Abstract
Caffeine, the most widely used psychoactive compound, is an adenosine receptor antagonist. It promotes wakefulness by blocking adenosine A2A receptors (A2ARs) in the brain, but the specific neurons on which caffeine acts to produce arousal have not been identified. Using selective gene deletion strategies based on the Cre/loxP technology in mice and focal RNA interference to silence the expression of A2ARs in rats by local infection with adeno-associated virus carrying short-hairpin RNA, we report that the A2ARs in the shell region of the nucleus accumbens (NAc) are responsible for the effect of caffeine on wakefulness. Caffeine-induced arousal was not affected in rats when A2ARs were focally removed from the NAc core or other A2AR-positive areas of the basal ganglia. Our observations suggest that caffeine promotes arousal by activating pathways that traditionally have been associated with motivational and motor responses in the brain.
Introduction
Caffeine is the most consumed psychoactive compound in the world. It is readily available through dietary products, such as coffee, tea, soft drinks, and chocolate treats, but is also added to nonprescription medications, such as pain-relievers and cold remedies. Regardless of the source, the worldwide average caffeine consumption has been estimated to be just under 80 mg/d, although the levels of intake in countries such as Sweden and Finland are in the range of 400 mg of caffeine per day (Fredholm et al., 1999).
Caffeine is widely used to promote wakefulness and to counteract fatigue. Caffeine binds with very similar affinity to adenosine A1 (A1Rs) and A2A (A2ARs) receptors, and, at doses commonly consumed by humans, adenosine actions at both receptors are antagonized. Adenosine is an inhibitory neuromodulator involved in sleep–wake regulation (Porkka-Heiskanen et al., 1997; Huang et al., 2011). Using global genetic knock-outs of A1Rs and A2ARs, in which the receptor is deleted from the entire animal, we demonstrated previously that the A2AR, but not the A1R, mediates the arousal effect of caffeine (Huang et al., 2005). However, the neurons with A2ARs on which caffeine acts to produce wakefulness have not yet been identified.
A2ARs are densely expressed on striatopallidal neurons in the indirect pathway of the basal ganglia (BG), in which dopamine D2 receptors (D2Rs) are coexpressed with the A2ARs and contribute to the control of locomotor activity, motivation, and addiction, all activities that require wakefulness (Rosin et al., 1998; Svenningsson et al., 1999a). The striatopallidal neurons also facilitate movement by operating in parallel with dopamine D1 receptor (D1R)-bearing striatonigral neurons in the direct pathway of the BG. Abilities to maintain arousal are compromised under low dopamine conditions, such as Parkinson's disease (Arnulf et al., 2002; Qu et al., 2010), but the extent to which A2ARs in the BG contribute to the regulation of wakefulness is not known and the role of A2ARs in other brain regions is unclear.
In the present study, we used site-specific gene deletion strategies based on the Cre/loxP technology in mice and also silenced focally the expression of A2ARs in rats by using stereotaxic microinjections of adeno-associated virus (AAV) carrying short-hairpin RNA (shRNA). We found that the A2ARs in the shell region of the nucleus accumbens (NAc) are responsible for the effect of caffeine on wakefulness.
Materials and Methods
Genetic mouse models.
Animals were handled according to the NIH Guide for the Care and Use of Laboratory Animals and in accordance with the protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the Boston University School of Medicine, the Legacy Research Institute IACUC, and the Animal Research Committee at the Osaka Bioscience Institute. All mice (weighing 24–28 g, 11–13 weeks old) and male Sprague Dawley rats (weighing 150–180 g, 6 weeks old; Shizuoka Laboratory Animal Center) used in the present study were housed at a constant temperature (24 ± 0.5°C) with a relative humidity of 60 ± 2% on an automatically controlled 12 h light/dark cycle (light on at 7:00 A.M.). Three genetic mouse lines on a C57BL/6 background were used in the present study: (1) global A2AR knock-out mice (A2AR KO) (Chen et al., 1999), (2) basal ganglia–A2AR knock-out mice (BG–A2AR KO), exclusively lacking BG A2ARs (Shen et al., 2008), and (3) a mouse line with a loxP-site-inserted A2AR gene that is amenable to conditional disruption by the injection of Cre recombinase-expressing AAV.
Vigilance state assessment using electroencephalogram/electromyogram/locomotor activity recordings.
Assessment of vigilance states was performed on adult male conditional A2AR KO mice and their respective WT littermates (n = 4–5, per genotype and drug dose). Under anesthesia using 1.5% isoflurane in N2O/O2 (2:1), mice were implanted with electroencephalogram (EEG) and electromyogram (EMG) electrodes for polysomnographic recordings. To monitor EEG signals, two stainless steel EEG recording screws (Plastics One) were implanted epidurally over the frontal cortical area (1 mm anterior to bregma, 1.5 mm lateral to the midline) and over the parietal area (2 mm posterior to bregma, 3 mm lateral to midline) of the right hemisphere. EMG activity was monitored by stainless steel, Teflon-coated wires (0.2 mm in diameter; Plastics One) bilaterally placed into both trapezius muscles. Finally, the electrode assembly was anchored and fixed to the skull with Super6-Bond (Sun Medical Co.) and dental cement. After a 10 d recovery period, the mice were placed in experimental cages for a 4 d habituation/acclimatization period with connection of counterbalanced recording leads.
All mice that were subjected to EEG recordings received vehicle and drug treatment on 2 consecutive days. On day 1, mice were treated with vehicle (saline, intraperitoneally) at 9:00 A.M., and the 24 h recordings performed on day 1 were used as baseline data. On day 2, mice were treated with caffeine (intraperitoneally, in a volume of 10 ml/kg body weight), and EEG/EMG signals were recorded for 24 h. The EEG/EMG signals were amplified and filtered (EEG, 0.5–30 Hz; EMG, 20–200 Hz), then digitized at a sampling rate of 128 Hz, and recorded using SLEEPSIGN software (Kohtoh et al., 2008). In addition, locomotor activity (LMA) was recorded with an infrared photocell sensor (Biotex). The vigilance states were scored offline by 10 s epochs into three stages, including waking, rapid-eye movement (REM) sleep, and non-REM (NREM) sleep, according to standard criteria (Mizoguchi et al., 2001). As a final step, defined vigilance stages were examined visually and corrected when necessary.
Assessment of activity and inactivity.
Assessment of LMA and inactivity was performed in adult male A2AR KO mice, BG–A2AR KO mice, and their respective WT littermates (n = 8, per genotype and drug dose). At 9:00 A.M., all animals received an intraperitoneal injection of either vehicle or caffeine at one of the following doses: 2, 10, or 30 mg/kg. Inactivity/activity was used to assess sleep and wakefulness based on a previous report (Pack et al., 2007) after caffeine treatment. LMA was recorded in standard polypropylene cages with seven infrared photocell beams (San Diego Instrument) in 50 s bins during the two experimental days. Inactivity and activity were defined based on LMA as follows: time spent in inactivity (no beam break/50 s) was used as an approximation of sleep; each period of activity was subdivided into high (at least two beam breaks/50 s, assessing ambulation) versus low (one beam break/50 s, assessing rest activity with fine movements) LMA. These three levels of activity were used to analyze the motor stimulant effects of caffeine as opposed to the arousal effects of caffeine, which were assessed using polygraphic recordings as described above.
Generation of AAV vectors.
For the generation of the AAV–shRNA–mCherry vector plasmids, a U6–shA2AR cassette was amplified by PCR from the psiSTRIKE–hMGFP plasmid (Promega) containing the rat A2A receptor shRNA (target sequence 1913) (Chen et al., 2004) or a control shRNA (shCTRL) sequence (GTCAGGCTATCGCGTATCG) and was inserted into the MluI site of the pAAV–hrGFP plasmid (Stratagene). Subsequently, the hrGFP gene was replaced by the gene encoding mCherry. For the generation of the AAV–Cre plasmid, the hrGFP in the pAAV–hrGFP plasmid was replaced by the Cre recombinase coding sequence derived by PCR from the pBS185 plasmid (Sauer and Henderson, 1990). The AAVs of serotype rh10 were generated by tripartite transfection (AAV–rep2/caprh10 expression plasmid, adenovirus helper plasmid, and AAV–vector plasmid) into HEK293A cells and purified by iodixanol density step-gradient centrifugation, as described previously (Zolotukhin et al., 1999). The virus distributed in the 40% density step was concentrated and dialyzed against PBS with a centrifugal concentrator (molecular weight cutoff, 100 kDa; Sartorius) and then titered by quantitative PCR.
Stereotaxic AAV injection and placement of EEG/EMG electrodes.
Surgeries for AAV injections were conducted under pentobarbital anesthesia (50 mg/kg, i.p.). Using aseptic techniques, 6-week-old rats were injected stereotaxically into the NAc and other BG nuclei with recombinant AAV–shA2AR or AAV–shCTRL (250 nl/injection, 6 × 1012 particles/ml) with a glass micropipette and an air pressure injector system (Chamberlin et al., 1998). Also, 8- to 10-week-old conditional A2AR KO mice were injected with AAV–Cre or AAV–mCherry. Table 1 summarizes coordinates used for bilateral injections into selected BG nuclei of rats or conditional A2AR KO mice, according to the atlases of Paxinos and Watson (2001, 2007). At 3 weeks after the AAV injection, rats underwent surgery for implantation of electrodes for EEG and EMG recordings as described previously (Matsumura et al., 1994), whereas EEG/EMG electrodes in the conditional KO mice were implanted as described above. Postoperatively, animals were housed individually for 8–10 d. The caffeine treatment was performed as described above; intraperitoneal injections were made at 9:00 A.M. (mice) or 10:00 A.M. (rats). In addition, at least 1 week after the caffeine treatment, each animal received an injection of vehicle or modafinil on a 2 d schedule as described above. Modafinil (Sigma-Aldrich) was dissolved in saline containing 10% DMSO and 2% (w/v) cremophor immediately before use and administered intraperitoneally at 9:00 A.M. on the experimental day at a dose of 45 mg/kg.
Table 1.
Coordinates for bilateral injections of AAV–Cre or AAV–mCherry in rats and mice according to Paxinos and Watson (2001, 2007)
| Area | Coordinates (mm) |
||
|---|---|---|---|
| Anterior to bregma | Lateral to midline | Below dural surface | |
| Rat | |||
| OLT | 1.8 | 1.5 | 8 |
| 1.3 | 1.3 | 8 | |
| 0.8 | 1.3 | 8 | |
| NAc | 1.8 | 1.5 | 7 |
| 1.3 | 1.2 | 7 | |
| 0.8 | 1 | 6.8 | |
| CPu | 1.8 | 2.0 | 4.5 |
| 1.3 | 2.5 | 4 | |
| 0.8 | 2.5 | 4.5 | |
| GP | −0.4 | 2.2 | 7 |
| −0.9 | 2.8 | 6 | |
| Mouse | |||
| NAc | 1 | 0.75 | 4.2 |
| 1.4 | 0.8 | 3.9 | |
| SI/HDB | 0 | 1.4 | 4.6 |
Each animal received two or three bilateral injections using different sets of coordinates. A2AR-positive areas in the BG: CPu, NAc, OLT, and GP. Arousal-related areas in the basal forebrain: SI and HDB.
Immunohistochemistry.
After all of the above procedures, animals were deeply anesthetized with an overdose of chloral hydrate (500 mg/kg, i.p.) and perfused through the left ventricle of the heart with saline, followed by neutral buffered 10% Formalin. Brains were removed and placed in 10% sucrose in PBS overnight at 4°C to reduce freezing artifacts. The brains were then frozen on dry ice and sectioned at 30 μm (mice) or 40 μm (rats) on a freezing microtome. Immunohistochemistry was performed on free-floating sections as described previously (Estabrooke et al., 2001). In brief, sections were rinsed in PBS, incubated in 3% hydrogen peroxide in PBS for 30 min at room temperature, and then sequentially at room temperature in 3% normal donkey serum and 0.25% Triton X-100 in PBS (PBT) for 1 h and primary antibody diluted in PBT with 0.02% sodium azide overnight. Primary antibodies included rabbit anti-Cre (1:10,000; EMD Biosciences), rabbit anti-mCherry (1:10,000; Clontech), mouse anti-A2AR (1:2000; Millipore), goat anti-A2AR (1:1000; Santa Cruz Biotechnology), chicken anti-β-galactosidase (β-gal) (1:4000; Abcam), and mouse anti-neuronal-specific nuclear protein (NeuN) (1:2000; Millipore). After incubation with the primary antisera overnight, sections were rinsed and incubated for 2 h in biotinylated anti-rabbit, anti-goat, anti-chicken, or anti-mouse secondary antiserum (Jackson ImmunoResearch) at a dilution of 1:1000. Immunoreactions for D2R with a rabbit anti-D2R antibody (1:500; Millipore) were conducted over two nights at 4°C and one night at room temperature. All tissue sections were then treated with avidin–biotin complex (1:1000; Vectastain ABC Elite kit; Vector Laboratories) for 1 h, and immunoreactive cells were visualized by reaction with 3,3′-diaminobenzidine and 0.1% hydrogen peroxide. Tissue sections mounted on glass slides were scanned with Aperio ScanScope, and digital photomicrographs were analyzed with Aperio ImageScope software version 10. The region of A2AR knockdown by shA2AR was identified by the expression of mCherry and confirmed by the absence of A2AR immunoreactivity, whereupon the area of loss of A2ARs in the NAc was quantified on photomicrographs of sections containing the rostral, central, and caudal NAc. Digital photomicrographs were adjusted for optimal display for the output levels of the contained color values and then imported into NIH ImageJ 1.42 software for area measurements of mCherry expression in the NAc versus total nucleus extension. Double immunofluorescence staining for β-gal and choline acetyltransferase (ChAT) was also performed. Sections were incubated overnight at room temperature in a mixture of anti-β-gal and goat anti-ChAT (1:200; Millipore) primary antibodies in PBT with donkey normal serum. On the next day, sections were incubated for 2 h in a mixture of biotinylated anti-chicken and Alexa Fluor-594-conjugated anti-goat secondary antibodies (Invitrogen) at a dilution of 1:500. After several washes, sections were incubated for 1 h in Alexa Fluor-488-conjugated streptavidin (Invitrogen) at a dilution of 1:500. Fluorescence microscopy with tissue sections mounted on glass slides was performed with a Carl Zeiss laser scanning confocal microscope.
Statistical analysis.
The data were presented as the mean ± SEM. Statistical comparisons between two groups were performed by using the unpaired Student's t test. Comparisons among combined multiple parameters (genotype, experimental conditions, and more than two groups) were performed by one-way ANOVA, followed by Bonferroni's post hoc comparisons.
Results
Deletion of A2ARs in the basal ganglia of mice abolishes the arousal effect of caffeine
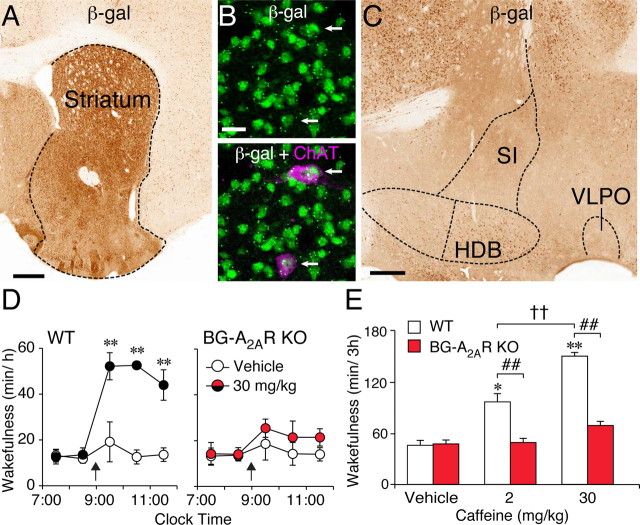
We first examined sleep–wake profiles in our previously developed BG–A2AR KO mice based on the Cre/loxP technology (Shen et al., 2008). The existence of A2ARs in arousal-related cell groups surrounding the striatum, such as the nucleus of the horizontal limb of the diagonal band of Broca (HDB), the substantia inominata (SI), or the ventrolateral preoptic area (VLPO), remains elusive (Svenningsson et al., 1997; Rosin et al., 1998). However, to assess Cre-dependent knock-out of A2ARs in these adjacent areas, we crossbred the Dlx5/6–Cre transgenic mouse, which was used to create the BG–A2AR KO mouse, with a Rosa26 reporter line (Soriano, 1999) expressing β-gal only in the presence of Cre recombinase (Rosa26–Dlx5/6–Cre). The limit of this method may, however, hinge on the barely existing expression of A2ARs in various regions of the brain. As shown in Figure 1A, the striatum, including the olfactory tubercle (OLT), caudate–putamen (CPu), and NAc, showed robust β-gal staining. The vast majority of neurons in the striatum are GABAergic medium-sized spiny output neurons, but double immunofluorescence staining for β-gal and ChAT (Fig. 1B) revealed that the A2AR knock-out in the striatum occurred also in the cholinergic interneurons. Outside of the striatum, sparse β-gal expression was detected in the HDB, whereas β-gal staining was absent in the SI and the VLPO (Fig. 1C). In addition, only scattered cells with β-gal immunostaining were observed in the septum, cerebral cortex, thalamic nuclei, and hippocampus (Fig. 1A or data not shown). Brain sections from Cre-negative mice of the Rosa26–Dlx5/6–Cre line did not show any β-gal staining (data not shown).
Figure 1.
Arousal effect of caffeine was abolished in BG–A2AR KO mice. A–C, Typical sections from the Rosa26–Dlx5/6–Cre reporter mouse were stained with mouse polyclonal antibodies against β-gal to visualize Cre-expressing neurons indirectly. A, Robust expression of β-gal is seen in the striatum of the reporter mouse. B, At a single-cell level, double immunofluorescence for β-gal (green) and ChAT (magenta) on an adjacent section to A shows that cholinergic interneurons in the striatum also express β-gal. The arrows in the top and bottom of B indicate neurons with dual immunolabeling for β-gal and ChAT. C, In the classical arousal/sleep-related cell groups of the basal forebrain and anterior hypothalamus, i.e., the nucleus of the HDB, SI, or VLPO, only moderate immunoreactivity for β-gal is detected in the HDB. β-gal immunolabeling is absent in neurons of the SI and VLPO. Scale bars: A, 500 μm; B, 20 μm; C, 250 μm. D, E, The BG–A2AR KO mice and WT littermates were treated with vehicle or caffeine (2 or 30 mg/kg, i.p.). Time course (D) and total time (E) of wakefulness during the first 3 h after caffeine injection (arrows) were assessed with EEG/EMG recordings. Data are presented as the mean ± SEM (n = 4–5). *p < 0.05, **p < 0.01 compared with vehicle treatment within corresponding genotype. ##p < 0.01 compared with corresponding WT littermates. ††p < 0.01 compared between caffeine doses.
We then recorded EEG and EMG for 2 consecutive days in the BG–A2AR KO mice and their wild-type littermates (Fig. 1D,E). On day 1, the mice were treated with vehicle (intraperitoneally) at 9:00 A.M. in the early phase of the light (inactive) period, and the recordings made on that day served as the baseline data. The animals were then treated with caffeine 24 h later (either 2 or 30 mg/kg, i.p.). The vigilance states were classified offline into three stages: waking, REM sleep, and NREM sleep. Caffeine dose-dependently increased wakefulness in control WT mice 2-fold and 3.2-fold after the 2 and 30 mg/kg doses, respectively (Fig. 1D,E). This arousal effect of caffeine was almost completely eliminated in the BG–A2AR KO mice.
Deletion of A2ARs in the basal ganglia of mice abolishes caffeine-induced locomotor activity
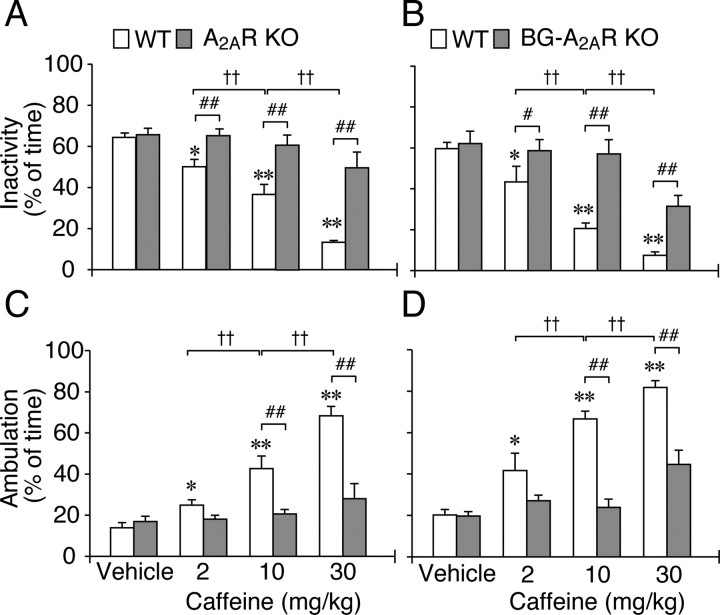
We measured activity in global A2AR KO (Chen et al., 1999) and BG–A2AR KO mice to determine whether caffeine induces in these mice the motor pattern that is typical for an animal during caffeine-induced wakefulness. We injected intraperitoneally male A2AR KO and BG–A2AR KO mice with vehicle (saline) or caffeine in a physiologically relevant range of 2–30 mg/kg at 9:00 A.M. and monitored the behavior of the mice in a field of infrared photocell beams to assess inactivity versus low and high levels of activity. Caffeine dose dependently decreased time spent in inactivity in the control WT mice but not in A2AR KO and BG–A2AR KO mice (Fig. 2A,B). Interestingly, although the time spent in low levels of locomotor activity (fine movement or quiet wakefulness) was not altered by caffeine in the A2AR KO and BG–A2AR KO mice (data not shown), the waking period characterized by high LMA (ambulation) was dose dependently increased by caffeine only in the control WT mice of both A2AR KO genotypes (global vs BG) (Fig. 2C,D). Thus, caffeine acts at A2ARs in the BG to promote wakefulness and associated locomotion.
Figure 2.
Locomotion in A2AR KO and BG–A2AR KO mice after caffeine treatment. Rest–activity assessment was performed based on the amount of LMA in 50 s bins for 3 h after caffeine (2, 10, or 30 mg/kg) treatment. Time spent in inactivity (A, B) or high LMA (ambulation; C, D) is presented as percentage of total time (3 h). Data are presented as mean ± SEM (n = 8, per genotype and drug dose rest–activity assessment). *p < 0.05, **p < 0.01 compared with vehicle treatment within corresponding genotypes. #p < 0.05, ##p < 0.01 compared with corresponding WT littermates. ††p < 0.01 compared between caffeine doses.
Selective deletion of A2ARs in the NAc of mice eliminates the arousal effect of caffeine
The A2AR agonist CGS21680 (2-p-(2-carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine), which induces sleep, also produces c-Fos expression in the NAc (Scammell et al., 2001). We therefore tested whether the arousal effect of caffeine depended on A2ARs in the NAc by using a mouse strain with a loxP-modified A2AR gene conditionally deletable by Cre recombinase. An AAV vector that contained the gene for Cre recombinase under the control of the cytomegalovirus (CMV) promoter was stereotaxically injected bilaterally into the NAc of loxP-modified A2AR mice to generate NAc–A2AR KO. At 3 weeks after the injection of AAV–Cre, immunohistochemistry for A2AR confirmed the loss of A2ARs in the NAc (Fig. 3A, right photomicrograph), whereas A2AR expression was unchanged in the control group of loxP-modified WT mice injected with the red fluorescent protein mCherry-expressing AAV (Fig. 3A, left photomicrograph). Microinjections of AAV vectors do not induce inflammation at the injection site, and tissue injury is minimal, as after the injection of saline (Lazarus et al., 2007). A2ARs are known to be coexpressed with D2Rs on neurons of the NAc (Svenningsson et al., 1997; Durieux et al., 2009), and the unaltered D2R staining confirmed the integrity of the NAc of AAV–mCherry- and AAV–Cre-injected mice except for the absence of the A2ARs (Fig. 3A,B).
Figure 3.
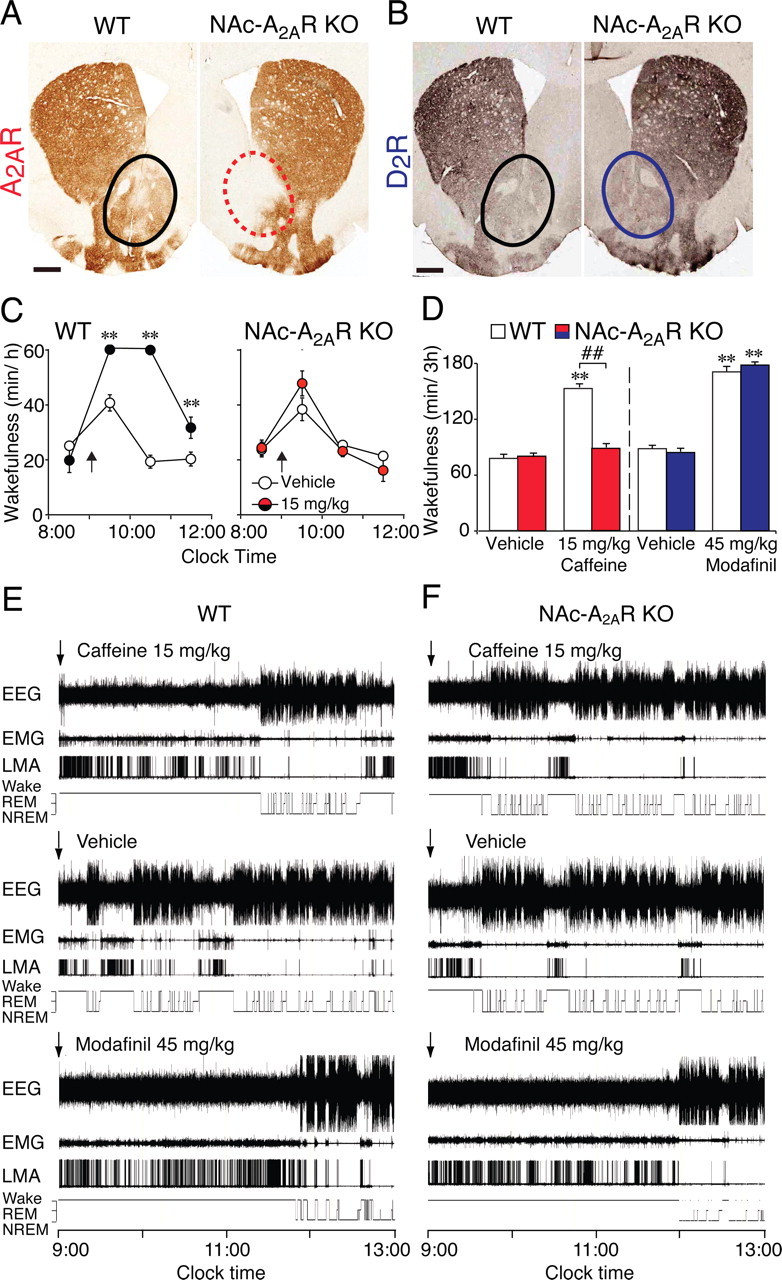
Arousal effect of caffeine was abolished in NAc–A2AR KO mice. A, B, Typical sections of conditional A2AR KO mice after injection with mCherry-expressing AAVs (WT; left photomicrograph) and AAV carrying Cre recombinase (NAc–A2AR KO; right photomicrograph) were stained with a goat polyclonal antibody against A2AR (Santa Cruz Biotechnology) to visualize the presence (black circle) or loss (red dashed circle) of A2ARs in the NAc. Immunostaining with a rabbit polyclonal antibody against D2R (Millipore) confirms the integrity of the NAc (black and blue circles) in the WT and NAc–A2AR KO mice (B). C, D, The NAc–A2AR KO and WT mice were treated with caffeine (15 mg/kg, i.p.) or modafinil (45 mg/kg, i.p.). The time course (C) and total time (D) of wakefulness for the first 3 h after caffeine treatment were assessed from EEG/EMG recordings, as was modafinil-induced wakefulness for 3 h (D, right). Arrows in C indicate the time of caffeine injection. E, F, Typical examples of EEG, EMG, LMA, and hypnograms after administration of caffeine (15 mg/kg, i.p., top panels), vehicle for caffeine administration (middle panels), or modafinil (45 mg/kg, i.p., bottom panels) in a WT (E) and NAc–A2AR KO (F) mouse. Arrows in E and F indicate the time of injection. Data are presented as the mean ± SEM (n = 4–5). *p < 0.05, **p < 0.01 compared with vehicle treatment within corresponding AAV injection. ##p < 0.01 compared with AAV treatment. Scale bars: A, B, 500 μm.
Next, we injected both mouse groups with caffeine (15 mg/kg, i.p.) and recorded their EEG and EMG (Fig. 3C,D). Typically, the effect of caffeine on wakefulness was strongly attenuated in the NAc–A2AR KO mice generated by the AAV–Cre injection compared with the control group injected with AAV–mCherry (Fig. 3C). The time spent in waking in the control mice was increased twofold during a 3 h period after the 15 mg/kg dose of caffeine but was indistinguishable from the vehicle injection in mice with a deletion of A2ARs in the NAc (Fig. 3D, left). In addition, modafinils (45 mg/kg, i.p.), a wakefulness-inducing compound that primarily requires D2R (Qu et al., 2008), induced strong arousal in the NAc–A2AR KO and control mice, causing almost complete insomnia during a 3 h period after injection (Fig. 3D, right). Typical examples of EEG, EMG, LMA, and hypnograms are shown in Figure 3, E and F, after the administration of caffeine (15 mg/kg, i.p.; top panels) or modafinil (45 mg/kg, i.p.; bottom panels) in a WT and NAc–A2AR KO mouse. The vehicle control is only shown for the caffeine administration (middle panels), because the vehicle response in the modafinil and caffeine experiment was similar. Caffeine increased wakefulness in the WT mouse but not in the mouse with a deletion of A2ARs in the NAc (Fig. 3F, top panel), whereas modafinil induced long-lasting suppression of sleep in both the NAc–A2AR KO and control mice. Because the possibility of a knock-out of A2ARs in the HDB of the BG–A2AR KO mice cannot be entirely excluded (Fig. 1C), we also injected AAV–Cre bilaterally into the basal forebrain (BF) region, including the HDB and SI, of loxP-modified A2AR mice and found that caffeine (15 mg/kg, i.p.) induced wakefulness (155 ± 7 min/3 h, n = 4) at similar levels as in WT mice (153 ± 3 min/3 h) (Fig. 1D). These experiments indicate that A2ARs in the NAc were specifically required for the caffeine-induced arousal and that dopamine D2R functions of these neurons were not affected by the deletion of the A2ARs in the same neurons.
Site-specific knockdown of A2ARs in the NAc of rats blocks caffeine-induced arousal
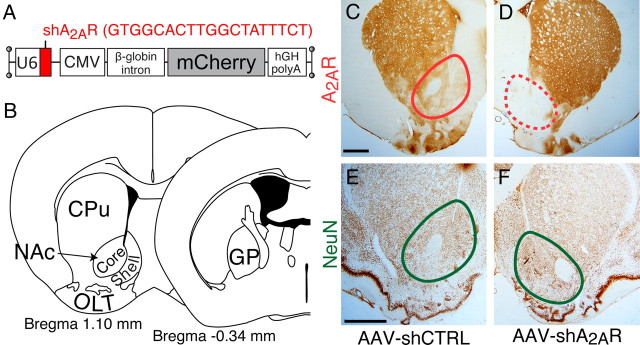
We next aimed to validate our findings by using a knockdown of A2ARs and to define the extent to which the A2AR-positive neurons in the core and shell regions of the NAc were required for the arousal effect of caffeine. Because rats are more suitable for anatomical work than mice, we used rats to dissect the contribution of A2AR-positive neurons in the core and shell of the NAc, as well as in several other BG regions [the OLT, CPu, and globus pallidus (GP)] by stereotaxically injecting AAV vectors that contained short-hairpin interfering RNA specific for A2AR (shA2AR) and the reporter gene mCherry (Fig. 4A,B). The A2AR shRNA sequence was derived from a previously validated small-interfering RNA target for A2AR (Chen et al., 2004). At 4 weeks after the injection, A2ARs were completely eliminated at the site of injection (Fig. 4D). A2AR expression was not attenuated when injections were made with shCTRL with no homology to any known sequences in the rat genome (Fig. 4C). NeuN staining confirmed the absence of neuronal toxicity at the injection site in the NAc of both AAV–shCTRL- and shA2AR-injected rats (Fig. 4E,F).
Figure 4.
Site-specific deletion of A2ARs in rats by focal RNA interference. A, Generation of AAV vectors that contained shA2AR and the red fluorescent protein mCherry as a reporter gene. B, AAV–shA2AR vectors were stereotaxically injected into the A2AR-positive core and shell of the NAc, as well as into the OLT, CPu, and GP. C, D, Typical sections from rats injected bilaterally with AAV carrying shCTRL or shA2AR into the NAc were stained with mouse monoclonal antibody against A2AR. Immunoreactivity for A2AR is depleted selectively in the NAc of the AAV–shA2AR-treated rats (D, dashed red circle), but it is unaffected in the AAV–shCTRL-treated rats (C, red circle). E, F, NeuN staining confirms the integrity of NAc neurons (green circles) in AAV–shCTRL-injected (E) and AAV-shA2AR-injected (F) rats. Scale bars: C, E, 500 μm (also apply to D, F, respectively).
We then examined the effects of an intraperitoneal injection of caffeine (15 mg/kg) in rats that had been bilaterally injected with AAV–shCTRL or AAV–shA2AR into the NAc (Fig. 5). EEG/EMG recordings made during a 3 h period after caffeine and vehicle injections were analyzed to obtain the total duration of wakefulness after each injection, and the difference in total amount of wakefulness after caffeine versus vehicle injection was defined as caffeine-induced wakefulness. We also measured the area of reporter expression as an indicator for the loss of A2A receptors, using tissue sections of the rostral, central, and caudal NAc that were immunostained for the reporter. A Pearson's correlation of r = 0.8 (p < 0.01) between the reporter immunoreactivity and the caffeine-induced wakefulness (Fig. 5A) showed that the disruption of A2ARs in the NAc was proportional to the loss of the arousal effect of caffeine. In contrast, the caffeine-induced arousal was not affected in rats when AAV–shA2AR was bilaterally injected into other A2AR-positive areas of the BG, including the CPu, OLT, and GP, or in AAV–shCTRL-treated or AAV-untreated rats (Fig. 5B).
Figure 5.
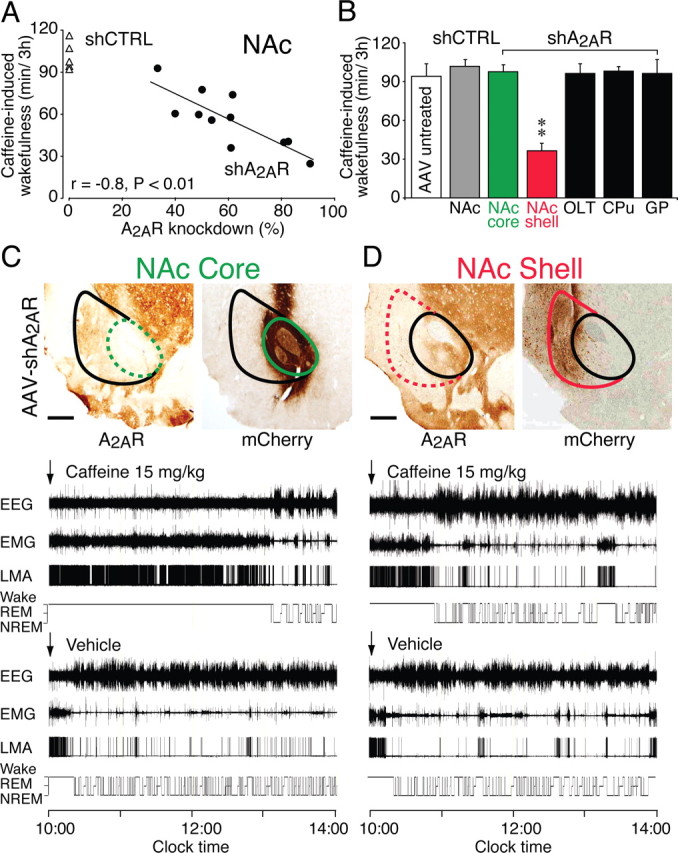
Arousal effect of caffeine was abolished in rats with site-specific deletion of A2ARs in the shell of the NAc. A, The loss of the arousal response to caffeine (15 mg/kg, i.p.) in rats that had received AAV–shA2AR injections (black circles) into the NAc correlated closely with the knockdown of A2ARs in the NAc, whereas there was very little variation in caffeine-induced wakefulness in control animals injected with shCTRL (white triangles). B, Caffeine-induced wakefulness for 3 h in AAV-untreated rats and in rats that received injection of AAV–shCTRL into the NAc or injection of AAV–shA2AR into the NAc shell and core, OLT, CPu, and GP. Caffeine was given intraperitoneally at 15 mg/kg. C, D, Typical sections from two rats injected bilaterally with AAV carrying shA2AR into the shell or core of the NAc that were stained with mouse monoclonal antibody against A2AR showed dominant depletion of A2ARs either in the shell (D, right photomicrograph) or the core (C, left photomicrograph) of the NAc. Adjacent sections to C and D were stained with rabbit polyclonal antibody against mCherry (Clontech) to confirm the extent to which neural cells in the NAc were transfected with AAV–shA2AR (C, D, right photomicrographs). The red circles in the photomicrographs in D outline the entire NAc, including both the core and shell region, whereas the green circles in the photomicrographs in C delineate the core of the NAc. The polysomnographic recordings in C and D show typical examples of EEG, EMG, LMA, and hypnograms after administration of caffeine at a dose of 15 mg/kg (top polysomnographic panel) or vehicle (bottom polysomnographic panel) in two rats with AAV–shA2AR infections of the shell (D) or the core (C) of the NAc. Data are presented as mean ± SEM (n = 5–6 per AAV-treatment). **p < 0.01 compared with AAV-untreated or AAV–shCTRL-injected rats, assessed by one-way ANOVA. Scale bars: C, D, 300 μm.
All injections into the NAc were aimed at the border area between the core and shell of the NAc (Fig. 5D), but occasionally injections fell more laterally. In several such cases, only neurons of the NAc core but not those of the shell portion were infected with AAV–shA2AR (Fig. 5C), and those rats showed a normal response to caffeine (Fig. 5B, green column). Figure 5, C and D, shows typical examples of EEG, EMG, LMA, and hypnograms after the administration of caffeine at a dose of 15 mg/kg (top polysomnographic panels) or vehicle (bottom polysomnographic panels) in two rats with AAV–shA2AR infections of the shell (Fig. 5D) or the core (Fig. 5C) of the NAc. The A2AR depletion in the NAc shell attenuated the effect of caffeine on wakefulness (Fig. 5D, top polysomnographic panel), whereas the rat with a loss of A2ARs only in the core portion of the NAc showed a normal response to caffeine (Fig. 5C, top polysomnographic panel). These results indicate that A2AR-positive neurons in the shell of the NAc were crucial for caffeine to induce wakefulness (Fig. 5B, red column).
Discussion
Our results, using a combination of different gene ablation strategies based on the Cre/loxP technology and RNA interference in two different species, clearly demonstrate that the expression of A2ARs by neurons in the shell of the NAc is essential for the caffeine-induced arousal. For caffeine to be effective as an A2AR antagonist, excitatory A2ARs on NAc shell neurons must be tonically activated by endogenous adenosine. Such tonic activation likely occurs because A2ARs are abundantly expressed in the NAc shell and, even under the most basal conditions, a finite level of adenosine is detected in the extracellular space (Svenningsson et al., 1999a,b). Thus, adenosine activates A2ARs on medium spiny projection neurons in the NAc shell and contributes to restrain the arousal system. As a consequence, caffeine clearly overrides the “adenosine brake” and promotes wakefulness. Therefore, based on a similarity between mouse and man, the area of the human brain in which caffeine acts to counteract fatigue, the shell of the NAc, is just about the astonishingly small size of a pea.
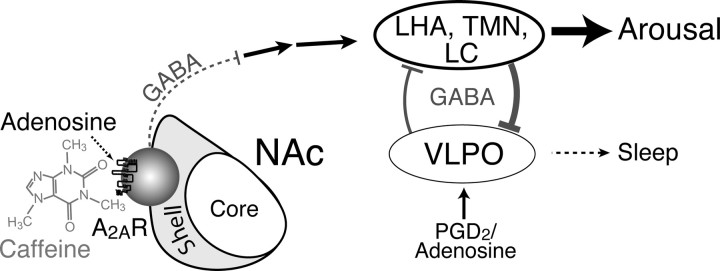
The depletion of A2ARs in the NAc shell diminished the caffeine-induced wakefulness but did not change the amount of wakefulness after the vehicle injection (Fig. 3C,D), indicating that the inhibition of A2ARs in the NAc shell is crucial for caffeine-induced, but not the basal, wakefulness. Therefore, the adenosine A2AR system in the NAc shell is considered to function as an accessory nucleus for regulation of the main sleep center in the VLPO, which is activated by prostaglandin D2 (Scammell et al., 1998) and adenosine acting via A2AR (Scammell et al., 2001). The neural network accounting for the arousal effect of caffeine on A2AR-expressing neurons in the NAc shell is summarized in Figure 6, in which blockade of the massive GABAergic output of NAc shell neurons activates classical arousal centers, such as the lateral hypothalamus (LHA), the tuberomammillary hypothalamic nucleus (TMN), and the locus ceruleus (LC), via direct or indirect projections from the NAc shell. Those arousal centers are reciprocally regulated by the primary sleep-promoting neurons in the VLPO via GABAergic inhibitory projections (Saper et al., 2005, 2010).
Figure 6.
A proposed wake-regulatory role of the A2AR-expressing neurons in the shell of the NAc that accounts for the wake-promoting effect of caffeine. Endogenous somnogens, such as adenosine and prostaglandin D2 (PGD2), promote sleep by activating sleep-promoting neurons of the VLPO (Saper et al., 2005, 2010; Urade, 2011), which, in a putative flip–flop arrangement, inhibit the arousal-promoting regions, including the LHA, TMN, and LC in the brainstem. Adenosine acting at A2ARs on medium spiny neurons in the shell of the NAc is hypothesized to exert inhibitory effects on the arousal systems via indirect (GABAergic and glutamatergic) pathways. Caffeine blocks the A2ARs in the NAc shell, thereby removing the restraint on the arousal systems to promote wakefulness.
The inability of caffeine (15 mg/kg) to induce any arousal effect in mice with A2AR gene deletions in the NAc shell (Figs. 3, 5) indicates that the blockade of A2ARs in the rest of the brain is not sufficient at this dose to promote arousal. However, this observation does not rule out the possibility that A2ARs in other brain regions may also contribute to caffeine-induced wakefulness. Selective reinsertion of A2ARs in the NAc shell of A2AR KO mice would be necessary to show that A2ARs in the NAc shell are sufficient to produce caffeine arousal. In fact, caffeine at 30 mg/kg decreased inactivity and increased LMA compared with the vehicle treatment for the BG–A2AR KO mice but not for the global A2AR KO mice (Fig. 2), suggesting that caffeine at high concentrations has psychomotor effects at A2ARs outside the BG. This minor wake-promoting effect in BG–A2AR KO mice with 30 mg/kg caffeine (Fig. 1D,E) may be attributable to the blockade of A2ARs in the leptomeninges near the VLPO, because these receptors are responsible for activation of sleep-promoting neurons in the VLPO (Scammell et al., 2001).
Adenosine clearly acts as an endogenous somnogen that regulates the homeostatic sleep drive (Urade and Hayaishi, 2010). There are multiple pathways through which sleep and wakefulness can be regulated. Because A2ARs on medium spiny neurons of the NAc are colocalized with D2Rs, which are essential in the maintenance of wakefulness (Qu et al., 2008, 2010), caffeine acting on neurons in the shell of the NAc may modulate neural substrates through which dopamine produces arousal. Adenosine acting via A1R has also been shown to induce sleep by inhibiting the cholinergic region of the BF (Basheer et al., 2004). For example, the unilateral infusion of the BF with an A1R-selective antagonist increased waking and decreased sleep (Strecker et al., 2000). Single-unit recording of BF neurons in conjunction with in vivo microdialysis of an A1R-selective agonist decreased, and an A1R antagonist increased, the discharge activity of the neurons in the BF (Alam et al., 1999). In addition, the activation of A1Rs expressed in the TMN inhibits the histaminergic system and promotes NREM sleep (Oishi et al., 2008). Although caffeine is an antagonist for both A1R and A2AR, it increased wakefulness in A1R KO and WT mice but not in A2AR KO mice (Huang et al., 2005), and, therefore, A1Rs are clearly not required for the effect of caffeine on wakefulness.
Instead of acting at the classical sleep–wake-regulatory neurons, such as the cholinergic BF neurons and the sleep-promoting preoptic neurons, caffeine appears to induce arousal by activating, at least initially, many neuronal pathways that have traditionally been associated with locomotion and motivational behaviors. The NAc shell has long been thought to activate, mainly through indirect pathways via the ventral pallidum and substantia innominata, midbrain–pontine areas that are involved in exploratory locomotion (Mogenson et al., 1983; Groenewegen and Trimble, 2007). In addition, reciprocal connections between the NAc shell and the ventral tegmental area (Zahm and Heimer, 1993), a site of dopamine neurons involved in motivation, reward, and motor control, promote arousal driven by motivation (Sesack and Grace, 2010).
The NAc shell is also well positioned to recruit the cortex, in particular the medial prefrontal cortex (mPFC), into sleep-regulatory circuits. The mPFC is a key executive interface between cognition and emotion but is also uniquely sensitive to sleep and sleep need (Muzur et al., 2002) and might promote sleep (Koenigs et al., 2010). In addition to its modulatory re-entrant projections to the NAc shell, the mPFC could provide a top-down modulation through its direct descending projections to sleep–wake-regulatory systems in the hypothalamus (e.g., the TMN containing histamine and the LHA containing orexins) and the brainstem, including the LC containing noradrenaline (Hurley et al., 1991; Saper et al., 2005). Many of these cortical and subcortical areas also directly or indirectly receive NAc shell outputs (Zahm and Heimer, 1993; Yoshida et al., 2006; Sano and Yokoi, 2007; Sesack and Grace, 2010) and produce strong c-Fos expression, a marker for neuronal activation, in response to systemic caffeine (Deurveilher et al., 2006). The critical role of the NAc shell and its A2ARs in caffeine-induced arousal suggests that this unique transition area between the striatum and the stress and anxiety systems within the extended amygdala may play a regulatory role for sleep mechanisms.
Different from amphetamine, A2AR antagonists (including caffeine) enhance motor and arousal activities but have minimal addictive potential (Fredholm et al., 1999). This difference is probably attributable to the unique cellular localization of the A2AR in the D2R-bearing neurons of the indirect pathway but not in the D1R-bearing striatonigral neurons, in which the psychostimulants amphetamine and cocaine predominantly act and which constitute the major therapeutic target site implicated in drug addiction and dependence. Caffeine may, however, influence the intake or actions of dependence-producing drugs, because the blockade of A2AR can synergize with agents that activate D1R pathways (Le Moine et al., 1997).
Footnotes
This work was supported by Japan Society for the Promotion of Science Grant 21300133, a grant from the Ministry of Education, Culture, Sports, Science, and Technology, a Health and Labor Science Research grant from the Ministry of Health, Labour and Welfare, a Research Grant for promoting Technological Seeds from the Japan Science and Technology Agency, the Takeda Science Foundation, the Sankyo Foundation, the Program of Basic and Applied Researches for Innovations in Bio-Oriented Industry of Japan, Ono Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. LTD., Osaka City, National Natural Science Foundation of China Grants 30625021 and 30821002, Ministry of Science and Technology Grants 2009CB5220004, 2011CB711000, and 2009ZX09303-006, Shanghai Leading Academic Discipline Project B119, NIH Grants NS048995, DA019362, DA024763, and MH083973, and Department of Defense Grant W81XWH-07-0012. We thank N. Matsumoto, E. Ko-Mitamura, M. Masaki, M. Nakamura, and X.-H. Xu for their technical assistance.
The authors declare no competing financial interests.
References
- Alam MN, Szymusiak R, Gong H, King J, McGinty D. Adenosinergic modulation of rat basal forebrain neurons during sleep and waking: neuronal recording with microdialysis. J Physiol. 1999;521:679–690. doi: 10.1111/j.1469-7793.1999.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnulf I, Konofal E, Merino-Andreu M, Houeto JL, Mesnage V, Welter ML, Lacomblez L, Golmard JL, Derenne JP, Agid Y. Parkinson's disease and sleepiness: an integral part of PD. Neurology. 2002;58:1019–1024. doi: 10.1212/wnl.58.7.1019. [DOI] [PubMed] [Google Scholar]
- Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Du B, de Lacalle S, Saper CB. Recombinant adeno-associated virus vector: use for transgene expression and anterograde tract tracing in the CNS. Brain Res. 1998;793:169–175. doi: 10.1016/s0006-8993(98)00169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A2A adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Epperson S, Makhsudova L, Ito B, Suarez J, Dillmann W, Villarreal F. Functional effects of enhancing or silencing adenosine A2B receptors in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2004;287:H2478–H2486. doi: 10.1152/ajpheart.00217.2004. [DOI] [PubMed] [Google Scholar]
- Deurveilher S, Lo H, Murphy JA, Burns J, Semba K. Differential c-Fos immunoreactivity in arousal-promoting cell groups following systemic administration of caffeine in rats. J Comp Neurol. 2006;498:667–689. doi: 10.1002/cne.21084. [DOI] [PubMed] [Google Scholar]
- Durieux PF, Bearzatto B, Guiducci S, Buch T, Waisman A, Zoli M, Schiffmann SN, de Kerchove d'Exaerde A. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci. 2009;12:393–395. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- Groenewegen HJ, Trimble M. The ventral striatum as an interface between the limbic and motor systems. CNS Spectr. 2007;12:887–892. doi: 10.1017/s1092852900015650. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Urade Y, Hayaishi O. The role of adenosine in the regulation of sleep. Curr Top Med Chem. 2011;11:1047–1057. doi: 10.2174/156802611795347654. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Holliday J, Solomon J, Grafman J. Left dorsomedial frontal brain damage is associated with insomnia. J Neurosci. 2010;30:16041–16043. doi: 10.1523/JNEUROSCI.3745-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohtoh S, Taguchi Y, Matsumoto N, Wada M, Huang ZL, Urade Y. Algorithm for sleep scoring in experimental animals based on fast Fourier transform power spectrum analysis of the electroencephalogram. Sleep Biol Rhythms. 2008;6:163–171. [Google Scholar]
- Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10:1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Svenningsson P, Fredholm BB, Bloch B. Dopamine-adenosine interactions in the striatum and the globus pallidus: inhibition of striatopallidal neurons through either D2 or A2A receptors enhances D1 receptor-mediated effects on c-fos expression. J Neurosci. 1997;17:8038–8048. doi: 10.1523/JNEUROSCI.17-20-08038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H, Nakajima T, Osaka T, Satoh S, Kawase K, Kubo E, Kantha SS, Kasahara K, Hayaishi O. Prostaglandin D2-sensitive, sleep-promoting zone defined in the ventral surface of the rostral basal forebrain. Proc Natl Acad Sci U S A. 1994;91:11998–12002. doi: 10.1073/pnas.91.25.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A, Eguchi N, Kimura K, Kiyohara Y, Qu WM, Huang ZL, Mochizuki T, Lazarus M, Kobayashi T, Kaneko T, Narumiya S, Urade Y, Hayaishi O. Dominant localization of prostaglandin D receptors on arachnoid trabecular cells in mouse basal forebrain and their involvement in the regulation of non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2001;98:11674–11679. doi: 10.1073/pnas.201398898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Swanson LW, Wu M. Neural projections from nucleus accumbens to globus pallidus, substantia innominata, and lateral preoptic-lateral hypothalamic area: an anatomical and electrophysiological investigation in the rat. J Neurosci. 1983;3:189–202. doi: 10.1523/JNEUROSCI.03-01-00189.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzur A, Pace-Schott EF, Hobson JA. The prefrontal cortex in sleep. Trends Cogn Sci. 2002;6:475–481. doi: 10.1016/s1364-6613(02)01992-7. [DOI] [PubMed] [Google Scholar]
- Oishi Y, Huang ZL, Fredholm BB, Urade Y, Hayaishi O. Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2008;105:19992–19997. doi: 10.1073/pnas.0810926105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack AI, Galante RJ, Maislin G, Cater J, Metaxas D, Lu S, Zhang L, Von Smith R, Kay T, Lian J, Svenson K, Peters LL. Novel method for high-throughput phenotyping of sleep in mice. Physiol Genomics. 2007;28:232–238. doi: 10.1152/physiolgenomics.00139.2006. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego: Academic; 2001. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Boston: Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu WM, Huang ZL, Xu XH, Matsumoto N, Urade Y. Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J Neurosci. 2008;28:8462–8469. doi: 10.1523/JNEUROSCI.1819-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu WM, Xu XH, Yan MM, Wang YQ, Urade Y, Huang ZL. Essential role of dopamine D2 receptor in the maintenance of wakefulness, but not in homeostatic regulation of sleep, in mice. J Neurosci. 2010;30:4382–4389. doi: 10.1523/JNEUROSCI.4936-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol. 1998;401:163–186. [PubMed] [Google Scholar]
- Sano H, Yokoi M. Striatal medium spiny neurons terminate in a distinct region in the lateral hypothalamic area and do not directly innervate orexin/hypocretin- or melanin-concentrating hormone-containing neurons. J Neurosci. 2007;27:6948–6955. doi: 10.1523/JNEUROSCI.0514-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B, Henderson N. Targeted insertion of exogenous DNA into the eukaryotic genome by the Cre recombinase. New Biol. 1990;2:441–449. [PubMed] [Google Scholar]
- Scammell T, Gerashchenko D, Urade Y, Onoe H, Saper C, Hayaishi O. Activation of ventrolateral preoptic neurons by the somnogen prostaglandin D2. Proc Natl Acad Sci U S A. 1998;95:7754–7759. doi: 10.1073/pnas.95.13.7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Gerashchenko DY, Mochizuki T, McCarthy MT, Estabrooke IV, Sears CA, Saper CB, Urade Y, Hayaishi O. An adenosine A2A agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience. 2001;107:653–663. doi: 10.1016/s0306-4522(01)00383-9. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HY, Coelho JE, Ohtsuka N, Canas PM, Day YJ, Huang QY, Rebola N, Yu L, Boison D, Cunha RA, Linden J, Tsien JZ, Chen JF. A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knock-outs. J Neurosci. 2008;28:2970–2975. doi: 10.1523/JNEUROSCI.5255-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Strecker RE, Morairty S, Thakkar MM, Porkka-Heiskanen T, Basheer R, Dauphin LJ, Rainnie DG, Portas CM, Greene RW, McCarley RW. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav Brain Res. 2000;115:183–204. doi: 10.1016/s0166-4328(00)00258-8. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Kull B, Sunahara R, Bloch B, Fredholm BB. Cellular expression of adenosine A2A receptor messenger RNA in the rat central nervous system with special reference to dopamine innervated areas. Neuroscience. 1997;80:1171–1185. doi: 10.1016/s0306-4522(97)00180-2. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999a;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Fourreau L, Bloch B, Fredholm BB, Gonon F, Le Moine C. Opposite tonic modulation of dopamine and adenosine on c-fos gene expression in striatopallidal neurons. Neuroscience. 1999b;89:827–837. doi: 10.1016/s0306-4522(98)00403-5. [DOI] [PubMed] [Google Scholar]
- Urade Y. Prostaglandin D2 and adenosine as endogenous somnogens. Sleep Biol Rhythms. 2011;9:10–17. [Google Scholar]
- Urade Y, Hayaishi O. Crucial role of prostaglandin D2 and adenosine in sleep regulation: experimental evidence from pharmacological approaches to gene-knockout mice. Future Neurol. 2010;5:363–376. [Google Scholar]
- Yoshida K, McCormack S, España RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell. J Comp Neurol. 1993;327:220–232. doi: 10.1002/cne.903270205. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ, Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]