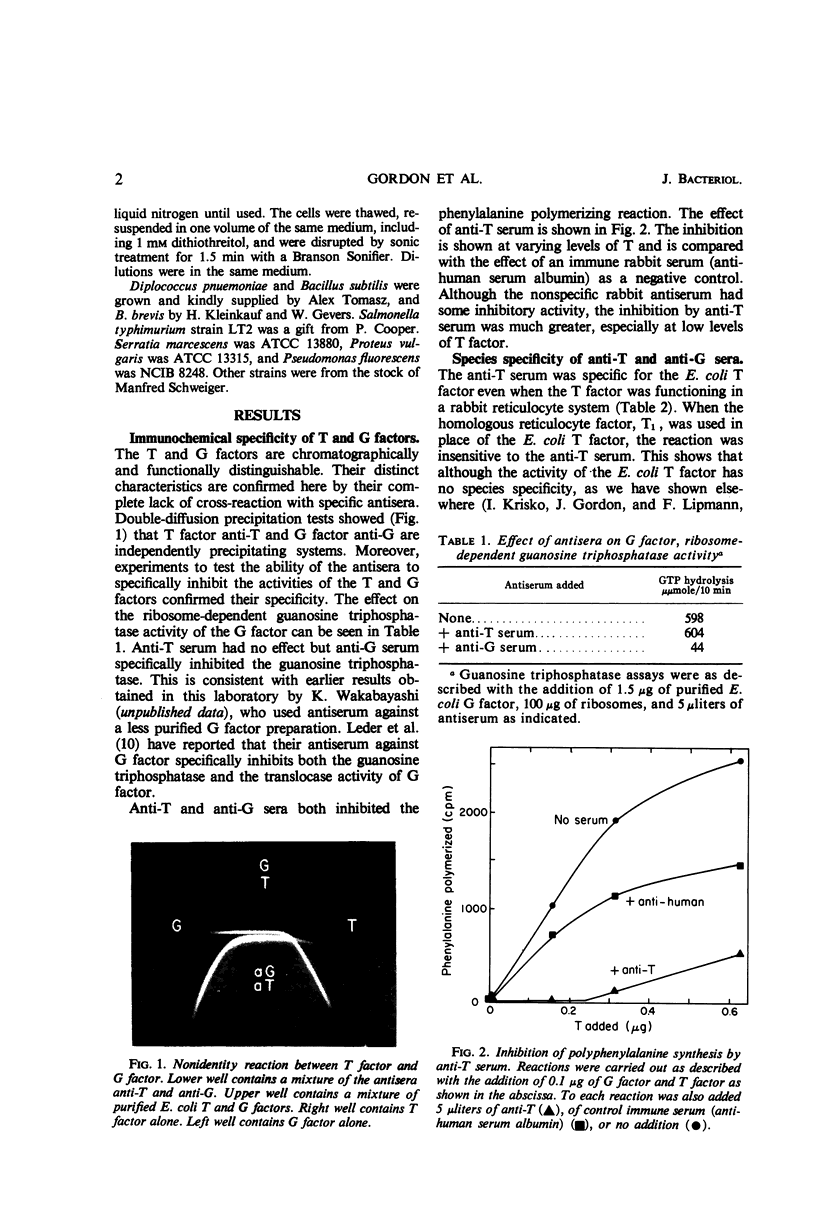
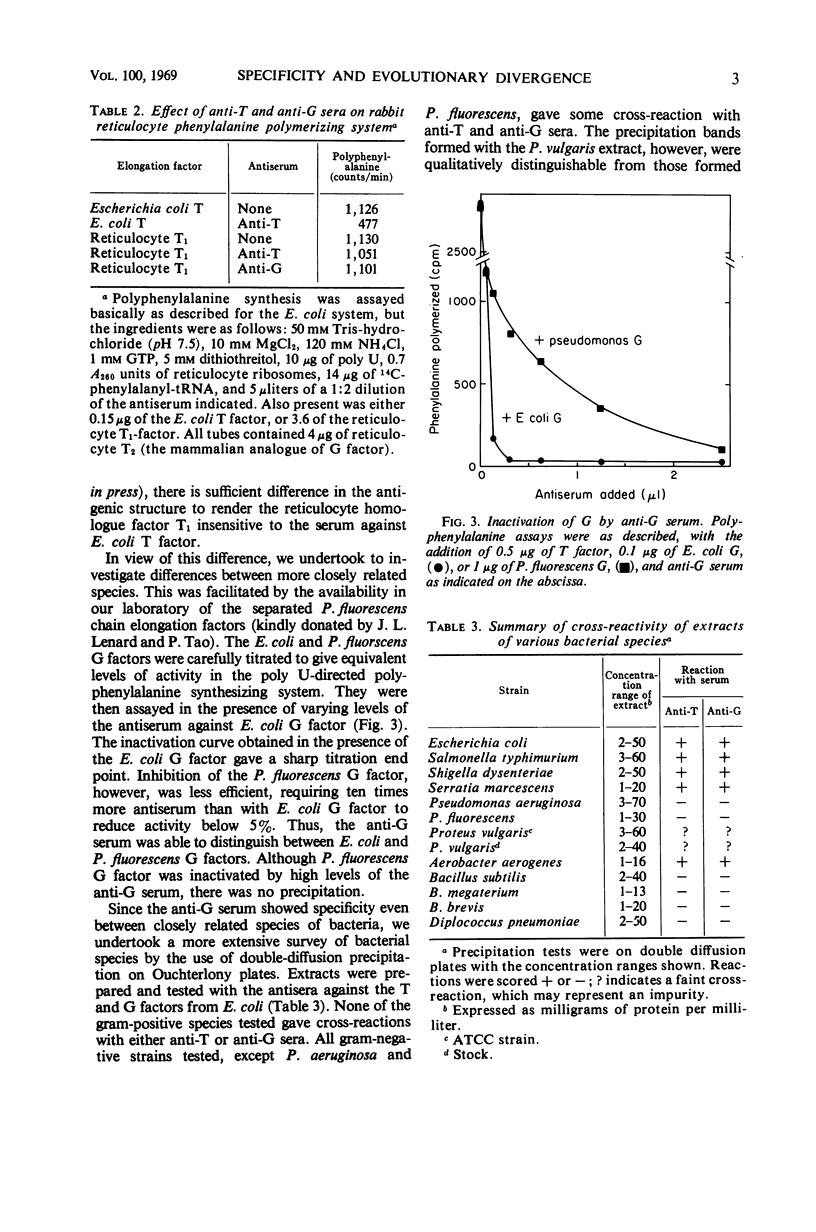
Abstract
Antisera have been prepared against two electrophoretically homogeneous “polypeptide chain elongation factors,” T and G, from Escherichia coli. Inactivation and precipitation tests showed that these two fractions were antigenically distinct with no cross-reaction. The immune inactivation curve of G factor from E. coli was distinguishable from that of G factor from Pseudomonas fluorescens. Mammalian factors were not inhibited by antibody directed against the E. coli T and G factors. By Ouchterlony diffusion tests, the antisera also detected significant antigenic variability among different bacterial species. It is concluded that the factors have undergone considerable evolutionary divergence in their antigenic structure.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brot N., Ertel R., Weissbach H. Effect of a soluble transfer factor on the reaction of aminoacyl-tRNA with puromycin. Biochem Biophys Res Commun. 1968 May 23;31(4):563–570. doi: 10.1016/0006-291x(68)90515-9. [DOI] [PubMed] [Google Scholar]
- Ciferri O., Parisi B., Perani A., Grandi M. Different specificity of yeast transfer enzymes for Escherichia coli ribosomes. J Mol Biol. 1968 Nov 14;37(3):529–533. doi: 10.1016/0022-2836(68)90121-6. [DOI] [PubMed] [Google Scholar]
- Erbe R. W., Leder P. Initiation and protein synthesis: translation of di- and tri-codon messengers. Biochem Biophys Res Commun. 1968 Jun 10;31(5):798–803. doi: 10.1016/0006-291x(68)90633-5. [DOI] [PubMed] [Google Scholar]
- Ertel R., Brot N., Redfield B., Allende J. E., Weissbach H. Binding of guanosine 5'-triphosphate by soluble factors required for polypeptide synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):861–868. doi: 10.1073/pnas.59.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Gordon J. A stepwise reaction yielding a complex between a supernatant fraction from E. coli, guanosine 5'-triphosphate, and aminoacyl-sRNA. Proc Natl Acad Sci U S A. 1968 Jan;59(1):179–183. doi: 10.1073/pnas.59.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenni A. L., Lucas-Lenard J. Stepwise synthesis of a tripeptide. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1363–1369. doi: 10.1073/pnas.61.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaziro Y., Inoue N. Crystalline G factor from Escherichia coli. J Biochem. 1968 Sep;64(3):423–425. doi: 10.1093/oxfordjournals.jbchem.a128913. [DOI] [PubMed] [Google Scholar]
- LEBOY P. S., COX E. C., FLAKS J. G. THE CHROMOSOMAL SITE SPECIFYING A RIBOSOMAL PROTEIN IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Dec;52:1367–1374. doi: 10.1073/pnas.52.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leder P., Skogerson L. E., Roufa D. J. Translocation of mRNA codons. II. Properties of an anti-translocase antibody. Proc Natl Acad Sci U S A. 1969 Mar;62(3):928–933. doi: 10.1073/pnas.62.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Lenard J., Haenni A. L. Release of transfer RNA during peptide chain elongation. Proc Natl Acad Sci U S A. 1969 May;63(1):93–97. doi: 10.1073/pnas.63.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Lenard J., Haenni A. L. Requirement of granosine 5'-triphosphate for ribosomal binding of aminoacyl-SRNA. Proc Natl Acad Sci U S A. 1968 Feb;59(2):554–560. doi: 10.1073/pnas.59.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Lenard J., Lipmann F. Separation of three microbial amino acid polymerization factors. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1562–1566. doi: 10.1073/pnas.55.6.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y., Lipmann F. Comparison of guanosine triphosphate split and polypeptide synthesis with a purified E. coli system. Proc Natl Acad Sci U S A. 1966 Jan;55(1):212–219. doi: 10.1073/pnas.55.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y., Lipmann F. The interrelationship between guanosine triphosphatase and amino acid polymerization. Arch Biochem Biophys. 1966 Sep 26;116(1):344–351. doi: 10.1016/0003-9861(66)90040-3. [DOI] [PubMed] [Google Scholar]
- Parisi B., Milanesi G., Van Etten J. L., Perani A., Ciferri O. Species specificity in protein synthesis. J Mol Biol. 1967 Sep 14;28(2):295–309. doi: 10.1016/s0022-2836(67)80011-1. [DOI] [PubMed] [Google Scholar]
- Parmeggiani A. Crystalline transfer factors from Escherichia coli. Biochem Biophys Res Commun. 1968 Mar 27;30(6):613–619. doi: 10.1016/0006-291x(68)90556-1. [DOI] [PubMed] [Google Scholar]
- Pestka S. Studies on the formation of trensfer ribonucleic acid-ribosome complexes. V. On the function of a soluble transfer factor in protein synthesis. Proc Natl Acad Sci U S A. 1968 Oct;61(2):726–733. doi: 10.1073/pnas.61.2.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel J. M. Demonstration of a guanosine triphosphate-dependent enzymatic binding of aminoacyl-ribonucleic acid to Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1811–1816. doi: 10.1073/pnas.57.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]



