system for studying normal mechanisms of consciousness. Impairments in consciousness during absence seizures are dynamic and rapidly reversible, and are relatively selective, without many of the motor or other manifestations seen in other seizure types. Several converging lines of evidence suggest that impaired consciousness during absence seizures is not a global phenomenon, but rather can be decomposed into specific deficits in a number of different cognitive functions, associated with impaired function in selective neuroanatomical networks. On the basis of the studies reviewed here, we hypothesize that loss of consciousness in absence seizures is not an “all-or-none” phenomenon resulting from involvement of the entire brain in the seizure discharge. Rather, focal involvement of the bilateral association cortex, perhaps most prominently in frontal neocortex and related subcortical structures, disrupts normal information processing in these brain regions leading to impairment of specific cognitive functions necessary for normal consciousness. The detailed spatial distribution of these changes, the physiological effects of spike-wave discharges on normal neuronal signaling, the molecular and cellular mechanisms underlying selective network involvement, and the relationship of these changes to behavior will need additional investigation.
Plate 20.1.
Fronto-parietal cortical involvement in generalized tonic-clonic seizures. Generalized tonic-clonic seizures were induced by electroconvulsive therapy for treatment of refractory depression (American Psychiatric Association, 2001) and cerebral blood flow (CBF) was imaged by single photon emission computed tomography (SPECT). Red represents relative increases in ictal compared to interictal CBF on SPECT scans, and green represents decreases. Despite the clinically generalized convulsions, focal relative signal increases are present in higher-order frontal and parietal association cortex, while many other brain regions are relatively spared. Ictal versus interictal SPECT images were analyzed with statistical parametric mapping. Reproduced with permission from Blumenfeld et al. (2003b)..
Plate 20.5.
fMRI during human absence seizures. 18-year-old patient with onset of absence seizures at age 8. EEG and fMRI were recorded simultaneously. (A) fMRI activation. (B) fMRI deactivation. Focal regions of increased and decreased signal are seen bilaterally. Color bars show t scores. Reproduced with permission from Aghakhani et al. (2003).
Acknowledgments
I gratefully thank Dr. Alain Hyman, Dr. David L. Blumenfeld, and Dr. Ulrich Schridde for translating of papers from French and German literature. I also thank George Varghese for assistance in preparing the figures. This work was supported by NIH R01 NS049307 and by the Betsy and Jonathan Blattmachr family.
References
- Aarts JHP, Binnie CD, Smit AM, Wilkins AJ. Selective cognitive impairment during focal and generalized epileptiform EEG activity. Brain. 1984;107:293–308. doi: 10.1093/brain/107.1.293. [DOI] [PubMed] [Google Scholar]
- Aghakhani Y, Bagshaw AP, Benar CG, Hawco C, Andermann F, Dubeau F, Gotman J. fMRI activation during spike- and wave-discharges in idiopathic generalized epilepsy. Brain. 2003;127:1127–1144. doi: 10.1093/brain/awh136. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association CoET. The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging. 2. American Psychiatric Association; Washington, DC: 2001. [Google Scholar]
- Archer JS, Abbott DF, Waites AB, Jackson GD. fMRI “deactivation” of the posterior cingulate during generalized spike and wave. Neuroimage. 2003;20:1915–1922. doi: 10.1016/s1053-8119(03)00294-5. [DOI] [PubMed] [Google Scholar]
- Avoli M, Gloor P, Kostopoulos G, Naquet T, editors. Generalized Epilepsy. Birkhauser; Basel, Boston: 1990. [Google Scholar]
- Bates JAV. A technique for identifying changes in consciousness. Electroen Clin Neuro. 1953;5:445–446. doi: 10.1016/0013-4694(53)90088-1. [DOI] [PubMed] [Google Scholar]
- Bell WL, Walczak TS, Shin C, Radtke RA. Painful generalised clonic and tonic-clonic seizures with retained consciousness. J Neurol Neurosur Ps. 1997;63:792–795. doi: 10.1136/jnnp.63.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. Ueber das Elektrenkephalogramm des Menschen. Arch Psychiat. 1929;87:527. [Google Scholar]
- Binnie CD, Lloyd DSL. A technique for measuring reaction times during paroxysmal discharges. Electroen Clin Neuro. 1973;35:420. [Google Scholar]
- Blumenfeld H. The thalamus and seizures. Arch Neurol. 2002;59:135–137. doi: 10.1001/archneur.59.1.135. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H. From molecules to networks: cortical/subcortical interactions in the pathophysiology of idiopathic generalized epilepsy. Epilepsia. 2003;44(2):7–15. doi: 10.1046/j.1528-1157.44.s.2.2.x. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, McCormick DA. Corticothalamic inputs control the pattern of activity generated in thalamo-cortical networks. J Neurosci. 2000;20:5153–5162. doi: 10.1523/JNEUROSCI.20-13-05153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H, McNally KA, Ostroff R, Zubal IG. Targeted prefrontal cortical activation with bifrontal ECT. Psychiat Res Neuroimag. 2003a;123:165–170. doi: 10.1016/s0925-4927(03)00073-8. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, McNally KA, Vanderhill SD, Paige AL, Chung R, Davis K, Norden AD, Stokking R, Studholme C, Novotny EJ, Zubal IG, Spencer SS. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex. 2004b;14:892–902. doi: 10.1093/cercor/bhh048. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Rivera M, McNally KA, Davis K, Spencer DD, Spencer SS. Ictal neocortical slowing in temporal lobe epilepsy. Neurology. 2004a;63:1015–1021. doi: 10.1212/01.wnl.0000141086.91077.cd. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Taylor J. Why do seizures cause loss of consciousness? The Neuroscientist. 2003;9:301–310. doi: 10.1177/1073858403255624. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Westerveld M, Ostroff RB, Vanderhill SD, Freeman J, Necochea A, Uranga P, Tanhehco T, Smith A, Seibyl JP, Stokking R, Studholme C, Spencer SS, Zubal IG. Selective frontal, parietal and temporal networks in generalized seizures. Neuroimage. 2003b;19:1556–1566. doi: 10.1016/s1053-8119(03)00204-0. [DOI] [PubMed] [Google Scholar]
- Boudin G, Barbizet J, Masson S. Etude de la dissolution de la conscience dans 3 cas de petit mal avec crises prolongees. Rev Neurol. 1958;99:483–487. [Google Scholar]
- Browne TR, Penry JK, Porter RJ, Dreifuss FE. Responsiveness before during and after spike-wave paroxysms. Neurology. 1974;24:659–665. doi: 10.1212/wnl.24.7.659. [DOI] [PubMed] [Google Scholar]
- Bureau M, Guey J, Dravet C, Roger J. A study of the distribution of petit mal absences in the child in relation to his activities. Electroen Clin Neuro. 1968;25:513. [PubMed] [Google Scholar]
- Chatrian GE, Lettich E, Miller LH, Green JR, Kupfer C. Pattern-sensitive epilepsy. 2 Clinical changes, tests of responsiveness and motor output, alterations of evoked potenials and therapeutic measures. Epilepsia. 1970;11:151–162. doi: 10.1111/j.1528-1157.1970.tb03877.x. [DOI] [PubMed] [Google Scholar]
- Coenen AM, Van Luijtelaar EL. Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav Genet. 2003;33:635–655. doi: 10.1023/a:1026179013847. [DOI] [PubMed] [Google Scholar]
- Coppola R. Topographic display of spike-and-wave discharges. In: Mysobodsky MS, Mirsky AF, editors. Elements of Petit Mal Epilepsy. Peter Lang; New York: 1988. pp. 105–130. [Google Scholar]
- Cornil L, Gastaut H, Corriol J. Appreciation du degre de conscience au cours des paroxysmes epileptiques ‘petit mal’. Rev Neurol. 1951;84:149–151. [PubMed] [Google Scholar]
- Courtois GA, Ingvar DH, Jasper HH. Nervous and mental defects during petit mal attacks. Electroen Clin Neuro. 1953;(Suppl 3):87. [Google Scholar]
- Crunelli V, Leresche N. Childhood absence epilepsy: genes channels neurons and networks. Nat Rev Neurosci. 2002;3:371–382. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- Davidoff RA, Johnson LC. Paroxysmal EEG activity and cognitive-motor performance. Electroen Clin Neuro. 1964;16:343–354. doi: 10.1016/0013-4694(64)90068-9. [DOI] [PubMed] [Google Scholar]
- Diehl B, Knecht S, Deppe M, Young C, Stodieck SR. Cerebral hemodynamic response to generalized spike-wave discharges. Epilepsia. 1998;39:1284–1289. doi: 10.1111/j.1528-1157.1998.tb01326.x. [DOI] [PubMed] [Google Scholar]
- Ebersole JS, Pedley TA. Current Practice of Clinical Electroencephalography. 3. Lippincott Williams & Wilkins; Philadelphia, PA: 2003. [Google Scholar]
- Engel J, Jr, Kuhl DE, Phelps ME. Patterns of human local cerebral glucose metabolism during epileptic seizures. Science. 1982;218:64–66. doi: 10.1126/science.6981843. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Lubens P, Kuhl DE, Phelps ME. Local cerebral metabolic rate for glucose during petit mal absences. Ann Neurol. 1985;17:121–128. doi: 10.1002/ana.410170204. [DOI] [PubMed] [Google Scholar]
- Fairweather H, Hutt SJ. Inter-relationships of EEG activity and information processing on paced and unpaced tasks in epileptic children. Electroen Clin Neuro. 1969;27:701. doi: 10.1016/0013-4694(69)91345-5. [DOI] [PubMed] [Google Scholar]
- Fernandez H, Robinson R, Taylor RR. A device for testing consciousness. Am J EEG Technol. 1967;7:77–78. [Google Scholar]
- Ferrie CD, Maisey M, Cox T, Polkey C, Barrington SF, Panayiotopoulos CP, Robinson RO. Focal abnormalities detected by 18FDG PET in epileptic encephalopathies [comment] Arch Dis Child. 1996;75:102–107. doi: 10.1136/adc.75.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischgold H. La conscience et ses modifications. Systemes de references en EEG clinique. Congres International des Sciences Neurologiques; 21–28 juillet; Bruxelles. 1957. pp. 181–208. [Google Scholar]
- Fischgold H, Mathis P. Polygraphies des modifications de la conscience. Electroen Clin Neuro. 1957;9:177. [Google Scholar]
- Gastaut H. The brain stem and cerebral electrogenesis in relation to consciousness. In: Delafresnaye JF, editor. Brain Mechanisms and Consciousness. Thomas, Springfield, ILH; 1954. pp. 249–283. [Google Scholar]
- Geller MR, Geller A. Brief amnestic effects of spike-wave discharges. Neurology (Section on Child Neurology, Abstract CN1) 1970;20:380–381. [PubMed] [Google Scholar]
- Gibbs FA, Davis H, Lennox WG. The electroencephalogram in epilepsy and in conditions of impaired consciousness. Arch Neurol Psychiatr. 1935;34:1133–1148. [Google Scholar]
- Gloor P. Consciousness as a neurological concept in epileptology: a critical review. Epilepsia. 1986;27:S14–S26. doi: 10.1111/j.1528-1157.1986.tb05737.x. [DOI] [PubMed] [Google Scholar]
- Gokygit A, Caliskan A. Diffuse spike-wave status of 9-year duration without behavioral change or intellectual decline. Epilepsia. 1995;36:210–213. doi: 10.1111/j.1528-1157.1995.tb00982.x. [DOI] [PubMed] [Google Scholar]
- Goldie L, Green JM. Spike-and-wave discharges and alterations of conscious awareness. Nature. 1961;191:200–201. doi: 10.1038/191200a0. [DOI] [PubMed] [Google Scholar]
- Goode DJ, Penry JK, Dreifuss FE. Effects of paroxysmal spike-wave on continuous visual-motor performance. Epilepsia. 1970;11:241–254. doi: 10.1111/j.1528-1157.1970.tb03888.x. [DOI] [PubMed] [Google Scholar]
- Grisell JL, Levin SM, Cohen BD, Rodin EA. Effects of subclinical seizure activity on overt behavior. Neurology. 1964;14:133–135. doi: 10.1212/wnl.14.2.133. [DOI] [PubMed] [Google Scholar]
- Guey J, Tassinari CA, Charles C, Coquery C. Variations du niveau d’efficience en relation avec des des-charges epileptiques paroxystiques. Rev Neurol. 1965;112:311–317. [PubMed] [Google Scholar]
- Hauser F. erception et responses motrices au cours des paroxysmes de pontesondes. Foulon; Paris: 1960. p. 52. [Google Scholar]
- Heikman P, Kalska H, Katila H, Sarna S, Tuunainen A, Kuoppasalmi K. Right unilateral and bifrontal electroconvulsive therapy in the treatment of depression: a preliminary study. J ECT. 2002;18:26–30. doi: 10.1097/00124509-200203000-00009. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Brown M, Tucker DM. Are “generalized” seizures truly generalized? Evidence of localized mesial frontal and frontopolar discharges in absence. Epilepsia. 2004;45(12):1568–1579. doi: 10.1111/j.0013-9580.2004.23204.x. [DOI] [PubMed] [Google Scholar]
- Holmes GL, McKeever M, Adamson M. Absence seizures in children: clinical and electroencephalographic features. Ann Neurol. 1987;21:268–273. doi: 10.1002/ana.410210308. [DOI] [PubMed] [Google Scholar]
- Hovey HB, Kooi KA. Transient disturbances of thought processes and epilepsy. AMA Arch Neurol Psy. 1955;74:287–291. doi: 10.1001/archneurpsyc.1955.02330150053007. [DOI] [PubMed] [Google Scholar]
- Hutt SJ. Experimental analysis of brain activity and behaviour in children with “minor” seizures. Epilepsia. 1972;13:520–534. doi: 10.1111/j.1528-1157.1972.tb04390.x. [DOI] [PubMed] [Google Scholar]
- Hutt SJ, Denner S, Newton J. Auditory thresholds during evoked spike-wave activity in epileptic patients. Cortex. 1976;12:249–257. doi: 10.1016/s0010-9452(76)80006-8. [DOI] [PubMed] [Google Scholar]
- Hutt SJ, Fairweather H. Spike-wave paroxysms and information processing. P Roy Soc Med. 1971a;64:918–919. [PMC free article] [PubMed] [Google Scholar]
- Hutt SJ, Fairweather H. Some effects of performance variables upon generalized spike-wave activity. Brain. 1971b;94:321–326. doi: 10.1093/brain/94.2.321. [DOI] [PubMed] [Google Scholar]
- Hutt SJ, Fairweather H. Paced and unpaced serial response performance during two types of EEG activity. J Neurol Sci. 1973;19:85–96. doi: 10.1016/0022-510x(73)90059-2. [DOI] [PubMed] [Google Scholar]
- Hutt SJ, Fairweather H. Information processing during two types of EEG activity. Electroen Clin Neuro. 1975;39:43–51. doi: 10.1016/0013-4694(75)90125-x. [DOI] [PubMed] [Google Scholar]
- Hutt SJ, Gilbert S. Effects of evoked spike-wave discharges upon short term memory in patients with epilepsy. Cortex. 1980;16:445–457. doi: 10.1016/s0010-9452(80)80045-1. [DOI] [PubMed] [Google Scholar]
- Hutt SJ, Newton J, Fairweather H. Choice reaction time and EEG activity in children with epilepsy. Neuropsychologia. 1977;15:257–267. doi: 10.1016/0028-3932(77)90034-3. [DOI] [PubMed] [Google Scholar]
- ILAE. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the commission on classification and terminology of the International League Against Epilepsy. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- Jung R. Uber vegetative Reaktionen und Hemmungswirkung von Sinnesreizen im kleinen epileptischen Anfall. Nervenarzt. 1939;12:169–185. [Google Scholar]
- Jung R. Correlation of biolectrical and autonomic phenomena with alterations of consciousness and arousal in man. In: Delafresnaye JF, editor. Brain Mechanisms and Consciousness. Thomas; Springfield, ILH: 1954. pp. 310–344. [Google Scholar]
- Jus A, Jus C. Etude electro-clinique des alterations de conscience dans le petit mal. Studii si cercetari de Neurol. 1960;5:243–254. [Google Scholar]
- Jus A, Jus K. Retrograde amnesia in petit mal. Arch Gen Psychiat. 1962;6:163–167. doi: 10.1001/archpsyc.1962.01710200055007. [DOI] [PubMed] [Google Scholar]
- Kakegawa Y. On the wave and spike formation of petit mal epilepsy. Folia Psychiat Neurol Jp. 1948;3:16–29. [Google Scholar]
- Klein JP, Khera DS, Nersesyan H, Kimchi EY, Waxman SG, Blumenfeld H. Dysregulation of sodium channel expression in cortical neurons in a rodent model of absence epilepsy. Brain Res. 2004;1000:102–109. doi: 10.1016/j.brainres.2003.11.051. [DOI] [PubMed] [Google Scholar]
- Kooi KA, Hovey HB. Alterations in mental function and paroxysmal cerebral activity. Arch Neurol Psychiatr. 1957;78:264–271. [PubMed] [Google Scholar]
- Kostopoulos GK. Involvement of the thalamocortical system in epileptic loss of consciousness. Epilepsia. 2001;42:13–19. doi: 10.1046/j.1528-1157.2001.042suppl.3013.x. [DOI] [PubMed] [Google Scholar]
- Lee KH, Meador KJ, Park YD, King DW, Murro AM, Pillai JJ, Kaminski RJ. Pathophysiology of altered consciousness during seizures: subtraction SPECT study. Neurology. 2002;59:841–846. doi: 10.1212/wnl.59.6.841. [DOI] [PubMed] [Google Scholar]
- Lehmann HJ. Praparoxysmale Weckreaktion bei pyknoleptischen Absencen. Arch Psychiatr Nervenkr. 1963;204:417–426. [PubMed] [Google Scholar]
- Lennox WG, Gibbs FA, Gibbs EL. Effect on the electroencephalogram of drugs and conditions which influence seizures. Arch Neurol Psychiatr. 1936;36:1236–1250. [Google Scholar]
- Mallin U, Stefan H, Penin H. Psychopathometrische Studien zum Verlauf epileptischer Absencen. Z EEG EMG. 1981;12:45–49. [PubMed] [Google Scholar]
- McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Ann Rev Physiol. 2001;63:815–846. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- McNally KA, Blumenfeld H. Focal network involvement in generalized seizures: new insights from electro-convulsive therapy. Epilepsy Behav. 2004;5:3–12. doi: 10.1016/j.yebeh.2003.10.020. [DOI] [PubMed] [Google Scholar]
- McNally KA, Davis K, Doernberg SB, Zubal IG, Spencer SS, Blumenfeld H. Cerebral blood flow changes during secondarily generalized tonic-clonic seizures. Soc Neurosci. 2004a Abstracts, Online at http://websfnorg/
- McNally KA, Davis K, Doernberg SB, Zubal IG, Spencer SS, Blumenfeld H. Epilepsia. 2004b. Ictal SPECT in secondarily generalized tonic-clonic seizures. AES abstracts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeren HKM, Pijn JPM, Van Luijtelaar ELJM, Coenen AML, da Silva FHL. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002;22:1480–1495. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel C, Grunwald F, Klemm E, Ruhlmann J, Elger CE, Biersack HJ. Inhibitory effects of mesial temporal partial seizures onto frontal neocortical structures. Acta Neurol Belgica. 1998;98:327–331. [PubMed] [Google Scholar]
- Milstein V, Stevens JR. Verbal and conditioned avoidance learning during abnormal EEG discharge. J Nerv Ment Dis. 1961;132:50–60. [PubMed] [Google Scholar]
- Mirsky AF, Buren JMV. On the nature of the “absence” in centrencephalic epilepsy: a study of some behavioral, electroencephalographic, and autonomic factors. Electroen Clin Neuro. 1965;18:334–348. doi: 10.1016/0013-4694(65)90053-2. [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Primac DW, Ajmone Marsan C, Rosvold HE, Stevens JR. A comparison of the psychological test performance of patients with focal nonfocal epilepsy. Exp Neurol. 1960;2:75–89. doi: 10.1016/0014-4886(60)90049-2. [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Rosvold HE. Behavioral and physiological studies in impaired attention. In: Zea V, editor. Psychopharmacological Methods: Proceedings of a Symposium on the Effects of Psychotropic Drugs on Higher Nervous Activity. Pergamon Press; Oxford: 1963. pp. 302–315. [Google Scholar]
- Mirsky A, Tecce J. The analysis of visual evoked potentials during spike-and-wave activity. Epilepsia. 1968;9:211–220. doi: 10.1111/j.1528-1157.1968.tb04621.x. [DOI] [PubMed] [Google Scholar]
- Nersesyan H, Hyder F, Rothman D, Blumenfeld H. Dynamic fMRI and EEG recordings during spike-wave seizures and generalized tonic-clonic seizures in WAG/Rij rats. J Cereb Blood Flow Metab. 2004a;24:589–599. doi: 10.1097/01.WCB.0000117688.98763.23. [DOI] [PubMed] [Google Scholar]
- Nersesyan H, Herman P, Erdogan E, Hyder F, Blumenfeld H. Relative changes in cerebral blood flow and neuronal activity in local microdomains during generalized seizures. J Cereb Blood Flow Metab. 2004b;24:1057–1068. doi: 10.1097/01.WCB.0000131669.02027.3E. [DOI] [PubMed] [Google Scholar]
- Norden AD, Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy Behav. 2002;3:219–231. doi: 10.1016/s1525-5050(02)00029-x. [DOI] [PubMed] [Google Scholar]
- Ochs RF, Gloor P, Tyler JL, Wolfson T, Worsley K, Andermann F, Diksic M, Meyer E, Evans A. Effect of generalized spike-and-wave discharge on glucose metabolism measured by positron emission tomography. Ann Neurol. 1987;21:458–464. doi: 10.1002/ana.410210508. [DOI] [PubMed] [Google Scholar]
- Opp J, Wenzel D, Brandl U. Visuomotor coordination during focal and generalized EEG discharges. Epilepsia. 1992;33:836–840. doi: 10.1111/j.1528-1157.1992.tb02191.x. [DOI] [PubMed] [Google Scholar]
- Panayiotopoulos CP, Chroni E, Daskalopoulos C, Baker A, Rowlinson S, Walsh P. Typical absence seizures in adults: clinical EEG video-EEG findings and diagnostic/syndromic considerations. J Neurol Neurosur Ps. 1992;55:1002–1008. doi: 10.1136/jnnp.55.11.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayiotopoulos C, Koutroumanidis M, Giannakodimos S, Agathonikou A. Idiopathic generalised epilepsy in adults manifested by phantom absences, generalised tonic-clonic seizures, and frequent absence status. J Neurol Neurosur Ps. 1997;63:622–627. doi: 10.1136/jnnp.63.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayiotopoulos CP, Obeid T, Waheed G. Differentiation of typical absence seizures in epileptic syndromes: a video-EEG study of 124 seizures in 20 patients. Brain. 1989;112:1039–1056. doi: 10.1093/brain/112.4.1039. [DOI] [PubMed] [Google Scholar]
- Park YD, Hoffman JM, Radtke RA, DeLong GR. Focal cerebral metabolic abnormality in a patient with continuous spike waves during slow-wave sleep. J Child Neurol. 1994;9:139–143. doi: 10.1177/088307389400900207. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Penry JK. Responsiveness at the onset of spike-wave bursts. Electroen Clin Neuro. 1973;34:239–245. doi: 10.1016/0013-4694(73)90251-4. [DOI] [PubMed] [Google Scholar]
- Prechtl HFR, Boeke PE, Schut T. The electroencephalogram and performance in epileptic patients. Neurology. 1961;11:296–304. doi: 10.1212/wnl.11.4.296. [DOI] [PubMed] [Google Scholar]
- Prevett MC, Duncan JS, Jones T, Fish DR, Brooks DJ. Demonstration of thalamic activation during typical absence seizures using H2(15)O and PET. Neurology. 1995;45:1396–1402. doi: 10.1212/wnl.45.7.1396. [DOI] [PubMed] [Google Scholar]
- Rodin E, Ancheta O. Cerebral electrical fields during petit mal absences. Electroen Clin Neuro. 1987;66:457–466. doi: 10.1016/0013-4694(87)90092-7. [DOI] [PubMed] [Google Scholar]
- Salek-Haddadi A, Lemieux L, Merschhemke M, Friston KJ, Duncan JS, Fish DR. Functional magnetic resonance imaging of human absence seizures. Ann Neurol. 2003;53:663–667. doi: 10.1002/ana.10586. [DOI] [PubMed] [Google Scholar]
- Schwab RS. Method of measuring consciousness in attacks of petit mal epilepsy. Arch Neurol Psychiat. 1939;41:215–217. (Society Transactions: Boston Society of Psychiatry and Neurology, presented May 19, 1938) [Google Scholar]
- Schwab RS. The influence of visual and auditory stimuli on the electroencephalographic tracing of petit mal. Amer J Pschiatr. 1941;97:1301–1312. [Google Scholar]
- Schwab RS. Reaction time in petit mal epilepsy. Res Publ Assoc Res Nerv Ment Dis. 1947:339–341. [Google Scholar]
- Sellden U. Psychotechnical performance related to paroxysmal discharges in EEG. Clin Electroencephal. 1971;2(1):18–27. [Google Scholar]
- Sengoku A, Kanazawa O, Kawai I, Yamaguchi T. Visual cognitive disturbance during spike-wave discharges. Epilepsia. 1990;31:47–59. doi: 10.1111/j.1528-1157.1990.tb05359.x. [DOI] [PubMed] [Google Scholar]
- Shimazono Y, Hirai T, Okuma T, Fukuda T, Yamamasu E. Disturbance of consciousness in petit mal epilepsy. Epilepsia. 1953;2:49–55. [Google Scholar]
- Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shulman RG, Hyder F. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. Proc Natl Acad Sci USA. 2002;99:10765–10770. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss U, Kole MH, Brauer AU, Pahnke J, Bajorat R, Rolfs A, Nitsch R, Deisz RA. An impaired neocortical Ih is associated with enhanced excitability and absence epilepsy. Eur J Neurosci. 2004;19:3048–3058. doi: 10.1111/j.0953-816X.2004.03392.x. [DOI] [PubMed] [Google Scholar]
- Theodore WH, Brooks R, Margolin R, Patronas N, Sato S, Porter RJ, Mansi L, Bairamian D, DiChiro G. Positron emission tomography in generalized seizures. Neurology. 1985;35:684–690. doi: 10.1212/wnl.35.5.684. [DOI] [PubMed] [Google Scholar]
- Tizard B, Margerison JH. Psychological functions during wave-spike discharges. Brit J Soc Clin Psychol. 1963a;3:6–15. [Google Scholar]
- Tizard B, Margerison JH. The relationship between generalized and paroxysmal EEG discharges and various test situations in two epileptic patients. J Neurol Neurosurg Psychiatry. 1963b;26:308–313. doi: 10.1136/jnnp.26.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuvo F. Contribution a l’etude des niveaux de conscience au cours des paroxysmes epileptiques infraclinique. Electroen Clin Neuro. 1958;10:715–718. doi: 10.1016/0013-4694(58)90076-2. [DOI] [PubMed] [Google Scholar]
- Van Paesschen W, Dupont P, Van Driel G, Van Billoen H, Maes A. SPECT perfusion changes during complex partial seizures in patients with hippocampal sclerosis. Brain. 2003;126:1103–1111. doi: 10.1093/brain/awg108. [DOI] [PubMed] [Google Scholar]
- Vergnes M, Marescaux C, Depaulis A. Mapping of spontaneous spike-and-wave discharges in Wistar rats with genetic generalized non-convulsive epilepsy. Brain Res. 1990;523:87–91. doi: 10.1016/0006-8993(90)91638-w. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Assal F, Blanke O, Jallon P. Distinct behavioral and EEG topographic correlates of loss of consciousness in absences. Epilepsia. 2000;41:687–693. doi: 10.1111/j.1528-1157.2000.tb00229.x. [DOI] [PubMed] [Google Scholar]
- Weir B. The morphology of the spike-wave complex. Electroen Clin Neuro. 1965;19:284–290. doi: 10.1016/0013-4694(65)90208-7. [DOI] [PubMed] [Google Scholar]
- Williams D. A study of thalamic and cortical rhythms in petit mal. Brain. 1953;76:50–69. doi: 10.1093/brain/76.1.50. [DOI] [PubMed] [Google Scholar]
- Yaqub BA. Electroclinical seizures in Lennox-Gastaut syndrome. Epilepsia. 1993;34:120–127. doi: 10.1111/j.1528-1157.1993.tb02383.x. [DOI] [PubMed] [Google Scholar]
- Yeager CL, Guerrant JS. Subclinical epileptic seizures; impairment of motor performance and derivative difficulties. Calif Med. 1957;86:242–247. [PMC free article] [PubMed] [Google Scholar]
- Yeni SN, Kabasakal L, Yalcinkaya C, Nisli C, Dervent A. Ictal and interictal SPECT findings in childhood absence epilepsy. Seizure. 2000;9:265–269. doi: 10.1053/seiz.2000.0400. [DOI] [PubMed] [Google Scholar]