Abstract
An anaerobic three-stage continuous culture model of the human colon (gut model), which represent different anatomical areas of the large intestine, was used to study the effect of S. aureus infection of the gut on the resident faecal microbiota. Studies on the development of the microbiota in the three vessels were performed and bacteria identified by culture independent fluorescence in situ hybridization (FISH). Furtheremore, short chain fatty acids (SCFA), as principal end products of gut bacterial metabolism, were measured along with a quantitative assessment of the predominant microbiota. During steady state conditions, numbers of S. aureus cells stabilised until they were washed out, but populations of indigenous bacteria were transiently altered; thus S. aureus was able to compromise colonisation resistance by the colonic microbiota. Furthermore, the concentration of butyric acid in the vessel representing the proximal colon was significantly decreased by infection. Thus infection by S. aureus appears to be able to alter the overall structure of the human colonic microbiota and the microbial metabolic profiles. This work provides an initial in vitro model to analyse interactions with pathogens.
Introduction
Staphylococcus aureus is a major opportunistic human pathogen, able to cause a wide variety of disease in humans and animals. It is the cause of a large burden of morbidity and mortality, globally, in both hospital and community settings [1]. Despite the plethora of antimicrobial agents available, infection continues to spread and many strains are resistant to an array of antibiotics. In particular, methicillin resistant S. aureus (MRSA) is an acute clinical problem, with many hospital and community isolates displaying resistance [1]. In response, measures have been developed to prevent the spread of this naturally ubiquitous organism, which lives as a commensal in the nares of 20–25% of the population at any one time [2], [3]. While nasal colonisation is a well-established risk factor for most types of infections, several recent studies have suggested that colonisation of the intestines, which occurs in c. 20% of individuals and which relatively, has been less intensively studied, could have important clinical implications [4]. Patients with S. aureus intestinal colonisation may serve as an important source of transmission, as they often contaminate the adjacent environment [5]. Factors such as faecal incontinence and diarrhea contribute to dissemination of the pathogen in the healthcare environment [6]. Similarly, such patients display increased frequencies of skin colonisation [7] and studies in intensive care and liver transplant units have shown that patients colonised by MRSA at the rectum and nares have a significantly higher risk of disease than patients with nasal colonisation alone [8]. Furthermore, hospitalised patients reported co-colonisation by S. aureus and vancomycin-resistant enterococci in >50% of the individuals studied [6]. Thus, intestinal colonisation by S. aureus provides the pathogen with the opportunity to acquire new antibiotic resistance genes.
The clinical implications of intestinal colonisation by S. aureus are still relatively ill-defined. It is assumed that carriage imposes a risk for intestinal infection. S. aureus can induce psuedomembranous colitis that is histologically distinct from that caused by Clostridium difficile [9]. Several studies have shown that intestinal colonisation occurs frequently in infants, particularly those that have been breast-fed [10] and is positively correlated with development of allergies and increased expression levels of the soluble immune modulator CD14, in such children [11]–[15]. The role of intestinal carriage in development of systemic S. aureus disease has not yet been established. However in mice, colonisation of the intestinal lumen can lead to S. aureus crossing the intestinal epithelial barrier and subsequent spread to the mesenteric lymph nodes [16], [17].
The human intestine represents a complex ecosystem with many commensal microflora species, various types of secretory fluids, fermentation metabolites of digested food and host defense molecules [18]. Survival and subsequent successful colonisation of pathogenic bacteria in the human intestinal tract requires them to resist innate defenses in the intestine. Colonisation resistance by normal commensal bacteria, acidic pH, fermentation metabolites like short chain fatty acids (SCFA), high osmolarity, local gut mucosal immunity by host defense molecules and bile acids in the intestine represent major obstacles for the survival and colonisation of invading pathogenic bacteria.
To date, no suitable in vivo models have been used to study carriage and survival of S. aureus in the human intestine. Laboratory mouse models of infection do not reproduce the complex microbial ecosystem or the physicochemical environment of the human gut [19].
An in vitro three-stage continuous culture system (gut model) is a useful tool to monitor the ecology and metabolic activities of the microbiota in the proximal, transverse and distal colon, in particular in relation to different environmental conditions, dietary intervention, as well as administration of drugs and antimicrobials [20], [21]. In this study, we investigated the impact of S. aureus on the normal intestinal flora, and survival of the pathogen, using a three-stage continuous culture model system of the human colon. We found that infection had a significant impact on the normal colonic microflora. Futhermore, short chain fatty acids (SCFA) generation, end products of gut bacterial metabolism, were measured and found to be altered significantly by the presence of S. aureus. Thus S. aureus is able to influence the composition in the human gut, of both the microbiota and SCFAs.
Results
Survival of S. aureus in a continuous culture model of the human gut
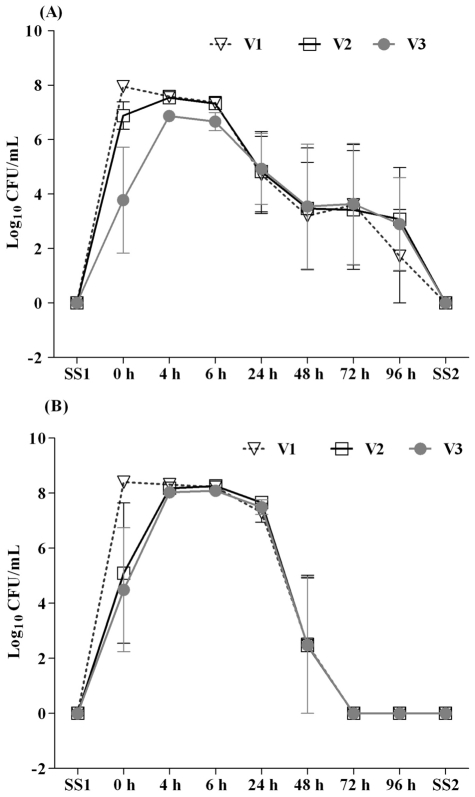
An in vitro three-stage culture system was used to model the conditions found in the human colon (Figure 1). Post-infection survival of S. aureus in three independent in vitro colonic models was quantified by viable colony count methods using selective BHI; all colonies displayed identical morphology. Prior to S. aureus inoculation into V1 of each colonic model, colony counts at SS1 revealed absence of detectable S. aurues in the colonic models (Figure 2A). After inoculating the colonic models with S. aureus to a concentration of c. 2×1010 CFU/mL, as a single dose, the S. aureus populations stabilised at 6 to 7 Log10 units over a period of 4 and 6 h in all the three vessels of colonic models (Figure 2A). Sub populations of 3 to 4 Log10 units were found over a period of 24, 48, and 72 h in all three vessels and less than 2 to3 Log10 units counts were found at 96 h after initial inoculation (Figure 2A). No S. aureus was detected in any of the three vessels at SS2.
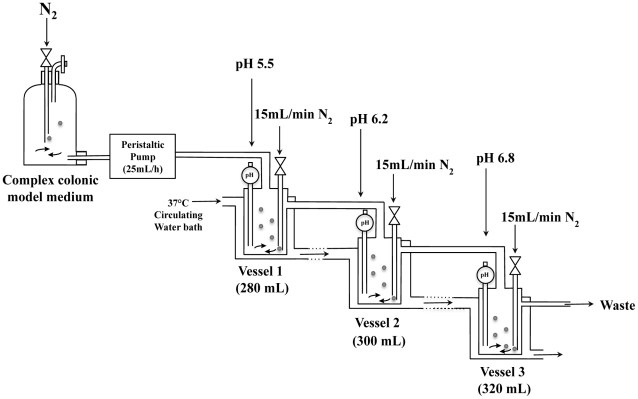
Figure 1. Schematic diagram of the in vitro three-stage culture colonic model system (human colonic model).
Each vessel was continually sparged with O2 free N2. The pH of each vessel was individually maintained via automated addition of 1M HCl or 1M NaOH, as required. The system was maintained at 37°C and stirred continuously.
Figure 2. S. aureus populations detected in culture broths recovered from the three different vessels (V1, V2 and V3) of the colonic model before (SS1) and after (0, 4, 6, 24, 48, 72, and 96 h and SS2) inoculation of S. aureus by viable plate count (A) and FISH method (B).
Results are reported as means (Log10 CFU/mL) of the data of three colonic models ± standard error of mean. The apparent
S. aureus populations were also quantified by FISH, using a Cy3 labelled-16S rRNA targeted oligonucleotide probe specific to S. aureus. Enumeration of S. aureus populations by FISH revealed similar patterns of survival in all the three vessels compared to the viable colony count method, although some differences occur due to the higher threshold of detection of FISH. The absence of S. aureus from the faecal samples used, was confirmed prior to the inoculation of S. aureus into the colonic models at SS1. After the initial inoculation, S. aureus achieved stabilsing populations of 7 to 8 Log10 units over a period of 4, 6, and 24 h (Figure 2B). No bacteria were detected at 24 h after inoculation due to a lower detection limit (6 Log10 CFU/mL) of the FISH method. In one gut model, 2 to 3 Log10 units of S. aureus were present after 48 h, in all three vessels (Figure 2B). Such variations in the survival could be influenced by indigenous faecal microflora and dietary habbits of the donors who contributed faecal samples for the study.
S. aureus colonies recovered on the selective agar were confirmed by PCR amplification using oligonucleotide primers specific to S. aureus specific genes. Interogation of the J. Craig Venter Institute Comprehensive Microbial Resource (http://cmr.jcvi.org) confirmed that efb (extracellular fibronogen binding protein) and spa (Staphylococcal protein A) genes were present only in the sequenced S. aureus strains. PCR products c. 0.5 and 1.5 kb representing efb and spa respectively, confirmed that the recovered colonies were indeed S. aureus (results not shown).
Impact of S. aureus survival on the colonic microbiota composition
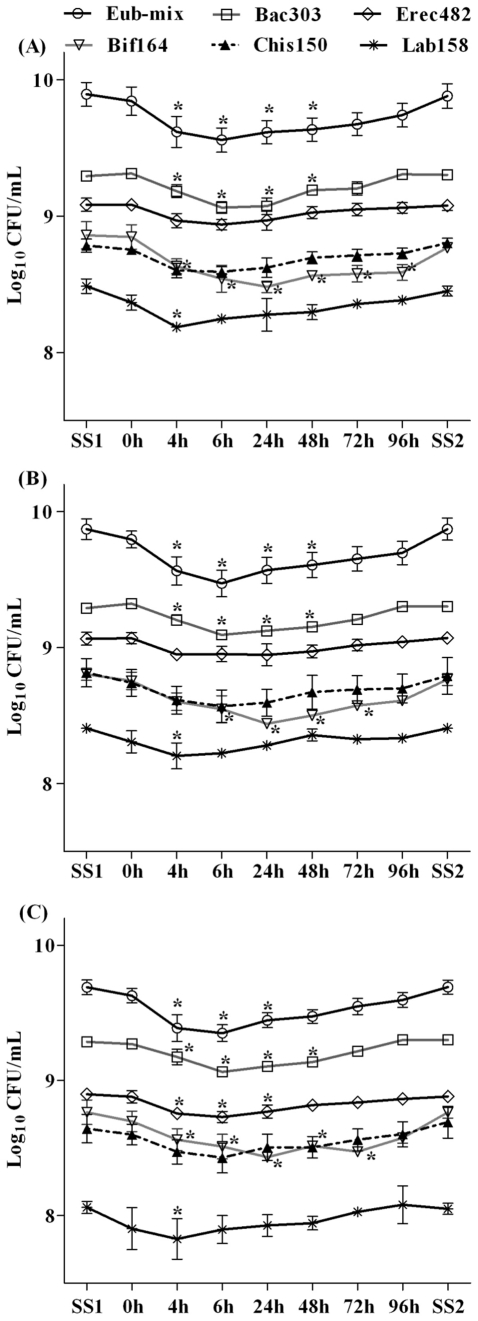
The effect of S. aureus survival on the 6 major colonic commensal bacterial groups, were determined for each vessel at SS1 (prior to the inoculation of S. aureus into colonic models) and 0, 4, 6, 24, 48, 72, and 96 h post inoculation as well as at SS2 by FISH. In all three model vessels, Bacteroides group (Bac303) was the predominant group at SS1, followed by Eubacterium rectale/Clostridium cluster XIVa (Erec482), Bifidobacterium (Bif164), Clostridium histolyticum (Chis150), and Lactobacillus (Lab158) (Figures 3A, 3B and 3C). A different behaviour was determined for the levels of Bifidobacterium, Bacteroides, Lactobacillus and total bacteria (Eub-mix) in all three vessels and Eubacterium rectale/Clostridium cluster XIVa for V3 (Figures 3A, 3B and 3C). FISH analysis showed that Bifidobacterium, a predominant health-promoting genus of the human gut microbiota [22], [23], decreased significantly from 4 to 96 h post inoculation (P<0.05) in V1, 6 to 72 h post inoculation (P<0.05) in V2, and 4 to 72 h post inoculation (P<0.05) in V3. At SS2, the bifidobacterial counts reverted back to the levels found in SS1.
Figure 3. Major colonic bacterial groups detected by FISH in the culture broths recovered from the vessel 1 (A), vessel 2 (B), and vessel 3 (C) of the colonic model before (SS1) and after (0, 4, 6, 24, 48, 72, and 96 h and SS2) inoculation of S. aureus.
Results are reported as means (Log10 CFU/mL) of the data of three colonic models ± standard error of mean. For each colonic model, measurements were performed in triplicate at SS1 (days 11, 12 and 13) and SS2 (days 21, 22 and 23). * P<0.05.
Bacteroides, another common genus of human gut microbiota involved in colonisation resistance to enteric pathogens [24], also decreased significantly from 4 to 48 h post inoculation (P<0.05) in all the three vessels and counts reverted back to the normal levels at SS2 as found in SS1. Lactobacillus counts decreased significantly only at 4 h post inoculation (P<0.05) and reverted back to normal levels there onwards in all three vessels. Total bacterial counts decreased significantly from 4 to 48 h post inoculation (P<0.05) in V1 and V2, and 4 to 24 h post inoculation (P<0.05) in V3. Total bacterial counts reverted back to normal levels at SS2 as found in SS1. A significant decrease in the Eubacterium rectale/Clostridium cluster XIVa counts were determined at 4 to 24 h post inoculation (P<0.05) in V3 only and no significant differences in the counts were found in V1 and V2 throughout the experiments. Clostridium histolyticum did not show any significant modification in counts at SS1 and post inoculation periods in all three vessels.
Impact of S. aureus survival on the SCFA production in the colonic models
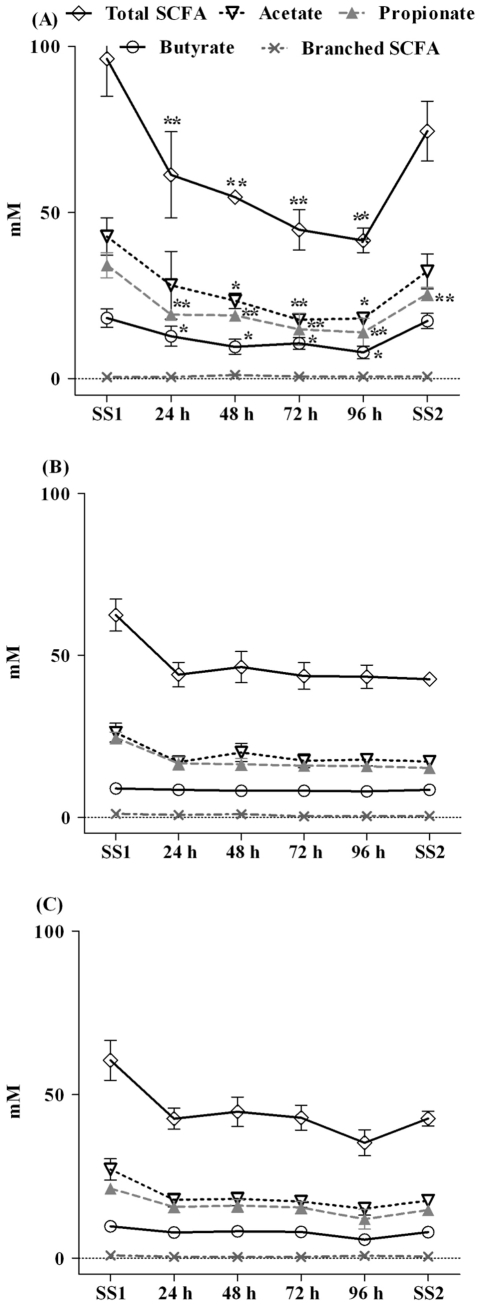
Profiles of SCFAs concentrations prior to the S. aureus inoculation (SS1) and during different time periods of post inoculation quantified by gas chromatography are shown in Figure 4. Acetate was the predominant SCFA detected during SS1 and through out different time periods of post inoculation, followed by propionate and butyrate in all three vessels. Significant differences in SCFA profiles were detected only in V1(Figure 4A), whereas changes in the SCFA profiles were insignificant during SS1 and different time periods of post inoculation in V2 and V3 (Figure 4B and 4C). Acetate concentrations decreased significantly from 48 to 96 h post inoculation (P<0.01), whereas decreases in concentrations at 24 h and SS2 were not significant in V1. Propionate levels decreased significantly from 24 to 96 h post inoculation and even at SS2 (P<0.01) in V1. Similarly, butyrate concentrations also decreased signifcantly from 24 to 96 h post inoculation (P<0.05) in V1. Total SCFA levels also decreased significantly from 24 to 96 h post inoculation (P<0.01). The difference between SS1 and SS2 in V1 was not statistically significant (Figure 4A). None of the changes in concentrations of acetate, propionate, butyrate, and total SCFAs in V2 and V3 were significant at any time point, when compared to SS1 (Figure 4B and 4C).
Figure 4. Short-chain fatty acids concentrations in the culture broths recovered from the vessel 1 (A), vessel 2 (B), and vessel 3 (C) of the colonic model before (SS1) and after (24, 48, 72, and 96 h and SS2) inoculation of S. aureus.
Results are reported as means (mM) of the data of three colonic models ± standard error of mean. For each colonic model, measurements were performed in triplicate at SS1 (days 11, 12 and 13) and SS2 (days 21, 22 and 23). * P<0.05; ** P<0.01.
Discussion
S. aureus is able to colonise multiple niches within humans. In doing so, the host is at significantly increased risk of developing disease. Colonisation of the human nares is a well studied risk factor which can predispose an individual to subsequent infection with the same strain [25]. Similarly, S. aureus rectal colonisation imparts an increased risk of disease [8]. Interestingly, USA300 S. aureus strains, which are responsible for a current outbreak of community acquired disease in the United States, colonise the rectum at rates higher than the nose [26]. A recent study to determine S. aureus genetic traits associated with these observed higher rectal carriage rates was inconclusive [27]. Thus it would be very interesting to study the effect that these strains have on gut microbial ecology.
The human intestinal tract is a highly complex ecosystem; whose diverse microbiota profoundly affects health of the host. It provides digestive functions, modulates host metabolism and stimulates angiogenesis, development of lymphatic tissue and the mucosal immune system [28]. Importantly, it can limit infection of the gut by other bacteria. This network of interactions is believed to stabilise the structure of the microbiota population and inhibit colonisation by invading pathogens [29]. Molecular interactions between different members of the microbiota, which prevent establishment of pathogen colonisation, remain to be determined. However, some pathogens are able to disrupt this colonisation resistance and infect the gut [29]. Similarly, the mechanisms that pathogens use to overcome these barriers, formed by the intrinsic microbiota, are poorly understood.
The intestinal microflora is able to ferment plant materials, such as celluloses, starches and sugars, which have been consumed by the host. Mammals lack the necessary enzymes to split such compounds and rely on microbial fermentation to convert indigestible carbohydrates and oligosaccharides into SCFAs, a process which occurs mainly in the proximal colon [30]. In the human colon, acetic, propionic and butyric acids predominate. Of these, butyrate has been particularly well studied because of the effects that it can have on gut health. Metabolism of butyrate represents the preferred energy source for colonocytes and is responsible for synthesis of many key components of the intestinal epithelium [31], [32].
SCFAs such as acetate, propionate and butryate are weak acids with bacteriostatic and bactericidal properties which, in part, depend on the physiological status of the bacteria and the physico-chemical characteristics of the external environment. They can influence the pattern of gene expression in bacteria. Studies on Salmonella typhimurium and Salmonella enteritidis have shown that SCFAs specifically downregulate genes of the Salmonella pathogenicity island (SPI)-1 [33], resulting in supressed invassion of epithelial cells. Conversely, butyrate has been shown to enhance expression of virulence associated genes in enterohaemorrhagic Escherichia coli [34].
In addition to direct effects on pathogenic bacteria, SCFAs are able to induce pathogen resistance in host cells [30]. Butyrate treatment of human epithelial cells has been shown to induce resistance to bacterial invasion by Campylobacter jejuni or S. aureus [35], [36]. Furthermore, butyrate treated epithelial cells produce increased levels of antimicrobial peptides and nitric oxide [36].
Our study demonstrates that S. arueus is able to survive the microbial environment of the human gut. Addition of S. aureus to the model to mimic infection, resulted in a significant, transient decrease in the numbers of eubacteria and specifically Bacteroides, Bifidobacterium and Lactobacillus/Enterococcus species in vessels 1 and 2, which simulate the proximal and transverse colons, respectively. In vessel 3, which reflects conditions in the distal colon, a broader impact on the microbiota was observed; this involving significant decreases in all of the genera or species measured, except for Clostridium histolyticum. The mechanisms by which S. aureus is able to colonise the human gut remain poorly understood, however it is now well recognised that a healthy resident microbiota provides good protection against invading pathogens. Given the findings of this study, it can be presumed that S. aureus is able to inhibit these bacteria in order to acquire a niche. The eventual loss of detectable levels of S. aureus from the model is assumed to be the result of washout from the continuous culture system which is constantly fed with growth medium.
Decreased butyrate in V1 is presumed to result from the altered microbiota, some members of which carry out the conversion of complex molecules to SCFAs. The effect that butyrate has on S. aureus remains unknown. While butyrate is bactericidal for some species, it has recently been reported to have no effect on S. aureus at neutral pH. However, SCFAs possess increased potency at low pH and any effect of butyrate on S. aureus physiology in conditions of the proximal colon (pH 5.5), remains to be determined. Furthermore, decreased butyrate concentrations in the human colon could result in increased localised inflammation, which is believed to facilitate pathogen colonisation [37]–[40].
The complex, beneficial interplay between the host and its microbiota can be interupted by pathogenic bacteria. However any invading pathogen must perturb this delicate association in order to overcome resistance and subsequently colonise and persist within its host. In this study we have demonstrated that S. aureus is able to disrupt the normal microbiota of the human colon. The physiological adaptations made by the pathogen in order that it can colonise the gut, remain to be determined.
Materials and Methods
Bacteria and Growth conditions
The routinely used laboratory strain S. aureus SH1000 [41] was grown on Brain Heart Infusion (BHI) (Oxoid) agar at 37°C.
Three-stage continuous culture colonic model system (human colonic model)
The three-stage continuous culture model of the human colon comprised of three glass fermenters of increasing working volume, simulating the proximal (V1, 280 mL), transverse (V2, 300 mL) and distal colonic areas (V3, 320 mL). The three fermenters were connected in series, which fed into each other sequentially and finally overflowing into a waste vessel. All vessels were kept at 37°C by means of a circulating water-bath. The pH was controlled and held at 5.5 (V1), 6.2 (V2) and 6.8 (V3) and the system was kept anaerobic by continuously sparging with oxygen free nitrogen gas (15 mL/min). Faecal healthy human samples were collected on site, kept in an anaerobic cabinet (10% H2, 10% CO2, 80% N2) and used within a maximum of 15 min after collection. This experiment was carried out in triplicate using faecal samples from three different volunteers (one faecal donor for each experimental set up). After obtaining verbal informed consent, a standard questionnaire to collect information regarding the health status, drugs use, clinical anamnesis, and lifestyle was administrated before the donor was ask to provide a faecal sample. The University of Reading ethics committee exempted this study from review because no donors were involved in any intervention and waived the need for written consent due to the fact the samples received were not collected by means of intervention. None of the volunteers had received antibiotics or probiotics for at least 3 months before sampling, or steroids or other drugs with a proven impact on gut microbiota composition over the preceding 12 weeks. A 1∶5 (w/w) dilution in anaerobic PBS [0.1 mol/L PBS (pH 7.4)] was prepared and the samples were homogenized in a stomacher (Seward, Worthing, UK) for 2 min. Each vessel was inoculated with 100 mL faecal slurry. Total system transit time was set at 36 h. V1 was fed by means of a peristaltic pump with complex colonic model medium as previously reported [21]. Following inoculation, the colonic model was run as a batch culture for a 24 h period in order to stabilise bacterial populations prior to or the initiation of medium flow. After 24 h (T0) the medium flow was initiated and the system was run for eight full volume turnovers to allow steady state to be achieved (SS1). At SS1, samples were obtained on three consecutive days to confirm steady state status by SCFA and fluorescence in situ hybridisation (FISH) based analyses of the microbiota. After the establishment of SS1, V1 of the colonic model was inoculated with S. aureus SH1000 (c. 2×1010 CFU/ml) as a single dose, suspended in colonic model media. Three samples were taken from each vessels of the colonic model at SS1, 0, 4, 6, 24, 48, 72 and 96 h after inoculation with S. aureus for viable plate counting and FISH analysis of major colonic bacteria. Samples were also collected on three consecutive days as described for SS1 for a further full eight volume turnovers upon which steady state 2 (SS2) was achieved. Samples for FISH were fixed immediately in 4% paraformaldehyde as previously described [42]. Another set of samples collected at SS1, 24, 48, 72, and 96 h after S. aureus inoculation as well as at SS2 were centrifuged at 13000 g and the supernatant stored at −20°C for gas chromatography analysis.
Enumeration of S. aureus on selective agar
Samples (100 µl) of the experimental sets at times SS1, 0, 4, 6, 24, 48, 72, and 96 h after infection as well as at SS2 from all three vessels of the in vitro colonic model were plated onto BHI agar containing 0.01% (w/v) potassium tellurite as a selective agent at different dilutions (from 102 to 109 CFU/ml) in triplicate for each time point to measure bacterial growth. Dilutions were prepared using phosphate buffered saline (PBS). The lowest dilution that had bacterial colonies growing on it was used to calculate CFU/mL. As all dilutions were carried out in triplicate, the mean of their log10 was calculated (Log10 CFU/mL = n×50× dilution factor; n = the number of colonies counted).
Species- specific PCR analysis
A random selection of single colonies from selective agar plates was inoculated into 10 ml BHI and grown overnight at 37°C. One mL of overnight culture was harvested and DNA samples extracted using the DNeasy Blood and Tissue kit (Qiagen). Extracted bacterial DNA was quantified using a NanoDrop ND-1000 Spectrophotometer (NanoDrop 116 Technologies, Wilmington, DE, USA) and stored at −20°C. The primers (synthesised by Eurofins, Germany) used and the PCR conditions were described in Table 1.
Table 1. Primers and PCR conditions for colony screening.
| Gene | Oligonucleotide sequences (5′ to 3′) | PCR product size | PCR conditions |
| efb | CGTCAACAGCACATATGAGCGAAGGATACG GCAACGATTGAACTCGAGTTTAACTAATCC | c. 0.5 kb | 1 min at 95°C; 30 cycles:1 min at 95°C, 1 min at 59°C,1 min at 72°C, and finally 2 min at 72°C |
| spa | CTAGGTGTAGGTATTGCATC CGCTGCACCTAAGGCTAATG | c. 1.5 kb | 1 min at 95°C; 30 cycles:1 min at 95°C, 1 min at 51°C,1 min at 72°C, and finally 2 min at 72°C |
Enumeration of major colonic bacterial populations by Fluorescence in Situ Hybridization (FISH)
FISH experiments were performed as previously described [42]. All probes were Cy3-labelled and synthesized by Sigma Aldrich (Sigma-Aldrich, UK). Table 2 gives the details of the probes used in this study. The composition of hybridization and wash buffers depends on the rRNA-targeted oligonucleotide probe as reported in probeBase (http://www.microbial-ecology.net/probebase) and was used accordingly.
Table 2. Oligonucleotide probes used in this study and hybridization conditions for FISH analysis.
| Short Name | Target genus | Sequences (5′ to 3′) | Pre-treatment | Temperature (°C) | Reference | |
| Hybridizing | Washing | |||||
| Bac303 | Most Bacteroides sensu stricto and Prevotella spp.; all Parabacteroides; Barnesiella viscericola and Odoribacter splanchnicus | CCAATGTGGGGGACCTT | None | 46 | 48 | [44] |
| Bif164 | Most Bifidobacterium spp. and Parascardovia denticolens | CATCCGGCATTACCACCC | None | 50 | 50 | [45] |
| Erec482 | Most members of Clostridium cluster XIVa; Syntrophococcus sucromutans, (Bacteroides) galacturonicus and (Bacteroides) xylanolyticus, Lachnospira pectinschiza and Clostridium saccharolyticum | GCTTCTTAGTCARGTACCG† | None | 50 | 50 | [46] |
| Lab158 | Most Lactobacillus, Leuconostoc and Weissella spp.; Lactococcus lactis; all Vagococcus, Enterococcus, Melisococcus, Tetragenococcus, Catellicoccus, Pediococcus and Paralactobacillus spp. | GTATTAGCAYCTGTTTCCA‡ | Lysozyme | 50 | 50 | [47] |
| Chis150 | Most members of Clostridium cluster I; all members of Clostridium cluster II; Clostridium tyrobutyricum; Adhaeribacter aquaticus and Flexibacter canadensis (family Flexibacteriaceae); (Eubacterium) combesii (family Propionibacteriaceae) | TTATGCGGTATTAATCTYCCTTT‡ | None | 50 | 50 | [46] |
| Sau* | Staphylococcus aureus | GAAGCAAGCTTCTCGTCCG | Lysozyme and Lysostaphin | 53 | 53 | [48] |
| EUB338** | Total Bacteria | GCTGCCTCCCGTAGGAGT | None | 46 | 48 | [49] |
| EUB338II** | Total Bacteria | GCAGCCACCCGTAGGTGT | None | 46 | 48 | [49] |
R = G/A; ‡Y = T/C.
*20% Formamide was incorporated in the hybridization buffer.
**These probes were used together in equimolar concentrations (50 ng/µL) and 35% formamide was incorporated in the hybridization buffer.
Short chain fatty acids analysis (SCFA) by gas chromatography
Aliquots of 1 mL collected from each vessel in microcentrifuge tubes were centrifuged at 13000 g for 5 min. The supernatants were transferred into fresh microcentrifuge tubes and stored at −20°C until use. Samples were derivatized as previously described [43]. Briefly, the supernatants stored at −20°C were thawed on ice and centrifuged at 13000 g for 10 min. 500 µl of each supernatant was transferred into fresh microcentrifuge tubes and 25 µL of internal standard (2-ethyl butyric acid) followed by 250 µl of concentrated HCl and 1 mL of ether added to each of the tubes. Tubes were vortexed for 1 min and centrifuged at 3000 g for 10 min. The top ether layer was collected and transferred into fresh microcentrifuge tubes. Aliquots (400 µl) of the ether extract were pipetted into a Wheaton vial and then 50 µl of N-tertButyldimethyl silyl N-methyltrifluoroacetamide (MTBSTFA) was added. The vials were sealed tightly by screwing after addition of MTBSTFA and heated at 80°C for 20 min in a water bath. Samples were transferred to Agilent crimp cap vials for gas chromatography analysis. Vials were capped with Crimp top natural rubber/PTFE seal type 7 aluminium silver 11 mm Chromacol caps and sealed using a crimper. The capped vials were left at room temperature for 48 h for derivatization.
Calibration was achieved using standard solutions of derivatized acetic, propionic, i-butyric, n-butyric, i-valeric, n-valeric, and n-caproic acids as described for test samples. The final concentrations of each standard was 25, 10, 5, 1, and 0.5 mM. The derivatized samples were run through a 5890 series II GC system (HP, Crawley, West Sussex, UK) fitted with SGE-HT5 column (0.32 mm×25 m×0.1 µm; J&W Scientific, Folsom, CA, USA) and flame ionisation detector. Helium was used a carrier gas and delivered at a flow rate of 14 mL/min. The head pressure was set at 10 psi with split ratio 10∶1. Injector, column and detector were set at 275, 250 and 275°C respectively. One micro liter quantity of each sample was injected with a run time of 10 min. Peaks were integrated using the Atlas Lab managing software (Thermo Lab Systems, Mainz, Germany). Fatty acid concentrations were quantified by comparing their peak areas with the standards and expressed in mM.
Statistical Analysis
All data were analyzed by a One-Way Anova method, using the Tukey post test analysis when overall P of the experiment is below the value of significance (P<0.05). Additional paired t-test was applied to understand significance of results of single pairs of data. Analyses were performed using GraphPad Prism 5.0 (GraphPad Software Inc., La Jolla, CA, USA).
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis. 2001;32(suppl 2):S114–S132. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- 2.Peacock SJ, de Silva I, Lowy FD. What determines nasal carriage of Staphylococcus aureus? Trends Microbiol. 2001;9:605–610. doi: 10.1016/s0966-842x(01)02254-5. [DOI] [PubMed] [Google Scholar]
- 3.Wertheim HFL, Melles D, Vos C, van Leeuwen W, van Belkum A, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 4.Acton DS, Tempelmans Plat-Sinnige MJ, Van Wamel W, de Groot N, Van Belkum A. Intestinal carriage of Staphylococcus aureus: how does its frequency compare with that of nasal carriage and what is its clinical impact. Eur J C Microbiol Infect Dis. 2009;28:115–127. doi: 10.1007/s10096-008-0602-7. [DOI] [PubMed] [Google Scholar]
- 5.Masaki H, Asoh N, Watanabe H, Tao M, Watanabe K, et al. Possible relationship between Staphylococcus aureus colonizing the respiratory tract and rectum and S. aureus isolated in a geriatric hospital environment. Intern Med. 2003;42:281–282. doi: 10.2169/internalmedicine.42.281. [DOI] [PubMed] [Google Scholar]
- 6.Ray AJ, Nicole JP, Bhalla A, David CA, Donskey CJ. Coexistence of vancomycin resistant Enterococci and Staphylococcus aureus in the intestinal tracts of hospitalized patients. Clin Infect Dis. 2003;37:875–881. doi: 10.1086/377451. [DOI] [PubMed] [Google Scholar]
- 7.Bhalla A, Aron DC, Donskey CJ. Staphylococcus aureus intestinal colonization is associated with increased frequency of S. aureus on skin of hospitalized patients. BMC Infect Dis. 2007;7:105. doi: 10.1186/1471-2334-7-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Squier C, John D, Kathleen JR, Sagnimeni A, Marilyn M, et al. Staphylococcus aureus rectal carriage and its association with infections in patients in a surgical intensive care unit and a liver transplant unit. Inf Cont Hosp Ep. 2002;23:495–501. doi: 10.1086/502095. [DOI] [PubMed] [Google Scholar]
- 9.Froberg MK, Palavecino E, Dykoski R, Gerding DN, Peterson LR, et al. Staphylococcus aureus and Clostridium difficile cause distinct pseudomembranous intestinal diseases. Clin Infect Dis. 2004;39:747–750. doi: 10.1086/423273. [DOI] [PubMed] [Google Scholar]
- 10.Vael C, Desager K. The importance of the development of the intestinal microbiota in infancy. Curr Opin Pediatr. 2009;21:794–800. doi: 10.1097/MOP.0b013e328332351b. [DOI] [PubMed] [Google Scholar]
- 11.Björkstén B, Naaber P, Sepp E, Mikelsaar, M The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy. 1999;29:342–346. doi: 10.1046/j.1365-2222.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 12.Lindberg E, Nowrouzian F, Adlerberth I, Wold AE. Long-time persistence of superantigen-producing Staphylococcus aureus strains in the intestinal microflora of healthy infants. Pediatr. Res. 2000;48:741–747. doi: 10.1203/00006450-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Lindberg E, Adlerberth I, Hesselmar B, Saalman R, Strannegård I-L, et al. High rate of transfer of Staphylococcus aureus from parental skin to infant gut flora. J Clin Microbiol. 2004;42:530–534. doi: 10.1128/JCM.42.2.530-534.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adlerberth I, Strachan DP, Matricardi PM, Ahrné S, Orfei L, et al. Gut microbiota and development of atopic eczema in 3 European birth cohort. J Allergy Clin Immunol. 2007;120:343–350. doi: 10.1016/j.jaci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Lundell A-C, Adlerberth I, Lindberg E, Karlsson H, Ekberg S, et al. Increased levels of circulating soluble CD14 but not CD83 in infants are associated with early intestinal colonization with Staphylococcus aureus. . Clin Exp Allergy. 2007;37:62–71. doi: 10.1111/j.1365-2222.2006.02625.x. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura Y, Aramaki Y, Kakiuchi T. A mouse model for postoperative fatal enteritis due to Staphylococcus infection. J Surg Res. 2001;96:35–43. doi: 10.1006/jsre.2000.6043. [DOI] [PubMed] [Google Scholar]
- 17.Hess DJ, Garni RM, Henry-Stanley MJ, Wells CL. Escherichia coli modulates extraintestinal spread of Staphylococcus aureus. Shock. 2005;24:376–381. doi: 10.1097/01.shk.0000180615.75822.fe. [DOI] [PubMed] [Google Scholar]
- 18.Sekirov I, Finlay, BB The role of the intestinal microbiota in enteric infection. J Physiol. 2009;587:4159–4167. doi: 10.1113/jphysiol.2009.172742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hapfelmeier S, Hardt WD. A mouse model for S. typhimurium–induced enterocolitis. Trends Microbiol. 2005;13:497–503. doi: 10.1016/j.tim.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Gibson GR, Cummings JH, Macfarlane, GT Use of a three-stage continuous culture system to study the effect of mucin on dissimilatory sulfate reduction and methanogenesis by mixed populations of human gut bacteria. Appl Environ Microbiol. 1988;54:2750–2755. doi: 10.1128/aem.54.11.2750-2755.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macfarlane GT, Macfarlane S, Gibson GR. Validation of a Three-Stage Compound Continuous Culture System for Investigating the Effect of Retention Time on the Ecology and Metabolism of Bacteria in the Human Colon. Microb Ecol. 1998;35:180–187. doi: 10.1007/s002489900072. [DOI] [PubMed] [Google Scholar]
- 22.Gibson GR, Wang, X Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol. 1994;77:412–420. doi: 10.1111/j.1365-2672.1994.tb03443.x. [DOI] [PubMed] [Google Scholar]
- 23.Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. 2004;28:405–440. doi: 10.1016/j.femsre.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Fons M, Gomez A, Karjalainen T. Mechansims of colonisation and colonisation resistance of the digestive tract: Part2: Bacteria/Bacteria interactions. Microb Ecol Health D. 2000;2:240–246. [Google Scholar]
- 25.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med. 2001;344:505–509. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 26.Faden H, Lesse AJ, Trask J, Hill JA, Hess DJ, et al. Importance of colonization site in the current epidemic of staphylococcal skin abscesses. Peadiatr. 2010;125:e618–e624. doi: 10.1542/peds.2009-1523. [DOI] [PubMed] [Google Scholar]
- 27.Lemmens N, van Wamel W, Snijders S, Lesse AJ, Faden H, et al. Genomic comparisons of USA300 Staphylococcus aureus colonizating the nose and rectum of children with skin abscesses. Microb Pathogenesis. 2011;50:192–199. doi: 10.1016/j.micpath.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Sekirov I, Russell SL Antunes CM, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 29.Stecher B, Hardt W-D. The role of microbiota in infectious disease. Trends Microbiol. 2007;16:107–117. doi: 10.1016/j.tim.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Guilloteau P, Martin L, Eekhaut V, Ducatelle R, Zabielski R, et al. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev. 2010;23:366–384. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- 31.Roediger WE. Utilization of nutrients by isolated epithelial cells of a rat colon. Gastroenterology. 1982;83:424–429. [PubMed] [Google Scholar]
- 32.Sakata T, ven Engelhardt W. Stimulatory effect of short chain fatty acids on the epithelial cell proliferation in rat large intestine. Comp Biochem Physiol A Comp Physiol. 1983;74:459–462. doi: 10.1016/0300-9629(83)90631-x. [DOI] [PubMed] [Google Scholar]
- 33.Gantois I, Ducatelle R, Pasmans F, Haesebrouck F, Hautefort I, et al. Butyrate specifically down-regulates Salmonella pathogenicity island 1 gene expression. Appl Environ Microbiol. 2006;72:946–949. doi: 10.1128/AEM.72.1.946-949.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakanishi N, Tashiro K, Kuhara S, Hayashi T, Sugimoto N, et al. Regulation of virulence by butyrate sensing in enterohaemorrhagic Escherichia coli. Microbiology. 2009;155:521–530. doi: 10.1099/mic.0.023499-0. [DOI] [PubMed] [Google Scholar]
- 35.Siavoshian S, Blottière HM, Le Foll E, Kaeffer B, Cherbut C, et al. Comparison of the effect of different short chain fatty acids on the growth and differentiation of human colonic carcinoma cell lines in vitro. Chem Biol Int. 1997;21:281–287. doi: 10.1006/cbir.1997.0153. [DOI] [PubMed] [Google Scholar]
- 36.Ochoa-Zarzosa A, Villarreal-Fernandez E, Cano-Camacho H, Lopez-Meza JE. Sodium butyrate inhibits Staphylococcus aureus internalization in bovine mammary epithelial cells and induces the expression of antimicrobial peptide genes. Microb Pathogenesis. 2009;47:1–7. doi: 10.1016/j.micpath.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Rosignoli P, Fabiani R, De Bartolomeo A, Spinozzi F, Agea E, et al. Protective activity of butyrate on hydrogen peroxide-induced DNA damage in isolated human colonocytes and HT29 tumour cells. Carcinogenesis. 2001;22:1675–1680. doi: 10.1093/carcin/22.10.1675. [DOI] [PubMed] [Google Scholar]
- 38.Heimesaat MM, Fischer A, Siegmund B, Kupz A, Niebergall J, et al. Shift towards pro-inflammatory intestinal bacteria aggravates acute murine colitis via Toll-like receptors 2 and 4. PLoS One. 2007;2:e662. doi: 10.1371/journal.pone.0000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119–129. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, et al. sigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J Bacteriol. 2002;184:5457–5467. doi: 10.1128/JB.184.19.5457-5467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martín-Peláez S, Gibson GR, Martín-Orúe SM, Klinder A, Rastall RA, et al. In vitro fermentation of carbohydrates by porcine fecal inocula and their influence on Salmonella typhimurium growth in batch culture systems. FEMS Microbiol Ecol. 2008;66:608–619. doi: 10.1111/j.1574-6941.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 43.Richardson A, Calder A, Stewart C, Smith A. Simultaneous determination of volatile and non-volatile acidic fermentation products of anaerobes by capillary gas chromatography. Letters in Applied Microbiology. 1989;9:5–8. [Google Scholar]
- 44.Manz W, Amann R, Ludwig W, Vancanneyt M, Schleifer KH. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum cytophaga-flavobacter-bacteroides in the natural environment. Microbiology. 1996;142:1097–1116. doi: 10.1099/13500872-142-5-1097. [DOI] [PubMed] [Google Scholar]
- 45.Langendijk PS, Schut F, Jansen GJ, Raangs GC, Kamphuis GR, et al. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl Environ Microbiol. 1995;61:3069–75. doi: 10.1128/aem.61.8.3069-3075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franks AH, Harmsen HJ, Raangs GC, Jansen GJ, Schut F, et al. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl Environ Microbiol. 1998;64:3336–3345. doi: 10.1128/aem.64.9.3336-3345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harmsen HJ, Elfferich P, Schut F, Welling GW. A 16S rRNA-targeted probe for detection of lactobacilli and enterococci in fecal samples by fluorescent in situ hybridization. Microbiol Ecol Health Dis. 1999;11:3–12. [Google Scholar]
- 48.Kempf VA, Trebesius K, Autenrieth IB. Fluorescent in situ hybridization allows rapid identification of microorganisms in blood cultures. J Clin Microbiol. 2000;38:830–838. doi: 10.1128/jcm.38.2.830-838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daims H, Bruhl A, Amann R, Schleifer KH, Wagner M. The domain-specific probe EUB338 is insufficient for the detection of all Bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol. 1999;22:434–44. doi: 10.1016/S0723-2020(99)80053-8. [DOI] [PubMed] [Google Scholar]