Abstract
Objectives
To determine if optical methods can estimate cervix function during pregnancy and if progestins modify this process.
Study Design
Photos of the external cervix of timed-pregnant rats were taken every other day from day 13 (d13) until pp5 (postpartum day 5) following daily treatments with vehicle (controls) or progestin treatments (progesterone, P4 s.c., or vaginally; 17-alpha-hydroxyprogesterone caproate, 17P s.c. and RU-486 s.c., once on d16). Surface area (SA) of the cervix was estimated from photos.
Results
The SA of cervix increases throughout pregnancy and reverses pp in controls. In P4 s.c. or 17P s.c. groups, increases in SA are lower (17P group until day 19 only) (P<.05). Vaginal P4 does not prevent SA increases. Only the P4 s.c. blocked delivery. RU-486 increases SA of the cervix (P<.05) during preterm delivery.
Conclusions
An optical method is useful for quantitative assessment of the cervix and evaluation of agents that modify cervical function.
Keywords: cervix, 17-alpha-hydroxyprogesterone caproate, preterm labor, progesterone, rats
Introduction
Preterm birth is a severe pregnancy complication that occurs in about 10 % of all pregnancies in the developed countries and even more often in developing countries.1, 2 Despite all efforts, at this date reliable tools that predict and diagnose preterm birth as well as successful treatment regiments for a better outcome of the pregnant woman and the baby are still missing. The development of effective therapies to prevent or reduce the occurrence of this difficult medical condition depends on the understanding of the circumstances that initiate labor.
After staying rigid and closed throughout most of pregnancy, to protect and secure the special environment created inside the uterus, the cervix switches to a soft and easy-to-open state that is essential for successful vaginal delivery. Many biochemical and functional changes occur in the cervical connective tissue during gestation, which are summarized in the term “cervical ripening”. It is a chronic process, which starts in the first trimester of pregnancy and progressively proceeds until term. It is usually described as a 3-step preparation process, which must occur in sequence and each step seems to be irreversible: First softening, then effacement and finally dilatation of the cervix.3 This sequence is associated with a dramatic reorganization of the extracellular matrix, which consists of elastin, proteoglycans and especially collagen that decreases by 30–70 % and is accountable by a change from insoluble to more soluble collagen.4, 5 Cervical ripening is an active biochemical process with similarities to an inflammatory like reaction (infiltration of leukocytes, increase of cytokines and metalloproteinases) and occurs independent of uterine contractions.6–9 This process also appears to be at least partially regulated by steroid hormones (in particular progesterone (P4) and estrogen), as antiprogestins successfully induce cervical ripening.10–12 Other hormones and mediators shown to be involved in cervical ripening are dihydrotestosterone13, prostaglandins14, and local mediators such as platelet-activating factor15 and nitric oxide14
The consistent and precise identification of the changes that occur in the cervix is one of the challenges obstetricians face today. Various methods have been used to identify this condition. Physical examination is one of the oldest techniques and a number of scoring systems to characterize the cervix (e.g. the Bishop Score) have been developed.16, 17 Other techniques to assess cervical changes are the measurement of the cervical length by transvaginal ultrasound and biochemical markers, like fetal fibronectin, a glycoprotein found at the chorionic-decidual interface, or Insulin-like growth factor binding protein-1, a protein synthesized by the maternal decidua.17 Our group has used light-induced fluorescence (LIF) of the cervix to estimate changes in cervical collagen and effects of treatments.18, 19 These studies show that parenteral or topical P4 are equally effective in inhibiting delivery in rats but these treatments only partially prevent cervical ripening.19
Recent studies have investigated the use of progestins as treatment for preterm delivery.20–26 Early studies also discussed the potential benefit of 17-α hydroxyprogesterone caproate (17P), a synthetic caproate ester of the naturally occurring metabolite of P4, for the treatment or prevention of preterm labor.27 Several recent randomized control trials have studied the effects of progestins on cervical length changes assessed by transvaginal ultrasound.24–26 Other possible treatments for woman at risk of cervical insufficency include bed rest, cervical cerclage and antibiotics.28–30 None is evidence-based and there is a controversy in the findings. Uterine contractions can be suppressed by tocolytic drugs but only for a very limited time and all compounds have considerable side-effects.31, 32
Pregnant rats, a well-known model to study pregnancy in animals, are exquisitely sensitive to changes in P4 with preterm delivery or prolonged gestation when P4 levels are manipulated or P4 receptor antagonists are used.33 Our hypothesis is that exogenous progestins inhibit cervical ripening and prevent term delivery, that there are differences in abilities of the progestins and that consideration of routes of administration is important.
The objective of this study was to determine if optical methods can be used to estimate changes of the properties of cervical tissue during pregnancy and whether progestins given by various routes can alter these properties. We also used light-induced fluorescence to assess cervical changes and compared them to an optical surface analysis of changes in area of the cervix during pregnancy and postpartum.
Study Design
Animals
Timed-pregnant Sprague-Dawley rats (200–250 g) from Charles-River Laboratories (Wilmington, MA, USA) were transferred to our animal care facilities on day 12 of gestation (day 1 being the day when a sperm plug was observed). The animals were housed separately, with free access to food and water and maintained on a constant 12-hour light-dark cycle. Control pregnant rats were spontaneously delivering on day 22 and 23 of gestation. For the measurements with the endoscopic camera and the collascope the animals were anaesthetized (i.p. injection) with a combination of xylazine (Gemini, Burns Veterinary Supply Inc, Rockville Center, NY, USA) and ketamine HCl (Ketaset; Fort Dodge Laboratories Inc, Fort Dodge, IO, USA). The animals were randomly allocated to one of the groups and sacrificed by carbon dioxide inhalation on day 5 postpartum or on day 25 of pregnancy in the groups with delayed delivery. All procedures were approved by the Animal Care and Use Committee of the St. Joseph’s Hospital and Medical Center in Phoenix.
Treatments
Pregnant rats (N = 6/ group) were treated, when not otherwise mentioned, from day 13 of pregnancy until delivery. Single daily treatments were performed at 8 a.m. and twice a day treatments at 8 a.m. and 8 p.m. All daily injections (4 mg P4 and 10 mg 17P) were by the subcutaneous route (s.c.) in sesame oil (0.2 ml), which was also used for the controls of the injection groups. Vaginal gels were applied twice a day with a blunt ball-top needle deep into the vagina. Crinone was used for the P4 vaginal group (we used equivalent volumes of Crinone for 2–15 mg P4/ treatment, all data presented show the results of the highest dose (total daily dose of 30 mg P4 = 1/3 of a applicator of 8% Crinone that contains 90 mg P4). The control rats for the vaginal groups were treated with Replens (0.18 ml/ treatment). RU-486 (3 mg in 0.2 ml sesame oil) was injected s.c. once on day 16 of gestation.
Reagents
Crystalline P4 (used for subcutaneous P4), RU-486, sesame oil and ethanol were purchased from Sigma (St Louis, MO, USA). 17P was obtained from MP Biomedicals (Solon, OH, USA). P4, 17P, and RU-486 were dissolved in ethanol and then mixed with sesame oil. Crinone (micronized P4 in Replens, a bioadhesive gel, used for vaginal P4) and Replens from Columbia Laboratories (Livingston, NJ, USA).
Assessment of cervical changes
Measurements with the endoscopic camera and the collascope were performed on every other day starting at day 13 until day 21 of gestation and on postpartum day 3 and/or postpartum day 5 and for some animals also on postpartum days 4, 8 and 10.
Optical evaluation with an endoscopic camera
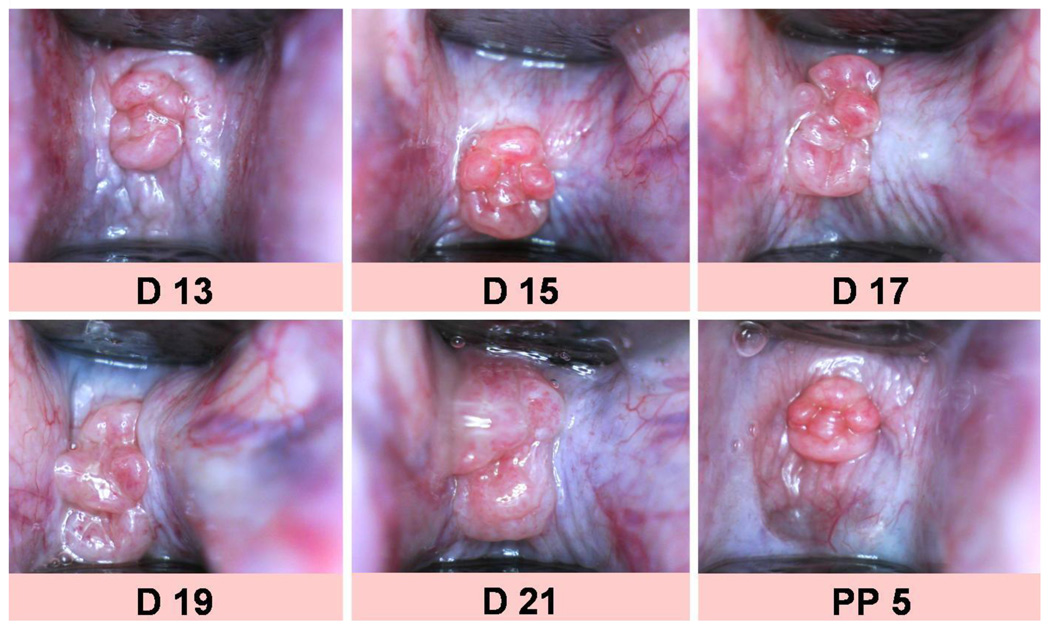
A small pediatric speculum was inserted into the vagina of the anesthetized animal and always opened to a certain level (distance between the top and the bottom part of the end of the speculum was set to standard width of 9 mm, which was used for calibration of the measurements of the surface area). An endoscopic camera was placed in front of the cervix at approximately 10 mm and photos were taken of the cervix and ends of the speculum (Figure 1). The surface area (SA in mm2) of the cervix was calculated from digitized photographs by morphometric methods using the software ImageJ 1.43, National Institute of Health, Bethesda, Maryland, USA (see at http://rsbweb.nih.gov/ij/).34, 35
Figure 1.
Photos of the external cervix throughout pregnancy (D13 = day 13 of gestation to D21) and postpartum (day 5 postpartum).
Evaluation with the collascope using light-induced-fluorescence
The amount of cervical collagen was evaluated by measurement of the autofluorescent properties of cross-linked collagen with a new prototype of an instrument, termed collascope (Reproductive Research Technologies, Houston, TX), as used previously with an earlier prototype.15, 18 After insertion of a small speculum into the vagina of the anesthetized animal, the optical probe of the collascope was placed on the surface of the exocervix. The probe, which is connected to the main unit of the instrument by a fiber optic cable, delivers excitation light (wavelength, 339 nm) onto the cervix and also carries the fluorescent light (mainly caused by pyridinoline cross-links of collagen with a maximum peak at 390 nm) back to the instrument to a charge-coupled device camera to display the full spectrum of fluorescence and analysis of the photons that are emitted by the cervix. The exposure time for excitation was 100 msec. The average of 20 measurements of the detected fluorescent intensity (photon count) at 390 nm was used for each animal at any given time.
Determining the changes in delivery time
Pregnant animals were checked for delivery 3 times per day (8 a.m., 12 a.m., 8 p.m.). The expulsion of one pup was defined as the start of delivery.
Analysis
The SA of the cervix and the LIF of control animals (Figure 2) obtained at different times of gestation were compared using one-way analysis of variance (ANOVA) and multiple pairwise comparison procedures (Dunn's Method for cervical SA and Holm-Sidak for cervical LIF). Student’s t-test was used to compare the SA of a treatment group to its specific control group at any time in gestation and postpartum (Figure 3) and also to determine the differences in delivery times (Figure 4). A two-tailed probability value of P<0.05 was considered statistically significant.
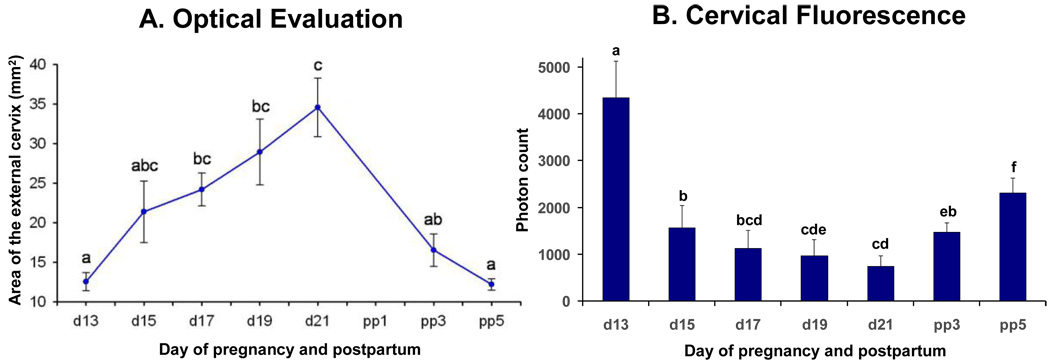
Figure 2.
Figure 2A: Figure showing surface area of cervix (means ± SD of the surface area of the cervix) obtained in vivo from pregnant rats (N = 6) at different days of pregnancy and postpartum treated daily with vehicle (controls). Different letters indicate significant differences between mean values (P < .05). Figure 2B: Bar graphs showing means ± SD of cervical light-induced fluorescence (LIF) obtained in vivo from pregnant rats (N = 6) at different days of pregnancy and postpartum treated daily with vehicle (controls). Significant differences (P <0 .05) between groups are marked with different letters.
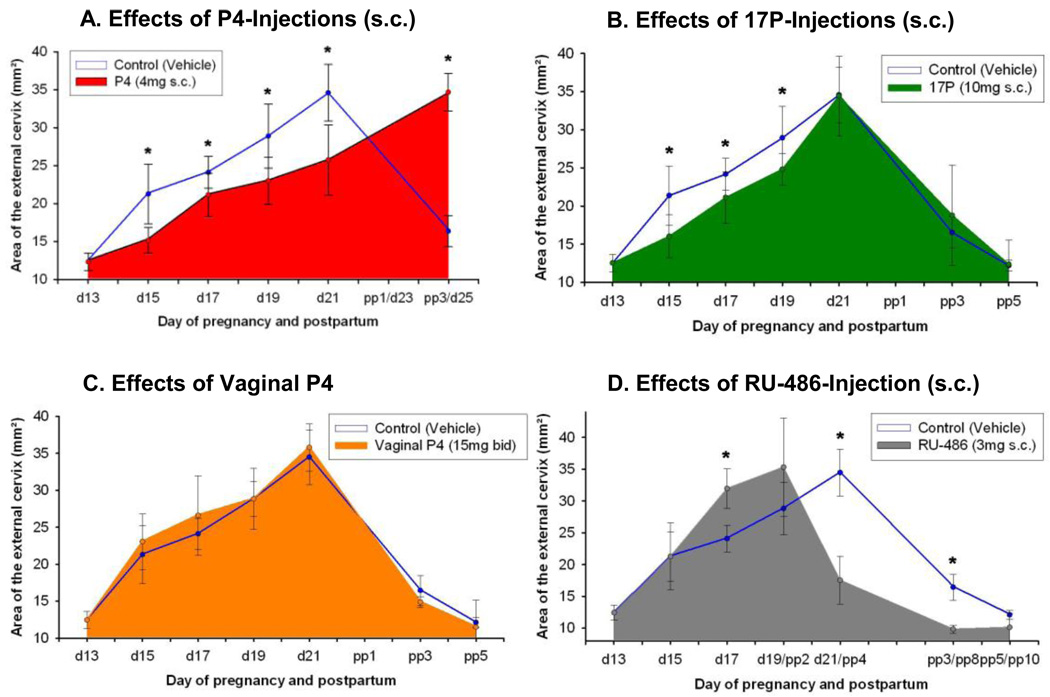
Figure 3.
Figure showing surface area of cervix (means ± SD of the surface area of the cervix) obtained in vivo from pregnant rats (N = 6/ group) at different days of pregnancy and postpartum treated with various progestins or vehicle. Figure 3A: Daily treatment with vehicle (controls) or P4 (4 mg, s.c.). Note that delivery is inhibited in the treatment group. Figure 3B: Daily treatment with vehicle (controls) or 17P (10 mg, s.c.). Note that significant differences are only observed until day 19 of gestation. Figure 3C: Twice a day treatment with vehicle (controls) or vaginal P4 (15 mg bid). Note that no significant differences are observed at any time between controls vs. treated animals. Figure 3D: Treatment once on day 16 with vehicle (controls) or RU-486 (3 mg s.c.).
Note that animals treated with RU-486 delivered prematurely.
Asterisks indicate P < 0.05 compared with controls.
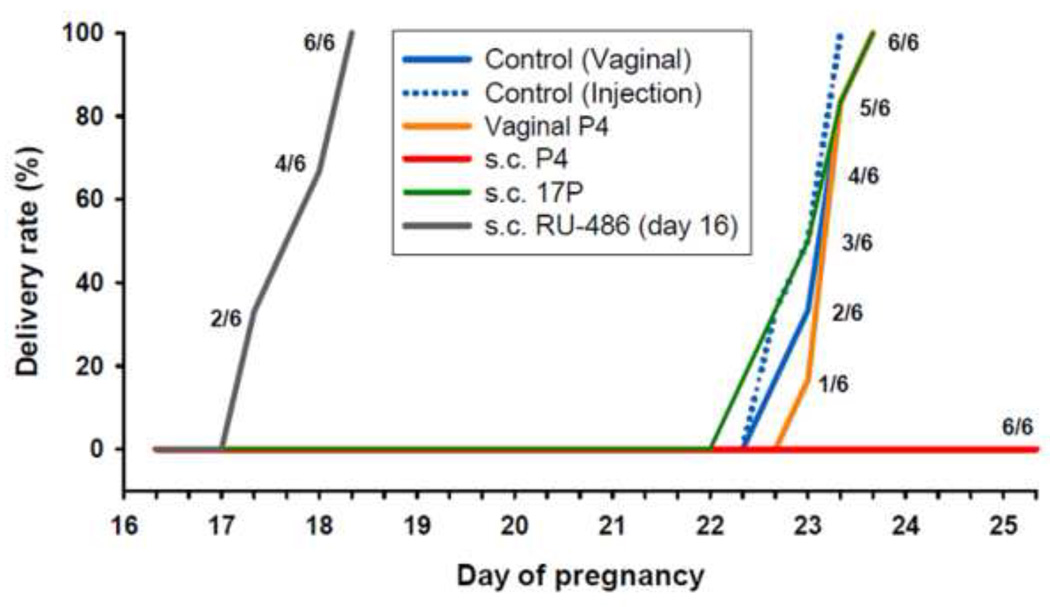
Figure 4.
Shown is the percent of animals delivering versus day of pregnancy after various treatments: RU-486 (single treatment, 3mg s.c., on day 16,), daily treatments from day 13 until delivery for P4 (4 mg s.c. or 15 mg vaginal bid), 17P (10 mg s.c.) and controls (0.2 ml sesame oil (vehicle) s.c. or 0.18 ml vaginal gel bid). Note that injections of P4 completely blocked delivery, whereas 17P or vaginal P4 had no effect on delaying term delivery. RU- 486 induced preterm delivery.
Results
The SA of the cervix in normal pregnancy continuously increases throughout gestation (d13: 12.6 ± 1.6 to 34.6 ± 3.7 at d21) and reverses pp (pp5: 12.2 ± 0.7) (Figure 2A). Measurements of cervical light-induced-fluorescence (LIF) in pregnant, non-treated animals show (Figure 2B) a continuously decreasing photon count throughout pregnancy and reversal postpartum. After significant (P<.05) changes from day 13 to day 15 the cervical SA and the LIF reaches a wider plateau of non-significant (P>.05) changes prior to delivery. LIF values progressively increase postpartum (P<.05), whereas cervical SA decreases respectively (P<0.05). In parenterally treated P4 (Figure 3A) or 17P (Figure 3B) groups increases in SA are lower (P<.05) (d15/d19: P4-group: 15.3 ± 1.7/23.5 ± 3.4; 17P-group: 16.0 ± 2.8 /24.2 ± 2.1; controls: 21.2± 3.9 /28.9± 4.2). Only for the P4-injection group is a lower (P<.05) SA noted on d21 (P4 vs. control: 25.8 ± 4.6 vs. 34.6 ± 3.7). Vaginal P4 (Figure 3C) does not prevent SA increases. RU-486 treatment increases the SA (P<.05) during preterm delivery (Figure 3D). Only parenteral P4 treatment blocked delivery (Figure 4). Neither vaginal P4 nor parenteral 17P delayed delivery significantly (Figure 4).
Comment
This study demonstrates that an optical evaluation can be useful to assess quantitative changes in cervical ripening in vivo. This method has never been described previously and may reveal a huge potential for the assessment of cervical changes in pregnancy as well as for other gynecological conditions. The hereby introduced method is not only helpful to observe the regular changes throughout pregnancy and postpartum, which have been investigated in other studies using different techniques, but also indicates preterm cervical changes and the success of pharmacotherapy and interventions. As shown in this study progestins have the ability to delay cervical ripening and delivery in term pregnant rats. These effects depend critically on the choice of the progestin and the route of administration. Cervical ripening can be assessed by an optical examination of the exterior of the cervix. The SA of the external cervix increases almost 300% from day 13 (12.6 ± 1.6mm2) of pregnancy to term (day 21: 34.6 ± 3.7 mm2) reflecting ripening (Figure 2A). Since we measure the overall surface area in photographs, not regarding folds, furrows etc. in the exterior of the cervix, it is undoubted that the absolute surface area is still underestimated and thus the method might be considered semiquantitative. Another method to assess early changes besides the optical evaluation is the use of light-induced fluorescence (LIF) of cross-linked collagen with an instrument called collascope.36 One of our previous studies used LIF to assess cervical changes during pregnancy and the influence of progestins and the results and the conclusions support the present study.19 Throughout gestation the collascope detects a decreasing photon count which describes the remodeling of the extracellular matrix including a decrease in collagen concentration and switch from_insoluble_to more soluble collagen (Figure 2B).18, 19, 37 This decrease of collagen could explain the softening of the cervix which is assessed by the endoscopic camera as an increase in SA of the cervix in consequence. As anticipated in the postpartal period the LIF increases progressively whereas the SA of the cervix decreases.
As described previously the tremendous changes in the cervix occur early in pregnancy, in midgestation. Following the concept of the 3-step process cervical ripening starts with a process called softening.3 This is a vital process and can not be assessed with the tools used at present in clinics to determine cervical problems in pregnancy. This optical evaluation reveals changes in the cervix earlier than many other techniques to assess the cervix. Ripening is associated with a strong reorganization of the extracellular matrix and an increase of proteoglycans.7, 38–40 Related with this there is an influx of water into the tissue41 that may contribute to the increase in cervical SA.
The increase in SA of the cervix is decelerated in the parenteral P4 (Figure 3A) and 17P (Figure 3B) groups and thus we conclude that these treatments delay cervical ripening but do not entirely prevent it, indicating the involvement of other control pathways. In analogy to the comparison of the control groups the collascope again supports the results of the endoscopic camera in the same way for the treatment groups (results not shown).19 Vaginal P4, even at 7.5 × the parenteral dose, does not inhibit ripening, as indicated in this study by changes in the surface area of the cervix, or delivery possibly because of reduced P4 uptake (Figure 3C). Parenteral P4, but not 17P, inhibits delivery and may be more effective for treatment of preterm labor (Figure 4). As the cervix manages to ripen also in the parenteral P4 treated group at the end of gestation we conclude that the inhibition of delivery is not due to an unripe cervix but must be due to an suppression of uterine contractions. Similar to the parenteral route we demonstrated in a previous study the block of delivery also for a topical (transdermal) route of administration of P4.19
Softening of the cervix is a chronic process, whereas effacement and dilatation are acute events.3 Techniques that measure early pathological changes of the cervix before the irreversible steps of reorganization of the extracellular matrix are accomplished could be of great value in the identification of patients that are in high-risk for prematurity. This may be the key why pharmacological interventions in clinical studies with P4 or 17P are not successful or with contrary results: Women in danger of prematurity might be treated too late when the drugs lose their influence to exert beneficial effects. Consequently many women could be exposed to steroids whether they needed them or not. One of our previous studies, where we used LIF to assess cervical changes during pregnancy and the influence of progestins supports the results and the conclusions of this study.19
In 1956 Csapo et al. developed the concept of a P4 withdrawal as a key step for parturition.42 Several groups have recently investigated the effects of vaginal P4 and intramuscular 17P to prevent preterm birth.20–26 Some studies showed a lower rate of preterm birth20, 21, 23 or attenuated cervical shortening24, 25 in the treatment groups. However other studies seemed to contradict the results of those trials.22, 26 The studies raise questions about the ability of P4 and 17P to prevent preterm labor and cervical shortening. Different study populations complicate the comparison of the studies and it is not established which of the progestins and which route of administration is superior and there is controversy in the findings.
Still, the ideal P4 formulation is unidentified, as well as the dosage and route of administration. The half-life of P4 is approximately 35 to 55 hours.43 Therefore P4 needs to be administered daily which clearly demonstrates the importance to find the least invasive route of administration to minimize side-effects and maximize safety and compliance of the patients without compromising the effectiveness of the treatment. Vaginal P4 was ineffective to inhibit delivery in this study and this questions the advantages of this common route for application of P4. In addition treatment of acute preterm labor by progestins has yet to be extensively studied and management is limited to the use of tocolytics, which suppress uterine contractility but do not reverse the labor process. Treatment of preterm labor might be greatly improved if methods of administration, compounds, and vehicles were optimized and if we had a better understanding of how the progestins affect the uterus and cervix. The optical evaluation of the cervix with an endoscopic camera instead of the so far used colposcope could be of value for assessment of other obstetrical problems and diseases like infections, dysplasias and cancers, especially in the context of follow-up examinations. The optical evaluation and the assessment of the SA of the cervix is a new, effective, non-invasive, objective, low-cost method to assess cervical changes during pregnancy as well as the success of pharmacological interventions.
Acknowledgments
Supported by NIH R01 HD037480 and the St. Joseph’s Foundation at St. Joseph’s Hospital and Medical Center, Phoenix, AZ
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 30th Annual Meeting of the Society for Maternal-Fetal Medicine, Chicago, IL, Feb. 2–6, 2010.
References
- 1.Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88:31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steer P. The epidemiology of preterm labour. BJOG. 2005;112 Suppl 1:1–3. doi: 10.1111/j.1471-0528.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- 3.Garfield RE, Maner WL, Maul H, Saade GR. Use of uterine EMG and cervical LIF in monitoring pregnant patients. BJOG. 2005;112 Suppl 1:103–108. doi: 10.1111/j.1471-0528.2005.00596.x. [DOI] [PubMed] [Google Scholar]
- 4.Uldbjerg N, Ekman G, Malmstrom A, Olsson K, Ulmsten U. Ripening of the human uterine cervix related to changes in collagen, glycosaminoglycans, and collagenolytic activity. Am J Obstet Gynecol. 1983;147:662–666. doi: 10.1016/0002-9378(83)90446-5. [DOI] [PubMed] [Google Scholar]
- 5.Rechberger T, Uldbjerg N, Oxlund H. Connective tissue changes in the cervix during normal pregnancy and pregnancy complicated by cervical incompetence. Obstet Gynecol. 1988;71:563–567. [PubMed] [Google Scholar]
- 6.Chwalisz K, Benson M, Scholz P, Daum J, Beier HM, Hegele-Hartung C. Cervical ripening with the cytokines interleukin 8, interleukin 1 beta and tumour necrosis factor alpha in guinea-pigs. Hum Reprod. 1994;9:2173–2181. doi: 10.1093/oxfordjournals.humrep.a138413. [DOI] [PubMed] [Google Scholar]
- 7.Junqueira LC, Zugaib M, Montes GS, Toledo OM, Krisztan RM, Shigihara KM. Morphologic and histochemical evidence for the occurrence of collagenolysis and for the role of neutrophilic polymorphonuclear leukocytes during cervical dilation. Am J Obstet Gynecol. 1980;138:273–281. doi: 10.1016/0002-9378(80)90248-3. [DOI] [PubMed] [Google Scholar]
- 8.Osmers R, Rath W, Adelmann-Grill BC, et al. Origin of cervical collagenase during parturition. Am J Obstet Gynecol. 1992;166:1455–1460. doi: 10.1016/0002-9378(92)91619-l. [DOI] [PubMed] [Google Scholar]
- 9.Liggins CG. Cervical ripening as an inflammatory reaction. In: Elwood DA, Andersson ABM, editors. Cervix in Pregnancy and Labour. Edinburgh: Churchill Livingstone; 1981. pp. 1–9. [Google Scholar]
- 10.Glassman W, Byam-Smith M, Garfield RE. Changes in rat cervical collagen during gestation and after antiprogesterone treatment as measured in vivo with light-induced autofluorescence. Am J Obstet Gynecol. 1995;173:1550–1556. doi: 10.1016/0002-9378(95)90648-7. [DOI] [PubMed] [Google Scholar]
- 11.Clark K, Ji H, Feltovich H, Janowski J, Carroll C, Chien EK. Mifepristone-induced cervical ripening: structural, biomechanical, and molecular events. Am J Obstet Gynecol. 2006;194:1391–1398. doi: 10.1016/j.ajog.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Chwalisz K, Garfield RE. Antiprogestins in the induction of labor. Ann N Y Acad Sci. 1994;734:387–413. doi: 10.1111/j.1749-6632.1994.tb21770.x. [DOI] [PubMed] [Google Scholar]
- 13.Ji H, Dailey TL, Long V, Chien EK. Androgen-regulated cervical ripening: a structural, biomechanical, and molecular analysis. Am J Obstet Gynecol. 2008;198 doi: 10.1016/j.ajog.2007.11.012. 543 e1-9. [DOI] [PubMed] [Google Scholar]
- 14.Shi L, Shi SQ, Saade GR, Chwalisz K, Garfield RE. Studies of cervical ripening in pregnant rats: effects of various treatments. Mol Hum Reprod. 2000;6:382–389. doi: 10.1093/molehr/6.4.382. [DOI] [PubMed] [Google Scholar]
- 15.Maul H, Shi L, Marx SG, Garfield RE, Saade GR. Local application of platelet-activating factor induces cervical ripening accompanied by infiltration of polymorphonuclear leukocytes in rats. Am J Obstet Gynecol. 2002;187:829–833. doi: 10.1067/mob.2002.126983. [DOI] [PubMed] [Google Scholar]
- 16.Bishop EH. Pelvic Scoring for Elective Induction. Obstet Gynecol. 1964;24:266–268. [PubMed] [Google Scholar]
- 17.Crane JM. Factors predicting labor induction success: a critical analysis. Clin Obstet Gynecol. 2006;49:573–584. doi: 10.1097/00003081-200609000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Fittkow CT, Shi SQ, Bytautiene E, Olson G, Saade GR, Garfield RE. Changes in light-induced fluorescence of cervical collagen in guinea pigs during gestation and after sodium nitroprusside treatment. J Perinat Med. 2001;29:535–543. doi: 10.1515/JPM.2001.074. [DOI] [PubMed] [Google Scholar]
- 19.Kuon RJ, Shi SQ, Maul H, et al. Pharmacologic actions of progestins to inhibit cervical ripening and prevent delivery depend on their properties, the route of administration, and the vehicle. Am J Obstet Gynecol. 2010;202 doi: 10.1016/j.ajog.2010.03.025. 455 e1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188:419–424. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- 21.Defranco EA, O'Brien JM, Adair CD, et al. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:697–705. doi: 10.1002/uog.5159. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien JM, Adair CD, Lewis DF, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:687–696. doi: 10.1002/uog.5158. [DOI] [PubMed] [Google Scholar]
- 23.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–469. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 24.Facchinetti F, Paganelli S, Comitini G, Dante G, Volpe A. Cervical length changes during preterm cervical ripening: effects of 17-alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2007;196 doi: 10.1016/j.ajog.2006.09.009. 453 e1-4; discussion 421. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien JM, Defranco EA, Adair CD, et al. Effect of progesterone on cervical shortening in women at risk for preterm birth: secondary analysis from a multinational, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2009;34:653–659. doi: 10.1002/uog.7338. [DOI] [PubMed] [Google Scholar]
- 26.Durnwald CP, Lynch CD, Walker H, Iams JD. The effect of treatment with 17 alpha-hydroxyprogesterone caproate on changes in cervical length over time. Am J Obstet Gynecol. 2009;201 doi: 10.1016/j.ajog.2009.07.009. 410 e1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson JW, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17alpha-hydroxyprogesterone caproate in the prevention of premature labor. N Engl J Med. 1975;293:675–680. doi: 10.1056/NEJM197510022931401. [DOI] [PubMed] [Google Scholar]
- 28.Incerti M, Ghidini A, Locatelli A, Poggi SH, Pezzullo JC. Cervical length < or = 25 mm in low-risk women: a case control study of cerclage with rest vs rest alone. Am J Obstet Gynecol. 2007;197 doi: 10.1016/j.ajog.2007.06.029. 315 e1-4. [DOI] [PubMed] [Google Scholar]
- 29.Jorgensen AL, Alfirevic Z, Tudur Smith C, Williamson PR. Cervical stitch (cerclage) for preventing pregnancy loss: individual patient data meta-analysis. BJOG. 2007;114:1460–1476. doi: 10.1111/j.1471-0528.2007.01515.x. [DOI] [PubMed] [Google Scholar]
- 30.Vidaeff AC, Ramin SM. From concept to practice: the recent history of preterm delivery prevention. Part II: Subclinical infection and hormonal effects. Am J Perinatol. 2006;23:75–84. doi: 10.1055/s-2006-931803. [DOI] [PubMed] [Google Scholar]
- 31.Blumenfeld YJ, Lyell DJ. Prematurity prevention: the role of acute tocolysis. Curr Opin Obstet Gynecol. 2009;21:136–141. doi: 10.1097/GCO.0b013e3283292455. [DOI] [PubMed] [Google Scholar]
- 32.Higby K, Xenakis EM, Pauerstein CJ. Do tocolytic agents stop preterm labor? A critical and comprehensive review of efficacy and safety. Am J Obstet Gynecol. 1993;168:1247–1256. doi: 10.1016/0002-9378(93)90376-t. discussion 1256-9. [DOI] [PubMed] [Google Scholar]
- 33.Garfield RE, Gasc JM, Baulieu EE. Effects of the antiprogesterone RU 486 on preterm birth in the rat. Am J Obstet Gynecol. 1987;157:1281–1285. doi: 10.1016/s0002-9378(87)80315-0. [DOI] [PubMed] [Google Scholar]
- 34.Rasband WS. Bethesda, Maryland, USA: National Institutes of Health; ImageJ. :1997–2009. http://rsb.info.nih.gov/ij/
- 35.Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 36.Maul H, Mackay L, Garfield RE. Cervical ripening: biochemical, molecular, and clinical considerations. Clin Obstet Gynecol. 2006;49:551–563. doi: 10.1097/00003081-200609000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Maul H, Olson G, Fittkow CT, Saade GR, Garfield RE. Cervical light-induced fluorescence in humans decreases throughout gestation and before delivery: Preliminary observations. Am J Obstet Gynecol. 2003;188:537–541. doi: 10.1067/mob.2003.94. [DOI] [PubMed] [Google Scholar]
- 38.Osmers R, Rath W, Pflanz MA, Kuhn W, Stuhlsatz HW, Szeverenyi M. Glycosaminoglycans in cervical connective tissue during pregnancy and parturition. Obstet Gynecol. 1993;81:88–92. [PubMed] [Google Scholar]
- 39.Osmers RG, Blaser J, Kuhn W, Tschesche H. Interleukin-8 synthesis and the onset of labor. Obstet Gynecol. 1995;86:223–229. doi: 10.1016/0029-7844(95)93704-4. [DOI] [PubMed] [Google Scholar]
- 40.Akins ML, Luby-Phelps K, Mahendroo M. Second harmonic generation imaging as a potential tool for staging pregnancy and predicting preterm birth. J Biomed Opt. 15 doi: 10.1117/1.3381184. 026020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breeveld-Dwarkasing VN, Te Koppele JM, Bank RA, Van Der Weijden GC, Taverne MA, Van Dissel-Emiliani FM. Changes in water content, collagen degradation, collagen content, and concentration in repeated biopsies of the cervix of pregnant cows. Biol Reprod. 2003;69:1608–1614. doi: 10.1095/biolreprod.102.012534. [DOI] [PubMed] [Google Scholar]
- 42.Csapo A. Progesterone block. Am J Anat. 1956;98:273–291. doi: 10.1002/aja.1000980206. [DOI] [PubMed] [Google Scholar]
- 43.Murray J. Natural progesterone: what role in women’s heatlh care? Women’s Health Primary Care. 1998;1:671–687. [Google Scholar]