Abstract
Neurons are born and become a functional part of the synaptic circuitry in adult brains. The proliferative phase of neurogenesis has been extensively reviewed. We therefore focus this review on a few topics addressing the functional role of adult-generated newborn neurons in the dentate gyrus. We discuss the evidence for a link between neurogenesis and behavior. We then describe the steps in the integration of newborn neurons into a functioning mature synaptic circuit. Given the profound effects of neural activity on the differentiation and integration of newborn neurons, we discuss the role of activity-dependent gene expression in the birth and maturation of newborn neurons. The differentiation and maturation of newborn neurons likely involves the concerted action of many genes. Thus we focus on transcription factors that can direct large changes to the transcriptome, and microRNAs, a newly-discovered class of molecules that can effect the expression of hundreds of genes. How microRNAs affect the generation and integration of newborn neurons is just being explored, but there are compelling clues hinting at their involvement.
Keywords: Neurogenesis, microRNA, integration, synapse, dentate gyrus
1. Introduction
In the adult mammalian brain newborn neurons are born and then are integrated into the functioning synaptic circuitry. There are two major neurogenic regions of the brain – the subgranular layer of the dentate gyrus and the subventricular zone adjacent to the lateral ventricles. Newborn neurons of the subgranular layer become granule cells (Overstreet-Wadiche and Westbrook, 2006) whereas those derived from the subventricular zone migrate via the rostral migratory stream to become granule and periglomerular cells in the olfactory bulb (Alvarez-Buylla and Garcia-Verdugo, 2002). In this review we discuss the evidence for dentate gyrus neurogenesis, how newborn neurons become integrated into the dentate gyrus, and some of the molecular mechanisms involved in this process. As newborn neurons integrate an array of cellular changes take place, therefore molecules that can orchestrate large-scale changes to the transcriptome such as transcription factors and possibly microRNAs are of particular interest.
2. Behavior and dentate gyrus neurogenesis
The proliferation of new neurons in the dentate gyrus and their chances of survival are influenced by environmental and endogenous factors, each of which can have profound effects on behavior. However, whether there is a causal relationship between the generation of new neurons and behaviors such as learning tasks is still debated. Two examples of a positive influence on neurogenesis are enriched environment and voluntary exercise or wheel running. Exposure to tunnels, toys, and running wheels increases the number of cells in the dentate gyrus (Brown et al., 2003; Kempermann et al., 1997; Kempermann et al., 1998). These manipulations also improve spatial learning and memory (Nilsson et al., 1999; van Praag et al., 1999) and decrease the stress response to anxiety-provoking conditions such as open field tests and predator odor exposure (Roy et al., 2001). These correlative data suggest that neurogenesis can influence anxiety and perhaps mediates the behavioral effects of environmental enrichment. However, after abolishing neurogenesis, Meshi et al. (2006) concluded that neurogenesis was not necessary for enrichment-associated improvements in latency to feed, time to hidden platform, and proportion of time in target quadrant. Similarly, Bartolomucci et al. (2002) showed that a stress-induced decrease in neurogenesis improved performance on hippocampus-dependent tasks and Van der Borght et al. did not find a correlation between strain-dependent differences in neurogenesis and spatial learning in rats (2005). Interpretation of these results is complicated because factors that influence neurogenesis also influence other aspects of mature and newborn neurons such as dendritic architecture, synaptogenesis, and synaptic plasticity. Because these factors can influence hippocampus-dependent learning, the role of neurogenesis in learning remains elusive.
2.1 Cognitive behaviors
Several studies have looked at the inverse relationship, i.e. whether learning increases the number and survival of new neurons. Training on hippocampus-dependent learning tasks such as trace eyeblink conditioning, the Morris water maze, and conditioned food preference all increase the number of newly generated granule cells in the adult dentate gyrus (Gould et al., 1999). Furthermore, Ambrogini et al. (2000) reported a correlation between actual learning and newborn cell survival. However, Dobrossy et al. (2003), differentiating between early- and late-phase learning, suggested that late-phase learning increased the number of newborn cells but decreased the number of neurons generated in the early phase. Interestingly, the decrease in new cells correlated with better performance in the Morris water maze. These data are congruent with Ambrogini et al. (2004b), in which hippocampus-dependent learning increased survival of newborn cells, but decreased the number of immature neurons.
To establish a relationship between cell number and learning performance requires investigation of whether neurogenesis is necessary for learning. Various studies have used antimitotic drugs, genetic manipulation, or radiation therapy to ablate neurogenesis. For example, knockdown of neurogenesis in the dentate gyrus (Jessberger et al., 2009) and chemotherapy (Mustafa et al., 2008) were shown to impair spatial memory. However, Ko et al. (2009) reported that inhibition of neurogenesis impaired only the formation of contextual fear memory, not its extinction. Similarly, Saxe et al. (2006) suggested that genetic ablation of neural progenitor cells or focal irradiation of the hippocampus impaired fear conditioning, but performance on spatial learning tasks and cued conditioning was unaffected. These data suggest that adult neurogenesis may be involved in only a subset of hippocampus-dependent functions.
2.2 Affective behaviors
Studies of depression have also suggested a behavioral role for neurogenesis. For example, stress, a causal factor for depression (Kendler et al., 1999), reduces neurogenesis. Because newborn neurons in the adult hippocampus are sensitive to increases in corticosteroid levels (Gould et al., 1992; McEwen, 1999) and adrenalectomy increases adult neurogenesis (Cameron and Gould, 1994), a stress-induced decrease in neurogenesis has been suggested as a trigger for depression (D’Sa and Duman, 2002; Duman et al., 2000; Jacobs et al., 2000; Jacobs, 2002; Kempermann, 2002; Kempermann and Kronenberg, 2003) This idea gains traction from successful treatment of depression with SSRIs, which increase serotonergic transmission and also stimulates neurogenesis (Brezun and Daszuta, 1999; Jacobs, 1998). Similarly, chronic treatment of animals with the antidepressant fluoxetine increases neurogenesis in the adult dentate gyrus (Czeh et al., 2001; Kempermann, 2002; Malberg et al., 2000; Malberg and Duman, 2003; Manev et al., 2001) as does lithium and electroconvulsive therapy (Boku et al., 2010; Chen et al., 2000; Madsen et al., 2000; Wexler et al., 2008).
Despite this correlative data, it is also clear that deficits in neurogenesis are not the sole cause of depression. Ablation of neurogenesis by irradiation or gene deletion does result in a blunted antidepressant response (Li et al., 2008; Santarelli et al., 2003). However, some behavioral responses to antidepressants do not require intact neurogenesis (David et al., 2009). Further, manipulations that decrease neurogenesis do not induce anhedonia outright (Jayatissa et al., 2009; Jayatissa et al., 2010; Taliaz et al., 2010). These data suggest that there is a strong correlative relationship between neurogenesis and both cognitive and affective behaviors. However specific manipulations disrupting neurogenesis do not necessarily abrogate behaviors that are correlated with enhanced neurogenesis. One possible interpretation arising from the disparity between the correlation of neurogenesis to antidepressant action and the lack of anhedonia in mice with disrupted neurogensis is that underlying circuit activity may contribute to both robust neurogenesis and positive affect. Therefore neurogenesis may serve as a sensor for this underlying activity. The underlying activity, itself, could more directly contribute to certain behaviors. Further, changes in underlying activity that promote hippocampal neurogenesis may also be occurring elsewhere in the brain. In such a model, global changes in activity in brain regions important for mood including the striatum, prefrontal cortex, and hippocampus could contribute to positive affect. In parallel, this activity promotes neurogenesis in the hippocampus which likely results in a functional feedback into limbic circuitries.
3. Integration of newborn neurons into the synaptic circuitry
In the adult rat, it has been estimated that 4000 to 9000 new cells are born every day (Cameron and McKay, 2001; Rao and Shetty, 2004). Of those surviving one week, about 75% differentiate into neurons (Rao and Shetty, 2004; Steiner et al., 2004). Of these, about half will die within the first month after birth whereas the other half appear to survive at least 6 months (Dayer et al., 2003). Thus it has been estimated that between 3.75% and 6% of neurons in the dentate gyrus are less than one month old (Cameron and McKay, 2001; Rao and Shetty, 2004).
Although earlier studies focused on precursor cell proliferation, differentiation, and survival, there also has been substantial progress in understanding how newborn neurons integrate into the synaptic circuitry. The basic properties of maturing newborn neurons in the adult were first described using untargeted whole-cell recordings from cells along the inner margin of the granule cell layer. Post-hoc morphological measurements and immunohistochemistry were then used to further characterize these cells. In many respects, newborn neurons of the adult show similar characteristics to their counterparts during early development. They have rudimentary dendritic arborization, high input resistance, and depolarized resting potentials. As they mature their dendritic arbors elaborate, input resistance decreases and the resting membrane potential becomes more hyperpolarized (Ambrogini et al., 2004a; Schmidt-Hieber et al., 2004).
A more detailed picture of newborn neuron development and integration has been established by studying newborn neurons labeled with retroviral particles, or transgenically labeled in POMC-GFP mice (Overstreet et al., 2004; van Praag et al., 2002). During the first week after differentiation, newborn neurons begin to extend dendritic processes through the granule cell layer and axons through the hilus towards the CA3 region (Esposito et al., 2005; Zhao et al., 2006). In whole-cell recording, these newborn neurons have very high input resistances (>4GΩ) and produce rudimentary action potentials, but mostly lack synaptic inputs (Esposito et al., 2005). During the second week after birth the newborn neurons begin to elaborate dendritic branches into the molecular layer and the axons have reached the CA3 region (Figure 1) (Esposito et al., 2005; Zhao et al., 2006). At this point the neurons have input resistances of about 1-5GΩ, depolarizing GABAergic inputs and only very few, if any, glutamatergic synaptic inputs (Figure 1) (Esposito et al., 2005; Markwardt et al., 2009; Overstreet Wadiche et al., 2005). During the third week of maturation dendrites grow through the outer molecular layer, the input resistance drops below 1GΩ, and there is a rapid increase in the formation of glutamatergic synapses and dendritic spines (Figure 1) (Esposito et al., 2005; Toni et al., 2007; Zhao et al., 2006). By four to five weeks post-mitosis newborn neurons mature and have lower input resistances (200-500mΩ) and fully elaborated dendrites with rich excitatory glutamatergic and inhibitory GABAergic inputs (Figure 1) (Esposito et al., 2005; Toni et al., 2007; Zhao et al., 2006). Between four and six weeks post-mitosis, long-term potentiation is more easily induced than in more mature neurons (Ge et al., 2007; Schmidt-Hieber et al., 2004).
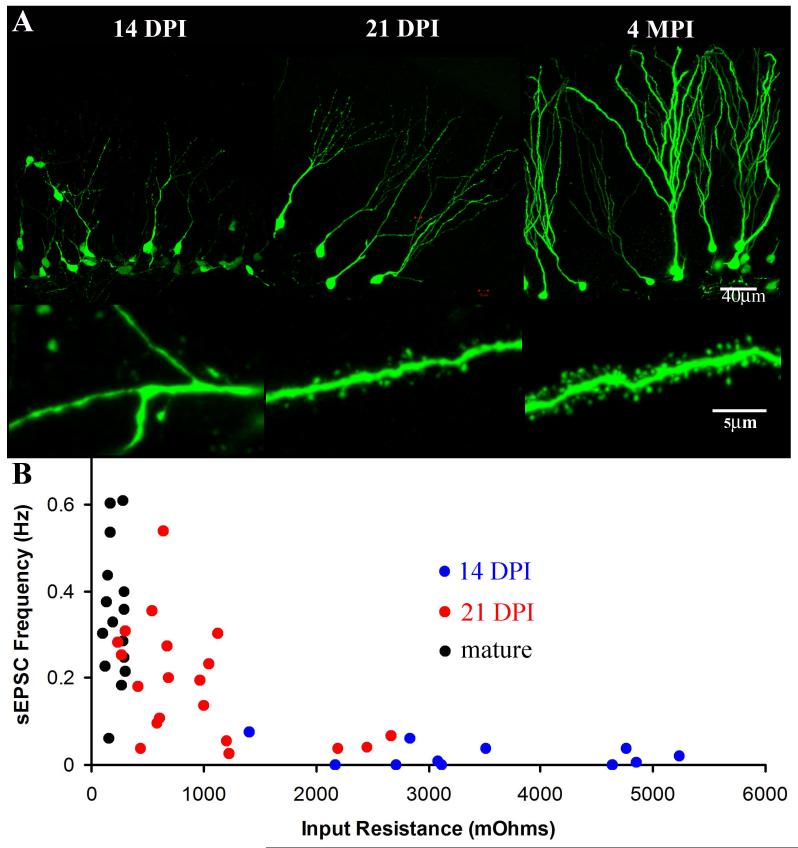
Figure 1.
The Integration of Newborn Neurons into the Adult Dentate Gyrus.
(A). The morphology of newborn neurons is depicted by labeling with a retrovirus expressing EGFP using the ubiquitin promoter (pRubi) in 6 to 8 week old mice. At 14 days post-injection (DPI) the aspiny dendrites (lower panel) have reached the inner molecular layer (upper panel). Only 1 week later at 21 DPI the majority of dendrites span the molecular layer (upper panel) and have numerous dendritic spines (lower panel). At 4 months post-injection (MPI) the neurons are fully mature with elaborate dendritic arbors (upper panel) and numerous mature dendritic spines (lower panel). (B) The stages of neuronal maturation depicted above can easily be discerned by the electrophysiological profile of the cells as well. Each point represents a single whole-cell recording of spontaneous excitatory post-synaptic currents (sEPSC) frequency plotted as a function of cellular input resistance. The sEPSC frequency correlates to the density of excitatory synaptic inputs onto the cell and the input resistance of the cell decreases as the size of the cell increases. At 14 DPI there is high input resistance and almost no excitatory synaptic currents. By 21 DPI there is a decrease in the input resistance and an increase in the sEPSC frequency. Mature neurons have the lowest input resistance and the greatest frequency of sEPSCs.
There are a number of parallels that exist between adult and developmental neurogenesis. In both situations, neurons differentiate and mature into functional components of the synaptic circuitry. In both development and in the adult newborn neurons undergo a critical period of enhanced plasticity characterized by slowly decaying NMDA receptor currents because of preferential expression of NR2B subunit containing receptors (Ge et al., 2007; Sheng et al., 1994; Tovar and Westbrook, 1999). The transition from depolarizing to hyperpolarizing GABA is also a developmental phenomenon (Leinekugel et al., 1999). However, in the adult the maturation of newborn neurons appears to be slower (Overstreet-Wadiche et al., 2006). It is difficult to discern whether differences between newborn neurons of the adult and in development result from cell-intrinsic dissimilarities or from differences in the environment into which the neurons are born. However this issues represents an important area of study in light of hopes for cell-based therapies to replace diseased neurons in adults.
4. Molecular mechanisms governing integration
4.1 Activity-dependent transcription
As progenitor cells undergo the transition into differentiated neurons and integrate into the synaptic circuitry, there are large-scale changes in gene expression (Flavell and Greenberg, 2008). Thus, there must be many molecular controls governing the stages of neurogenesis and subsequent circuit integration. One transcription factor governing this process, NeuroD1, is expressed in the dentate gyrus during the transition from precursor cells into neurons (Gao et al., 2009). Both overexpression and knockdown of NeuroD1 indicate that it is critical for the differentiation and survival of progenitor cells and neurons, respectively (Gao et al., 2009; Hsieh et al., 2004; Roybon et al., 2009). The reduction of NeuroD1 expression in β-catenin knockout mice underscores the importance of wnt signaling to set up this transcriptional program (Kuwabara et al., 2009). Because enhanced activity through enriched environment, exercise, or seizures positively influences neurogenesis and integration of newborn neurons, activity-dependent mechanisms that modulate transcription factor expression are of particular interest. Activity facilitates synaptic release of wnt (Ataman et al., 2008) and could therefore enhance NeuroD1 non cell-autonomously. Precursor cells may detect ambient circuit activity through NMDA receptors and L-type Ca++ channels (Deisseroth et al., 2004). This ion channel activity results in the suppression of pro-glial genes Hes1 and Id2 and the induction of NeuroD1 (Deisseroth et al., 2004). Thus activity can affect the transcriptional program whereby NeuroD promotes the neuronal fate of precursor cells.
As newborn neurons begin to receive GABAergic inputs another activity dependent transcriptional program begins. During developmental and adult neurogenesis newborn neurons are initially depolarized by GABA due to high intracellular chloride. The high levels of intracellular chloride are driven by expression of the Na+-K+-2Cl− transporter NKCC1 (Ge et al., 2006). As the neurons mature, NKCC1 expression decreases and the expression of the K+Cl− cotransporter KCC2 increases. This results in a shift of the chloride gradient and a transition from depolarizing to hyperpolarizing GABA responses. Neural activity appears to be necessary for this molecular switch (Fiumelli and Woodin, 2007). Further, elimination of depolarizing GABA via knockdown of NKCC1 results in impaired integration of newborn neurons into the adult synaptic circuitry (Ge et al., 2006). Interestingly, peak activation of the activity-dependent transcription factor CREB parallels the appearance of depolarizing GABAergic inputs, and retroviral mediated knockdown of CREB in adult-generated newborn neurons decreases their growth and survival. Thus depolarizing GABA results in CREB activation that is necessary for the normal integration of newborn neurons (Jagasia et al., 2009). Further, antidepressant medications and seizures enhance integration and also result in increased CREB phosphorylation (Fujioka et al., 2004; Lee et al., 2007; Overstreet-Wadiche et al., 2006). Thus CREB is ideally situated to contribute to large-scale changes in gene expression that occur during maturation of newborn granule neurons.
Though activity dependent changes in NeuroD1- and CREB-mediated transcription have been most studied in the context of adult neurogenesis, it is likely that other such transcriptional shifts occur at various stages throughout the birth and integration of newborn neurons. For example Klf-9 expression is induced by activity and is necessary for the maturation of granule neurons in vivo (Scobie et al., 2009). Activity dependent transcription factors such as Npas4 and Mef2 regulate synapse formation and this function could be tapped in the context of adult neurogenesis (Barbosa et al., 2008; Flavell et al., 2006; Lin et al., 2008). It is also likely that we will uncover activity-dependent mechanisms regulating transcription factors such as Ascl1, Pax6 and Ngn2 which are found in the adult dentate gyrus neurogenic niche (Kim et al., 2007; Ozen et al., 2007; Roybon et al., 2009).
4.2 Potential for activity dependent microRNAs
Like transcription factors, microRNAs can cause large-scale changes in the proteome of a cell, thus regulating processes such as differentiation and maturation. MicroRNAs are short ~21 nucleotide RNAs that are processed from endogenous genomic loci. These short RNAs are incorporated into the miRNA-induced silencing complex (miRISC), bind to target sequences of a transcript, and induce the translational repression or degradation of that transcript (Krol et al., 2010). Inhibition of microRNA biogenesis by knockout of the microRNA processing enzyme, dicer, has a profound impact on brain development (De Pietri Tonelli et al., 2008). However the specific influence of individual microRNAs in adult dentate gyrus neurogenesis is largely unexplored. To screen for microRNAs that may contribute to the process of integration we induced seizures in mice and used microarrays to screen for activity-dependent microRNAs (Figure 2). Although the microRNAs identified in this screen have not been extensively validated, we identified a number of microRNAs known to be activity-dependent. This list included miRs 329, 453, and 495 that are members of the miR-134 cluster. MiR-134 can regulate synapse development (Christensen et al., 2010; Schratt et al., 2006), thus it and other members of this gene family may play a role in newborn neuron integration.
Figure 2.
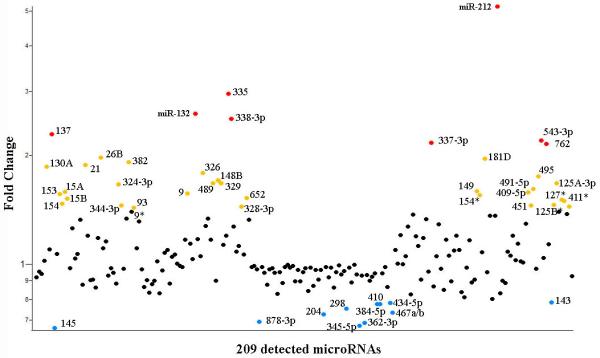
Activity-dependent microRNAs in the Adult Dentate Gyrus.
Seizures were elicited in 8-week-old mice using pilocarpine injection. At four hours post status epilepticus the dentate gyrus was acutely isolated, RNA extracted, and a microarray was used to identify microRNAs that are regulated by activity in vivo. The results displayed are the average transcript fold change (y-axis) from duplicate microarrays probing for all known microRNAs. While most of the individual microRNAs that appear to be regulated have not been experimentally verified, we find that there are many potential activity dependent microRNAs in the dentate gyrus. These microRNAs are interesting candidates that may contribute to the mechanisms by which newborn neurons integrate into the dentate gyrus.
Two of the most highly upregulated microRNAs in our screen, miR-132 and miR-212, are produced from the same transcript and show CREB-dependent expression (Impey et al., 2004; Vo et al., 2005). We and others have observed that its expression is induced by seizure activity in vivo (unpublished data (Nudelman et al., 2010). Further, miR-132 can enhance dendritic outgrowth and spine formation in vitro (Wayman et al., 2008). A number of downstream targets for miR-132 have been identified including p250-GAP, SirT1, MeCP2, and p300 (Impey et al., 2009; Klein et al., 2007; Lagos et al., 2010; Strum et al., 2009). Although it is difficult to discern which target mediates specific aspects of its function, miR-132 likely contributes to the epigenetic changes downstream of CREB activation. Further, its function in vitro makes it a strong candidate to enhance the integration of newborn neurons into the adult synaptic circuitry. We have recently found that knockdown of miR-132 using in vivo retroviral injection results in decreased synaptic inputs onto granule neurons (unpublished data). Further, cre-mediated deletion of miR-132/212 results in decreased dendritic arborization and dendritic spine density of newborn dentate gyrus neurons in vivo (Magill et al., 2010).
Some of the activity dependent microRNAs identified in our array have been studied in the context of neurogenesis. For example, viral methods have been used to test whether miR-137 plays a role in adult neurogenesis. The retroviral over-expression of miR-137 in the dentate gyrus inhibits the growth of newborn neurons (Smrt et al., 2010). Specifically, miR-137 over-expression resulted in decreased arborization and spine density of newborn neurons in vivo. The effects of miR-137 expression were, at least partially, a result of repression of the ubiquitin ligase mind bomb one (Mib1). That miR-137 expression is induced by seizures yet decreases the maturation of newborn neurons may indicate that it negatively regulates the pathological increases in growth that occur in newborn neurons after seizures.
Another microRNA identified on our array, miR-9, promotes neurogenesis in the mid-hindbrain domain of zebrafish (Leucht et al., 2008), promotes Cajal Retzius cell differentiation (Shibata et al., 2008), reduces proliferation and increases differentiation of mouse neural stem cells (Zhao et al., 2009), and enhances proliferation and differentiation of human neural stem cells (Delaloy et al., 2010). Although these studies all implicate miR-9 in neurogenesis-related phenotypes, they diverge in the proposed mechanisms with different miR-9 targets implicated. For example, the proposed targets are fgf signaling components (Leucht et al., 2008), FoxG1 (Shibata et al., 2008), TLX (Zhao et al., 2009), and stathmin (Delaloy et al., 2010). This divergence could result from the different systems and methods of miR-9 manipulation in these studies. However, it may also emphasize the fact that microRNAs target hundreds of genes, which makes it difficult to pinpoint a phenotype to a single target or even a small group of targets (Baek et al., 2008; Selbach et al., 2008).
Although we have been particularly intrigued by the possible role of activity-dependent microRNAs in the integration of newborn neurons, activity-dependence is certainly not necessary for a microRNA to regulate neurogenesis and/or circuit integration. One clue that a microRNA may play a role in neuronal differentiation is simply its tissue specific expression in the CNS (Sempere et al., 2004). For example, miR-124 is highly brain enriched and has been extensively studied in the context of neurogenesis. The repression of miR-124 in primary cortical neuron cultures resulted in upregulation of non-neuronal genes, suggesting that miR-124 contributes to the neuronal phenotype (Conaco et al., 2006). Makeyev et al. (2007) reported that miR-124 promotes the neuronal phenotype by repressing the RNA-binding protein PTB1 and promoting neuron-specific alternative splicing. MiR-124 can also regulate neurite outgrowth in cultures and a microarray-based approach has been used to identify hundreds of targets that could potentially mediate this phenotype (Yu et al., 2008). MiR-124 knockdown in the subventricular zone inhibited the differentiation of neural progenitors and Sox9 was reported as a target mediating this effect (Cheng et al., 2009). However the overexpression and knockdown of miR-124 in the chick using in ovo electroporation did not result in overt changes in neuronal differentiation (Cao et al., 2007). In this study, miR-124 overexpression resulted in subtle basal lamina defects with laminin γ1 and integrin β1 repression. Although these studies point to a role for miR-124 in differentiation, they suggest mechanisms that diverge from one another based on a handful of potential targets.
As individual microRNAs result in small expression changes of large arrays of genes, it may be useful to identify overlapping targets of co-regulated microRNAs. For example, an array of microRNAs, including miR-9 and miR-124, are regulated by REST and are thus co-expressed during the transition from a progenitor cell into a neuron (Conaco et al., 2006). Whereas each of these miRs individually did not result in significant repression of a BAF53a BAC transgenic reporter, the combined expression of miR-9* and miR-124 results in significant repression of this target (Yoo et al., 2009). Thus the combinatorial action of these two co-regulated microRNAs contributes to a fundamental switch in chromatin-remodeling that occurs during the transition of a progenitor cell into a mature neuron. In light of these results it will be of interest to identify overlapping targets of co-regulated microRNAs.
Identifying arrays of regulated genes may be useful in studying the mechanisms through which microRNAs exert their action. For example miR-125b promotes the differentiation of SY5Y cells. Using a microarray, 164 genes were suppressed by miR-125b leading to a model including 10 potential direct targets (Le et al., 2009). This approach was also used to unravel the mechanisms by which mutations in miR-96 promote progressive hearing loss in mice (Lewis et al., 2009). However, despite being informative, these microarray-based approaches cannot differentiate between direct microRNA targets and downstream indirect actions. One alternative approach to defining all targets of a particular microRNA is to overexpress or knock down a particular RNA and to sequence mRNAs crosslinked to the machinery responsible for microRNA action (Chi et al., 2009). Such unbiased methods to identify targets of individual microRNAs will be invaluable in understanding how families of activity dependent microRNAs function.
5. Conclusion
There is increasing evidence linking animal behavior to dentate gyrus neurogenesis in the adult animal. This link is exemplified by the ability of enriched environments, learning, and exercise to enhance adult neurogenesis and data suggesting that the behavioral effects of antidepressants are partially mediated by neurogenesis. However, understanding how adult neurogenesis contributes to learning and behavior is complicated by the difficulty in manipulating neurogenesis in isolation. That the correlation between behavior and increased neurogenesis appears stronger than the necessity for neurogenesis in certain behaviors could indicate that the role for neurogenesis in behavior is functionally redundant. However, it may also be that processes important for neurogenesis are, in parallel, important for hippocampal-dependent behaviors. For example increased activity enhances activity-dependent plasticity, which is necessary for both increased neurogenesis and certain behaviors.
The exact relationship between plasticity, neurogenesis, and behavior is still under debate. However there is no doubt that neural activity enhances the differentiation and integration of newborn neurons. Thus the differentiation and integration of newborn neurons is itself a form of activity-dependent plasticity. Mechanisms governing this process may also regulate plasticity in other brain regions. Newborn neurons are particularly attractive for such studies because their stereotyped integration into the synaptic circuitry has been well described. Furthermore, retroviruses allow for the specific genetic manipulation of adult-generated newborn neurons. Of particular interest are mechanisms whereby neuronal activity can make large-scale changes to the cellular transcriptome. MicroRNAs are a new class of molecules poised to regulate many genes in response to activity. However, an individual microRNA may only have a small effect on the expression of hundreds of genes. Therefore the concerted action of several microRNAs with overlapping targets may represent the most biologically relevant scheme through which microRNAs regulate adult neurogenesis.
Supplementary Material
Acknowledgements
This work was supported by T32NS007381, F32MH079548, and a NARSAD Young Investigator Award (BWL), and MH46613 (GLW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–34. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrogini P, Cuppini R, Cuppini C, Ciaroni S, Cecchini T, Ferri P, Sartini S, Del Grande P. Spatial learning affects immature granule cell survival in adult rat dentate gyrus. Neurosci Lett. 2000;286:21–4. doi: 10.1016/s0304-3940(00)01074-0. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Lattanzi D, Ciuffoli S, Agostini D, Bertini L, Stocchi V, Santi S, Cuppini R. Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res. 2004a;1017:21–31. doi: 10.1016/j.brainres.2004.05.039. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Orsini L, Mancini C, Ferri P, Ciaroni S, Cuppini R. Learning may reduce neurogenesis in adult rat dentate gyrus. Neurosci Lett. 2004b;359:13–6. doi: 10.1016/j.neulet.2003.12.123. [DOI] [PubMed] [Google Scholar]
- Ataman B, Ashley J, Gorczyca M, Ramachandran P, Fouquet W, Sigrist SJ, Budnik V. Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling. Neuron. 2008;57:705–18. doi: 10.1016/j.neuron.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa AC, Kim MS, Ertunc M, Adachi M, Nelson ED, McAnally J, Richardson JA, Kavalali ET, Monteggia LM, Bassel-Duby R, Olson EN. MEF2C, a transcription factor that facilitates learning and memory by negative regulation of synapse numbers and function. Proc Natl Acad Sci U S A. 2008;105:9391–6. doi: 10.1073/pnas.0802679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomucci A, de Biurrun G, Czeh B, van Kampen M, Fuchs E. Selective enhancement of spatial learning under chronic psychosocial stress. Eur J Neurosci. 2002;15:1863–6. doi: 10.1046/j.1460-9568.2002.02043.x. [DOI] [PubMed] [Google Scholar]
- Boku S, Nakagawa S, Koyama T. Glucocorticoids and lithium in adult hippocampal neurogenesis. Vitam Horm. 2010;82:421–31. doi: 10.1016/S0083-6729(10)82021-7. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17:2042–6. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–9. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–17. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cao X, Pfaff SL, Gage FH. A functional study of miR-124 in the developing neural tube. Genes Dev. 2007;21:531–6. doi: 10.1101/gad.1519207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Masana MI, Manji HK. Lithium regulates PKC-mediated intracellular cross-talk and gene expression in the CNS in vivo. Bipolar Disord. 2000;2:217–36. doi: 10.1034/j.1399-5618.2000.20303.x. [DOI] [PubMed] [Google Scholar]
- Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460:479–86. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen M, Larsen LA, Kauppinen S, Schratt G. Recombinant Adeno-Associated Virus-Mediated microRNA Delivery into the Postnatal Mouse Brain Reveals a Role for miR-134 in Dendritogenesis in Vivo. Front Neural Circuits. 2010;3:16. doi: 10.3389/neuro.04.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaco C, Otto S, Han JJ, Mandel G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc Natl Acad Sci U S A. 2006;103:2422–7. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98:12796–801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Sa C, Duman RS. Antidepressants and neuroplasticity. Bipolar Disord. 2002;4:183–94. doi: 10.1034/j.1399-5618.2002.01203.x. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–93. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–72. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- De Pietri Tonelli D, Pulvers JN, Haffner C, Murchison EP, Hannon GJ, Huttner WB. miRNAs are essential for survival and differentiation of newborn neurons but not for expansion of neural progenitors during early neurogenesis in the mouse embryonic neocortex. Development. 2008;135:3911–21. doi: 10.1242/dev.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–52. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Delaloy C, Liu L, Lee JA, Su H, Shen F, Yang GY, Young WL, Ivey KN, Gao FB. MicroRNA-9 coordinates proliferation and migration of human embryonic stem cell-derived neural progenitors. Cell Stem Cell. 2010;6:323–35. doi: 10.1016/j.stem.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrossy MD, Drapeau E, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Differential effects of learning on neurogenesis: learning increases or decreases the number of newly born cells depending on their birth date. Mol Psychiatry. 2003;8:974–82. doi: 10.1038/sj.mp.4001419. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S, D’Sa C. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000;48:732–9. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–86. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumelli H, Woodin MA. Role of activity-dependent regulation of neuronal chloride homeostasis in development. Curr Opin Neurobiol. 2007;17:81–6. doi: 10.1016/j.conb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–12. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. 2008;31:563–90. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka T, Fujioka A, Duman RS. Activation of cAMP signaling facilitates the morphological maturation of newborn neurons in adult hippocampus. J Neurosci. 2004;24:319–28. doi: 10.1523/JNEUROSCI.1065.03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, Eisch AJ, Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12:1090–2. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–66. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 1992;12:3642–50. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–5. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci U S A. 2004;101:16659–64. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, McCorkle SR, Cha-Molstad H, Dwyer JM, Yochum GS, Boss JM, McWeeney S, Dunn JJ, Mandel G, Goodman RH. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell. 2004;119:1041–54. doi: 10.1016/j.cell.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Impey S, Davare M, Lasiek A, Fortin D, Ando H, Varlamova O, Obrietan K, Soderling TR, Goodman RH, Wayman GA. An activity-induced microRNA controls dendritic spine formation by regulating Rac1-PAK signaling. Mol Cell Neurosci. 2009 doi: 10.1016/j.mcn.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, van Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry. 2000;5:262–9. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Jacobs BL. Adult brain neurogenesis and depression. Brain Behav Immun. 2002;16:602–9. doi: 10.1016/s0889-1591(02)00015-6. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Tanaparat P, Reeves AJ, Gould E. Serotonin stimulates the production of new hippocampal granule neurons via the 5HT1A receptor in the adult rat. Soc. Neurosci. Abs. 1998:1992. [Google Scholar]
- Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, Gage FH, Song H, Lie DC. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci. 2009;29:7966–77. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayatissa MN, Henningsen K, West MJ, Wiborg O. Decreased cell proliferation in the dentate gyrus does not associate with development of anhedonic-like symptoms in rats. Brain Res. 2009;1290:133–41. doi: 10.1016/j.brainres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Jayatissa MN, Henningsen K, Nikolajsen G, West MJ, Wiborg O. A reduced number of hippocampal granule cells does not associate with an anhedonia-like phenotype in a rat chronic mild stress model of depression. Stress. 2010;13:95–105. doi: 10.3109/10253890902951786. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr., Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–54. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–5. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Brandon EP, Gage FH. Environmental stimulation of 129/SvJ mice causes increased cell proliferation and neurogenesis in the adult dentate gyrus. Curr Biol. 1998;8:939–42. doi: 10.1016/s0960-9822(07)00377-6. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Regulation of adult hippocampal neurogenesis - implications for novel theories of major depression. Bipolar Disord. 2002;4:17–33. doi: 10.1034/j.1399-5618.2002.40101.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kronenberg G. Depressed new neurons--adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry. 2003;54:499–503. doi: 10.1016/s0006-3223(03)00319-6. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–41. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Leung CT, Reed RR, Johnson JE. In vivo analysis of Ascl1 defined progenitors reveals distinct developmental dynamics during adult neurogenesis and gliogenesis. J Neurosci. 2007;27:12764–74. doi: 10.1523/JNEUROSCI.3178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein ME, Lioy DT, Ma L, Impey S, Mandel G, Goodman RH. Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat Neurosci. 2007;10:1513–4. doi: 10.1038/nn2010. [DOI] [PubMed] [Google Scholar]
- Ko HG, Jang DJ, Son J, Kwak C, Choi JH, Ji YH, Lee YS, Son H, Kaang BK. Effect of ablated hippocampal neurogenesis on the formation and extinction of contextual fear memory. Mol Brain. 2009;2:1. doi: 10.1186/1756-6606-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12:1097–105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos D, Pollara G, Henderson S, Gratrix F, Fabani M, Milne RS, Gotch F, Boshoff C. miR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nat Cell Biol. 2010;12:513–9. doi: 10.1038/ncb2054. [DOI] [PubMed] [Google Scholar]
- Le MT, Xie H, Zhou B, Chia PH, Rizk P, Um M, Udolph G, Yang H, Lim B, Lodish HF. MicroRNA-125b promotes neuronal differentiation in human cells by repressing multiple targets. Mol Cell Biol. 2009;29:5290–305. doi: 10.1128/MCB.01694-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Dziema H, Lee KH, Choi YS, Obrietan K. CRE-mediated transcription and COX-2 expression in the pilocarpine model of status epilepticus. Neurobiol Dis. 2007;25:80–91. doi: 10.1016/j.nbd.2006.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinekugel X, Khalilov I, McLean H, Caillard O, Gaiarsa JL, Ben-Ari Y, Khazipov R. GABA is the principal fast-acting excitatory transmitter in the neonatal brain. Adv Neurol. 1999;79:189–201. [PubMed] [Google Scholar]
- Leucht C, Stigloher C, Wizenmann A, Klafke R, Folchert A, Bally-Cuif L. MicroRNA-9 directs late organizer activity of the midbrain-hindbrain boundary. Nat Neurosci. 2008;11:641–8. doi: 10.1038/nn.2115. [DOI] [PubMed] [Google Scholar]
- Lewis MA, Quint E, Glazier AM, Fuchs H, De Angelis MH, Langford C, van Dongen S, Abreu-Goodger C, Piipari M, Redshaw N, Dalmay T, Moreno-Pelayo MA, Enright AJ, Steel KP. An ENU-induced mutation of miR-96 associated with progressive hearing loss in mice. Nat Genet. 2009;41:614–8. doi: 10.1038/ng.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47:1043–9. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Magill ST, Cambronne XA, Luikart BW, Lioy DT, Leighton BH, Westbrook GL, Mandel G, Goodman RH. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc Natl Acad Sci U S A. 2010;107:20382–7. doi: 10.1073/pnas.1015691107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell. 2007;27:435–48. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–10. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–71. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Manev H, Uz T, Smalheiser NR, Manev R. Antidepressants alter cell proliferation in the adult brain in vivo and in neural cultures in vitro. Eur J Pharmacol. 2001;411:67–70. doi: 10.1016/s0014-2999(00)00904-3. [DOI] [PubMed] [Google Scholar]
- Markwardt SJ, Wadiche JI, Overstreet-Wadiche LS. Input-specific GABAergic signaling to newborn neurons in adult dentate gyrus. J Neurosci. 2009;29:15063–72. doi: 10.1523/JNEUROSCI.2727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–31. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Mustafa S, Walker A, Bennett G, Wigmore PM. 5-Fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. Eur J Neurosci. 2008;28:323–30. doi: 10.1111/j.1460-9568.2008.06325.x. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569–78. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Nudelman AS, DiRocco DP, Lambert TJ, Garelick MG, Le J, Nathanson NM, Storm DR. Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus. 2010;20:492–8. doi: 10.1002/hipo.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bensen AL, Westbrook GL. Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci. 2006;26:2326–34. doi: 10.1523/JNEUROSCI.4111-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Westbrook GL. Functional maturation of adult-generated granule cells. Hippocampus. 2006;16:208–15. doi: 10.1002/hipo.20152. [DOI] [PubMed] [Google Scholar]
- Overstreet LS, Hentges ST, Bumaschny VF, de Souza FS, Smart JL, Santangelo AM, Low MJ, Westbrook GL, Rubinstein M. A transgenic marker for newly born granule cells in dentate gyrus. J Neurosci. 2004;24:3251–9. doi: 10.1523/JNEUROSCI.5173-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet Wadiche L, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signaling to newborn neurons in dentate gyrus. J Neurophysiol. 2005;94:4528–32. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- Ozen I, Galichet C, Watts C, Parras C, Guillemot F, Raineteau O. Proliferating neuronal progenitors in the postnatal hippocampus transiently express the proneural gene Ngn2. Eur J Neurosci. 2007;25:2591–603. doi: 10.1111/j.1460-9568.2007.05541.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–46. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Roy V, Belzung C, Delarue C, Chapillon P. Environmental enrichment in BALB/c mice: effects in classical tests of anxiety and exposure to a predatory odor. Physiol Behav. 2001;74:313–20. doi: 10.1016/s0031-9384(01)00561-3. [DOI] [PubMed] [Google Scholar]
- Roybon L, Hjalt T, Stott S, Guillemot F, Li JY, Brundin P. Neurogenin2 directs granule neuroblast production and amplification while NeuroD1 specifies neuronal fate during hippocampal neurogenesis. PLoS One. 2009;4:e4779. doi: 10.1371/journal.pone.0004779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–6. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–7. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–9. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- Scobie KN, Hall BJ, Wilke SA, Klemenhagen KC, Fujii-Kuriyama Y, Ghosh A, Hen R, Sahay A. Kruppel-like factor 9 is necessary for late-phase neuronal maturation in the developing dentate gyrus and during adult hippocampal neurogenesis. J Neurosci. 2009;29:9875–87. doi: 10.1523/JNEUROSCI.2260-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–7. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Shibata M, Kurokawa D, Nakao H, Ohmura T, Aizawa S. MicroRNA-9 modulates Cajal-Retzius cell differentiation by suppressing Foxg1 expression in mouse medial pallium. J Neurosci. 2008;28:10415–21. doi: 10.1523/JNEUROSCI.3219-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrt RD, Szulwach KE, Pfeiffer RL, Li X, Guo W, Pathania M, Teng ZQ, Luo Y, Peng J, Bordey A, Jin P, Zhao X. MicroRNA miR-137 regulates neuronal maturation by targeting ubiquitin ligase mind bomb-1. Stem Cells. 2010;28:1060–70. doi: 10.1002/stem.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner B, Kronenberg G, Jessberger S, Brandt MD, Reuter K, Kempermann G. Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia. 2004;46:41–52. doi: 10.1002/glia.10337. [DOI] [PubMed] [Google Scholar]
- Strum JC, Johnson JH, Ward J, Xie H, Feild J, Hester A, Alford A, Waters KM. MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1. Mol Endocrinol. 2009;23:1876–84. doi: 10.1210/me.2009-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry. 2010;15:80–92. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–34. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–8. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Borght K, Wallinga AE, Luiten PG, Eggen BJ, Van der Zee EA. Morris water maze learning in two rat strains increases the expression of the polysialylated form of the neural cell adhesion molecule in the dentate gyrus but has no effect on hippocampal neurogenesis. Behav Neurosci. 2005;119:926–32. doi: 10.1037/0735-7044.119.4.926. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–4. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, Goodman RH, Impey S. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci U S A. 2005;102:16426–31. doi: 10.1073/pnas.0508448102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Davare M, Ando H, Fortin D, Varlamova O, Cheng HY, Marks D, Obrietan K, Soderling TR, Goodman RH, Impey S. An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc Natl Acad Sci U S A. 2008;105:9093–8. doi: 10.1073/pnas.0803072105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler EM, Geschwind DH, Palmer TD. Lithium regulates adult hippocampal progenitor development through canonical Wnt pathway activation. Mol Psychiatry. 2008;13:285–92. doi: 10.1038/sj.mp.4002093. [DOI] [PubMed] [Google Scholar]
- Yoo AS, Staahl BT, Chen L, Crabtree GR. MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature. 2009;460:642–6. doi: 10.1038/nature08139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JY, Chung KH, Deo M, Thompson RC, Turner DL. MicroRNA miR-124 regulates neurite outgrowth during neuronal differentiation. Exp Cell Res. 2008;314:2618–33. doi: 10.1016/j.yexcr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr., Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Sun G, Li S, Shi Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol. 2009;16:365–71. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.