Abstract
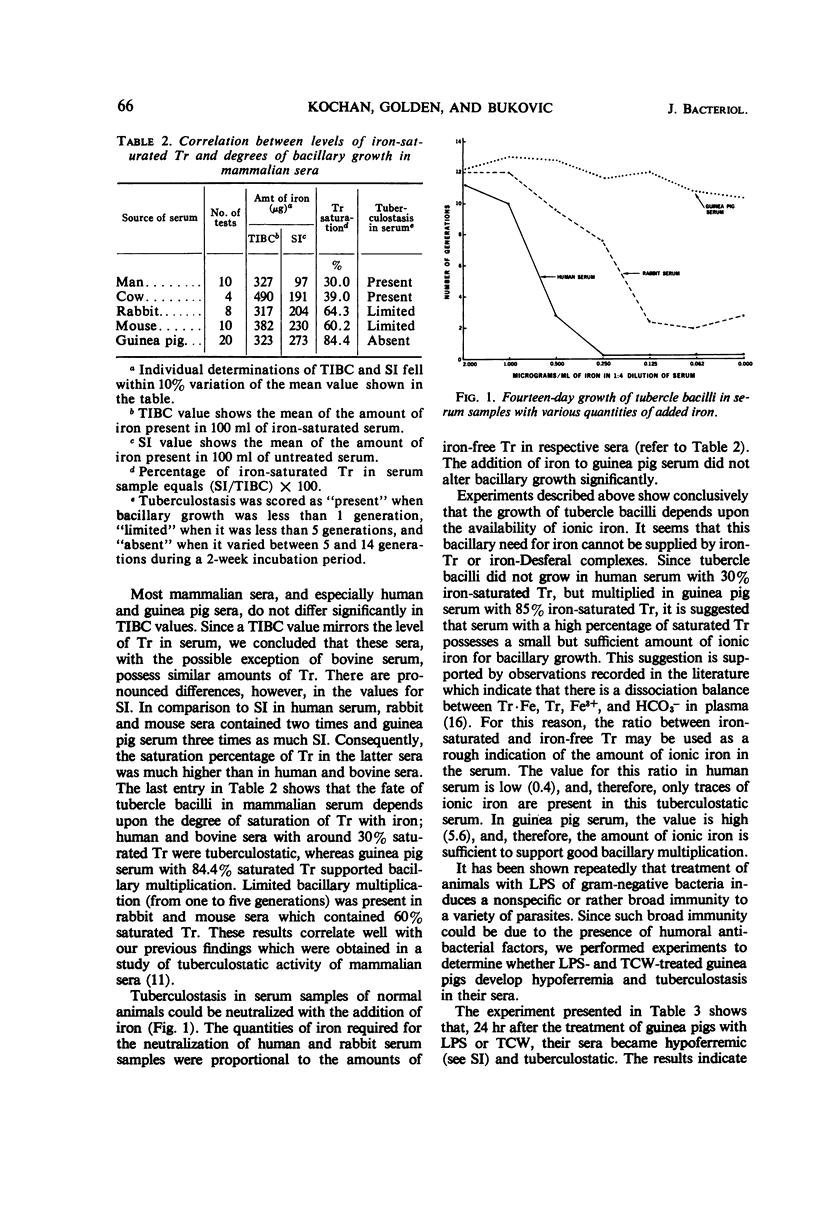
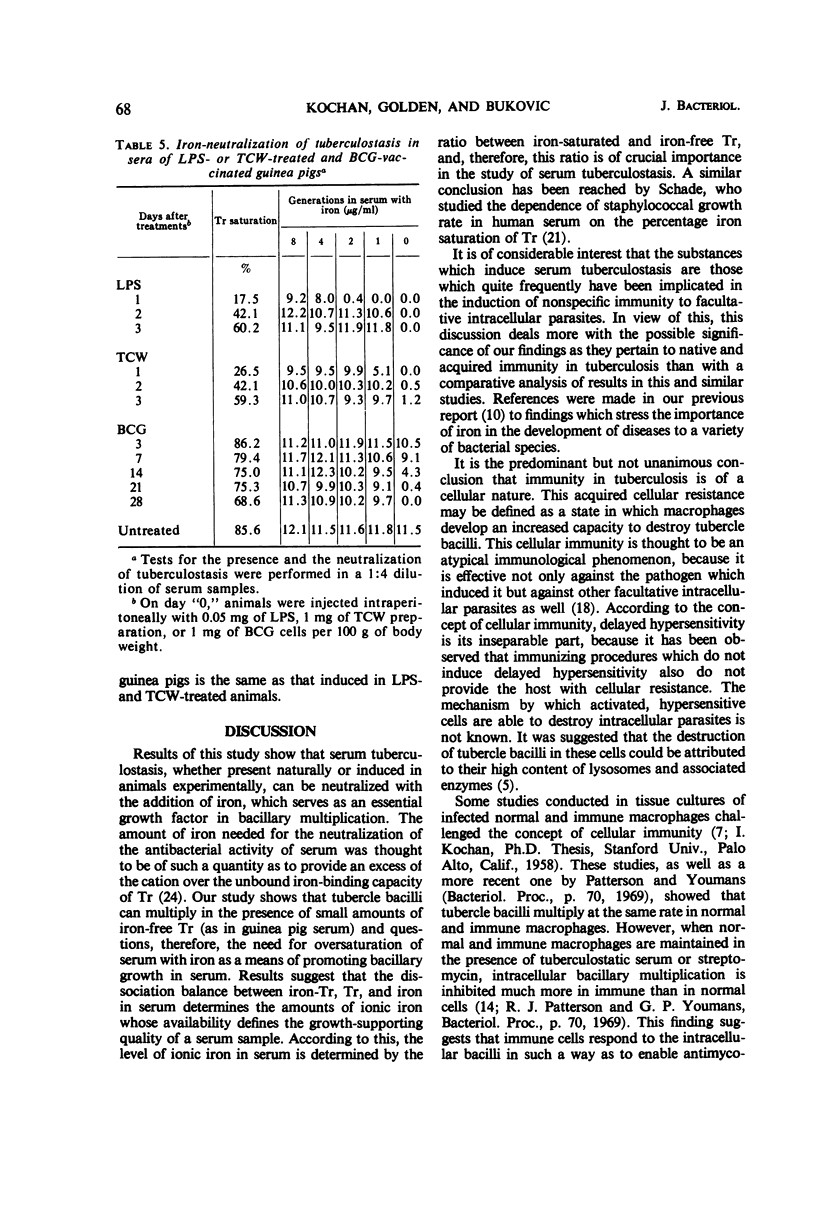
The growth of tubercle bacilli in serum samples of untreated animals depends upon the availability of ionic iron which serves as a growth factor in supporting bacillary multiplication. The amount of available iron in serum is determined by the ratio between iron-saturated and iron-free transferrin; a low value for the ratio is associated with tuberculostasis (e.g., human serum, 0.4), whereas a high value is associated with the growth-supporting quality (e.g., guinea pig serum, 5.6). The treatment of guinea pigs with lipopolysaccharide of Escherichia coli or tuberculous cell wall material consistently and significantly reduced serum iron levels; a similar but less striking effect was observed in BCG-vaccinated animals. Pronounced differences were observed in the time of appearance and duration of serum hypoferremia; in lipopolysaccharide-treated animals, it appeared in 1 day and lasted for several days, whereas in BCG-vaccinated animals it appeared in about 2 weeks and lasted for much longer time periods. The induced hypoferremia was always associated with the concomitant development of serum tuberculostasis which could be neutralized by the addition of iron. These results indicate, therefore, that the mechanism of induced serum tuberculostasis in lipopolysaccharide- or tuberculous cell wall-treated and BCG-vaccinated guinea pigs is the same as that present in tuberculostatic sera of untreated animals.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J., Wilson J. B. Hypoferremia in mice and its application to the bioassay of endotoxin. J Bacteriol. 1965 Oct;90(4):903–910. doi: 10.1128/jb.90.4.903-910.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Cushnie G. H., Rogers H. J. The abolition of the protective effect of Clostridium welchii type A antiserum by ferric iron. Immunology. 1967 Mar;12(3):303–312. [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Wilson A. B., Cushnie G. H., Rogers H. J. The abolition of the protective effect of Pasteurella septica antiserum by iron compounds. Immunology. 1968 Jun;14(6):889–898. [PMC free article] [PubMed] [Google Scholar]
- Coppel S., Youmans G. P. Specificity of the anamnestic response produced by Listeria monocytogenes or Mycobacterium tuberculosis to challenge with Listeria monocytogenes. J Bacteriol. 1969 Jan;97(1):127–133. doi: 10.1128/jb.97.1.127-133.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOS R. J., SCHAEDLER R. W. Reversible changes in the susceptibility of mice to bacterial infections. I. Changes brought about by injection of pertussis vaccine or of bacterial endotoxins. J Exp Med. 1956 Jul 1;104(1):53–65. doi: 10.1084/jem.104.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Cellular hypersensitivity and cellular immunity in the pathogensis of tuberculosis: specificity, systemic and local nature, and associated macrophage enzymes. Bacteriol Rev. 1968 Jun;32(2):85–102. doi: 10.1128/br.32.2.85-102.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELBERG S. S., FONG J., SCHNEIDER P. Studies on tubercle bacillus-monocyte relationship. I. Quantitative analysis of effect of serum of animals vaccinated with BCG upon bacterium-monocyte system. J Exp Med. 1956 Oct 1;104(4):455–465. doi: 10.1084/jem.104.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELBERG S. S., FONG J., SCHNEIDER P. Studies on tubercle bacillus-monocyte relationship. II. Induction of monocyte degeneration by bacteria and culture filtrate: specificity of serum and monocyte effects on resistance to degeneration. J Exp Med. 1957 Jan 1;105(1):25–37. doi: 10.1084/jem.105.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMPSCHMIDT R. F., UPCHURCH H. F. EFFECT OF ENDOTOXIN UPON TOTAL IRON-BINDING CAPACITY OF THE SERUM. Proc Soc Exp Biol Med. 1964 Jun;116:420–422. doi: 10.3181/00379727-116-29266. [DOI] [PubMed] [Google Scholar]
- KOCHAN I., ISHAK K., SAID M., STOTTS J. STUDY ON THE TUBERCULOSTATIC FACTOR OF MAMMALIAN SERUM. Am Rev Respir Dis. 1963 Dec;88:818–826. doi: 10.1164/arrd.1963.88.6.818. [DOI] [PubMed] [Google Scholar]
- KOCHAN I., PATTON C., ISHAK K. TUBERCULOSTATIC ACTIVITY OF NORMAL HUMAN SERA. J Immunol. 1963 May;90:711–719. [PubMed] [Google Scholar]
- KOCHAN I., RAFFEL S. A property of immune sera inhibitory for the growth of the tubercle bacillus. J Immunol. 1960 Apr;84:374–383. [PubMed] [Google Scholar]
- KOCHAN I., SMITH L. ANTIMYCOBACTERIAL ACTIVITY OF TUBERCULOSTATIC FACTOR ON INTRACELLULAR BACILLI. J Immunol. 1965 Feb;94:220–227. [PubMed] [Google Scholar]
- Kochan I. Mecahnism of tuberculostasis in mammalian serum. I. Role of transferrin in human serum tuberculostasis. J Infect Dis. 1969 Jan;119(1):11–18. doi: 10.1093/infdis/119.1.11. [DOI] [PubMed] [Google Scholar]
- LANDERS J. W., ZAK B. Determination of serum copper and iron in a single small sample. Am J Clin Pathol. 1958 Jun;29(6):590–592. doi: 10.1093/ajcp/29.6_ts.590. [DOI] [PubMed] [Google Scholar]
- MILLMAN I. Nonspecific resistance to tuberculosis. Am Rev Respir Dis. 1961 May;83:668–675. doi: 10.1164/arrd.1961.83.5.668. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The immunology of antituberculous immunity. Am Rev Respir Dis. 1968 Mar;97(3):337–344. doi: 10.1164/arrd.1968.97.3.337. [DOI] [PubMed] [Google Scholar]
- Sword C. P. Mechanisms of pathogenesis in Listeria monocytogenes infection. I. Influence of iron. J Bacteriol. 1966 Sep;92(3):536–542. doi: 10.1128/jb.92.3.536-542.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHEELER W. C., HANKS J. H. UTILIZATION OF EXTERNAL GROWTH FACTORS BY INTRACELLULAR MICROBES: MYCOBACTERIUM PARATUBERCULOSIS AND WOOD PIGEON MYCOBACTERIA. J Bacteriol. 1965 Mar;89:889–896. doi: 10.1128/jb.89.3.889-896.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON J. F., LAHEY M. E. Studies on iron metabolism. II. Observations on Ramsay's method for determination of the iron-binding capacity of the serum. Am J Dis Child. 1963 Jun;105:635–642. [PubMed] [Google Scholar]
- Weinberg E. D. Roles of metallic ions in host-parasite interactions. Bacteriol Rev. 1966 Mar;30(1):136–151. doi: 10.1128/br.30.1.136-151.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans G. P., Youmans A. S. Allergenicity of mycobacterial ribosomal and ribonucleic acid preparations in mice and guinea pigs. J Bacteriol. 1969 Jan;97(1):134–139. doi: 10.1128/jb.97.1.134-139.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans G. P., Youmans A. S. Immunizing capacity of viable and killed attenuated mycobacterial cells against experimental tuberculous infection. J Bacteriol. 1969 Jan;97(1):107–113. doi: 10.1128/jb.97.1.107-113.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



