Abstract
The shared epitope (SE) - an HLA-DRB1-encoded 5-amino acid sequence motif carried by the vast majority of rheumatoid arthritis (RA) patients - is a risk factor for severe disease. The mechanistic basis of RA-SE association is unknown. This group has previously demonstrated that the SE acts as a signal transduction ligand that activates nitric oxide and reactive oxygen species production. SE-activated signaling depends on cell surface calreticulin, a known innate immunity receptor previously implicated in immune regulation, autoimmunity and angiogenesis. Recent evidence that the SE enhances the polarization of Th17 cells, which is a key mechanism in autoimmunity, is discussed highlighting one of several potential functional effects of the SE in RA.
Keywords: Calreticulin, Nitric oxide, Oxidative stress, Rheumatoid arthritis, Shared epitope, Th17
1. Introduction
Rheumatoid arthritis (RA) affects 0.5–1.0% of the population [1]. The disease is characterized by chronic inflammatory changes in both articular and extra-articular tissues. Due to its high prevalence and debilitating nature, RA inflicts a major economic burden on society. In recent years it has been realized that in addition to causing pain and disability, the disease significantly shortens life expectancy due to accelerated atherosclerosis [2].
Although genes play a major role in RA risk, the disease appears to have low sibling occurrence with a concordance rate of 12 to 15% in monozygotic twins [3]. Overall, the contribution of genetic factors to RA risk is calculated at approximately 60%, while the remaining 40% are believed to be contributed by environmental factors. The observations that RA is more common in urban versus rural populations, a recent decline in the incidence of the disease in high-incidence of populations, and the effect of birth cohort on disease incidence are all indirectly supporting environmental influences. Importantly, over the past few years it has been conclusively shown that the disease is strongly associated with environmental pollutants, such as cigarette smoking [4].
Among the genetic risk factors, the HLA-DRB1 locus is the most significant one. RA has long been shown to associate with human leukocyte antigen (HLA) genes. The pioneering studies on HLA-RA association were carried out in the late 1970s by Peter Stastny [5] and Robert Winchester’s group [6], who independently concluded that HLA-DR4 is significantly more common among patients with RA. It was subsequently found that other HLA-DR serotypes, for example, HLA-DR1 in Mediterranean, or HLA-DR14 in Native Americans, are also associated with the disease. With the advent of modern DNA sequencing techniques it had become apparent in the 1980’s that there is no RA-specific HLA-DR sequence. Instead, it was found that the majority of RA patients share a short sequence motif coded by several HLA-DRB1 alleles. This revelation had prompted the Shared Epitope Hypothesis [7].
2. The RA shared epitope
The term “Shared Epitope” (SE) most commonly refers to a five amino acid sequence motif in residues 70–74 of the DR chain coded by several HLA-DRB1 alleles that are over-represented among RA patients (Fig. 1). The SE motif consists of three homologous amino acid sequence variants: 1. QKRAA, the SE variant that is the most common motif among Caucasian, is coded primarily by the HLA-DRB1*0401 allele; 2. The second most common motif, QRRAA, is coded by several alleles, among them HLA-DRB1*0404, HLA-DRB1*0101, and HLA-DRB1*0405; 3. The third motif, RRRAA, coded by allele HLA-DRB1*1001, is the rarest. In addition to increasing RA risk, SE-coding HLA-DRB1 alleles have been shown to associate with more severe disease [8] and to exhibit allele-dose effect, i.e. patients with 2 SE-coding alleles tend to experience more severe disease than patients with 1 allele, who, in turn, have more severe RA than SE-negative patients.
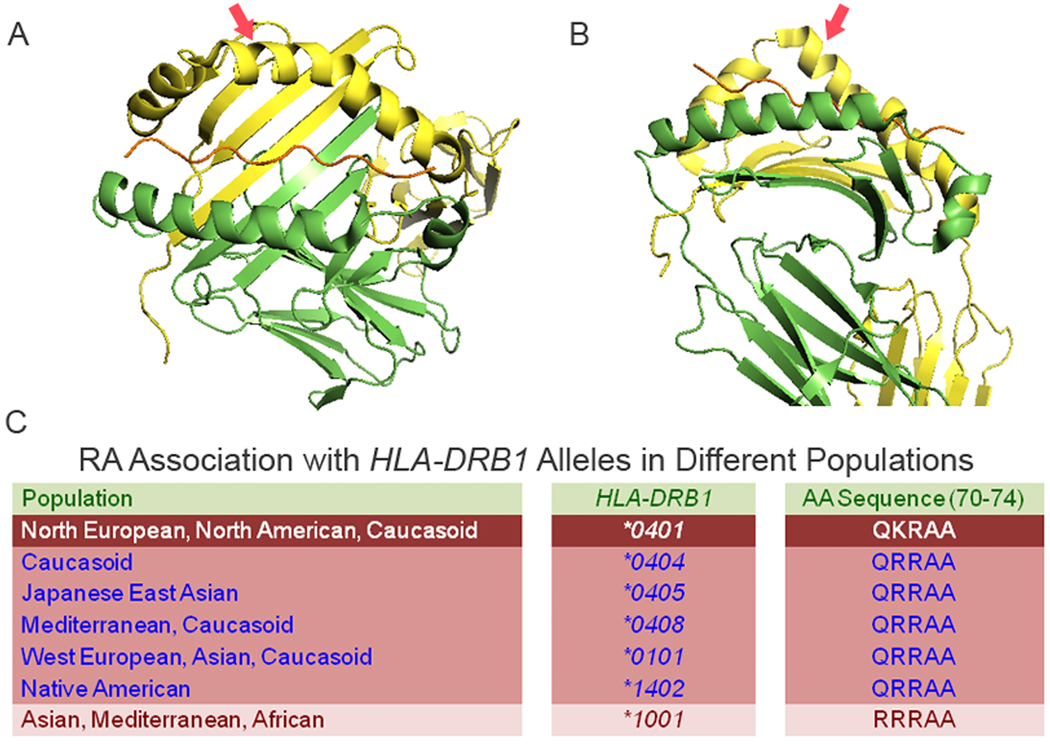
Figure 1.
Structure and epidemiology of the RA SE. A. ‘top’ view of a SE-expressing (HLA-DR1) molecule. The chain is shown in green, the chain is in yellow and the groove peptide is in brown. The red arrow points at the SE-containing helical loop. B. ‘side’ view of the same molecule. Note the localization of the SE near the chain ‘kink’. C. Ethnic and geographic distribution of RA-associated HLA-DRB1 alleles and their SE products.
The mechanism underlying the effect of the SE is unclear. Based on the known role of MHC class II molecules in antigen presentation, the prevailing paradigms postulate that presentation of arthritogenic self-peptides [9], molecular mimicry with foreign antigens [10], or T cell repertoire selection [11] are involved. While these hypotheses are all plausible, they are difficult to reconcile with the fact that data supporting antigen-specific responses as the primary event in RA are inconclusive. Additionally, several other human diseases have also been shown to be associated with SE-encoding DRB1 alleles, including polymyalgia rheumatica [12], giant cell arteritis [12], Type I diabetes [13], erosive bone changes in psoriatic arthritis [14] and lupus [15], autoimmune hepatitis [16] and early-onset chronic lymphoid leukemia [17], among other conditions. The SE is also associated with spontaneous arthritis in dogs [18] and, in HLA-DRB1*0401 transgenic mice it increases the incidence of spontaneous diabetes [19] and the severity of both collagen-induced arthritis (CIA) [20] and experimental autoimmune encephalomyelitis (EAE) [21]. Thus, although it is best known for its involvement in RA, the SE associates with several pathogenically unrelated diseases and experimental disease models, and its effect seems to lack antigen- or species-specificity. These promiscuities are incongruent with fundamental tenets of MHC-restricted antigen presentation theory.
3. Activation of Innate Signaling by the SE: A New Paradigm
Given the inconsistencies of SE-RA association with antigen presentation-based theories, over the past few years, our laboratory has examined an alternative hypothesis concerning the role of the SE in RA [22–28]. Based the known tri-dimensional homology among products of the MHC gene family, we postulated that similar to class I MHC-coded molecules [29], the SE may be acting as a ligand that can trigger innate immune signaling. The rationale of this antithetic hypothesis relates to the fact that the SE is located near the apex of helical tri-dimensional structural motif that has been preserved throughout the entire MHC gene family and seems to be enriched in signal transduction ligands.
The first crystal structure of a class II MHC molecule, published in 1993 by Don Wiley’s group [30], revealed a remarkable tri-dimensional similarity to a previously reported class I MHC molecule. The degree of the similarity was surprising, given a substantial evolutionary distance between the two molecules and the fact that the peptide-binding groove in class I molecules is coded by a single gene, while in class II it is formed jointly by the products of two distinct genes. The extent of evolutionary ‘choreography’ required to bring these two disparate MHC molecules to form a near-identical tri-dimensional structure, is staggering. One of the notable features of the similarity is a ‘kink’ in the 2 domain of the class I MHC molecule, which could be almost perfectly superimposed on a similar structure in the 1 domain of the class II molecule. The ‘kink’ region in both molecules involves allele-diversity regions. Subsequent crystal analyses have shown very similar tridimensional structures in the entire MHC gene product family, irrespective of whether or not they can present antigens [31]. In all cases, this region forms a sharp protrusion ‘above’ the MHC groove plane (Fig. 1).
The remarkable conservation of a similarly-shaped ‘kink’ in the midst of allele diversity regions in MHC molecules independent of their antigen presentation capabilities suggests that this region may possess important allele-specific, conformationally-dependent, non-antigen presentation functions. Indeed, there are some indications that this region performs such functions. For example, in both classical and non-classical (HLA-E) class I MHC molecules, this region contains ligands for natural killer (NK) cell receptors [32]; in HFE (an empty-grooved human class I-like molecule), it interacts with transferrin receptor [33]; In M10 (a mouse class I-like molecule), the same region has been proposed as an interaction site with a pheromone receptor [34].
These considerations have led us to pursue a novel hypothesis which postulates that similar to its structural homologue in the class I MHC molecule, the SE functions as a signal transduction ligand that interacts with an evolutionarily-conserved receptor. Our studies [22–28] have indeed demonstrated that the SE, whether expressed in its native conformation on the cell surface; as a cell-free HLA-DR tetrameric molecule; engineered into large recombinant proteins; or as a short synthetic peptide, activated in all cases nitric oxide (NO)-mediated signaling in trans in a strictly allele-specific manner. A consensus motif comprising of the 70Q/R-K/R-x-x-A74 sequence was found to be necessary for triggering the signal.
Given the known pro-oxidative effect of NO and the proposed role of oxidative stress in the pathogenesis of RA, we have explored whether SE-triggered signaling can increase cellular oxidative stress. These studies have shown that cells exposed to cell surface SE-positive HLA-DR molecules, to cell-free recombinant proteins genetically engineered to express the SE motif, or to SE-positive synthetic peptide showed diminished cyclic AMP-dependent signaling, increased reactive oxygen species (ROS) levels, and higher vulnerability to oxidative DNA damage. The SE effect is critically dependent on amino acids Q/R70, K/R71 and A74 of the DR chain. The pro-oxidative effect of the SE could be reversed by inhibiting NO production. Thus, these studies demonstrate that the SE acts as a signaling ligand that activates an NO-mediated pro-oxidative pathway.
SE-triggered signaling is transduced via cell surface calreticulin (CRT), a known innate immunity receptor. The role of CRT and the dependence on CD91 have been published [25]. In brief, cell surface CRT was identified as the SE-binding molecule using affinity chromatography purification, cell-binding assays, surface plasmon resonance (SPR) and time-resolved fluorescence resonance energy transfer techniques. SE-triggered signaling could be blocked by anti-CRT antibodies or antibodies against CD91 and by CRT-specific anti-sense or small interfering RNA (siRNA) oligonucleotides. Murine embryonic fibroblasts from Crt−/− or cd91-deficient mice failed to transduce SE-activated signals. Exogenously added soluble CRT attached to the cell surface and restored signaling responsiveness in Crt−/− cells.
More recently we have mapped the SE binding site on CRT [28]. SPR experiments with domain deletion mutants suggested that the SE binding site is located in the P-domain of CRT. The role of this domain as a SE-binding region was further confirmed by a photoactive cross-linking and mass spectrometry methods. In silico analysis of docking interactions between a conformationally intact SE ligand and the CRT P-domain predicted the region within amino acid residues 217–224 as a potential SE binding site. Site-directed mutagenesis demonstrated involvement of residues Glu217 and Glu223 and, to a lesser extent, residue Asp220 in cell-free SPR-based binding and signal transduction assays.
4. Potential Functional Consequences of SE-activated Signaling
As illustrated in Figure 2, the SE acts as a signal transduction ligand that interacts with cell surface CRT and activates signal transduction in trans. Although the SE-CRT pathway has not been fully mapped, our data to date indicate that it involves activation of NO synthase, and production of ROS. We have also demonstrated that the SE-activated pathway blocks a cyclic AMP-mediated pathway [23].
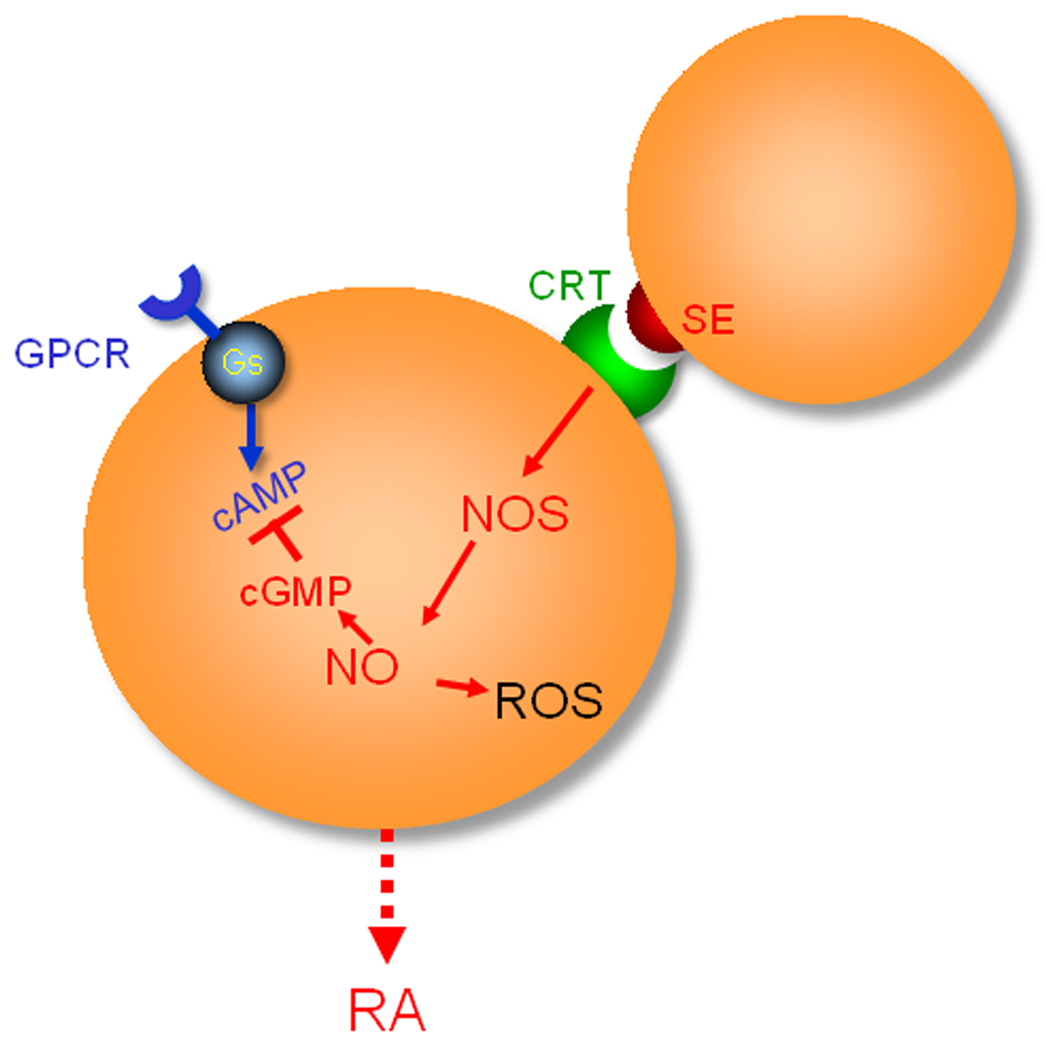
Figure 2.
The SE-CRT pathway. See text for details. Abbreviations: cAMP, cyclic AMP; cGMP, cyclic GMP; GPCR, G protein-coupled receptor; Gs, stimulatory G protein; NOS, NO synthase.
NO is a ubiquitous signaling molecule with versatile effects in the immune system. In RA, increased NO levels correlate significantly with inflammatory markers [35] and anti-rheumatic agents have been shown to suppress NO production [35,36]. NO has also been implicated in the pathogenesis of experimental autoimmune models in mice. For example, SJL mice (H-2s) are known for their NO overproduction [37]. These mice are susceptible to many autoimmune diseases, including EAE, myasthenia, myositis, inflammatory bowel disease and CIA [38], and their autoimmune tendencies are attributed to their NO overproduction [39]. Similar to human RA, SJL mice display aging-associated increase in disease incidence [40], excessive DNA damage [41], higher mutation rates [42] and higher incidence of spontaneous lymphoma [43].
There are many ways by which NO could contribute to RA pathogenesis. The salient cellular mechanisms by which SE-activated NO could be involved in RA are depicted in Figure 3. For example:
NO has been shown to modulate apoptosis, a process that has been extensively implicated in RA and other autoimmune conditions [44].
NO plays a major role in angiogenesis [45], a key pathogenic mechanism in the inflammatory pannus. Blood vessels are critically important for both nourishing the proliferative synovial tissue and for the ingress of inflammatory leukocytes into the joint. Anti-angiogenic agents have been shown to modulate arthritis in animal models of RA [46]. Additionally, certain disease modifying anti-rheumatic drugs, such as methotrexate or anti-TNF antibodies, have been found to be angiostatic [47]. Thus, SE-activated NO overproduction could conceivably enhance angiogenesis in RA.
The pathogenesis of RA pannus involves activation of matrix metalloproteinases (MMPs), with MMP-13 being of particular interest. The relevance of this MMP to RA relates to its high potency and specificity for type II collagen [48]. In addition to its direct tissue degrading effects, MMP-13 is a likely contributor to RA pathogenesis due to its central position in the MMP activation cascade [49] and its pro-angiogenic effect [50]. MMP-13 is upregulated by inflammatory cytokines, such as IL-1, IL-6, TNF and IL-17 [51]. Consistent with the model discussed here, NO has been shown to increase MMP-13 expression and activity [52]. Directly relevant to the focus of this review, NO has been recently shown to potently inhibit indoleamine 2,3 dioxygenase (IDO), a tolerogenic enzyme [53, 54]. Our recent studies have indeed demonstrated that the SE, which trigger NO signaling in dendritic cells (DCs) has an inhibitory effect on IDO activation, with immune dysregulatory effect, both in vitro and in vivo (See below).
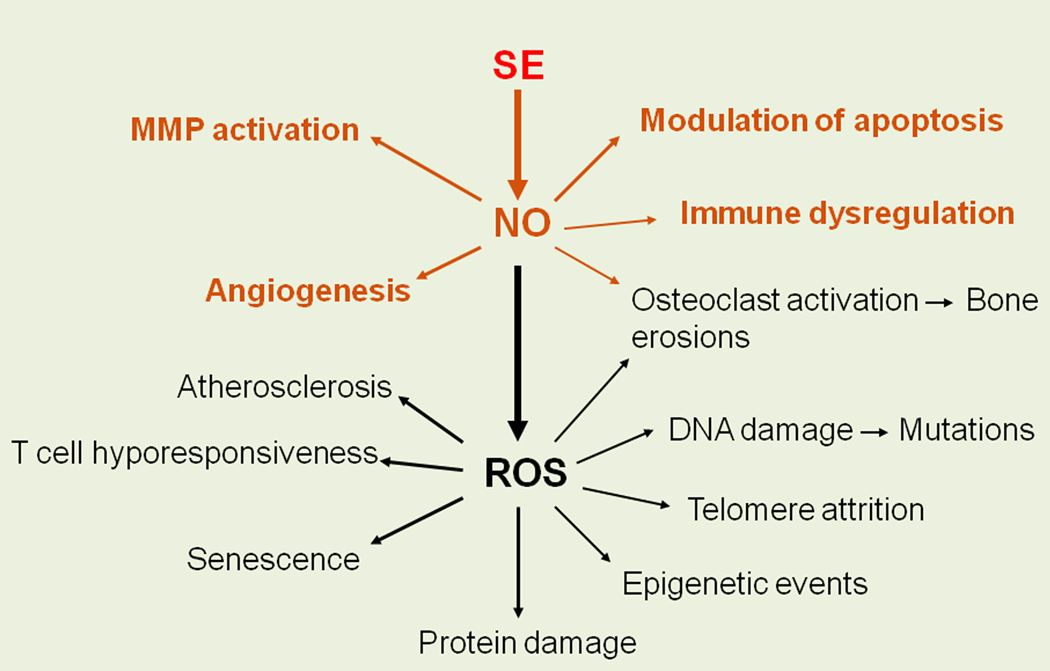
Figure 3.
Potential RA-relevant functional consequences of SE-activated signaling.
As Figure 3 shows, SE-activated NO production leads to oxidative stress. The role of ROS in RA has been extensively studied [55]. There are many potential RA-relevant cellular and molecular mechanisms that could be affected by excessive production of ROS. Among them: protein and DNA damage, epigenetic modifications, telomere attrition, cell senescence and T cell hyporesponsiveness, all of which have been implicated in the pathogenesis of RA [56–61]. Among the many potential pathogenic effects, two ROS-mediated disease processes are of particular interest: atherosclerosis (AS) and bone erosions.
As mentioned above, RA patients have long been noticed to have shorter life expectancy. It is now becoming increasingly apparent that AS is the most common cause of premature death in RA with a relative risk of about 2, compared with age-matched control populations [2]. Several studies have noted the fact that AS risk in RA exists in a rate greater than would be expected from the profile of classical cardiovascular risk factors, such as diabetes mellitus, hypercholesterolemia, obesity or hypertension [62]. The prevalent hypothesis for the increased risk of AS in RA attributes the association to inflammation. However, there is substantial evidence to suggest that the inflammatory milieu may not be the sole culprit. For example, in RA patients without established cardiovascular risk factors, the erythrocyte sedimentation rate was found to lack correlation with the carotid intima-media thickness, a known biomarker of AS [63]. Additionally, in RA patients chronically treated with TNF blockers, endothelial cell dysfunction continues to exist [64] and AS continues to progress [65] despite reversal of the inflammatory state and improvement of the arthritis. Finally, patients with many other inflammatory conditions are not known to be at a higher AS risk, suggesting that disease-specific factors may contribute to the increased AS risk in RA. Given the unequivocal role of oxidative stress in AS on one hand, and anecdotal reports suggesting that SE association with AS may exist also in the non-RA population [66–68] on the other, the possibility that SE-activated ROS production might be a direct contributing factor to premature AS development in RA is worth consideration.
Osteoclast-driven destruction of juxta-articular bone is a hallmark of RA. The severity of bone destruction is RA has been linked to the SE [69] with indications for allele-dose effect in certain populations [70]. Interestingly, the SE has also been implicated in erosive changes in non-RA conditions, such as psoriatic arthritis [14], SLE [15] and periodontal disease [71]. Thus, the SE may be involved in bone destruction irrespective of the underlying disease. The mechanism discussed here involving SE-activated NO and ROS production might provide a mechanistic basis for these associations. It has been previously shown that NO activates osteoclasts in a biphasic dose-dependent fashion [72]. ROS, likewise, have been shown to activate osteoclastogenesis [73] and bone resorption [75]. Accordingly, one scenario to consider is that the SE may contribute to osteoclast activation by stimulating higher production of NO and ROS, thereby increasing osteoclast-mediated bone destruction.
5. A Case in Point: SE-Activated Immune Dysregulation
Using the diagram shown in Figure 3 as a ‘blueprint’, this laboratory has been investigating RA-relevant SE effects in several pathogenic system with encouraging results. Here we discuss our recent findings in one of the areas of our current research interest: SE-activated immune dysregulation.
As discussed in greater detail in a recent review [27] CRT has been previously implicated in immune regulation. CRT is a 60 kDa protein which is expressed on the surface of many cells [75,76] and functions as an important innate immune system receptor [77–79]. It serves as the signal-transducing receptor for members of the collectin family [80]. Collectins bind foreign organisms or apoptotic cells through their globular heads, while their collagen-like tails bind to cell surface CRT. This leads to CRT-dependent phagocytosis. Different from elimination of foreign organisms, events which are associated with an intense inflammatory reaction [81], safe elimination of apoptotic cells is critically dependent on suppressing the inflammatory response [82]. The decision whether a pro- or anti-inflammatory reaction should be activated depends on the presence or absence of second signals that are triggered uniquely by apoptotic cells [83]. Thus, CRT plays a pivotal role in the junction between tolerance and autoimmunity due to its critical role in elimination of apoptotic cells [80]. Aberrant activation of the CRT-mediated pathway can lead to autoimmunity as exemplified by conditions that involve defective CRT-mediated clearance of apoptotic cells [84].
Our research interest in the effect of the SE on DCs is partly based on the fact that these cells are known to express functional CRT receptors on their surface [76]. DCs are professional antigen presenting cells strategically positioned in the interface between the innate and adaptive immune systems. In addition to their role in antigen presentation, DCs also induce tolerance through a variety of mechanisms, including a direct cross talk with regulatory T (Treg) cells [85]. A growing body of evidence indicates that the tolerogenic effect of DCs is mediated partly by IDO, an enzyme that catalyzes the catabolism of tryptophan [86,87]. The precise mechanism by which IDO exerts its effect is unknown, but may involve tryptophan depletion and/or pro-apoptotic or anti-proliferative effects of tryptophan downstream metabolites [88]. IDO is inducible in DCs by the Th1 cytokine IFN [89] and by Treg-expressed CTLA4 through ligation of cell surface CD80/CD86 molecules [90]. Activation of IDO in DCs by Treg has been shown to inhibit IL17-producing helper T (Th17) cells [91], a T cell subset that is believed to play a key role in autoimmunity (discussed below).
It is worth noting that in contrast to the IDO-inducing effect of IFN and CTLA4, NO is a potent inhibitor of IDO [53,54]. Given our findings that the SE activates NO production in many cell types, including DCs, we examined whether the SE ligand could affect IDO enzymatic activity. Using murine fibroblast L-cells transfectants expressing structurally intact and functionally HLA-DR / heterodimeric molecules through cDNA transfection we demonstrated that transfectants expressing SE-positive HLA-DR molecules on their surface produced significantly less kynurenine (an IDO-dependent tryptophan metabolite) in response to IFN , compared to transfectants expressing SE-negative HLA-DR molecules. An identical pattern was seen when human fibroblasts were stimulated with a soluble SE ligand in the form of SE-expressing peptides. Importantly, the SE ligand IDO inhibitory activity was restricted to CD11c+CD8+ DCs, a subset known to express IDO [92]. Thus, the SE ligand effectively and specifically inhibits the activity of the tolerogenic enzyme IDO in DCs.
In addition to IDO-mediated T cell regulation, DCs can affect immune reactions by production of cytokines that can activate or expand particular subsets of T cells. In mice, the combination of IL-6 and TGF facilitates differentiation of Th17 cells, while IL-23 is involved in the expansion of this subset [93]. Accordingly, we have studied supernatants of SE-stimulated DCs. Our data showed that in the CD11c+CD8− DC subset, but not in the CD11c+CD8+ subset, a SE peptidic ligand activated a robust production of IL-6. An SE-negative ligand did not trigger any cytokine production. Other cytokines (IL-4, IL-10, IL-12, IL-1 , TGF ) did not show any increased production, indicating the specificity of SE effect. Interestingly, IL-23 levels in DCs did not increase following stimulation with the SE ligand. However, in the presence of suboptimal concentrations of LPS (100 ng/ml), the SE had a prolonged synergistic effect on IL-23 production. The effect was specific for IL-23, since no synergism was found in the production of another LPS-inducible cytokine, IL-6.
It has been previously demonstrated that IDO inhibition [94] or increased IL-6 levels [95] inhibit Treg cells. As discussed above, the SE inhibited IDO activity in CD11c+CD8+ DCs and increased IL-6 production in CD11c+CD8− DCs. We therefore determined whether the SE interferes with Treg differentiation. Mouse CD11c+DCs were first incubated overnight with SE-positive or SE-negative peptides, or with medium. DCs were then co-cultured with purified syngeneic CD4+ T cells or CD4+CD25−CD62L+CD44− naïve T cells in the presence of TGF-β and anti-CD3 Ab. After 5 days, CD4+CD25+Foxp3+ Treg abundance was determined by FACS analysis. Our data showed that the SE-positive, but not an SE-negative ligand, significantly inhibited Treg cell differentiation. Similarly, splenic CD4+CD25−CD62L+CD44−naïve T cells cultured with CD11c+DCs, treated with a SE-positive HLA-DR tetramer, but not with SE-negative HLA-DR tetramers demonstrated markedly reduced Treg cell differentiation.
Since SE ligands were found to enhance production of Th17-promoting cytokines. IL-6 and IL-23, we determined whether they can facilitate Th17 differentiation. To this end, CD11c+DCs were first stimulated overnight with SE-expressing peptides or tetramers. CD4+CD25− CD62L+CD44− naïve T cells were added and cultured in the presence of a Th17-polarizing cocktail of cytokines and antibodies. After 6 days cells were collected and analyzed by flow cytometry. The results showed that SE-positive ligands, in particular when presented as HLA-DR tetramers, had a robust enhancement of Th17 differentiation. The SE showed similar effect when expansion of Th17 cells was studied [26].
To determine the biologic significance of the in vitro data shown above, we have undertaken to characterize the SE polarizing effect in vivo. Our findings showed that mice immunized with collagen type II in the presence of a cell-free SE ligand displayed much higher abundance of Th17 cells in the draining lymph nodes, compared to mice immunized with the same antigen in the presence or absence of a control ligand. Similarly, splenocytes from mice immunized with collagen in the presence of the SE ligand produced much higher levels of IL-17 compared to the control groups [26]. Thus, the SE facilitates Th17 polarization both in vitro and in vivo.
It is worth noting that IL-17 has been shown to enhance several pro-arthritogenic processes, including angiogenesis, MMPs production, osteoclastogenesis, leukocyte recruitment and inflammation [96]. Both IL-17-producing cells and IL-17 are abundantly expressed in the RA joint [97], and neutralizing IL-17 prevents experimental arthritis development, while deficiency of Treg cells has been shown to increase cellular and humoral immune responses and disease severity in CIA [98]. Thus, SE-activated Th17 polarization, observed by us both in vitro and in vivo, suggests a potential mechanism by which the SE could affect RA pathogenesis.
6. A Proposed Model
Different from the prevailing paradigms, which attribute the role of the SE in RA to presentation of a putative self or foreign antigen, the considerations discussed above and our experimental findings implicate an allele-specific, antigen presentation-independent mechanism. We have shown that the SE activates RA-relevant signaling events in several immune and non-immune cell types. The SE signaling effect is independent of the antigen presentation function of the parent HLA-DR molecule and could be seen using synthetic SE ligands, which do not possess any antigen presentation capability. It is worth mentioning, however, that using X-ray crystallography-based analyses others have predicted that amino acid residues K71 [99], or Q70 and K71 [100] of the HLA-DRB1*0401-coded SE may participate in the binding to human collagen type II-derived groove peptides. These predictions seemingly conflict with the model proposed here, however, it should be explained that: 1. The identity of the target antigen in RA is unknown. The role of collagen type II as a target self antigen has been disputed by many. It is therefore unclear whether the proposed interactions between SE residues and collagen II epitopes are pathogenically relevant; 2. Different groove peptides may have different affinities to the SE. It is therefore conceivable that CRT could displace certain types of peptides; 3. Another scenario to consider is that CRT interacts primarily with ‘empty’-grooved HLA-DR molecules; 4. Our preliminary data using SE-positive HLA-DR tetrameric molecules loaded with two distinct groove peptides suggest that the identity of the peptide does not have any significant impact on SE-CRT interaction or signaling (unpublished results); 5. Finally, as discussed bellow, the antigen presentation paradigm and the SE signaling hypothesis are not mutually exclusive. We cannot rule out a scenario in which certain groove peptides and CRT could co-interact with the SE.
Based on the experimental and theoretical considerations discussed above, we peopose the following model: In healthy individuals carrying SE-coding HLA-DRB1 alleles, the SE ligand, expressed on antigen presenting cells or lymphocytes interacts at low affinity with cell surface CRT. As our data show, SE activation of DCs promotes Th17 polarization, which in healthy individuals could be advantageous against pathogens. We further propose that over time, due to environmentally-triggered stochastic events, the affinity of SE-CRT interaction could increase with resultant aberrant activation of the SE pathway, excessive Th17 polarization and development of RA. It is worth mentioning here that different from humans, mice do not express class II MHC molecules on the surface of activated T cells. We therefore posit that the reported SE-promoted autoimmunity in HLA-DRB1 transgenic mice could be due to activation of proinflammatory signaling by SE expressed on cells other than T lymphocytes (i.e. macrophages, B lymphocytes or DCs).
This model is non-exclusive with other hypotheses in the field. One scenario to consider is that while SE-expressing HLA-DR molecules are uniquely capable of presenting joint-specific antigens, thereby determining the tissue-specificity of the immune response and its anatomical distribution, it is the SE ligand function that determines the outcome of this response by facilitating Th17 polarization. Finally, we wish to clarify that given the focus of this review on Th17, the simplified model proposed here highlights the SE effect on DCs. However, the SE could conceivably activate RA-associated functional consequences in other cell systems (some of which are listed in Fig. 4).
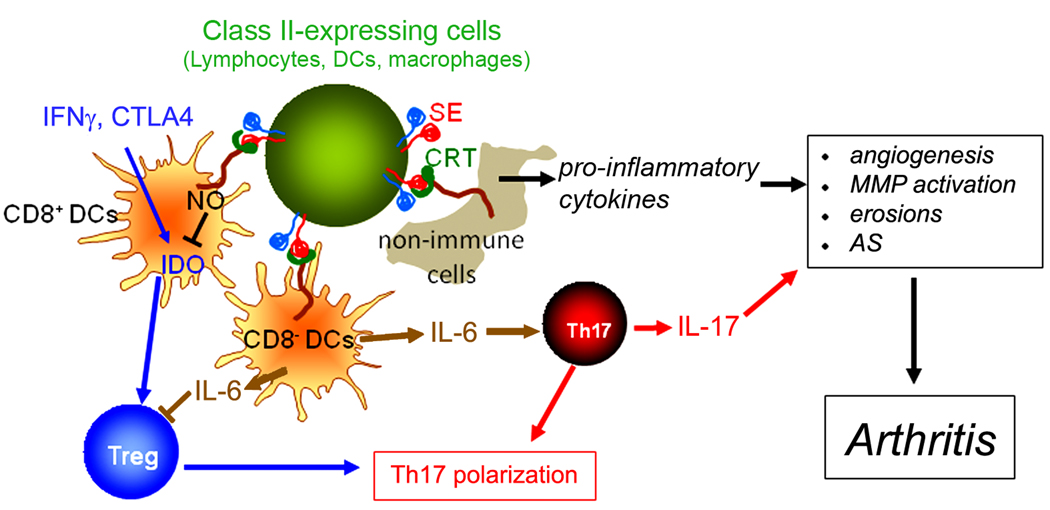
Figure 4.
A proposed model. The SE ligand, expressed on antigen presenting cells or lymphocytes interacts with cell surface CRT. In DCs this interaction promotes Th17 polarization, which in healthy individuals could be advantageous against pathogens. In RA, due to environmentally-triggered non-genetic changes, the affinity of SE-CRT interaction could increase with resultant aberrant activation of the SE pathway, excessive Th17 polarization and disease development. IL-17 has been previously shown to promote autoimmunity through its effect on many target tissues. In addition to its Th17 polarizing effect through activation of DCs, the SE could conceivably directly activate functional consequences in other cell systems (shown in italics) known to contribute to RA pathogenesis.
7. Summary
We have previously demonstrated that the SE acts as a signal transduction ligand that activates NO and ROS production in other cells. SE signaling is transduced by cell surface CRT, a known innate immunity receptor previously implicated in immune regulation, autoimmunity and angiogenesis. There are multiple RA-relevant cellular and molecular mechanisms that could conveivably exert pathogenic influences secondary to activation of the newly discovered pathway. An example of SE-activated aberrant immune regulation that involves inhibition of the tolerogenic enzyme IDO on one hand and enhanced Th17 expansion on the other is presented here for illustration. Other conceivable SE-activated pathogenic mechanisms are being currently studied by this group.
Acknowledgements
Dr. Holoshitz has been supported by the US National Institutes of Health (AR55170, UL1RR02498, AI47331, GM88560, AR20557, AR48310, AR56786), the University of Michigan Global Reach Collaborative Research Fund, by a Basic Research Grant from the Arthritis Foundation and an Innovative Basic Science Award from the American College of Rheumatology Research and Education Foundation. Special thanks to the NIH Tetramer Core Facility for providing valuable research reagents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 2.Gabriel SE. Heart disease and rheumatoid arthritis: understanding the risks. Ann Rheum Dis. 2010;69 Suppl 1:i61–i64. doi: 10.1136/ard.2009.119404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ollier WE, MacGregor A. Genetic epidemiology of rheumatoid disease. Br Med Bull. 1995;51:267–285. doi: 10.1093/oxfordjournals.bmb.a072960. [DOI] [PubMed] [Google Scholar]
- 4.Baka Z, Buzas E, Nagy G. Rheumatoid arthritis and smoking: putting the pieces together. Arthritis Res Ther. 2009;11:238. doi: 10.1186/ar2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stastny P. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. N Engl J Med. 1978;298:869–871. doi: 10.1056/NEJM197804202981602. [DOI] [PubMed] [Google Scholar]
- 6.Gibofsky A, et al. Contrasting patterns of newer histocompatibility determinants in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum. 1978;21:S134–S138. doi: 10.1002/art.1780210920. [DOI] [PubMed] [Google Scholar]
- 7.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 8.Mewar D, et al. Association between radiographic severity of rheumatoid arthritis and shared epitope alleles: differing mechanisms of susceptibility and protection. Ann Rheum Dis. 2008;67:980–983. doi: 10.1136/ard.2007.075382. [DOI] [PubMed] [Google Scholar]
- 9.Wucherpfennig KW, Strominger JL. Selective binding of self peptides to disease-associated major histocompatibility complex (MHC) molecules: a mechanism for MHC-linked susceptibility to human autoimmune diseases. J Exp Med. 1995;181:1597–1601. doi: 10.1084/jem.181.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Cava A, et al. Genetic bias in immune responses to a cassette shared by different microorganisms in patients with rheumatoid arthritis. J Clin Invest. 1997;100:658–663. doi: 10.1172/JCI119577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhayani HR, Hedrick SM. The role of polymorphic amino acids of the MHC molecule in the selection of the T cell repertoire. J Immunol. 1991;146:1093–1098. [PubMed] [Google Scholar]
- 12.Weyand CM, Hunder NN, Hicok KC, Hunder GG, Goronzy JJ. HLA-DRB1 alleles in polymyalgia rheumatica, giant cell arteritis, and rheumatoid arthritis. Arthritis Rheum. 1994;37:514–520. doi: 10.1002/art.1780370411. [DOI] [PubMed] [Google Scholar]
- 13.Tait BD, Drummond BP, Varney MD, Harrison LC. HLA-DRB1*0401 is associated with susceptibility to insulin-dependent diabetes mellitus independently of the DQB1 locus. Eur J Immunogenet. 1995;22:289–297. doi: 10.1111/j.1744-313x.1995.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 14.Korendowych E, Dixey J, Cox B, Jones S, McHugh N. The Influence of the HLA-DRB1 rheumatoid arthritis shared epitope on the clinical characteristics and radiological outcome of psoriatic arthritis. J Rheumatol. 2003;30:96–101. [PubMed] [Google Scholar]
- 15.Chan MT, Owen P, Dunphy J, Cox B, Carmichael C, Korendowych E, McHugh NJ. Associations of erosive arthritis with anti-cyclic citrullinated peptide antibodies and MHC Class II alleles in systemic lupus erythematosus. J Rheumatol. 2008;35:77–83. [PubMed] [Google Scholar]
- 16.Doherty DG, et al. Allelic sequence variation in the HLA class II genes and proteins in patients with autoimmune hepatitis. Hepatology. 1994;19:609–615. doi: 10.1002/hep.1840190311. [DOI] [PubMed] [Google Scholar]
- 17.Dorak MT, Machulla HK, Hentschel M, Mills KI, Langner J, Burnett AK. Influence of the major histocompatibility complex on age at onset of chronic lymphoid leukaemia. Int J Cancer. 1996;65:134–139. doi: 10.1002/(SICI)1097-0215(19960117)65:2<134::AID-IJC2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Ollier WE, et al. Dog MHC alleles containing the human RA shared epitope confer susceptibility to canine rheumatoid arthritis. Immunogenetics. 2001;53:669–673. doi: 10.1007/s002510100372. [DOI] [PubMed] [Google Scholar]
- 19.Wen L, Chen NY, Tang J, Sherwin R, Wong FS. The regulatory role of DR4 in a spontaneous diabetes DQ8 transgenic model. J Clin Invest. 2001;107:871–880. doi: 10.1172/JCI11708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosloniec EF, et al. An HLA-DR1 transgene confers susceptibility to collagen-induced arthritis elicited with human type II collagen. J Exp Med. 1997;185:1113–1122. doi: 10.1084/jem.185.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forsthuber TG, et al. T cell epitopes of human myelin oligodendrocyte glycoprotein identified in HLA-DR4 (DRB1*0401) transgenic mice are encephalitogenic and are presented by human B cells. J Immunol. 2001;167:7119–7125. doi: 10.4049/jimmunol.167.12.7119. [DOI] [PubMed] [Google Scholar]
- 22.Ling S, Lai A, Borschukova O, Pumpens P, Holoshitz J. Activation of nitric oxide signaling by the rheumatoid arthritis shared epitope. Arthritis Rheum. 2006;54:3423–3432. doi: 10.1002/art.22178. [DOI] [PubMed] [Google Scholar]
- 23.Ling S, Li Z, Borschukova O, Xiao L, Pumpens P, Holoshitz J. The rheumatoid arthritis shared epitope increases cellular susceptibility to oxidative stress by antagonizing an adenosine-mediated anti-oxidative pathway. Arthritis Res Ther. 2007;9:R5. doi: 10.1186/ar2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holoshitz J, Ling S. Nitric oxide signaling triggered by the rheumatoid arthritis shared epitope: a new paradigm for MHC disease association? Ann N Y Acad Sci. 2007;1110:73–83. doi: 10.1196/annals.1423.009. [DOI] [PubMed] [Google Scholar]
- 25.Ling S, Pi X, Holoshitz J. The rheumatoid arthritis shared epitope triggers innate immune signaling via cell surface calreticulin. J Immunol. 2007;179:6359–6367. doi: 10.4049/jimmunol.179.9.6359. [DOI] [PubMed] [Google Scholar]
- 26.De Almeida DE, Ling S, Pi X, Hartmann-Scruggs AM, Pumpens P, Holoshitz J. Immune dysregulation by the rheumatoid arthritis shared epitope. J Immunol. 2010;185:1927–1934. doi: 10.4049/jimmunol.0904002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holoshitz J, De Almeida DE, Ling S. A role for calreticulin in the pathogenesis of rheumatoid arthritis. Ann N Y Acad Sci. 2010;1209:91–98. doi: 10.1111/j.1749-6632.2010.05745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ling S, Cheng A, Pumpens P, Michalak M, Holoshitz J. Identification of the rheumatoid arthritis shared epitope binding site on calreticulin. PLoS One. 2010;5:e11703. doi: 10.1371/journal.pone.0011703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201–214. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 30.Brown JH, Jardetzky TS, Gorga JC, Stern LJ, Urban RG, Strominger JL, Wiley DC. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993;364:33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- 31.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 32.Brooks AG, Boyington JC, Sun PD. Natural killer cell recognition of HLA class I molecules. Rev Immunogenet. 2000;2:433–448. [PubMed] [Google Scholar]
- 33.Bennett MJ, Lebron JA, Bjorkman PJ. Crystal structure of the hereditary haemochromatosis protein HFE complexed with transferrin receptor. Nature. 2000;403:46–53. doi: 10.1038/47417. [DOI] [PubMed] [Google Scholar]
- 34.Olson R, Dulac C, Bjorkman PJ. MHC homologs in the nervous system--they haven't lost their groove. Curr Opin Neurobiol. 2006;16:351–357. doi: 10.1016/j.conb.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Yki-Jarvinen H, Bergholm R, Leirisalo-Repo M. Increased inflammatory activity parallels increased basal nitric oxide production and blunted response to nitric oxide in vivo in rheumatoid arthritis. Ann Rheum Dis. 2003;62:630–634. doi: 10.1136/ard.62.7.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grant DD, Goldstein R, Karsh J, Birnboim HC. Nitric oxide donors induce large-scale deletion mutations in human lymphoblastoid cells: implications for mutations in T-lymphocytes from arthritis patients. Environ Mol Mutagen. 2001;38:261–267. doi: 10.1002/em.1080. [DOI] [PubMed] [Google Scholar]
- 37.Ables GP, Hamashima N, Watanabe T. Analysis of genetic factors associated with nitric oxide production in mice. Biochem Genet. 2001;39:379–394. doi: 10.1023/a:1013859502862. [DOI] [PubMed] [Google Scholar]
- 38.Chiba A, Oki S, Miyamoto K, Hashimoto H, Yamamura T, Miyake S. Suppression of collagen-induced arthritis by natural killer T cell activation with OCH, a sphingosine-truncated analog of alpha-galactosylceramide. Arthritis Rheum. 2004;50:305–313. doi: 10.1002/art.11489. [DOI] [PubMed] [Google Scholar]
- 39.Ding M, Zhang M, Wong JL, Rogers NE, Ignarro LJ, Voskuhl RR. Antisense knockdown of inducible nitric oxide synthase inhibits induction of experimental autoimmune encephalomyelitis in SJL/J mice. J Immunol. 1998;160:2560–2564. [PubMed] [Google Scholar]
- 40.Weller AH, Magliato SA, Bell KP, Rosenberg NL. Spontaneous myopathy in the SJL/J mouse: pathology and strength loss. Muscle Nerve. 1997;20:72–82. doi: 10.1002/(sici)1097-4598(199701)20:1<72::aid-mus10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 41.Nair J, Gal A, Tamir S, Tannenbaum SR, Wogan GN, Bartsch H. Etheno adducts in spleen DNA of SJL mice stimulated to overproduce nitric oxide. Carcinogenesis. 1998;19:2081–2084. doi: 10.1093/carcin/19.12.2081. [DOI] [PubMed] [Google Scholar]
- 42.Gal A, Wogan GN. Mutagenesis associated with nitric oxide production in transgenic SJL mice. Proc Natl Acad Sci U S A. 1996;93:15102–15107. doi: 10.1073/pnas.93.26.15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamir S, deRojas-Walker T, Gal A, Weller AH, Li X, Fox JG, Wogan GN, Tannenbaum SR. Nitric oxide production in relation to spontaneous B-cell lymphoma and myositis in SJL mice. Cancer Res. 1995;55:4391–4397. [PubMed] [Google Scholar]
- 44.Nagy G, Clark JM, Buzas EI, Gorman CL, Cope AP. Nitric oxide, chronic inflammation and autoimmunity. Immunol Lett. 2007;111:1–5. doi: 10.1016/j.imlet.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 45.Pyriochou A, Vassilakopoulos T, Zhou Z, Papapetropoulos A. cGMP-dependent and -independent angiogenesis-related properties of nitric oxide. Life Sci. 2007;81:1549–1554. doi: 10.1016/j.lfs.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 46.Mould AW, Tonks ID, Cahill MM, Pettit AR, Thomas R, Hayward NK, Kay GF. Vegfb gene knockout mice display reduced pathology and synovial angiogenesis in both antigen-induced and collagen-induced models of arthritis. Arthritis Rheum. 2003;48:2660–2669. doi: 10.1002/art.11232. [DOI] [PubMed] [Google Scholar]
- 47.Hirata S, Matsubara T, Saura R, Tateishi H, Hirohata K. Inhibition of in vitro vascular endothelial cell proliferation and in vivo neovascularization by low-dose methotrexate. Arthritis Rheum. 1989;32:1065–1073. doi: 10.1002/anr.1780320903. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Opdenakker G, et al. Remnant epitopes, autoimmunity and glycosylation. Biochim Biophys Acta. 2006;1760:610–615. doi: 10.1016/j.bbagen.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 50.Burbridge MF, Coge F, Galizzi JP, Boutin JA, West DC, Tucker GC. The role of the matrix metalloproteinases during in vitro vessel formation. Angiogenesis. 2002;5:215–226. doi: 10.1023/a:1023889805133. [DOI] [PubMed] [Google Scholar]
- 51.Borden P, Solymar D, Sucharczuk A, Lindman B, Cannon P, Heller RA. Cytokine control of interstitial collagenase and collagenase-3 gene expression in human chondrocytes. J Biol Chem. 1996;271:23577–23581. doi: 10.1074/jbc.271.38.23577. [DOI] [PubMed] [Google Scholar]
- 52.Zaragoza C, Lopez-Rivera E, Garcia-Rama C, Saura M, Martinez-Ruiz A, Lizarbe TR, Martin-de-Lara F, Lamas S. Cbfa-1 mediates nitric oxide regulation of MMP-13 in osteoblasts. J Cell Sci. 2006;119:1896–1902. doi: 10.1242/jcs.02895. [DOI] [PubMed] [Google Scholar]
- 53.Thomas SR, Mohr D, Stocker R. Nitric oxide inhibits indoleamine 2,3-dioxygenase activity in interferon-gamma primed mononuclear phagocytes. J Biol Chem. 1994;269:14457–14464. [PubMed] [Google Scholar]
- 54.Alberati-Giani D, Malherbe P, Ricciardi-Castagnoli P, Kohler C, Denis-Donini S, Cesura AM. Differential regulation of indoleamine 2,3-dioxygenase expression by nitric oxide and inflammatory mediators in IFN-gamma-activated murine macrophages and microglial cells. J Immunol. 1997;159:419–426. [PubMed] [Google Scholar]
- 55.Hitchon CA, El-Gabalawy HS. Oxidation in rheumatoid arthritis. Arthritis Res Ther. 2004;6:265–278. doi: 10.1186/ar1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ueda M, Mashiba S, Uchida K. Evaluation of oxidized alpha-1-antitrypsin in blood as an oxidative stress marker using anti-oxidative alpha1-AT monoclonal antibody. Clin Chim Acta. 2002;317:125–131. doi: 10.1016/s0009-8981(01)00765-3. [DOI] [PubMed] [Google Scholar]
- 57.Lee SH, Chang DK, Goel A, Boland CR, Bugbee W, Boyle DL, Firestein GS. Microsatellite instability and suppressed DNA repair enzyme expression in rheumatoid arthritis. J Immunol. 2003;170:2214–2220. doi: 10.4049/jimmunol.170.4.2214. [DOI] [PubMed] [Google Scholar]
- 58.Lee KW, Lee HJ. Biphasic effects of dietary antioxidants on oxidative stress-mediated carcinogenesis. Mech Ageing Dev. 2006;127:424–431. doi: 10.1016/j.mad.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 59.Schonland SO, Lopez C, Widmann T, Zimmer J, Bryl E, Goronzy JJ, Weyand CM. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc Natl Acad Sci U S A. 2003;100:13471–13476. doi: 10.1073/pnas.2233561100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goronzy JJ, Henel G, Sawai H, Singh K, Lee EB, Pryshchep S, Weyand CM. Costimulatory pathways in rheumatoid synovitis and T-cell senescence. Ann N Y Acad Sci. 2005;1062:182–194. doi: 10.1196/annals.1358.022. [DOI] [PubMed] [Google Scholar]
- 61.Cemerski S, van Meerwijk JP, Romagnoli P. Oxidative-stress-induced T lymphocyte hyporesponsiveness is caused by structural modification rather than proteasomal degradation of crucial TCR signaling molecules. Eur J Immunol. 2003;33:2178–2185. doi: 10.1002/eji.200323898. [DOI] [PubMed] [Google Scholar]
- 62.del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–2745. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 63.del Rincon I, Freeman GL, Haas RW, O'Leary DH, Escalante A. Relative contribution of cardiovascular risk factors and rheumatoid arthritis clinical manifestations to atherosclerosis. Arthritis Rheum. 2005;52:3413–3423. doi: 10.1002/art.21397. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez-Juanatey C, Testa A, Garcia-Castelo A, Garcia-Porrua C, Llorca J, Gonzalez-Gay MA. Active but transient improvement of endothelial function in rheumatoid arthritis patients undergoing long-term treatment with anti-tumor necrosis factor alpha antibody. Arthritis Rheum. 2004;51:447–450. doi: 10.1002/art.20407. [DOI] [PubMed] [Google Scholar]
- 65.Gonzalez-Juanatey C, Llorca J, Garcia-Porrua C, Martin J, Gonzalez-Gay MA. Effect of anti-tumor necrosis factor alpha therapy on the progression of subclinical atherosclerosis in severe rheumatoid arthritis. Arthritis Rheum. 2006;55:150–153. doi: 10.1002/art.21707. [DOI] [PubMed] [Google Scholar]
- 66.Mas A, Blanco E, Monux G, Urcelay E, Serrano FJ, de la Concha EG, Martinez A. DRB1-TNF-alpha-TNF-beta haplotype is strongly associated with severe aortoiliac occlusive disease, a clinical form of atherosclerosis. Hum Immunol. 2005;66:1062–1067. doi: 10.1016/j.humimm.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 67.Rasmussen TE, Hallett JW, Jr, Metzger RL, Richardson DM, Harmsen WS, Goronzy JJ, Weyand CM. Genetic risk factors in inflammatory abdominal aortic aneurysms: polymorphic residue 70 in the HLA-DR B1 gene as a key genetic element. J Vasc Surg. 1997;25:356–364. doi: 10.1016/s0741-5214(97)70358-6. [DOI] [PubMed] [Google Scholar]
- 68.Palikhe A, Sinisalo J, Seppanen M, Valtonen V, Nieminen MS, Lokki ML. Human MHC region harbors both susceptibility and protective haplotypes for coronary artery disease. Tissue Antigens. 2007;69:47–55. doi: 10.1111/j.1399-0039.2006.00735.x. [DOI] [PubMed] [Google Scholar]
- 69.Marotte H, Farge P, Gaudin P, Alexandre C, Mougin B, Miossec P. The association between periodontal disease and joint destruction in rheumatoid arthritis extends the link between the HLA-DR shared epitope and severity of bone destruction. Ann Rheum Dis. 2006;65:905–909. doi: 10.1136/ard.2005.036913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gorman JD, Lum RF, Chen JJ, Suarez-Almazor ME, Thomson G, Criswell LA. Impact of shared epitope genotype and ethnicity on erosive disease: a meta-analysis of 3,240 rheumatoid arthritis patients. Arthritis Rheum. 2004;50:400–412. doi: 10.1002/art.20006. [DOI] [PubMed] [Google Scholar]
- 71.Bonfil JJ, Dillier FL, Mercier P, Reviron D, Foti B, Sambuc R, Brodeur JM, Sedarat C. A "case control" study on the role of HLA DR4 in severe periodontitis and rapidly progressive periodontitis. Identification of types and subtypes using molecular biology (PCR.SSO) J Clin Periodontol. 1999;26:77–84. doi: 10.1034/j.1600-051x.1999.260203.x. [DOI] [PubMed] [Google Scholar]
- 72.Evans DM, Ralston SH. Nitric oxide and bone. J Bone Miner Res. 1996;11:300–305. doi: 10.1002/jbmr.5650110303. [DOI] [PubMed] [Google Scholar]
- 73.Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, Lee SY. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106:852–859. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- 74.Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, Urry ZL, Chambers TJ. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest. 2003;112:915–923. doi: 10.1172/JCI18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 76.Zeng G, et al. Dendritic cell surface calreticulin is a receptor for NY-ESO-1: direct interactions between tumor-associated antigen and the innate immune system. J Immunol. 2006;177:3582–3589. doi: 10.4049/jimmunol.177.6.3582. [DOI] [PubMed] [Google Scholar]
- 77.Cho JH, Homma K, Kanegasaki S, Natori S. Activation of human neutrophils by a synthetic anti-microbial peptide, KLKLLLLLKLK-NH2, via cell surface calreticulin. Eur J Biochem. 1999;266:878–885. doi: 10.1046/j.1432-1327.1999.00920.x. [DOI] [PubMed] [Google Scholar]
- 78.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 79.Luo X, Weber GA, Zheng J, Gendelman HE, Ikezu T. C1q-calreticulin induced oxidative neurotoxicity: relevance for the neuropathogenesis of Alzheimer's disease. J Neuroimmunol. 2003;135:62–71. doi: 10.1016/s0165-5728(02)00444-7. [DOI] [PubMed] [Google Scholar]
- 80.Gardai SJ, Bratton DL, Ogden CA, Henson PM. Recognition ligands on apoptotic cells: a perspective. J Leukoc Biol. 2006;79:896–903. doi: 10.1189/jlb.1005550. [DOI] [PubMed] [Google Scholar]
- 81.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 82.Pittoni V, Valesini G. The clearance of apoptotic cells: implications for autoimmunity. Autoimmun Rev. 2002;1:154–161. doi: 10.1016/s1568-9972(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 83.Henson PM, Bratton DL, Fadok VA. The phosphatidylserine receptor: a crucial molecular switch? Nat Rev Mol Cell Biol. 2001;2:627–633. doi: 10.1038/35085094. [DOI] [PubMed] [Google Scholar]
- 84.Donnelly S, et al. Impaired recognition of apoptotic neutrophils by the C1q/calreticulin and CD91 pathway in systemic lupus erythematosus. Arthritis Rheum. 2006;54:1543–1556. doi: 10.1002/art.21783. [DOI] [PubMed] [Google Scholar]
- 85.Paust S, Cantor H. Regulatory T cells and autoimmune disease. Immunol Rev. 2005;204:195–207. doi: 10.1111/j.0105-2896.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- 86.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 87.Kolodziej LR, Paleolog EM, Williams RO. Kynurenine metabolism in health and disease. Amino Acids. 2010 doi: 10.1007/s00726-010-0787-9. Oct 23. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 88.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Terness P, Chuang JJ, Opelz G. The immunoregulatory role of IDO-producing human dendritic cells revisited. Trends Immunol. 2006;27:68–73. doi: 10.1016/j.it.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 90.Fallarino F, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 91.De Luca A, et al. Functional yet balanced reactivity to Candida albicans requires TRIF, MyD88, and IDO-dependent inhibition of Rorc. J Immunol. 2007;179:5999–6008. doi: 10.4049/jimmunol.179.9.5999. [DOI] [PubMed] [Google Scholar]
- 92.Fallarino F, et al. Functional expression of indoleamine 2,3-dioxygenase by murine CD8 alpha(+) dendritic cells. Int Immunol. 2002;14:65–68. doi: 10.1093/intimm/14.1.65. [DOI] [PubMed] [Google Scholar]
- 93.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007;19:409–417. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharma MD, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, Miossec P. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–970. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 98.Morgan ME, et al. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452–1460. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- 99.Dessen A, Lawrence CM, Cupo S, Zeller DM, Wiley DC. X-Ray Crystal Structure of HLA-DR4 (DRA*0101, DRB1*0401) Complexed with a Peptide from Human Collagen II. Immunity. 1997;7:473–481. doi: 10.1016/s1074-7613(00)80369-6. [DOI] [PubMed] [Google Scholar]
- 100.De Rosa MC, Giardina B, Bianchi C, Carelli Alinovi C, Pirolli D, Ferraccioli G, De Santis M, Di Sante G, Ria F. Modeling the ternary complex TCR-Vbeta/CollagenII(261–273)/HLA-DR4 associated with rheumatoid arthritis. PLoS One. 2010;5:e11550. doi: 10.1371/journal.pone.0011550. [DOI] [PMC free article] [PubMed] [Google Scholar]