Abstract
Purpose of review
The promise of islet transplantation for type 1 diabetes has been hampered by the lack of a renewable source of insulin-producing cells. However, steadfast advances in the field have set the stage for stem cell-based approaches to take over in the near future. This review focuses on the most intriguing findings reported in recent years, which include not only progress in adult and embryonic stem cell differentiation, but also the direct reprogramming of non-endocrine tissues into insulin-producing beta cells.
Recent findings
In spite of their potential for tumorigenesis, human embryonic stem (hES) cells are poised to be in clinical trials within the next decade. This is mainly due to the preclinical success of a differentiation method that recapitulates beta cell development. In contrast, adult stem cells still need one such gold standard of differentiation, and progress is somewhat impeded by the lack of consensus on the best source. A concerted effort is necessary to bring their potential to clinical fruition. In the meantime, reported success in reprogramming might offer a “third way” towards the rescue of pancreatic endocrine function.
Summary
Here we discuss the important strategic decisions that need to be made in order to maximize the therapeutic chances of each of the presented approaches.
Keywords: Human embryonic stem (hES) cells, induced pluripotent stem (iPS) cells, mesenchymal stem cells (MSCs), beta cell differentiation, reprogramming, islet transplantation
Introduction
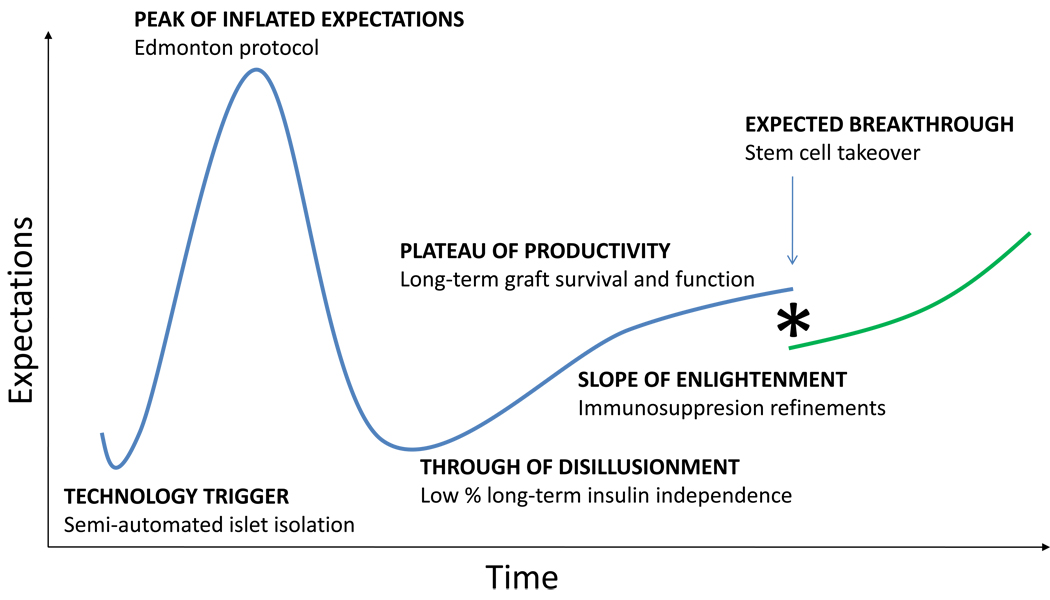
The evolution of islet transplantation has visibly followed each one of the steps of the often cited “Gartner hype cycle” [1]. There is little argument that the invention of the semi-automated method for islet isolation [2] was the “technology trigger” that helped popularize the procedure throughout the world. Indeed, this breakthrough was universally saluted as the onset of a new era of cell therapy for the treatment of autoimmune diabetes. Few disputed that this safe and conceptually simple procedure may lead to the relegation of whole pancreas transplantation for the radical treatment of type 1 diabetes. The “peak of inflated expectations” was reached around 2000 with the clinical implementation of a steroid-free immunosuppressive regime that resulted in long-term graft survival (the Edmonton protocol) [3]. Unfortunately, a subsequent follow-up study revealed a much more somber panorama. The realization that only about 20% of the patients remained insulin-independent five years after the procedure [4] dragged the field through a long “trough of disillusionment” that stoked the perception that islet transplantation had taken us as far as it could –which was nowhere close to a cure. Many of the numerous centers that had mushroomed after the first announcements of long-term engraftment closed or drastically reduced operations, and overall funding decreased. A slow “slope of enlightenment” amidst this hostile climate has quietly led to a “plateau of productivity” in which T-cell depleting strategies and other novel immunological interventions now ensure graft survival and function at rates comparable to those observed with whole organ transplantation [5]. While the expectations for this therapy are now considerably lower than just a decade ago, our progressive understanding of its limitations has helped us pave the way for the new generation of cell therapeutics. In fact, it is thanks to islet transplantation that we can now accurately predict not only the nature but also the likely course of the next breakthrough. While stem cell-based advances for other conditions will have to blaze new trails, at the time we manage to effectively and safely convert stem cells into insulin-producing cells, we will already be familiar with the learning curve. The groundwork is laid for the next generation of scientists to dramatically expand the clinical applicability of islet transplantation beyond its current reach (figure 1).
Figure 1.
Gartner innovation curve representing the defining steps of the evolution of clinical islet transplantation.
There is a broad consensus on the idea that stem cells will just take the place of islets in the near future, riding along the beaten track set by decades of research on the latter. However, the jury is still out regarding the candidate cell type/s and approach that will ultimately succeed. Since the subject has been recently reviewed in great depth by us [6, 7] and others [8–14], we will focus this article in three of the most rapidly evolving trends, namely the differentiation of embryonic stem (ES)/induced pluripotent stem (iPS) cells, the characterization of some of the most promising additions to the arsenal of adult stem cells (neural crest-derived and adipose cells), and the prospects of direct reprogramming of adult tissues.
ES and iPS cells
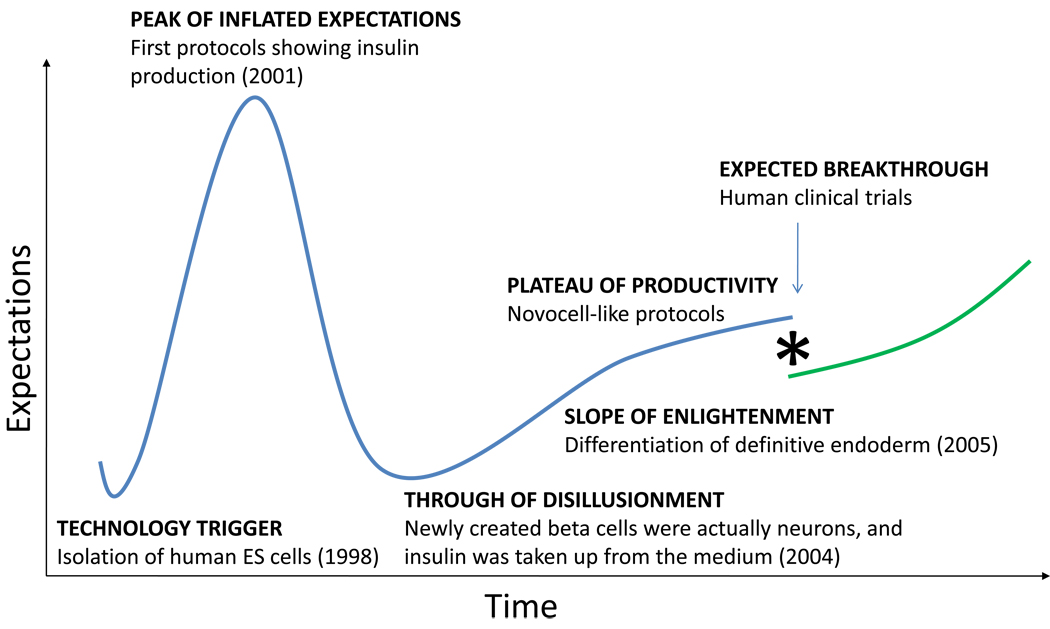
By almost any measure, embryonic stem (ES) cells are the gold standard of all stem cells. Both their replicative potential and ability to become any cell type remain unmatched. Detractors of this technology often cite the lack of ongoing clinical trials with ES cells as evidence of the purported superiority of adult stem cells, which undoubtedly are more widely represented in the clinical arena. This assessment is misleading on several counts, chiefly the fact that adult stem cells have been in use for more than half a century, whereas human ES cells were first isolated just twelve years ago [15]. In fact, a careful review of the literature indicates that insulin-producing cells of ES cell origin are closer to true beta cells than any other cell differentiation product from adult stem cells. This is so despite a Gartner hype cycle curve that has fallen precipitously after a peak of overinflated expectations (see figure 2). Following a careful recapitulation of the earlier events of specification towards definitive endoderm [16], D’Amour and colleagues initiated the “slope of enlightenment” by showing that a small percentage of these cells can be converted into beta-like cells with an insulin content similar to that seen in native islets [17]. These studies were followed by others in which transplantation into immunocompromised mice was done halfway through the differentiation process. When the cells were allowed to mature in vivo, the efficiency of differentiation was significantly improved, and chemically induced diabetes prevented [18]. California-based ViaCyte (formerly Novocell) has reported preclinical success with Pro-Islet™, which is based on the above strategy in conjunction with a durable, retrievable encapsulation device. Efforts at translating this strategy into clinical therapies have been highlighted by the recent award of more than $20 million from the California Institute of Regenerative Medicine (CIRM). This makes the goal of having ES cell-based therapies in clinical trials within the next 5–10 years an attainable one. Inasmuch as the formation of teratomas is a common occurrence in pre-clinical experiments [18, 19], safety remains a concern. It is argued, however, that the use of immunoisolation devices will allow for the use of allogeneic material in fully immunocompetent recipients, because any tumorigenic escapees would be promptly destroyed by the host’s immune response.
Figure 2.
Gartner innovation curve representing the key milestones of embryonic stem cell differentiation along the insulin-producing cell lineage.
iPS cells have recently stormed in the field almost by surprise [20–27]. Although some claim that the hype associated with these non-controversial ES cell surrogates is now starting to wane [28, 29], there is little not to love about this perfect combination of the best features of adult and embryonic stem cells. Although they are virtually indistinguishable from ES cells, their procurement does not entail the destruction of human embryos, and the fact they could be generated from the prospective patient only adds to their appeal for the design of autologous therapies. Following the main steps of another successful beta cell differentiation protocol[30], Tateishi and colleagues [31] reported recently the generation of insulin-producing islet-like clusters. There is no doubt that ES and iPS cell-based protocols will be largely interchangeable in the near future. Original concerns about the use of integrative viruses for their generation have been addressed by means of the progressive introduction of transducible proteins [32, 33], episomal constructs [26], DNA minicircles [34], modified mRNAs [35] and even small molecules with reprogramming properties [36]. On the negative side, the risks inherent to the use ES cells are also common to iPS cells, and a recent report warned about the potential premature senescence of iPS cell-derived hemangioblasts [37].
Adipose and neural crest-derived stem cells
Mesenchymal stem cells (MSCs) have long been considered the workhorse of adult stem cell research. Easily procurable from virtually every tissue [38], they have been favored by their remarkable (if not unlimited) expandability in plastic surfaces and their readiness to differentiate into a variety of therapeutically valuable tissues, chiefly those of the connective family [39]. Despite the fact that clear-cut criteria should in theory unify MSCs of every possible origin [40, 41], to date there is no consensus on whether MSCs are one and the same. The study of the properties of MSCs from different tissues is additionally complicated by the observation that even discrete clones from the same tissue exhibit intrinsic variability in their differentiation potential [42]. Therefore, the quest for an elusive “best” source for beta cell differentiation may actually entail the screening of hundreds of potential candidates. Considering the current dispersion of differentiation methods for adult stem cells (which span numerous signal-driven approaches [43–52], genetic manipulation [53–58] and even in vivo maturation after systemic administration [48, 49, 59–61]), the fact that we still do not have a gold-standard of MSC-to-beta cell differentiation similar to what the ViaCyte protocol [17, 18] represents for hES cells is reason for concern. However, before making a decision to bet on any given MSC source and try to develop a universal protocol that could later be applied to others, we must also consider that the ViaCyte method was indeed optimized for one particular hES cell line, and does not seem to work as efficiently with other lines. As laboratories around the world juggle the centrifugal needs of defining a gold-standard protocol and identifying the ideal MSC source, we anticipate a period of tentativeness before a true breakthrough is reported. Until such time, banking patient-matched MSCs seems like a sensible idea. Those patients for whom the MSC-rich fraction of the umbilical cord blood was not preserved [62–65] may still have the option of harvesting their own adipose stem cells by liposuction later in life. In this context, adipose MSCs obtained from blepharoplastic procedures have recently shown great promise at reversing diabetes in preclinical models [66]. Success in these studies was attributed to the use of a specific MSC lineage claimed to derive from the neural crest, which is home to highly multipotent cells [67]. This would also be consistent with the reported ability of another neural crest-derived MSC, the periodontal ligament, to differentiate into insulin-producing cells [68]. Whether or not MSCs hold the key to an unlimited supply of beta cells, at the very least they are known to have strong pro-angiogenic [69, 70] and immunomodulatory [71–74] properties, which makes them extremely attractive from a therapeutic perspective. Some investigators even go as far as maintaining that some MSCs will not be rejected in allogeneic and even xenogeneic settings [66]. It is indeed a common misconception that, since MSCs are negative for class II major histocompatibility complex molecules, they should not be rejected. This is not true, as class I mismatches are known to invariably result in rejection in immunocompetent hosts. However, the possibility that these cells are actively hiding from the immune system through other yet-to-be-elucidated mechanisms cannot be ruled out.
Direct reprogramming
The basic idea behind reprogramming (also termed transdifferentiation) is that even a terminally differentiated tissue might be converted into another under the appropriate conditions. Following Waddington’s imagery [75, 76], if the determinants of normal differentiation were like boulders rolling downhill until they found their final accommodation, reprogramming would require a rearrangement of the cell’s epigenetic landscape akin to pushing the boulders over the top of the mountain and down another valley. Such relocation is possible, and indeed has been described under specific circumstances between the pancreas and the liver [77–82], perhaps because of the shared ancestry of both organs [83–91]. However, it is clear by now that accomplishing this in a consistent fashion entails much more aggressive interventions (namely, the introduction of “master genes” from the desired tissue) than those used for standard differentiation. Earlier this decade, Ferber and colleagues pioneered this approach by delivering the Pdx1 gene (a vital regulator of pancreatic development [92] and beta cell homeostasis [93]) into recipient mice by means of adenoviral vehicles. Ectopic expression in the liver led to the activation of beta cell genes and dramatic reductions in blood glucose levels, which outlived the period during which the adenovirus was expected to remain in the system [94, 95]. Subsequent studies confirmed that an initial trigger was sufficient to unfold a self-sustainable beta cell differentiation program [96]. Following their lead, other groups reported similar results either with Pdx1 alone or together with other reprogramming genes [97–104]. However, with the possible exception of the transgenic frog setting described by Horb et al [105] (which, strictly speaking, was a “redirecting” of embryonic development rather than proper transdifferentiation), the majority of these approaches did not yield true, glucose-responsive beta cells. This changed recently with a report showing that the transfer of three factors (Pdx1, Ngn3 and MafA) led to the reprogramming of pancreatic acinar tissue towards beta cells [106]. Although similar combinations of genes had already been tested with only moderate success in liver [84, 99, 107–110], the developmental proximity between pancreatic acinar and endocrine cells may have made the difference. These results are very exciting from a therapeutic perspective, because the harnessing of direct reprogramming may offer a less cumbersome alternative to differentiation, especially if methods that do not rely on the use of viruses (such as protein transduction [111]) are developed. Although in vivo reprogramming strategies would be a stretch in a therapeutic context, the plentiful leftover tissue that is currently discarded after islet isolation procedures could be an excellent source of newly created beta cells. Alternatively, candidate tissues could be biopsied from the patient, reprogrammed ex vivo and then implanted back. While autoimmunity would still have to be addressed separately, allorejection would no longer be of concern. This is further discussed in the next section.
Conclusion
To the long-standing controversy between the use of adult or embryonic stem cells, a key decision to be made is whether to bank on autologous cells customized from the patient we intend to treat, or to focus on a universal donor cell line that could be taken “over the counter” in the context of a robust tolerance induction or immunoisolation method. This question is most timely. Millions are invested each year in strategies that will potentially involve the use of patient-derived autologous adult stem or iPS cells. But are these approaches scientifically justified for diabetes? Are they really worth the trouble? On the one hand, it could be argued that taking allorejection out of the picture would single out autoimmunity as the only remaining challenge, one that perhaps could be amenable to more targeted interventions. On the other, wouldn’t meeting such challenge be akin to stopping the onset of the disease on its tracks –in other words, curing the disease? If we follow this reasoning, years and millions of dollars spent on the development of autologous beta cell transplantation strategies may just lead us to the realization that we would still need to find a cure for diabetes. Despite the uniqueness of this condition when compared to other possible targets of stem cell therapies, the momentum of novel techniques such as nuclear reprogramming may have contributed to the perception that some approaches must be pursued just because they can be pursued. Gathering the necessary data to decide between allogeneic and autologous is a strategic decision that should be made now, to prevent precious resources from being spread too thin in too many directions. The prospect of personalizing regenerative medicine strategies for type 1 diabetes is appealing, but complicated and, at the end of the day, perhaps useless from the perspective of getting us closer to a cure. The alternative of a well-defined allogeneic stem cell substrate may enormously simplify the problem –not because the patient would not need immunosuppresion, but because immunosuppresion would be also needed for autologous transplantation anyway, or because advances in immunoisolation [112, 113] will render the entire autologous approach obsolete.
Acknowledgements
The authors state no conflict of interest and acknowledge the funding of the National Institutes of Health, The Juvenile Diabetes Research Foundation, the American Diabetes Association, the Foundation for Diabetes Research and the Diabetes Research Institute Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fenn J, Raskino M. Mastering the hype cycle: how to choose the right innovation at the right time. Boston, Mass: Harvard Business Press; 2008. [Google Scholar]
- 2. Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413–420. doi: 10.2337/diab.37.4.413. (**) The semi-automated method soon became the standard of islet isolation around the world.
- 3. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. (**) This is the first report on long-term islet function in humans. It was based on a steroid-free immunosuppressive regimen (the “Edmonton protocol”). Anti-rejection drugs used prior to this clinical breakthrough usually resulted in graft death shortly after transplantation.
- 4. Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060–2069. doi: 10.2337/diabetes.54.7.2060. (*) This report revealed the limitations of the Edmonton protocol, with progressive loss of graft function between 1 and 5 years post-transplantation.
- 5.Bellin MD, Kandaswamy R, Parkey J, Zhang HJ, Liu B, Ihm SH, et al. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am J Transplant. 2008;8(11):2463–2470. doi: 10.1111/j.1600-6143.2008.02404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atala A. Principles of regenerative medicine. Amsterdam; Boston [Mass.]: Academic Press; 2008. [Google Scholar]
- 7.Domínguez-Bendala J. Pancreatic stem cells. New York: Humana Press; 2009. SpringerLink (Online service) [Google Scholar]
- 8.Noguchi H. Production of pancreatic beta-cells from stem cells. Curr Diabetes Rev. 6(3):184–190. doi: 10.2174/157339910791162934. [DOI] [PubMed] [Google Scholar]
- 9.Aguayo-Mazzucato C, Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol. 6(3):139–148. doi: 10.1038/nrendo.2009.274. [DOI] [PubMed] [Google Scholar]
- 10.Guo T, Hebrok M. Stem cells to pancreatic beta-cells: new sources for diabetes cell therapy. Endocr Rev. 2009;30(3):214–227. doi: 10.1210/er.2009-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borowiak M, Melton DA. How to make beta cells? Curr Opin Cell Biol. 2009;21(6):727–732. doi: 10.1016/j.ceb.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kordowich S, Mansouri A, Collombat P. Reprogramming into pancreatic endocrine cells based on developmental cues. Mol Cell Endocrinol. 323(1):62–69. doi: 10.1016/j.mce.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Mishra PK, Singh SR, Joshua IG, Tyagi SC. Stem cells as a therapeutic target for diabetes. Front Biosci. 15:461–477. doi: 10.2741/3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hori Y. Insulin-producing cells derived from stem/progenitor cells: therapeutic implications for diabetes mellitus. Med Mol Morphol. 2009;42(4):195–200. doi: 10.1007/s00795-009-0471-x. [DOI] [PubMed] [Google Scholar]
- 15. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. (*) First report on human embryonic stem cell isolation
- 16. D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23(12):1534–1541. doi: 10.1038/nbt1163. (*) First description of the conditions leading to the efficient in vitro generation of definitive endoderm from human embryonic stem cells, which opened the door to the differentiation of insulin-producing beta cells.
- 17. D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006 doi: 10.1038/nbt1259. (**) First report on the generation of bona fide beta cells from stem cells.
- 18. Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–452. doi: 10.1038/nbt1393. (**) Follow-up on the previous report, showing that transplanting immature pancreatic progenitor cells in recipient mice prevents hyperglycemia upon chemical induction of diabetes.
- 19.Fujikawa T, Oh SH, Pi L, Hatch HM, Shupe T, Petersen BE. Teratoma formation leads to failure of treatment for type I diabetes using embryonic stem cell-derived insulin-producing cells. Am J Pathol. 2005;166(6):1781–1791. doi: 10.1016/S0002-9440(10)62488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewitzky M, Yamanaka S. Reprogramming somatic cells towards pluripotency by defined factors. Curr Opin Biotechnol. 2007;18(5):467–473. doi: 10.1016/j.copbio.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2007 doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 22.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of Mouse Induced Pluripotent Stem Cells Without Viral Vectors. Science. 2008 doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2(12):3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, et al. Human Induced Pluripotent Stem Cells Free of Vector and Transgene Sequences. Science. 2009 doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 28.Belmonte JC, Ellis J, Hochedlinger K, Yamanaka S. Induced pluripotent stem cells and reprogramming: seeing the science through the hype. Nat Rev Genet. 2009;10(12):878–883. doi: 10.1038/nrg2700. [DOI] [PubMed] [Google Scholar]
- 29.Mason C, Manzotti E. Induced pluripotent stem cells: an emerging technology platform and the Gartner hype cycle. Regen Med. 2009;4(3):329–331. doi: 10.2217/rme.09.20. [DOI] [PubMed] [Google Scholar]
- 30.Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, et al. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25(8):1940–1953. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- 31.Tateishi K, He J, Taranova O, Liang G, D'Alessio AC, Zhang Y. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008 doi: 10.1074/jbc.M806597200. [DOI] [PubMed] [Google Scholar]
- 32.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou H, Wu S, Young Joo J, Zhu S, Wook Han D, Lin T, Trauger S, Bien G, Yao S, Zhu Y, Siuzdak G, Scholer H, Duan L, Ding S. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009 doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, et al. A nonviral minicircle vector for deriving human iPS cells. Nat Methods. 7(3):197–199. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, et al. Highly Efficient Reprogramming to Pluripotency and Directed Differentiation of Human Cells with Synthetic Modified mRNA. Cell Stem Cell. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi Y, Desponts C, Do JT, Hahm HS, Scholer HR, Ding S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3(5):568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Feng Q, Lu SJ, Klimanskaya I, Gomes I, Kim D, Chung Y, et al. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells. 28(4):704–712. doi: 10.1002/stem.321. [DOI] [PubMed] [Google Scholar]
- 38.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(Pt 11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 39.Trounson A. New perspectives in human stem cell therapeutic research. BMC Med. 2009;7:29. doi: 10.1186/1741-7015-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 41.Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 42.Paredes B, Santana A, Arribas M, Vicente-Salar N, de Aza P, Roche E. Phenotypic differences during the osteogenic differentiation of single-cell derived clones isolated from human lipoaspirates. Journal of Tissue Engineering and regenerative Medicine. 2010 doi: 10.1002/term.351. (in press) [DOI] [PubMed] [Google Scholar]
- 43.Chen LB, Jiang XB, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol. 2004;10(20):3016–3020. doi: 10.3748/wjg.v10.i20.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi KS, Shin JS, Lee JJ, Kim YS, Kim SB, Kim CW. In vitro trans-differentiation of rat mesenchymal cells into insulin-producing cells by rat pancreatic extract. Biochem Biophys Res Commun. 2005;330(4):1299–1305. doi: 10.1016/j.bbrc.2005.03.111. [DOI] [PubMed] [Google Scholar]
- 45.Baertschiger RM, Bosco D, Morel P, Serre-Beinier V, Berney T, Buhler LH, et al. Mesenchymal stem cells derived from human exocrine pancreas express transcription factors implicated in beta-cell development. Pancreas. 2008;37(1):75–84. doi: 10.1097/MPA.0b013e31815fcb1e. [DOI] [PubMed] [Google Scholar]
- 46.Chang CF, Hsu KH, Chiou SH, Ho LL, Fu YS, Hung SC. Fibronectin and pellet suspension culture promote differentiation of human mesenchymal stem cells into insulin producing cells. J Biomed Mater Res A. 2008;86(4):1097–1105. doi: 10.1002/jbm.a.31767. [DOI] [PubMed] [Google Scholar]
- 47.Chang C, Niu D, Zhou H, Li F, Gong F. Mesenchymal stem cells contribute to insulin-producing cells upon microenvironmental manipulation in vitro. Transplant Proc. 2007;39(10):3363–3368. doi: 10.1016/j.transproceed.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 48.Chang C, Niu D, Zhou H, Zhang Y, Li F, Gong F. Mesenchymal stromal cells improve hyperglycemia and insufficient insulin upon diabetic pancreatic microenvironment in pigs. Cytotherapy. 2008:1–10. doi: 10.1080/14653240802461924. [DOI] [PubMed] [Google Scholar]
- 49.Chang C, Wang X, Niu D, Zhang Z, Zhao H, Gong F. Mesenchymal Stem Cells Adopt beta-Cell Fate Upon Diabetic Pancreatic Microenvironment. Pancreas. 2008 doi: 10.1097/MPA.0b013e318191521c. [DOI] [PubMed] [Google Scholar]
- 50.Hisanaga E, Park KY, Yamada S, Hashimoto H, Takeuchi T, Mori M, et al. A simple method to induce differentiation of murine bone marrow mesenchymal cells to insulin-producing cells using conophylline and betacellulin-delta4. Endocr J. 2008;55(3):535–543. doi: 10.1507/endocrj.k07e-173. [DOI] [PubMed] [Google Scholar]
- 51.Chandra V, G S, Phadnis S, Nair PD, Bhonde RR. Generation of pancreatic hormone-expressing islet-like cell aggregates from murine adipose tissue-derived stem cells. Stem Cells. 2009;27(8):1941–1953. doi: 10.1002/stem.117. [DOI] [PubMed] [Google Scholar]
- 52.Vikash C, Swetha G, Smruti P, Prabha DN, Ramesh RB. Generation of Pancreatic Hormone-Expressing Islet-Like Cell Aggregates from Murine Adipose Tissue-Derived Stem Cells. Stem Cells. 2009;27(8):1941–1953. doi: 10.1002/stem.117. [DOI] [PubMed] [Google Scholar]
- 53.Sun J, Yang Y, Wang X, Song J, Jia Y. Expression of Pdx-1 in bone marrow mesenchymal stem cells promotes differentiation of islet-like cells in vitro. Sci China C Life Sci. 2006;49(5):480–489. doi: 10.1007/s11427-006-2016-z. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Zhang R, Qiao H, Zhang H, Wang Y, Yuan H, et al. Generation of insulin-producing cells from PDX-1 gene-modified human mesenchymal stem cells. J Cell Physiol. 2007;211(1):36–44. doi: 10.1002/jcp.20897. [DOI] [PubMed] [Google Scholar]
- 55.Moriscot C, de Fraipont F, Richard MJ, Marchand M, Savatier P, Bosco D, et al. Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro. Stem Cells. 2005;23(4):594–603. doi: 10.1634/stemcells.2004-0123. [DOI] [PubMed] [Google Scholar]
- 56.Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells. 2007;25(11):2837–2844. doi: 10.1634/stemcells.2007-0164. [DOI] [PubMed] [Google Scholar]
- 57.Masaka T, Miyazaki M, Du G, Hardjo M, Sakaguchi M, Takaishi M, et al. Derivation of hepato-pancreatic intermediate progenitor cells from a clonal mesenchymal stem cell line of rat bone marrow origin. Int J Mol Med. 2008;22(4):447–452. [PubMed] [Google Scholar]
- 58.Xu J, Lu Y, Ding F, Zhan X, Zhu M, Wang Z. Reversal of diabetes in mice by intrahepatic injection of bone-derived GFP-murine mesenchymal stem cells infected with the recombinant retrovirus-carrying human insulin gene. World J Surg. 2007;31(9):1872–1882. doi: 10.1007/s00268-007-9168-2. [DOI] [PubMed] [Google Scholar]
- 59.Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yanez AJ, Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant. 2008;14(6):631–640. doi: 10.1016/j.bbmt.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 60.Dong QY, Chen L, Gao GQ, Wang L, Song J, Chen B, et al. Allogeneic diabetic mesenchymal stem cells transplantation in streptozotocin-induced diabetic rat. Clin Invest Med. 2008;31(6):E328–E337. doi: 10.25011/cim.v31i6.4918. [DOI] [PubMed] [Google Scholar]
- 61.Estrada E, Valacchi F, Nicora E, Brieva S, Esteve C, Echevarria L, Froud T, Bernetti K, Messinger Cayetano S, Velazquez O, Alejandro R, Ricordi C. Combined Treatment of Intrapancreatic Autologous Bone Marrow Stem Cells and Hyperbaric Oxygen in Type 2 Diabetes Mellitus. Cell Transplant. 2008;17(12):1295–1304. doi: 10.3727/096368908787648119. [DOI] [PubMed] [Google Scholar]
- 62.Ballen K. Challenges in umbilical cord blood stem cell banking for stem cell reviews and reports. Stem Cell Rev. 2010;6(1):8–14. doi: 10.1007/s12015-009-9105-x. [DOI] [PubMed] [Google Scholar]
- 63.Hollands P, McCauley C. Private cord blood banking: current use and clinical future. Stem Cell Rev. 2009;5(3):195–203. doi: 10.1007/s12015-009-9082-0. [DOI] [PubMed] [Google Scholar]
- 64.Newcomb JD, Sanberg PR, Klasko SK, Willing AE. Umbilical cord blood research: current and future perspectives. Cell Transplant. 2007;16(2):151–158. [PMC free article] [PubMed] [Google Scholar]
- 65.Samuel GN, Kerridge IH, O'Brien TA. Umbilical cord blood banking: public good or private benefit? Med J Aust. 2008;188(9):533–535. doi: 10.5694/j.1326-5377.2008.tb02198.x. [DOI] [PubMed] [Google Scholar]
- 66.Kang HM, Kim J, Park S, Kim J, Kim H, Kim KS, et al. Insulin-secreting cells from human eyelid-derived stem cells alleviate type I diabetes in immunocompetent mice. Stem Cells. 2009;27(8):1999–2008. doi: 10.1002/stem.127. [DOI] [PubMed] [Google Scholar]
- 67.Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131(19):4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- 68.Huang CY, Pelaez D, Dominguez-Bendala J, Garcia-Godoy F, Cheung HS. Plasticity of stem cells derived from adult periodontal ligament. Regen Med. 2009;4(6):809–821. doi: 10.2217/rme.09.55. [DOI] [PubMed] [Google Scholar]
- 69.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 70.Ball SG, Shuttleworth CA, Kielty CM. Mesenchymal stem cells and neovascularization: role of platelet-derived growth factor receptors. J Cell Mol Med. 2007;11(5):1012–1030. doi: 10.1111/j.1582-4934.2007.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57(7):1759–1767. doi: 10.2337/db08-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mishra PK. Bone marrow-derived mesenchymal stem cells for treatment of heart failure: is it all paracrine actions and immunomodulation? J Cardiovasc Med (Hagerstown) 2008;9(2):122–128. doi: 10.2459/JCM.0b013e32820588f0. [DOI] [PubMed] [Google Scholar]
- 73.Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262(5):509–525. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 74.Ozaki K, Sato K, Oh I, Meguro A, Tatara R, Muroi K, et al. Mechanisms of immunomodulation by mesenchymal stem cells. Int J Hematol. 2007;86(1):5–7. doi: 10.1532/IJH97.07003. [DOI] [PubMed] [Google Scholar]
- 75.Slack JM. Conrad Hal Waddington: the last Renaissance biologist? Nat Rev Genet. 2002;3(11):889–895. doi: 10.1038/nrg933. [DOI] [PubMed] [Google Scholar]
- 76.Waddington CH. Organisers & genes. Cambridge Eng.: The University Press; 1940. [Google Scholar]
- 77.Rao MS, Dwivedi RS, Subbarao V, Usman MI, Scarpelli DG, Nemali MR, et al. Almost total conversion of pancreas to liver in the adult rat: a reliable model to study transdifferentiation. Biochem Biophys Res Commun. 1988;156(1):131–136. doi: 10.1016/s0006-291x(88)80814-3. [DOI] [PubMed] [Google Scholar]
- 78.Rao MS, Reddy JK. Hepatic transdifferentiation in the pancreas. Semin Cell Biol. 1995;6(3):151–156. doi: 10.1006/scel.1995.0021. [DOI] [PubMed] [Google Scholar]
- 79.Rao MS, Subbarao V, Reddy JK. Induction of hepatocytes in the pancreas of copper-depleted rats following copper repletion. Cell Differ. 1986;18(2):109–117. doi: 10.1016/0045-6039(86)90005-9. [DOI] [PubMed] [Google Scholar]
- 80.Shen CN, Slack JM, Tosh D. Molecular basis of transdifferentiation of pancreas to liver. Nat Cell Biol. 2000;2(12):879–887. doi: 10.1038/35046522. [DOI] [PubMed] [Google Scholar]
- 81.Wolf HK, Burchette JL, Jr, Garcia JA, Michalopoulos G. Exocrine pancreatic tissue in human liver: a metaplastic process? Am J Surg Pathol. 1990;14(6):590–595. doi: 10.1097/00000478-199006000-00011. [DOI] [PubMed] [Google Scholar]
- 82.Lee BC, Hendricks JD, Bailey GS. Metaplastic pancreatic cells in liver tumors induced by diethylnitrosamine. Exp Mol Pathol. 1989;50(1):104–113. doi: 10.1016/0014-4800(89)90060-9. [DOI] [PubMed] [Google Scholar]
- 83.Melton D. Signals for tissue induction and organ formation in vertebrate embryos. Harvey Lect. 1997;93:49–64. [PubMed] [Google Scholar]
- 84. Deutsch G, Jung J, Zheng M, Lora J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128(6):871–881. doi: 10.1242/dev.128.6.871. (*) Seminal study describing the molecular basis of the ontological relationship between the pancreas and the liver.
- 85.Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284(5422):1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- 86.Lemaigre F, Zaret KS. Liver development update: new embryo models, cell lineage control, and morphogenesis. Curr Opin Genet Dev. 2004;14(5):582–590. doi: 10.1016/j.gde.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 87.Tremblay KD, Zaret KS. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev Biol. 2005;280(1):87–99. doi: 10.1016/j.ydbio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 88.Yoshitomi H, Zaret KS. Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development. 2004;131(4):807–817. doi: 10.1242/dev.00960. [DOI] [PubMed] [Google Scholar]
- 89.Zaret KS. Hepatocyte differentiation: from the endoderm and beyond. Curr Opin Genet Dev. 2001;11(5):568–574. doi: 10.1016/s0959-437x(00)00234-3. [DOI] [PubMed] [Google Scholar]
- 90.Zaret KS. Liver specification and early morphogenesis. Mech Dev. 2000;92(1):83–88. doi: 10.1016/s0925-4773(99)00326-3. [DOI] [PubMed] [Google Scholar]
- 91.Wells JM, Melton DA. Vertebrate endoderm development. Annu Rev Cell Dev Biol. 1999;15:393–410. doi: 10.1146/annurev.cellbio.15.1.393. [DOI] [PubMed] [Google Scholar]
- 92.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371(6498):606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 93.Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12(12):1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6(5):568–572. doi: 10.1038/75050. (*) One of the pioneering studies on forced liver-to-pancreas transdifferentiation based on the ectopic expression of pancreatic genes.
- 95.Ber I, Shternhall K, Perl S, Ohanuna Z, Goldberg I, Barshack I, et al. Functional, persistent, and extended liver to pancreas transdifferentiation. J Biol Chem. 2003;278(34):31950–31957. doi: 10.1074/jbc.M303127200. [DOI] [PubMed] [Google Scholar]
- 96.Meivar-Levy I, Sapir T, Gefen-Halevi S, Aviv V, Barshack I, Onaca N, et al. Pancreatic and duodenal homeobox gene 1 induces hepatic dedifferentiation by suppressing the expression of CCAAT/enhancer-binding protein beta. Hepatology. 2007;46(3):898–905. doi: 10.1002/hep.21766. [DOI] [PubMed] [Google Scholar]
- 97.Tang DQ, Lu S, Sun YP, Rodrigues E, Chou W, Yang C, et al. Reprogramming liver-stem WB cells into functional insulin-producing cells by persistent expression of Pdx1- and Pdx1-VP16 mediated by lentiviral vectors. Lab Invest. 2006;86(1):83–93. doi: 10.1038/labinvest.3700368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang AY, Ehrhardt A, Xu H, Kay MA. Adenovirus transduction is required for the correction of diabetes using Pdx-1 or Neurogenin-3 in the liver. Mol Ther. 2007;15(2):255–263. doi: 10.1038/sj.mt.6300032. [DOI] [PubMed] [Google Scholar]
- 99.Kaneto H, Matsuoka TA, Nakatani Y, Miyatsuka T, Matsuhisa M, Hori M, et al. A crucial role of MafA as a novel therapeutic target for diabetes. J Biol Chem. 2005;280(15):15047–15052. doi: 10.1074/jbc.M412013200. [DOI] [PubMed] [Google Scholar]
- 100.Kaneto H, Miyatsuka T, Fujitani Y, Noguchi H, Song KH, Yoon KH, et al. Role of PDX-1 and MafA as a potential therapeutic target for diabetes. Diabetes Res Clin Pract. 2007;77(Suppl 1):S127–S137. doi: 10.1016/j.diabres.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 101.Kaneto H, Miyatsuka T, Shiraiwa T, Yamamoto K, Kato K, Fujitani Y, et al. Crucial role of PDX-1 in pancreas development, beta-cell differentiation, and induction of surrogate beta-cells. Curr Med Chem. 2007;14(16):1745–1752. doi: 10.2174/092986707781058887. [DOI] [PubMed] [Google Scholar]
- 102.Matsuoka TA, Kaneto H, Stein R, Miyatsuka T, Kawamori D, Henderson E, et al. MafA regulates expression of genes important to islet beta-cell function. Mol Endocrinol. 2007;21(11):2764–2774. doi: 10.1210/me.2007-0028. [DOI] [PubMed] [Google Scholar]
- 103.Miyatsuka T, Kaneto H, Kajimoto Y, Hirota S, Arakawa Y, Fujitani Y, et al. Ectopically expressed PDX-1 in liver initiates endocrine and exocrine pancreas differentiation but causes dysmorphogenesis. Biochem Biophys Res Commun. 2003;310(3):1017–1025. doi: 10.1016/j.bbrc.2003.09.108. [DOI] [PubMed] [Google Scholar]
- 104.Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, Maeda M, et al. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003;9(5):596–603. doi: 10.1038/nm867. [DOI] [PubMed] [Google Scholar]
- 105.Horb ME, Shen CN, Tosh D, Slack JM. Experimental conversion of liver to pancreas. Curr Biol. 2003;13(2):105–115. doi: 10.1016/s0960-9822(02)01434-3. [DOI] [PubMed] [Google Scholar]
- 106. Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455(7213):627–632. doi: 10.1038/nature07314. (**) First report on acinar-to-beta cell differentiation using a minimal combination of reprogramming genes
- 107.Aramata S, Han SI, Kataoka K. Roles and regulation of transcription factor MafA in islet beta-cells. Endocr J. 2007;54(5):659–666. doi: 10.1507/endocrj.kr-101. [DOI] [PubMed] [Google Scholar]
- 108.Aramata S, Han SI, Yasuda K, Kataoka K. Synergistic activation of the insulin gene promoter by the beta-cell enriched transcription factors MafA, Beta2, and Pdx1. Biochim Biophys Acta. 2005;1730(1):41–46. doi: 10.1016/j.bbaexp.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 109.Zhao L, Guo M, Matsuoka TA, Hagman DK, Parazzoli SD, Poitout V, et al. The islet beta cell-enriched MafA activator is a key regulator of insulin gene transcription. J Biol Chem. 2005;280(12):11887–11894. doi: 10.1074/jbc.M409475200. [DOI] [PubMed] [Google Scholar]
- 110.Kim HI, Ahn YH. Role of peroxisome proliferator-activated receptor-gamma in the glucose-sensing apparatus of liver and beta-cells. Diabetes. 2004;53(Suppl 1):S60–S65. doi: 10.2337/diabetes.53.2007.s60. [DOI] [PubMed] [Google Scholar]
- 111.Domínguez-Bendala J, Ricordi C, Pastori R. Protein transduction: a novel approach to induce in vitro pancreatic differentiation. Cell Transplantation. 2006;15(Supp. 15):85–90. doi: 10.3727/000000006783982359. [DOI] [PubMed] [Google Scholar]
- 112.Lee SH, Hao E, Savinov AY, Geron I, Strongin AY, Itkin-Ansari P. Human beta-cell precursors mature into functional insulin-producing cells in an immunoisolation device: implications for diabetes cell therapies. Transplantation. 2009;87(7):983–991. doi: 10.1097/TP.0b013e31819c86ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fort A, Fort N, Ricordi C, Stabler CL. Biohybrid devices and encapsulation technologies for engineering a bioartificial pancreas. Cell Transplant. 2008;17(9):997–1003. doi: 10.3727/096368908786991498. [DOI] [PubMed] [Google Scholar]