Abstract
Objectives
Cognitive impairment affects up to 80% of systemic lupus erythematosus (SLE) patients within ten years of diagnosis. Memantine, a seronergic receptor and nicotine acetylcholine receptor antagonist, acts on the glutamatergic system through the NMDA receptor, and is used to treat dementia. We investigated whether it had benefit for SLE cognitive impairment.
Methods
A randomized double-blind, placebo-controlled single center 12 week trial of memantine titrated to 20mg/day was performed, using a 2:1 randomization ratio, in 51 SLE patients. The primary outcome measures were change in the Automated Neuropsychological Assessment Metrics (ANAM) throughput scores at 12 weeks.
Results
There were no statistically significant differences between treatment groups on change from baseline in any of the ANAM throughput scores at 6 or 12 weeks. For the ACR cognitive battery, the only statistically significant finding was for the Controlled Oral Word Association Test - S words at 6 and 12 weeks. At 12 weeks, the memantine group exhibited greater improvement compared to the placebo group (3.6 ±1.8 vs. 0.5 ± 3.8 words, p=0.03). In a subset analysis limited to patients that scored ≥ 1 standard deviation below normal controls at baseline, no significant differences between treatment groups were found.
Conclusions
In this first clinical trial of memantine in SLE, patients treated with memantine did not exhibit significant improvement in cognitive performance compared to the placebo group, regardless of the degree of impairment at baseline, with the exception of controlled oral word association.
Keywords: SLE, cognitive impairment, memantine
INTRODUCTION
Neuropsychiatric systemic lupus erythematosus (NP-SLE) occurs in 30 to 75% of SLE patients [1–6]. Of the case definitions for NP-SLE [1], cognitive impairment occurs most frequently [7]. Ten years after diagnosis, 80% of a mostly Hispanic-American SLE cohort had cognitive impairment [8]. SLE patients can have psychomotor and mental tracking deficits similar to those seen in patients with subcortical brain disease, even in the absence of gross neurologic involvement [9].
Factors significantly associated with cognitive decline in SLE include persistently positive antiphospholipid levels, prednisone use, diabetes, higher depression scores and less education [10]. Verbal memory deficits, decreased psychomotor speed, and decreased overall productivity have all been significantly correlated to elevated antiphospholipid levels [10, 11, 12]. Menon and colleagues reported that SLE patients with persistently elevated IgG aCL levels over a period of two to three years performed significantly worse than SLE patients with occasionally elevated or never elevated titers on a variety of neuropsychological tests [12]. Hanly and colleagues followed 51 female SLE patients over a five year period and found that persistent anticardiolipin IgG elevations were associated with decreased psychomotor speed, while persistent anticardiolipin IgA elevations were correlated with problems with executive functioning and reasoning abilities [11]. Of four cross-sectional studies, two found a relationship between lupus anticoagulant positivity and cognitive dysfunction [9, 13], one found no such relationship [14] and one found no relationship between anticardiolipin and cognitive dysfunction [15].
An advance in the understanding of cognitive impairment in murine SLE has been the recognition of a subset of anti-DNA antibodies that cross-react with the anti-NR2 glutamic receptor. At low concentrations, the antibodies are positive modulators of receptor function (by increasing excitatory postsynaptic potentials), and at high concentrations they promote excitotoxicity (through enhanced mitochondrial permeability transition) [16]. These antibodies mediate apoptotic cell death of neurons. Both the presence of the autoantibodies and a break in the blood brain barrier are necessary to lead to cognitive impairment in the murine model [17]. The agent used to break the blood brain barrier determines what area of the brain is vulnerable to the antibodies [18]. Memantine prevents cognitive impairment from anti-NR2 in the murine model. Anti-NR2 autoantibody has been investigated in human SLE cognitive impairment. Three studies found no association with cognitive impairment [19–21]; one found an association with learning memory deficits [22].
Memantine (1-amino-3, 5-dimethyl-adamantase) is FDA-approved for Alzheimer’s disease [23–25]. It is an NMDA receptor antagonist (as well as a 5 HT 3 receptor antagonist and nicotine acetylcholine receptor antagonist). In Alzheimer’s, most of the benefit is in slowing down progression of symptoms, rather than a readily detectable improvement above baseline [26] Because of its known benefit in Alzheimer’s disease and vascular dementia, we hypothesized that it might have benefit in cognitive impairment in SLE, regardless of baseline anti-NR2 status.
METHODS
The study was approved by the Johns Hopkins University School of Medicine Institutional Review Board and was registered with clinicaltrials.gov (clinical trials.gov identifier NCT00181298). All patients gave signed informed consent.
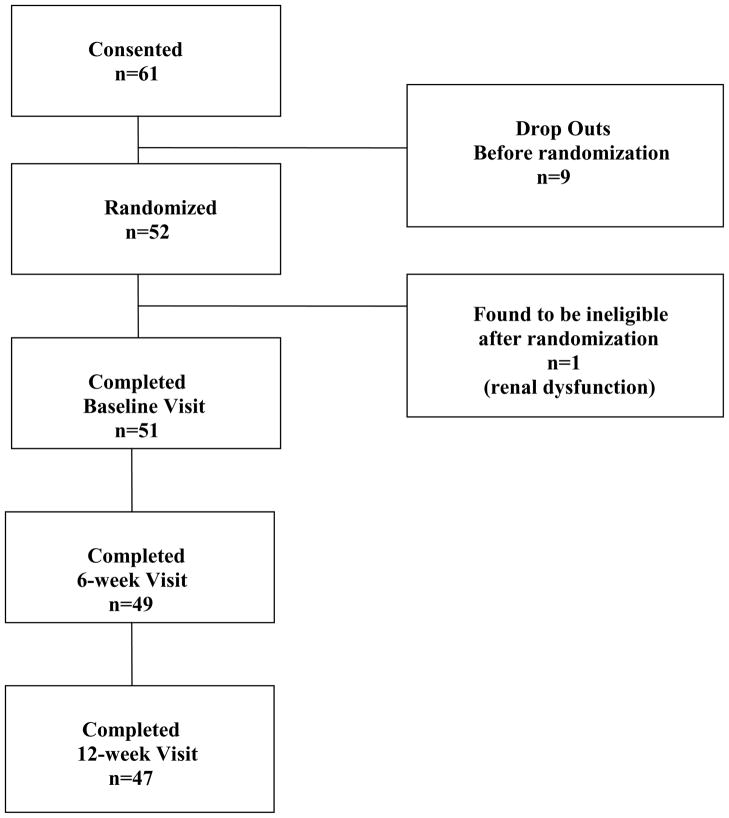
The study was a single center (Hopkins Lupus Center) double-blind, randomized, placebo-controlled phase 2 trial. The randomization allocation was 2:1 memantine to placebo. Enrollment took place between March 2006 and February 2007 at the Johns Hopkins Hospital. The consort diagram is shown in Figure 1. Fifty-one SLE patients were randomized and completed the baseline visit.
Figure 1.
CONSORT diagram
Inclusion/Exclusion criteria
Inclusion criteria included the diagnosis of SLE by the treating rheumatologist, confirmed by ACR classification criteria. Cognitive impairment was reported on multiple visits prior to the study and confirmed by the treating rheumatologist (MP), using the targeted questions proposed in the EULAR guidelines [27,28]. Exclusion criteria were age less than 18 years; history of noncompliance, pregnancy, liver failure or renal insufficiency (calculated creatinine clearance <50 cc); severe SLE flare in the last 6 weeks (defined by SELENA flare index) [29], recent (within four weeks) change in any medication relevant to cognitive function including prednisone, anti-depressants, medication for insomnia, narcotics or attention deficit disorder; current alcohol or illicit drug abuse; and current use of memantine, donepezil, or modafinil.
Automated Neuropsychological Assessment Metrics (ANAM)
ANAM is a set of computer-administered neuropsychological tests, selected from a larger battery developed by the Department of Defense [30]. It is administered in a single session, taking about 20 to 30 minutes. Because it is computer-generated, there is no repetition of individual tests. ANAM assesses the following nine domains: continuous performance (vigilance/sustained attention), code substitution (visual scanning and learning), code substitution with immediate memory (nonverbal memory), code substitution with delayed memory (nonverbal memory), simultaneous spatial processing (visual perception and mental rotation), Sternberg test (sustained attention and working memory), mathematical processing (simple mental arithmetic), matching to sample (visuospatial perception and working memory) and simple reaction time. Throughput is the number of correct responses per minute. It is the best outcome measure because it combines lapses, reaction time, accuracy and consistency. ANAM has previously been shown to be highly correlated with standard tests of cognitive performance [31] in both non-SLE and SLE [32,33] populations.
American College of Rheumatology (ACR) Neuropsychological Battery
Representative tests from the ACR neuropsychological test battery were selected from each of five major cognitive domains. Verbal functioning was assessed with the Woodcock-Munoz Test Battery [34], which assesses broad language abilities as well as picture vocabulary, verbal analogies, and literacy. Executive functioning was assessed with the Controlled Oral Word Association Test [35] and Part B of the Trail Making Test [35]. Memory was assessed with the immediate/delayed/recognition trials of the Rey Complex Figure Test [36] and California Verbal Learning Test [37]. Visuomotor processing and motor speed were assessed with the Rey Complex Figure (time/copy scores), Finger Tapping Test [35, 36], Part A of the Trail Making Test [35], and WAIS-R Coding and Block Design Subtests [38]. Attention was assessed with the WAIS-R Digit Span subtest [38]. Visual perception and speed were assessed by the symbol search test [38]. Tests were given in a single session and in a fixed order. This battery took 2–3 hours to administer.
Anti-NR2
Serum analyzed for the study was collected from the participants prior to study drug administration at baseline. Serum was stored at −70° C until it was shipped to Stavanger University Hospital, Norway (Prof. Omdal’s laboratory) with an adequate supply of dry ice.
A decapeptide (DWEYSVWLSN) was synthesized as previously described [39]. Ninety-six well microtiter plates (655001 Greiner bio-one, GmbH; Frickenhausen, Germany) were coated with 1μg synthetic decapeptide in 100μl phosphate buffered saline (PBS) (pH 7.4) in each well and kept overnight at 4°C. The next morning the wells were blocked with 300μl 10% fetal calf serum (FCS) in PBS for 1 hour at room temperature. Serum was then incubated for 2 hours at 1:50 dilution with 10% FCS. To detect antibodies bound to the antigen, peroxidase labeled anti-human immunoglobulins (A-8400, Sigma, St. Louis, Mo, USA) were used, incubated for 1 hour in room temperature, and washed with PBS.
For developing, o-phenyldiamine dihydrochloride (OPD, S-2045, Dako, Denmark) was used as a substrate and after 30 minutes incubation in room temperature the reaction was stopped by adding 100μl 1M H2SO4 to each well. The plates were read at 492 nm using a microplate reader. The cut-off OD value was set to 0.538 by analyzing serum samples from 25 healthy blood-donors and calculating the mean + 2SD (0.325 + 2*0.107). Anti-NR2 antibody positivity was defined as OD>0.538.
Study procedures
Patients were seen at baseline, 6 weeks and 12 weeks. At each visit ANAM, the SELENA SLEDAI [29], Krupp Fatigue Inventory [40], Calgary Depression Scale [41], mini-mental status examination [42] and fibromyalgia tender points were assessed. ACR Cognitive battery was also conducted on the patients. The starting dose of memantine/placebo was 5 mg/day. It was increased by 5 mg weekly to 20 mg/day by week 4. Matching drug/placebo was donated by Forest Laboratories Inc. (Jersey City, New Jersey, U.S.A.).
Statistical considerations
The a priori sample size and power analysis was based on a difference in means of 0.75 standard deviations (SDs) between the treatment groups on the outcome measures to be clinically significant using a two-sided test with significance set at 0.05. A total of 60 patients was needed for 80% power, so the power achieved for the study patients with follow-up data was 70%.
Demographic and clinical characteristics were summarized using appropriate descriptive statistics. Categorical data were summarized with frequencies and percentages and treatment groups were compared using Fisher’s exact tests. Continuous data were summarized with means and SDs and compared using two-sample t-tests. Cognitive function, as measured by the ANAM and the ACR Cognitive battery, at baseline were compared across treatment groups to test for differences. Change in each cognitive measure between baseline and 6 weeks and also at 12 weeks was calculated. Two-sample t-tests were used to test for differences in change in cognitive function between treatment groups. Mixed effects regression analysis was also used to assess treatment effect over visits, while accounting for within patient correlation and adjusting for anti-NR2 status. A subset analysis was performed on only those patients that performed at least 1 SD below a control sample at baseline on at least one ANAM subtest for the ANAM measures and one ACR cognitive test for the cognitive battery. Analysis was performed using SAS versions 9.1.3 and 9.2 (SAS Institute Inc., Cary, NC, USA). All reported P values are two-sided, and P < 0.05 was considered significant.
RESULTS
The consort diagram is shown in Figure 1. One patient was found to be ineligible after randomization (due to renal dysfunction). Two patients withdrew from the study after one month. Demographic and clinical characteristics of the 51 patients that completed the baseline visit are presented in Table 1. The memantine and placebo groups did not differ in demographic characteristics, or in the eleven ACR criteria. The only historical clinical characteristics that significantly differed between treatment groups at baseline were polyneuropathy, which had occurred in 29% of the placebo group and only 3% of the memantine group (p=0.01), and acute confusional state, which occurred in the past in 24% of the placebo group and only 3% of the memantine group (p=0.04). No patients had acute confusional state or polyneuropathy at baseline or during the trial itself.
Table 1.
Demographic and clinical characteristics of the patients at baseline by treatment group. Mean ± standard deviation or frequency (%).
| Characteristic | Memantine | Placebo | P Valuea |
|---|---|---|---|
| n=33 | n=18 | ||
| Age, years | 50.8 ± 13.2 | 50.3 ± 11.0 | 0.89 |
| Gender, female | 30 (91) | 15 (88) | 0.65 |
| Race | 1.0 | ||
| White | 23 (70) | 13 (72) | |
| African American | 7 (21) | 4 (22) | |
| Other | 3 (9) | 1 (6) | |
| Education, years | 14.5 ± 2.8 | 14.2 ± 2.5 | 0.70 |
| Fibromyalgia | 19 (58) | 12 (67) | 0.56 |
| Neurologic abnormalities:b | |||
| Cerebrovascular disease | 0 (0) | 2 (11) | 0.12 |
| Headache | 5 (15) | 5 (28) | 0.30 |
| Mononeuropathy | 1 (3) | 3 (17) | 0.12 |
| Polyneuropathy | 1 (3) | 5 (29) | 0.01 |
| Seizure disorder | 5 (15) | 1 (6) | 0.65 |
| Acute confusional state | 1 (3) | 4 (24) | 0.04 |
| Cognitive dysfunction | 33 (100) | 17 (94) | 0.35 |
| Mood disorder | 14 (42) | 9 (50) | 0.77 |
| SLICC Damage | 1.9 ± 2.0 | 1.7 ± 1.9 | 0.71 |
| VAS scale | 3.9 ± 1.6 | 3.5 ± 1.5 | 0.31 |
| Krupp fatigue | 5.7 ± 1.2 | 5.3 ± 1.2 | 0.28 |
| Calgary depression scale | 7.2 ± 5.8 | 7.3 ± 5.9 | 0.97 |
| Selena SLEDAI | 2.0 ± 2.1 | 1.2 ± 1.8 | 0.20 |
| Mini-mental | 29.2 ± 1.1 | 29.2 ± 0.9 | 0.96 |
| ACR Criteria: | |||
| Malar rash | 14 (42) | 8 (44) | 1.0 |
| Discoid rash | 5 (15) | 3 (17) | 1.0 |
| Photosensitivity | 21 (64) | 1 (61) | 1.0 |
| Oral ulcers | 20 (61) | 9 (50) | 0.56 |
| Arthritis | 24 (73) | 12 (67) | 0.75 |
| Serositis | 17 (52) | 10 (56) | 1.0 |
| Renal disorder | 5 (15) | 2 (11) | 1.0 |
| Neurological disorder | 4 (12) | 1 (6) | 0.64 |
| Hematological disorder | 20 (61) | 9 (50) | 0.56 |
| Immunologic disorder | 23 (70) | 11 (61) | 0.55 |
| Abnormal ANA titer | 32 (97) | 15 (83) | 0.12 |
From two-sample t-test for continuous and Fisher’s exact test for categorical measures
Only those neurologic abnormalities that occurred in at least two patients are presented.
Baseline ANAM subtest throughput scores and ACR cognitive battery results are summarized in Table 2. The treatment groups did not significantly differ on any of the scores at baseline. For ANAM, 31% (16/51) were ≥ 2 SD below controls on at least one ANAM test and for the ACR Neuropsychiatric battery, 59% (30/51) were ≥ 2 SD below controls on at least one ACR NP test.
Table 2.
Mean ± standard deviation of baseline neuropsychiatric tests by treatment group.
| Neuropsychiatric Measure | Memantine | Placebo | P Valuea |
|---|---|---|---|
| n=33 | n=18 | ||
| ANAM throughput score: | |||
| Code substitution, delayed memory | 29.2 ± 13.8 | 23.3 ± 10.9 | 0.12 |
| Code substitution, immediate memory | 27.8 ± 14.4 | 24.5 ± 10.9 | 0.40 |
| Code substitution | 36.3 ± 9.9 | 33.8 ± 8.4 | 0.36 |
| Continuous performance test | 72.1 ± 21.8 | 72.8 ± 18.3 | 0.91 |
| Matching to sample | 21.7 ± 6.4 | 19.5 ± 7.1 | 0.26 |
| Mathematical processing | 17.0 ± 6.9 | 15.6 ± 6.1 | 0.50 |
| Simultaneous spatial processing | 18.6 ± 7.0 | 18.4 ± 6.6 | 0.93 |
| Simple reaction time | 181.6 ± 48.2 | 189.0 ± 50.1 | 0.61 |
| Sternberg memory recall | 56.0 ± 16.9 | 54.4 ± 15.0 | 0.74 |
| ACR neuropsychiatric battery: | |||
| Minimental State Exam | 29.2 ± 1.1 | 29.2 ± 0.9 | 0.96 |
| Woodcock total | 54.5 ± 3.7 | 55.0 ± 2.2 | 0.51 |
| Trails A | 37.8 ± 16.7 | 41.6 ± 22.5 | 0.50 |
| Trails B | 85.5 ± 44.0 | 103.9 ± 65.6 | 0.30 |
| Rey total - copy | 32.1 ± 6.4 | 31.3 ± 8.3 | 0.68 |
| Rey total – immediate recall | 14.0 ± 7.6 | 13.9 ± 7.2 | 0.99 |
| Rey total – delayed recall | 13.9 ± 7.5 | 13.4 ± 7.1 | 0.83 |
| Symbol score | 29.6 ± 8.8 | 27.2 ± 11.3 | 0.40 |
| California Delay LDFR | 10.3 ± 3.9 | 8.4 ± 4.1 | 0.11 |
| California Delay LDCR | 10.8 ± 3.8 | 9.4 ± 3.5 | 0.21 |
| California Delay – Hits | 14.8 ± 2.4 | 13.6 ± 3.1 | 0.13 |
| California Delay – FP | 1.5 ± 1.8 | 1.4 ± 1.7 | 0.76 |
| Block total | 38.8 ± 9.6 | 39.8 ± 9.3 | 0.74 |
| COWAT - F | 13.1 ± 4.6 | 11.2 ± 5.3 | 0.19 |
| COWAT - A | 11.4 ± 4.6 | 8.9 ± 3.9 | 0.07 |
| COWAT – S | 13.2 ± 4.7 | 13.1 ± 5.9 | 0.96 |
| Coding score | 66.7 ± 18.6 | 65.8 ± 17.3 | 0.87 |
| Tap dominant | 47.2 ± 11.8 | 47.5 ± 9.7 | 0.92 |
| Tap nondominant | 42.8 ± 10.4 | 43.5 ± 13.8 | 0.85 |
| Digit forward | 10.5 ± 2.3 | 11.0 ± 2.4 | 0.42 |
| Digit backward | 6.4 ± 2.3 | 6.4 ± 2.6 | 0.99 |
From two-sample t-test.
There were three serious adverse events during the study: 1) one patient (on memantine) was hospitalized for lupus flare during week 7; 2) one patient (on placebo) was hospitalized for ankle injury; and 3) one patient (on memantine) had raised intraocular pressure during week 6. None of the serious adverse events were attributed to memantine. The patient with raised intraocular pressure withdrew after week 6 and the other two patients completed the study.
Anti-NR2
Only five study patients were positive for serum anti-NR2 (4 in the memantine and 1 in the placebo group). Therefore, comparisons of anti-NR2 across treatment groups were not possible.
Change in Cognitive Performance
There were no statistically significant differences between treatment groups on change from baseline in any of the ANAM throughput scores at 6 weeks (data not shown) or 12 weeks (Table 3). The only ACR cognitive battery measure that was significantly different between the groups was the Controlled Oral Word Association Test - S words. The memantine group had a mean (SD) improvement of 2.0 (4.1) words compared to a worsening of 1.1 (4.7) words in the placebo group at 6 weeks (p=0.02). Similarly at 12 weeks, the memantine group had a mean improvement of 3.6 (4.7) words compared to only a 0.5 (3.8) word improvement in the placebo group at 12 weeks (p=0.03; Table 3).
Table 3.
Change in Neuropsychiatric Test Scores at 12 Weeks by Treatment Group. Mean ± SD
| Neuropsychiatric Measure | Memantine | Placebo | P Valuea |
|---|---|---|---|
| n=30 | n=17 | ||
| ANAM throughput score: | |||
| Code substitution, delayed memory | −0.4 ± 14.4 | 2.8 ± 10.0 | 0.41 |
| Code substitution, immediate memory | 4.8 ± 15.2 | 7.6 ± 8.9 | 0.44 |
| Code substitution | 7.3 ± 7.2 | 6.2 ± 8.2 | 0.63 |
| Continuous performance test | 12.8 ± 12.9 | 2.9 ± 26.5 | 0.16 |
| Matching to sample | 0.6 ± 6.2 | 2.7 ± 4.6 | 0.23 |
| Mathematical processing | 3.2 ± 7.2 | 2.0 ± 3.9 | 0.46 |
| Simultaneous spatial processing | 4.1 ± 8.1 | 3.9 ± 3.2 | 0.93 |
| Simple reaction time | 14.0 ± 41.0 | 5.1 ± 50.4 | 0.51 |
| Sternberg memory recall | 7.0 ± 12.9 | 7.0 ± 11.4 | 0.99 |
| ACR neuropsychiatric battery: | |||
| Minimental State Exam | 0.2 ± 1.3 | 0.5 ± 1.1 | 0.41 |
| Woodcock total | −0.2 ± 2.7 | −0.1 ± 3.2 | 0.96 |
| Trails A | −3.6 ± 10.5 | −5.7 ± 14.6 | 0.56 |
| Trails B | −6.9 ± 26.2 | −13.8 ± 26.7 | 0.40 |
| Rey total - copy | 0.3 ± 3.7 | 1.7 ± 7.6 | 0.49 |
| Rey total – immediate recall | 6.7 ± 5.7 | 5.1 ± 6.2 | 0.39 |
| Rey total – delayed recall | 5.9 ± 5.6 | 6.4 ± 8.9 | 0.86 |
| Symbol score | 3.0 ± 7.4 | 2.1 ± 9.7 | 0.71 |
| California Delay LDFR | 2.6 ± 2.7 | 1.8 ± 3.8 | 0.40 |
| California Delay LDCR | 2.8 ± 2.4 | 1.6 ± 2.5 | 0.14 |
| California Delay – Hits | 0.6 ± 1.7 | 0.4 ± 2.4 | 0.71 |
| California Delay – FP | −0.7 ± 1.8 | 0.2 ± 1.7 | 0.12 |
| Block total | 2.7 ± 7.2 | 6.5 ± 6.0 | 0.07 |
| COWAT - F | 2.0 ± 4.4 | 2.5 ± 4.4 | 0.71 |
| COWAT - A | 0.6 ± 4.3 | 1.6 ± 3.0 | 0.41 |
| COWAT – S | 3.6 ± 4.7 | 0.5 ± 3.8 | 0.03 |
| Coding score | 5.5 ± 17.5 | −2.0 ± 21.1 | 0.20 |
| Tap dominant | 5.2 ± 9.4 | 3.0 ± 7.5 | 0.42 |
| Tap nondominant | 5.1 ± 9.7 | 2.2 ± 10.3 | 0.36 |
| Digit forward | 0.6 ± 2.1 | 0.6 ± 2.3 | 0.97 |
| Digit backward | 1.0 ± 1.7 | −0.1 ± 2.3 | 0.07 |
From two-sample t-test.
A mixed effects regression analysis, modeling the fixed effects of anti-NR2 status, visit, treatment group and the interaction of visit and treatment group on ANAM throughput scores, was performed. There were significant learning effects over time on 6 of the 9 ANAM subtests (all but code substitution with delayed memory, matching to sample, and simple reaction time). However, no statistically significant differences were seen between treatment groups across visits.
We performed a subset analysis on those patients whose baseline cognitive performance on at least one of the ANAM subtests was at least 1 SD below the mean of a control group [43, 44] and a similar subset analysis on those patients whose baseline performance was at least 1 SD below the mean of the control group on at least 1 of the ACR cognitive battery. There were no statistically significant differences between the memantine and the placebo groups for any of the subset analyses (Table 4).
Table 4.
Change in ANAM throughput scores at 12 weeks for patients that were at least 1 standard deviation below normal control data on at least one of the 9 ANAM subtests or one of the 21 ACR Neuropsychiatric battery at baseline
| Neuropsychiatric Measure | Memantine | Placebo | P Valuea |
|---|---|---|---|
| n=24 | n=13 | ||
| ANAM throughput score: | |||
| Code substitution, delayed memory | 0.4 ± 15.0 | 4.7 ± 9.8 | 0.36 |
| Code substitution, immediate memory | 6.8 ± 15.7 | 7.0 ± 9.5 | 0.97 |
| Code substitution | 7.7 ± 7.6 | 7.0 ± 8.9 | 0.78 |
| Continuous performance test | 14.5 ± 13.5 | 3.2 ± 29.8 | 0.21 |
| Matching to sample | 1.2 ± 6.1 | 2.5 ± 4.9 | 0.51 |
| Mathematical processing | 3.6 ± 7.6 | 1.7 ± 3.0 | 0.31 |
| Simultaneous spatial processing | 5.0 ± 6.3 | 4.0 ± 3.6 | 0.55 |
| Simple reaction time | 23.2 ± 36.9 | −0.3 ± 48.1 | 0.11 |
| Sternberg memory recall | 6.3 ± 13.5 | 5.9 ± 11.4 | 0.92 |
| ACR neuropsychiatric battery: | n=26 | n=16 | |
| Minimental State Exam | 0.2 ± 1.4 | 0.5 ± 1.1 | 0.41 |
| Woodcock total | −0.2 ± 2.9 | 0.0 ± 3.2 | 0.81 |
| Trails A | −3.5 ± 11.3 | −5.6 ± 15.0 | 0.62 |
| Trails B | −6.8 ± 27.0 | −15.5 ± 26.6 | 0.32 |
| Rey total - copy | 0.1 ± 4.0 | 1.7 ± 7.9 | 0.45 |
| Rey total – immediate recall | 6.5 ± 4.2 | 4.9 ± 6.3 | 0.41 |
| Rey total – delayed recall | 5.9 ± 5.7 | 6.7 ± 9.0 | 0.76 |
| Symbol score | 2.7 ± 7.7 | 1.9 ± 10.0 | 0.76 |
| California Delay LDFR | 2.7 ± 0.6 | 1.5 ± 3.8 | 0.24 |
| California Delay LDCR | 3.0 ± 2.5 | 1.6 ± 2.6 | 0.09 |
| California Delay – Hits | 0.7 ± 1.8 | 0.4 ± 2.5 | 0.68 |
| California Delay – FP | −0.8 ± 2.0 | 0.1 ± 1.7 | 0.13 |
| Block total | 2.8 ± 7.7 | 6.8 ± 6.0 | 0.09 |
| COWAT - F | 2.2 ± 4.7 | 2.4 ± 4.6 | 0.85 |
| COWAT - A | 0.2 ± 4.2 | 1.6 ± 3.1 | 0.29 |
| COWAT – S | 3.1 ± 4.5 | 0.6 ± 3.9 | 0.08 |
| Coding score | 4.5 ± 18.4 | −3.9 ± 20.1 | 0.17 |
| Tap dominant | 6.7 ± 9.4 | 2.7 ± 7.7 | 0.16 |
| Tap nondominant | 6.3 ± 10.2 | 2.0 ± 10.6 | 0.16 |
| Digit forward | 0.6 ± 2.1 | 0.9 ± 2.0 | 0.64 |
| Digit backward | 1.0 ± 1.9 | −0.1 ± 2.4 | 0.11 |
From two-sample t-test.
DISCUSSION
This trial is the first randomized trial for mild cognitive impairment in SLE. As such, important lessons were learned. One unexpected finding was that twelve patients (24%) with cognitive impairment by self-report and physician targeted questioning using EULAR guidelines [28] did not score at least a 1 SD below normative data on at least one ANAM test and six (12%) patients did not on at least one ACR cognitive test. The ACR Neuropsychiatric Battery appears to be more sensitive than ANAM in detecting mild cognitive impairment in SLE. We believe, however, that future clinical trials of cognitive impairment will need to require ANAM and ACR Neuropsychiatric Battery as part of screening.
The analysis that included all patients found a significant effect of memantine treatment for only the S words subtest of the Controlled Oral Word Association Test at both 6- and 12-week follow-ups. This would not have remained statistically significant, if corrected for multiple comparison testing. Although not statistically significant, the most marked improvement for the memantine group compared to placebo, in the analysis of the subset of patients with demonstrated cognitive impairment at baseline, occurred in the continuous performance test and the test of simple reaction time. This suggests that future studies might enroll patients with deficits in these domains to determine whether memantine use should be considered as part of clinical care.
Anti-NR2 was very infrequent in our patient population. We did not measure anti-NR2 in the cerebrospinal fluid where it might be produced in CNS-SLE. Our study strongly suggests that serum anti-NR2 is not correlated with mild cognitive impairment in SLE, in agreement with previous reports [19–21]. The percent positivity for anti-NR2 in our study was 10% (95% confidence interval, 3–21%). This is somewhat less than in the Brain CONECTIONS study of newly diagnosed SLE patients (20%) [45], and in other studies (25.8% (Harrison), 35% (Hanly), 19.0% (Omdal)). There is no enrichment of anti-NR2 when one selects for SLE patients with cognitive impairment, as in our study.
There are limitations to our study that should be addressed. First, the study used self-reported cognitive impairment confirmed by the physician using targeted questions. Twelve percent of patients did not exhibit significant impairment at baseline on any of the neuropsychiatric tests. This contributed to the study being slightly underpowered to detect a clinically meaningful improvement. Our subset analyses of patients who scored > 1 SD below a control sample at baseline also failed to detect any benefit of memantine. Second, another limitation is the very low prevalence of anti-NR-2 abnormality in the study population. However, multiple groups have not found any association of anti-NR2 with cognitive impairment in SLE [19–21]. The benefit of memantine in Alzheimer’s, in addition, is completely independent of anti-NR2. Third, it is possible that any benefit of memantine might take years to be apparent, if its role is to prevent worsening of cognitive impairment.
CONCLUSION
In conclusion, the trial was slightly under-powered, because 12% of patients with self-reported and physician-confirmed cognitive impairment did not exhibit significant impairment at baseline on any of the cognitive measures. Possible improvement was seen in two cognitive domains: continuous performance and simple reaction time, with significant improvement in one domain: oral word association. These results can be used to select appropriate patients and to power future clinical trials for cognitive impairment in SLE. However, memantine cannot be recommended for mild cognitive impairment in SLE, given the overall negative results of our trial.
Acknowledgments
ROLE OF FUNDING SOURCES: The study was partly supported by Forest Laboratories Inc.: supporting the research personnel and supplying the study drug. The study was also supported by NIH RO1 AR049125 and some biostatistical support was made available through the Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research.
Footnotes
COMPETING INTERESTS STATEMENT
There were no conflicts of interest with the authors. Each author’s ICMJE Form for Disclosure of Potential Conflicts of Interest is submitted with the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michelle Petri, Email: mpetri@jhmi.edu, 1830 East Monument Street Suite 7500, Baltimore MD 21205, USA, Telephone number: 410-955-3823, Fax number: 410-614-0498.
Mohammad Naqibuddin, Dept. of Medicine, Div. of Rheumatology, Johns Hopkins University, USA.
Margaret Sampedro, Dept. of Medicine, Div. of Rheumatology, Johns Hopkins University, USA.
Roald Omdal, Department of Internal Medicine, Clinical Immunology Unit, Stavanger University Hospital, Norway.
Kathryn A. Carson, Dept. of Epidemiology, Johns Hopkins Bloomberg School of Public Health, USA.
References
- 1.ACR ad hoc committee on neuropsychiatric lupus. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndrome. Arthritis Rheum. 1999;42:599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Hanson VG, Horowitz M, Rosenbluth D, Spiera H, Puszkin S. Systemic lupus erythematosus patients with central nervous system involvement show autoantibodies to a 50kd neuronal membrane protein. J Exp Med. 1992;176:565–573. doi: 10.1084/jem.176.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Futrell N, Schultz LR, Millikan C. Central nervous system disease in patients with systemic lupus erythematosus. Neurology. 1992;42:1649–1657. doi: 10.1212/wnl.42.9.1649. [DOI] [PubMed] [Google Scholar]
- 4.Kael AT, Shetty M, Lee BCP, Lockshin MD. The diversity of neurologic events in systemic lupus erythematosus. Prospective clinical classification and computed tomographic or 82 events in 71 patients. Arch Neurol. 1986;43:273–276. doi: 10.1001/archneur.1986.00520030063016. [DOI] [PubMed] [Google Scholar]
- 5.McNicholl J, Glynn D, Mongey A, Hutchinson M, Bresnihan B. A prospective study of neurophysiologic, neurologic and immunologic abnormalities in systemic lupus erythematosus. J Rheumatol. 1994;21:1061–1066. [PubMed] [Google Scholar]
- 6.West SG, Emlen W, Wener MH, Kotzin BL. Neuropsychiatric lupus erythematosus: a 10- year prospective study on the value of diagnostic tests. Am J Med. 1995;99:153–163. doi: 10.1016/s0002-9343(99)80135-1. [DOI] [PubMed] [Google Scholar]
- 7.Ainiala H, Hietaharju A, Loukkola J, Peltola J, Korpela M, Metsanoja R, et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation. Arthritis Care Res. 2001;45:419–423. doi: 10.1002/1529-0131(200110)45:5<419::aid-art360>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Brey RL, Holliday SL, Saklad AR, Navarrete MG, Hermosillo–Romo D, Stallworth CL. Neuropsychiatric syndromes in SLE: Prevalence using standardized definitions. Neurology. 2002;58(8):1214–20. doi: 10.1212/wnl.58.8.1214. [DOI] [PubMed] [Google Scholar]
- 9.Leritz E, Brandt J, Minor M, Reis-Jensen F, Petri M. “Subcortical” cognitive impairment in patients with systemic lupus erythematosus. J Int Neuropsychol” Soc. 2000;6(7):821–825. doi: 10.1017/s1355617700677093. [DOI] [PubMed] [Google Scholar]
- 10.McLaurin EY, Holliday SL, Williams P, Brey RL. Predictors of cognitive dysfunction in patients with systemic lupus erythematosus. Neurology. 2005;64:297–303. doi: 10.1212/01.WNL.0000149640.78684.EA. [DOI] [PubMed] [Google Scholar]
- 11.Hanly JG, Hong C, Smith S, Fisk JD. A prospective analysis of cognitive function and anticardiolipin antibodies in systemic lupus erythematosus. Arthritis Rheum. 1999;42(4):728–734. doi: 10.1002/1529-0131(199904)42:4<728::AID-ANR16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 12.Menon S, Jameson-Shortall E, Newman SP, Hall-Craggs MR, Chinn R, Isenberg DA. A longitudinal study of anticardiolipin antibody levels and cognitive functioning in systemic lupus erythematosus. Arthritis Rheum. 1999;42(4):735–741. doi: 10.1002/1529-0131(199904)42:4<735::AID-ANR17>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 13.Denburg SD, Carbotte RM, Ginsberg JS, Denburg JA. The relationship of antiphospholipid antibodies to cognitive function in patients with systemic lupus erythematosus. Journal of the International Neuropsychological Society. 1997;3:377–386. [PubMed] [Google Scholar]
- 14.Afeltra A, Garzia P, Mitterhofer AP, Vadacca M, Galluzzo S, Del Porto F. Neuropsychiatric lupus Syndromes: relationship with antiphospholipid Antibodies. Neurology. 2003;61:108–110. doi: 10.1212/01.wnl.0000058904.94330.a7. [DOI] [PubMed] [Google Scholar]
- 15.Hanly JG, Walsh NM, Fisk JD, Eastwood B, Hong C, Sherwood G. Cognitive impairment and autoantibodies in systemic lupus erythematosus. Br J Rheumatol. 1993;32:291–296. doi: 10.1093/rheumatology/32.4.291. [DOI] [PubMed] [Google Scholar]
- 16.Faust TWE, Change EH, et al. Neurotoxic lupus autoantibodies alter brain function through two distinct mechanisms. Proc Natl Acad Sci USA. 2010;107:18549–18574. doi: 10.1073/pnas.1006980107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nature Medicine. 2001;7:1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 18.Huerta PT, Kowal C, et al. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci USA. 2006;103:678–683. doi: 10.1073/pnas.0510055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanly JG, Robichaud J, Fisk JD. Anti-NR2 glutamate receptor antibodies and cognitive function in systemic lupus erythematosus. J Rheumatol. 2006;33(8):1553–1558. [PubMed] [Google Scholar]
- 20.Harrison MJ, Ravdin LD, Lockshin MD. Relationship between serum NR2a antibodies and Cognitive dysfunction in systemic lupus erythematosus. Arthritis Rheum. 2006;54(8):2515–2522. doi: 10.1002/art.22030. [DOI] [PubMed] [Google Scholar]
- 21.Lapteva L, Nowak M, Yarboro C, Takada K, Roebuck-Spencer T, Weickert T, et al. Anti-N-methyl-D-asparate receptor antibodies, cognitive dysfunction, and depression in systemic lupus erythematosus. Arthritis & Rheumatism. 2006;54(8):2505–2514. doi: 10.1002/art.22031. [DOI] [PubMed] [Google Scholar]
- 22.Omdal R, Brokstad K, Waterloo K, Koldingsnes W, Jonsson R, Mellgren SI. Neuropsychiatric disturbances in SLE are associated with antibodies against NMDA receptors. Eur J Neurol. 2005;12:392–398. doi: 10.1111/j.1468-1331.2004.00976.x. [DOI] [PubMed] [Google Scholar]
- 23.Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine treatment in patients with moderate to severe Alzheimer Disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291:317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 24.Winblad B, Poritis N. Memantine in severe dementia: results of the 9M-BEST study (benefit and efficacy in severely demented patients during treatment with memantine) Int J Geriatr Psychiatry. 1999;14:135–146. doi: 10.1002/(sici)1099-1166(199902)14:2<135::aid-gps906>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003;348(14):1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 26.Gauthier S, Scheltens P. Can we do better in developing new drugs for Alzheimer’s disease? Alzheimers Dement. 2009;5:489–491. doi: 10.1016/j.jalz.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Mosca M, Tani C, Aringer M, Bombardieri S, Boumpas D, Brey R, et al. Development of recommendations for monitoring systemic lupus erythematosus patients in clinical practice and in observational studies. Arthrits Rheum. doi: 10.1136/ard.2009.117200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertsias G, Ioannidis JPA, Boletis J, Bombardieri S, Cervera R, Dostal C, et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a task for for the EULAR standing committee for international clinical studies including therapeutics. Ann Rheum Dis. 2008;67:195–205. doi: 10.1136/ard.2007.070367. [DOI] [PubMed] [Google Scholar]
- 29.Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353(24):2550–2558. doi: 10.1056/NEJMoa051135. [DOI] [PubMed] [Google Scholar]
- 30.Reeves D, Bleiberg J, Spector J. Validation of the ANAM Battery in Multi-center head injury studies. Arch Clin Neuropsychol. 1993;8:356. [Google Scholar]
- 31.Bleiberg J, Kane RL, Reeves DL, Garmoe WS, Halpern EL. Factor analysis of computerized and traditional tests used in mild brain injury research. Clin Neuropsychologist. 2000;14:287–294. doi: 10.1076/1385-4046(200008)14:3;1-P;FT287. [DOI] [PubMed] [Google Scholar]
- 32.Roebuck-Spencer TM, Yarboro C, et al. Use of computerized assessment to predict neuropsychological functioning and emotional distress in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;55:434–441. doi: 10.1002/art.21992. [DOI] [PubMed] [Google Scholar]
- 33.Hanly JG, Omisade A, Su L, Farewell V, Fisk JD. Assessment of cognitive function in systemic lupus erythematosus, rheumatoid arthritis, and multiple sclerosis by computerized neuropsychological tests. Arthritis Rheum. 2010;62:1478–1486. doi: 10.1002/art.27404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodcock RW, Munoz-Sandoval AF. Woodcock-Munoz Language Survey. Comprehensive manual. Itasca, IL: The Riverside Publishing Company; 1993. [Google Scholar]
- 35.Spreen O, Strauss E. A compendium of neuropsychological tests. New York: Oxford University Press; 1991. [Google Scholar]
- 36.Meyers JE, Meyers KR. Rey Complex Figure Test and Recognition Trial. Professional Manual. Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- 37.Fridlund AJ, Delis DC. California Verbal learning Test, Adult Version 1.2. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 38.Wechsler D. Administration and Scoring Manual. 3. San Antonio, TX: The Psychological Corporation; 1997. Wechsler Adult Intelligence Scale. [Google Scholar]
- 39.Putterman C, Diamond B. Immunization with a peptide surrogate for double- stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J Exp Med. 1998;1:29–38. doi: 10.1084/jem.188.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang B, Gladman DD, Urowitz MB. Fatigue in lupus is not correlated with disease activity. J Rheumatol. 1998;25:892–5. [PubMed] [Google Scholar]
- 41.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Folstein MF, Folstein SE, McHugh PR. “Mini mental state” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatry Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 43.Naqibuddin M, Wallace DJ, Weisman MH, Holliday SL, Wing NF, Brey RL, et al. Cognitive functioning in recently-diagnosed systemic lupus erythematosus. Arthritis and Rheumatism. 2004;50(9):S195–S195. [Google Scholar]
- 44.Petri M, Naqibuddin M, Carson KA, Sampedro M, Wallace DJ, Weisman MH, et al. Cognitive function in a systemic lupus erythematosus inception cohort. J of Rheumatol. 2008;35(9):1776–1781. [PubMed] [Google Scholar]
- 45.Petri M, Naqibuddin M, Carson KA, Wallace DJ, Weisman MH, Holliday SL, et al. Depression and cognitive impairment in newly diagnosed systemic lupus erythematosus. J Rheumatol. 2010 Jul 15; doi: 10.3899/jrheum.091366. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]