Abstract
Damage to the motor cortex of one hemisphere has classically been associated with contralateral upper limb paresis, but recent patient studies have identified deficits in both upper limbs. In non-human primates, we tested the hypothesis that the severity of ipsilesional upper limb motor impairment in the early post-injury phase depends on the volume of gray and white matter damage of the motor areas of the frontal lobe. We also postulated that substantial recovery would accompany minimal task practice and that ipsilesional limb recovery would be correlated with recovery of the contralesional limb. Gross (reaching) and fine hand motor functions were assessed for 3-12 months post-injury using two motor tests. Volumes of white and gray matter lesions were assessed using quantitative histology. Early changes in post-lesion motor performance were inversely correlated with white matter lesion volume indicating that larger lesions produced greater decreases in ipsilesional hand movement control. All monkeys showed improvements in ipsilesional hand motor skill during the post-lesion period, with reaching skill improvements being positively correlated with total lesion volume indicating larger lesions were associate with greater ipsilesional motor skill recovery. We suggest that reduced trans-callosal inhibition from the lesioned hemisphere may play a role in the observed skill improvements. Our findings show that significant ipsilesional hand motor recovery is likely to accompany injury limited to frontal motor areas. In humans, more pronounced ipsilesional motor deficits that invariably develop after stroke may, in part, be a consequence of more extensive subcortical white and gray matter damage.
Keywords: brain injury, hand, dexterity
Introduction
Although it was once thought that unilateral motor cortex damage did not affect motor function of the ipsilateral upper limb, many contemporary human studies have shown measurable deficits in strength and speed of ipsilesional upper limb movements (Colebatch and Gandevia, 1989, Desrosiers, et al., 1996, Haaland, et al., 2009, Hermsdorfer, et al., 1999, Kim, et al., 2003, Noskin, et al., 2008, Pohl, et al., 2003, Schaefer, et al., 2007, Wetter, et al., 2005, Yarosh, et al., 2004, Yelnik, et al., 1996) including problems with ipsilesional precision grip initiation, grip release and digit force direction (Seo, et al., 2009, Seo, et al., 2010). Most studies to date have been cross-sectional, comparing post-lesion hand strength/motor performance to undamaged controls, but a few have examined post-stroke motor function longitudinally (Jones, et al., 1989, Laufer, et al., 2001, Marque, et al., 1997, Noskin, et al., 2008). The longitudinal studies indicate that motor function of the ipsilesional arm/hand improves with time after stroke in terms of strength and movement speed, but some deficits extend into the chronic phase of recovery. Indeed, it was recently reported that there are persistent deficits in scaling of ipsilesional peak elbow extensor acceleration for movements of different amplitude after unilateral stroke (Haaland, et al., 2009). These data suggest both hemispheres have important roles in controlling each upper limb. However, these ipsilesional deficits may also be related to a tendency to match the movement kinematics of both upper limbs (Al-Senawi and Cooke, 1985).
Although many studies in animal models have examined motor and/or sensory function of the contralesional limb before and after unilateral brain damage induced surgically (e.g., (Black, et al., 1975, Denny-Brown and Botterell, 1948, Gonzalez and Kolb, 2003, Travis, 1955, Travis, 1955), chemically (e.g., (Kermadi, et al., 1997, Liu and Rouiller, 1999) or by vascular occlusion (Carmichael, et al., 2005, Gao, et al., 2006, Luke, et al., 2004, Marshall, et al., 2003, Nudo, et al., 1996, O'Bryant, et al., 2007, Roitberg, et al., 2003), only a few have investigated function of the ipsilesional limb during single limb motor tasks (Brinkman, 1984, Bury and Jones, 2002, Gonzalez, et al., 2004, Luke, et al., 2004, Roitberg, et al., 2003). Unlike findings in humans, it has been shown that unilateral surgical (electrolytic) and chemical (ibotenic acid) lesions affecting motor cortex can actually facilitate learning and performance of a reaching motor skill by the ipsilesional limb of rats (Bury and Jones, 2002, Luke, et al., 2004) and monkeys (Kaeser, et al., 2010). However, one rodent study has reported severe acute and chronic deficits in control of ipsilesional forelimb reaching following motor cortex lesions (Gonzalez, et al., 2004). Studies in monkeys have also shown deficits in ipsilesional fine hand motor performance, but these persist for only a few weeks following a supplementary motor area (SMA or M2) lesion (Brinkman, 1984). In contrast, other non-human primate work has shown that a much larger lesion involving primary motor cortex, subcortical white matter and basal ganglia caused small speed deficits in an ipsilesional dexterity board task and larger deficits in complex ipsilesional hand motor tasks that persisted for several months (Roitberg, et al., 2003). Thus, effects of unilateral brain damage on ipsilesional upper limb movement control are not clear for relatively simple fine motor tasks and may depend on the amount of damage sustained by cortical and subcortical structures.
There is also physiological evidence demonstrating that the motor cortex ipsilateral to the hand/finger plays an important role in controlling its movements. In non-human primates, activity of M1 and M2 neurons ipsilateral to the moving arm is modulated in accordance with movement kinematics (Brinkman and Porter, 1979, Cisek, et al., 2003, Donchin, et al., 1998). Moreover, there are populations of M1 neurons that encode contralateral arm movement kinematics and, with a 60 ms greater delay, also encode ipsilateral arm movements (Ganguly, et al., 2009). Also in humans, “virtual lesions” induced by high frequency repetitive transcranial magnetic stimulation to the primary motor cortex disrupt ipsilateral upper limb reaching and grip/lift motions by altering timing of muscle activations (Davare, et al., 2007).
The primary purpose of the present work was to evaluate the volumetric effects of unilateral lesions targeting frontal lobe motor areas on reaching and fine hand motor function in the ipsilesional limb in an animal model similar to humans. Thus, isolated cortical lesions were induced in Rhesus monkeys contralateral to the preferred upper limb. The preferred limb was identified prior to brain injury using a fine motor task in which the monkey could choose which hand to use. We hypothesized that larger lesions encompassing more frontal lobe motor areas would cause greater initial impairments in performance of ipsilesional dexterous movements but expected the monkeys would eventually recover to similar, or higher than, pre-lesion performance levels. We also expected recovery of the ipsilesional limb to be directly correlated with recovery of the contralesional limb (Kaeser, et al., 2010) and inversely correlated with lesion volume. Finally, we examined whether monkeys with a strong hand preference would show greater improvements in ipsilesional motor abilities after the cortical lesion than monkeys with a weak hand preference. Strength of hand preference may be an indicator of cerebral hemispheric dominance and it has been shown that the dominant hemisphere exerts stronger trans-callosal inhibition on the non-dominant hemisphere (Lewis and Perreault, 2007). If so, dominant motor cortex injury may release inhibition from the less dominant hemisphere to permit the non-injured hemisphere to better control movements of the ipsilesional hand. We wish to also point out that this work is part of a large-scale project examining multiple behavioral, kinematic and kinetic components of the motor recovery process (Darling, et al., 2010, Darling, et al., 2009), structural plasticity of the corticofugal connections from spared motor areas (McNeal, et al., 2010) and molecular events which accompany recovery of upper limb movements (Nagamoto-Combs, et al., 2007, Nagamoto-Combs, et al., 2010).
Methods
Experimental Animals
Ten adult rhesus monkeys (Macaca mulatta – SDM38, 45, 46, 48, 50, 55, 56, 64, 67, and 70) were subjects for these experiments (see Table 1 of Darling et al., 2010). The animals were housed, cared for, and maintained in a United States Department of Agriculture (USDA) and Association for Assessment and Accreditation of Animal Laboratory Care (AAALAC) approved and inspected facility. All behavioral and surgical protocols were approved by the University of South Dakota (USD) Institutional Animal Care and Use Committee (IACUC), and conducted in accordance with USDA, National Institutes of Health, and Society for Neuroscience guidelines for the ethical treatment of experimental animals. Prior to beginning the study, each monkey was evaluated by a primate veterinarian and judged to be healthy and free of any neurological deficit. Proximal and distal movements and range of motion at the joints in both upper extremities of all animals were normal with the exception of SDM55. In this case the interphalangeal joints of digit 3 of the right hand were permanently extended and there were no abnormalities in the left hand. Despite this abnormality, this animal preferred the right hand and ably performed precision grip with digits 1 and 2.
Experimental Apparatus
Two devices were used to test fine hand/finger motor function. The modified movement assessment panel (mMAP) measures temporal movement variables and 3-dimensional forces applied while acquiring a carrot ring (carrot chip with a central hole) from a flat surface and over straight and curved rods (Darling, et al., 2006). The second apparatus is a modified dexterity board (mDB) which measures kinematic variables while acquiring a small food pellet from wells of different size (Pizzimenti et al., 2007). Different levels of fine-digit motor control are required in the mDB task depending on the size of the well (diameters ranging from 10 to 25 mm, 1 cm deep; 1 well was a shallow dimple designed to hold the pellet but not restrict digit motions needed to acquire the pellet). Both devices attach to the monkey's cage and direct, without restraint, which hand the monkey can use to successfully perform the tasks. The monkeys were allowed to move freely about the cage between trials. Food targets were used to minimize training requirements.
Data Acquisition
Forces applied during manipulation of the carrot chip in the mMAP task were recorded at 200 samples/s using Datapac 2k2 (Run Technologies). Movements of the hand during the mMAP task were recorded using a single digital video camera (Sony, model DCR-DVD301) placed directly in front of the cage. These recordings were used for qualitative ratings of the movement strategy and to assess success/failure on each trial.
Four digital video cameras interfaced with the SIMI Motion data acquisition package (SIMI Reality Motion Systems, Unterschleissheim, Germany) were used to record hand movements at 100 frames/s during the mDB task to assess spatial and temporal variables (e.g., accuracy and duration of the initial reach, grip aperture at touchdown of the hand, etc.) as described previously (Pizzimenti, et al., 2007). Video data collection began when the portal door was opened to allow the monkey to reach toward the food pellet and continued until the pellet was either retrieved into the cage, knocked off of the platform, or a 60 s time limit had expired. Further details are provided in our previous work (Pizzimenti, et. al, 2007).
Behavioral Procedures
Prior to an experimental session the monkey was food restricted for 18-24 hours. The initial training sessions for the dexterity board test used a “standard” rectangular dexterity board to assess the preferred hand for each monkey as described previously (McNeal, et al., 2010, Nudo, et al., 1992). Briefly, there were 100 randomly ordered reach trials conducted over two consecutive days (20 pellets placed in each of 4 wells and 20 pellets placed on the flat surface). The number of initial reaches and subsequent reaches with each hand on each trial was recorded and used to compute an index of hand preference as described previously (McNeal, et al., 2010, Nudo, et al., 1992)(see Table 1 of (Darling, et al., 2009)). Then the monkeys were trained to use the modified movement assessment panel (mMAP) (Darling, et al., 2006) and modified dexterity board (mDB) (Pizzimenti, et al., 2007). After initial training on these devices, the subjects were only exposed to them during the pre- and post-lesion testing sessions.
Specifically, training with the mMAP and mDB devices commenced after hand preference was determined and was performed in block fashion for each task. For the mMAP test, full testing sessions included blocks of 5 trials at each of the three difficulty levels with each hand. Thus, each monkey had a total of 15 opportunities to retrieve the carrot chips with each hand. In the prelesion mMAP tests, testing for both limbs proceeded from the most difficult task (curved) to easiest task (flat surface). During post-lesion tests, the more impaired (contralesional) hand was always tested first to ensure high motivation. Furthermore, during the first few post-lesion tests, the more impaired hand was tested first on the easiest task (flat surface). Then once the monkey began successfully retrieving the carrot chips, this hand was tested first on the curved rod task as done in the pre-lesion trials. For the mDB test, in both pre- and post-lesion situations full testing sessions included 5 retrieval attempts for each of the wells (A-E) proceeding from the easiest well (E) to the most difficult (A), for a total of 25 trials with each hand. After the lesion the more impaired (contralesional) hand was tested first.
Pre-lesion data were collected every 1-3 weeks. The number of training and testing sessions varied according to each monkey's ability to learn the task and perform consistently. The final five consecutive pre-lesion experiments that demonstrated relatively stable levels of performance were used to determine readiness for lesions to cortical motor areas (see Results). Post-lesion data were collected from both limbs during weekly experimental sessions for the first two months after the surgery and thereafter, tests were conducted every 2 weeks.
Surgical Procedure
Preoperative, surgical and postoperative procedures were the same as those described previously in a report on effects of the lesions on contralesional hand motor function (Darling, et al., 2009). All lesions were placed in the hemisphere contralateral to the preferred limb (as determined from hand preference testing sessions and calculated handedness index score). The planned surgical lesions targeted the arm areas of primary motor cortex (M1) (category F1 lesion – e.g., Fig. 1); M1 + the adjacent lateral premotor cortex (LPMC) (category F2 lesion – Fig 2, see also Fig. 6 of McNeal et al. 2010); M1 + LMPC + the supplementary motor cortex (M2) (category F3 lesion – e.g., Fig. 3). Two additional lesions cases are also presented that initially involved the surgical removal of motor cortex, but spread rostrally to involve medial prefrontal cortex (multifocal lesion – see Darling et al., 2009, Figs. 2 and 3). Extensive descriptions of the lesions of all 10 monkeys are included in our previous reports (Darling, et al., 2009, McNeal, et al., 2010).
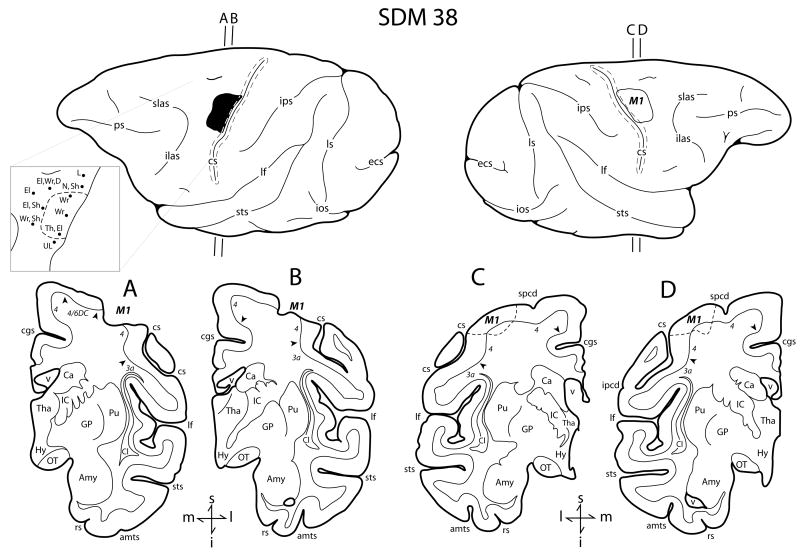
Figure 1.
Line drawings of the lateral surface of the hemisphere of case SDM38 which received a category F1 lesion. Representative coronal sections are located immediately below the lateral views. The left hemisphere illustrates the location of the cortical lesion (blackened area) and the right hemisphere the location of the superimposed lesion (outlined area) that was used to calculate the gray and white matter lesion volumes. Coronal panels A and B are through the lesioned hemisphere. Panels C and D show the location of the superimposed lesion site on the non-lesioned hemisphere (dashed line). In each coronal section, the region of extirpated cortex is identified by the bold italicized conventions (i.e., M1). Pertinent Brodmann's cytoarchitectonic areas are indicated on the coronal sections immediately below the gray matter and the respective boundaries are identified by the solid black arrow heads. The pullout on the left hemisphere illustrates the microstimulation map used to guide lesion localization. On the map each black dot represents a stimulation point with the affiliated movement(s) observed following stimulation. Abbreviations: amts, anterior medial temporal sulcus; Amy, amygdala; Ca, caudate nucleus; cgs, cingulate sulcus; Cl, claustrum; cs, central sulcus; D, digit; ecs, ectocalcarine sulcus; El, elbow; GP, globus pallidus; Hy, hypothalamus; I, inferior; IC, internal capsule; ilas, inferior limb of the arcuate sulcus; ios, inferior occipital sulcus; ipcd, inferior precentral dimple; ips, intraparietal sulcus; l, lateral; L, leg; lf, lateral fissure; ls, lunate sulcus; m, medial; M1, primary motor cortex; N, neck; OT, optic tract; ps, principle sulcus; Pu, putamen; rs, rhinal sulcus; s, superior; Sh, shoulder; slas, superior limb of the arcuate sulcus; spcd, superior precentral dimple; sts, superior temporal sulcus; Th, thumb; Tha, thalamus; UL, upper lip; v, ventricle; Wr, wrist. (Lateral hemispheric surface images are reproduced with permission from online supplemental materials of Darling et al. 2009).
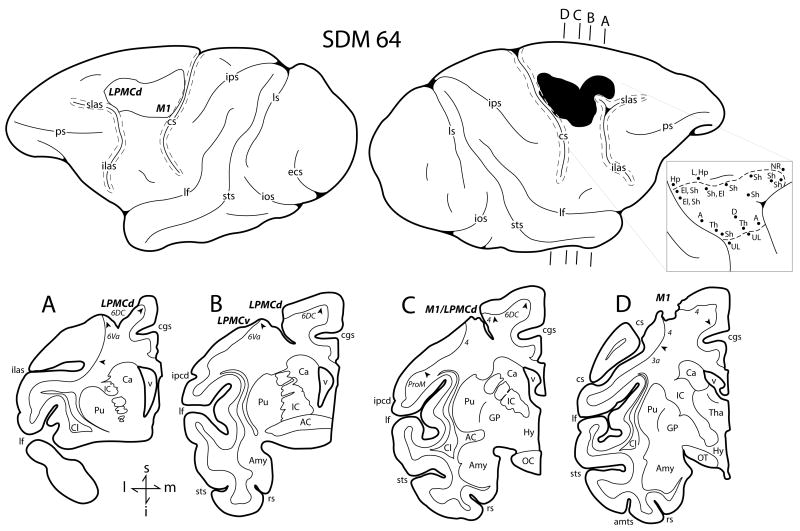
Fig. 2.
Line drawings of the lateral surface of the hemisphere of case SDM64 which received a category F2 lesion. Representative coronal sections are located immediately below the lateral views. The right hemisphere illustrates the location of the cortical lesion (blackened area) and the left hemisphere the location of the superimposed lesion (outlined area) that was used to calculate the respective gray and white matter lesion volumes. Coronal panels A through D are all through the lesion site on the right hemisphere. In each coronal section, the region of extirpated cortex is identified by the bold italicized conventions. Pertinent Brodmann's cytoarchitectonic areas are indicated on the coronal sections immediately below the gray matter and the respective boundaries are identified by the arrow heads. The pullout illustrates on the right hemisphere the microstimulation map. On the map each black dot represents a stimulation point with the affiliated movement(s) observed following stimulation. Abbreviations: A, arm; AC, anterior commissure; Hp, hip; LPMCd, dorsal lateral premotor cortex; LPMCv, ventral lateral premotor cortex; NR, no response; OC, optic chiasm. For other abbreviations see caption of Figure 1. (Lateral hemispheric surface images are reproduced with permission from online supplemental materials of Darling et al. 2009).
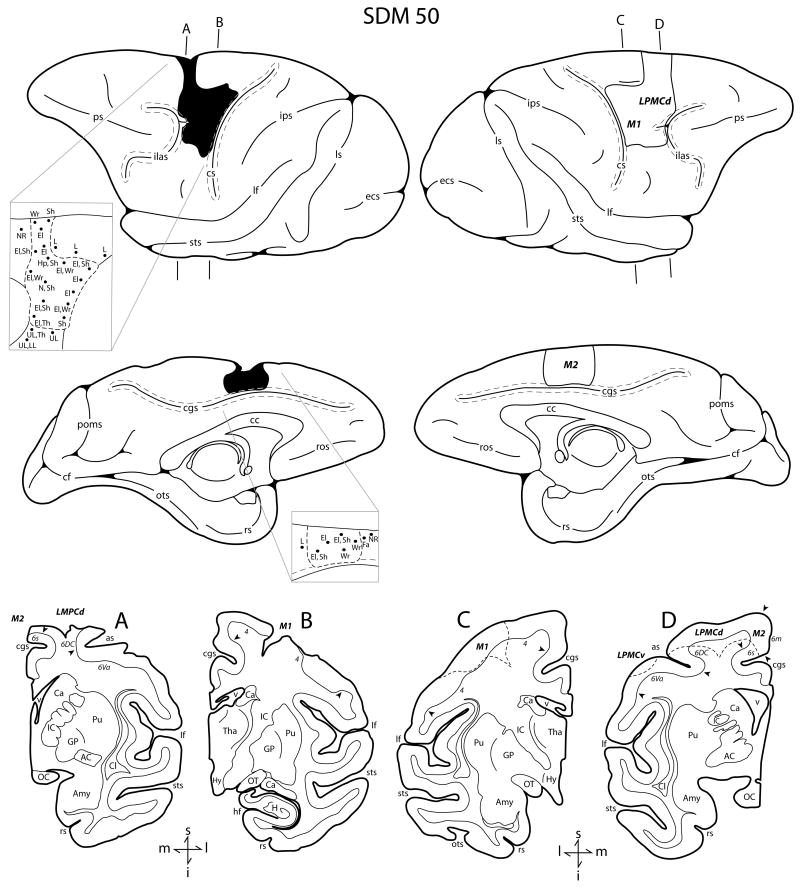
Fig. 3.
Line drawings of the lateral surface of the hemisphere of case SDM50 which received a category F3 lesion. The left hemisphere (both lateral and medial views) illustrates the location of the cortical lesion (blackened area) and the right hemisphere (both lateral and medial views) shows the location of the superimposed lesion (outlined area) that was used to calculate the gray and white matter lesion volumes. Coronal panels A and B are through the lesioned hemisphere. Coronal panels C and D show the location of the superimposed lesion site on the non-lesioned hemisphere (dashed line). In each coronal section, the region of extirpated cortex is identified by the bold italicized conventions. Pertinent Brodmann's cytoarchitectonic areas are indicated on the coronal sections immediately below the gray matter and the respective boundaries are identified by the arrow heads. The top pullout on the right hemisphere illustrates the microstimulation map used to guide lesion localization on the lateral surface of the hemisphere. The bottom pullout on the right hemisphere illustrates the microstimulation map used to guide the lesion location on the medial surface of the hemisphere. On both stimulation maps, each black dot represents a stimulation point with the affiliated movement(s) observed following stimulation. Abbreviations: as, spur of arcuate sulcus; cc, corpus callosum; cf, calcarine fissure; Fa, face; H, hippocampus; hf, hippocampal fissure; LL, lower lip ; M2, supplementary motor cortex; ots, occipito-temporal sulcus; poms, medial parieto-occipital sulcus; ros, rostral sulcus. For other abbreviations see captions of Figures 1 and 2.
Histological Procedures
Following the predetermined survival/recovery period, each monkey was deeply anesthetized with an overdose of pentobarbital (50 mg/kg or more) and perfused transcardially with 0.9% saline, followed by 2 liters of 4% paraformaldehyde in 0.1M phosphate buffer at pH 7.4 (PB), then one liter each of 10% and 30% sucrose in 0.1M PB for cryoprotection. The brain was removed, blocked into cortical, brainstem and spinal cord components. The tissue was placed in 30% sucrose in 0.1M PB for 2 to 5 days at 4° C and then processed for histochemical visualization of the cortical lesion (Morecraft, et al., 2002, Morecraft, et al., 2001, Morecraft, et al., 2007). To accomplish this, the cortical tissue was frozen sectioned in the coronal plane on a sliding microtome (American Optical 860, Buffalo, NY, USA) at a thickness of 50 μm in cycles of 10, forming 10 complete series of evenly spaced tissue sections respectively. In case SDM56, the cortex was sectioned at a thickness of 60 μm in cycles of 10. To identify the cytoarchitectonic organization of each brain, one complete series of tissue sections was processed for Nissl substance using our previously described histochemical methods (Morecraft, et al., 2004, Morecraft, et al., 1992) and used for the lesion volume analysis in the present report.
Estimation of Lesion Volume
The methods for estimating gray and white matter lesion volume were described previously (see Figs. 3 and 4 of Pizzimenti et al. 2007) and slightly modified to examine every Nissl stained section at 500 μm intervals (600 μm intervals in SDM56) through the lesion site (instead of 1000 μm intervals) (Darling, et al., 2009). We minimized the effects of post-injury atrophic distortion on estimating gray and white matter lesion volume by superimposing an outline of the lesion site onto the undamaged hemisphere to calculate the total gray matter and total white matter volume of the lesion. This was accomplished using metrically calibrated images of the cortical surface produced immediately before and after the lesion that included principal anatomical landmarks (e.g., central, arcuate and cingulate sulci) to assist the postmortem reconstruction and transfer of the lesion site boundaries onto a surface image of the non-lesioned hemisphere (Figs. 1,2,3). The superimposed lesion on the intact hemisphere in conjunction with the Nissl stained sections were also used to evaluate the laminar involvement (depth and width) of the lesion and report the specific cytoarchitectonic areas involved in the lesion. The cytoarchitectonic criteria used to evaluate the frontal lobe were based upon the original report of (Barbas and Pandya, 1987); see also Figs 1 and 2 of (Morecraft, et al., 2004). The same general method was used to estimate the white matter lesion area and volume. Briefly, the external boundary of the white matter region of interest coincided with the plane of interface between layer VI and the subcortical white matter. Similarly, the internal boundaries of the white matter ROI corresponded to the width and depth of the lesion as determined from Nissl stained sections through the lesioned hemisphere. The boundaries obtained from the lesioned hemisphere were then transferred to a matching coronal section in the non-lesioned hemisphere (e.g., Figs. 1C,A-D; 3A-D). Subcortical structures and fiber pathways that were spared as well as involved in the lesion site were identified using the atlas and nomenclature of Schmahmann and Pandya (Schmahmann and Pandya, 2006). Gray and white matter lesion volumes are reported in Table 1 of (Darling, et al., 2009).
mMAP Data Analysis
Force data from the mMAP task were analyzed visually in Datapac 2k2 to identify the first touch of the carrot chip or plate/rod supporting the carrot chip to the end of force application (i.e., when the carrot chip was removed from plate supported by the load cell or the rod) on each trial using recordings of applied forces in Datapac 2k2. The accompanying video data was also analyzed to verify these times and to identify trial outcome (see equation 1). The duration and total applied 3-D absolute impulse (see equation 1) were computed for each trial. The total absolute impulse was computed by integrating the rectified 3-dimensional force signals over the duration of force application. It represents the total force-time impulse applied during target acquisition and is larger if greater forces are applied over longer durations (Darling, et al., 2006). Low impulse on successful trials (outcome ≥ 2) represents greater skill because the subject uses less force and removes the carrot chip faster.
After pre-lesion data collection was completed, performance scores normalized to each individual monkey's abilities (i.e., minimum and maximum applied impulse and duration) for each trial at each difficulty level were computed (see equation 2). Also note in the performance score calculations that if the monkey attempts, but fails to successfully acquire the carrot chip (i.e., if outcome = 1) that higher scores are given if the monkey exerts larger impulses for longer time periods while attempting to acquire it. This typically occurs in the early post-lesion period when the monkey is recovering and less coordinated and may abandon the attempt quickly, resulting in very low applied impulse and duration (compared to during pre-lesion testing). With this scoring mechanism, a higher score is awarded for strong attempts (but limited to a maximum score of 200, which is also the minimum score for a successful trial).
| (1) |
TAImp (n) – Total Absolute Impulse of trial n
∫ - integral over duration of trial t with respect to time (dt)
Fx - Force applied in left/right direction
Fy - Force applied in anterior-posterior direction
Fz - Force applied in vertical direction
If outcome ≥ 2 (i.e., successful grasp and lift/manipulation of the carrot chip) then
| (2) |
if PSmMAP(n) < 200 then PSmMAP(n) = 200
Else
PSmMAP(n) = {100*((MaxTAImp − TAImp(n))/TAImp Range) + 100*((MaxDur − Dur(n))/DurRange)}*Outcome(n)
If PSmMAP(n) > 200 then PSmMAP(n) = 200
If PSmMAP(n) < 50 then PS mMAP(n) = 50
Where:
| PSmMAP(n) | – performance score on mMAP trial n |
| Outcome(n) | – success on trial n (0 for no attempt with the correct hand, 1 for unsuccessful attempt with the correct hand, 2 if the carrot chip is successfully grasped and lifted over the rod but then dropped and not removed from the food chamber, 3 if the carrot chip is successfully grasped and lifted over the rod but then dropped and removed from the food chamber, 4 for successful acquisition without dropping the carrot chip) |
| MinTAImp | – minimum single trial pre-lesion total absolute impulse within a difficulty level for either hand |
| MaxTAImp | - maximum single trial pre-lesion total absolute impulse within a difficulty level for either hand |
| TAImp Range | –MaxTAImp – MinTAImp |
| Dur(n) | – duration of trial n |
| MinDur | – minimum single trial duration during pre-lesion tests with either hand within a difficulty level |
| MaxDur | - maximum single trial duration during pre-lesion tests with either hand within a difficulty level |
| DurRange | – MaxDur – MinDur |
mDB Data Analysis
Temporal characteristics of reaching, manipulation, and 3D locations of the index finger and thumb tips were determined from the digital video files as described previously and used to compute a performance score on each pre- and post-lesion trial (Pizzimenti et al. 2007). Video recordings of hand/digit motion were digitized to compute reach duration, accuracy, grip aperture, manipulation duration and manipulation attempts (# of times contact between the pellet and a digit was lost and then re-established) on each trial. These measurements were normalized to the pre-lesion performance ranges for each monkey (i.e., to maximum/minimum reach and manipulation duration, least/most accurate reach, largest/smallest grip aperture, maximum/minimum number of manipulation attempts) and used to compute a performance score for each trial (see equation 1 of Pizzimenti et al. 2007). The lowest score is zero if no attempt was made to acquire the food pellet. Similar to the mMAP scoring, the minimum score of a trial in which the monkey attempted to retrieve the pellet was awarded 50 to ensure it is clearly better than if there is no attempt. The minimum score for a successful trial was 200. Scores are higher for shorter reach and manipulation durations, better accuracy, smaller grip aperture and fewer grasp attempts (maximum of 1000 for a single trial with the best score for each component among pre-lesion scores).
Analysis of Hand Motor Skill
Highly skilled reaching and grasping behavior should demonstrate consistent high performance scores with low variability. To quantify overall motor skill in our tasks we computed the mean and then divided it by the standard deviation of performance scores over 5 consecutive testing sessions (i.e., 25 trials over an approximately 5 week period) (Pizzimenti, et al., 2007). This measure of skill was computed for the performance score on the mMAP curved rod task and for the manipulation, reach and overall scores on the mDB task (best well, with highest pre-lesion skill, and a smaller diameter 2nd well with a lower pre-lesion skill for each monkey). Reach and manipulation performance scores were computed as described previously (Pizzimenti, et al., 2007). These skill measures were computed for the last 5 pre-lesion test sessions and for the 5 consecutive test sessions when skill was highest during post-lesion recovery. We also identified the post-lesion week when highest skill was attained in the mDB 2nd well task and mMAP curved rod task and computed average skill over subsequent weeks to assess whether skill was maintained at a level higher than pre-lesion levels. It should also be noted that we were unable to digitize some video recordings from some post-lesion mDB testing sessions for some monkeys because of technical problems. Specifically, if one of the four camera did not function the remaining three cameras were setup for collection from the contralesional limb (the primary target of our studies), which was not ideally positioned in some experimental sessions. In these cases, we computed skill levels only from sessions in which full digitized data were available (mean post-lesion sessionsavailable for all monkeys: 90%, range: 71%-100%). Manipulation and reach duration were analyzed for almost all post-lesion mDB sessions because temporal data (i.e., frames at which hand exit from cage, fingertip touchdown, manipulation onset and termination) could be analyzed.
Qualitative Analysis of Hand Movements
We qualitatively analyzed selected pre- and post-lesion movement trials to assess the basic movement strategies used in the mDB (best well and 2nd well) and mMAP (curved rod) tasks using similar methods to those described previously (Darling, et al., 2009). Briefly, one evaluator studied videos of performances in these tasks associated with high, average and below average performance scores during pre and post-lesion phases to assess hand posture and digits used to manipulate the food objects. We were primarily looking for changes in strategy due to altered hand postures and changes in digit use during manipulation.
Statistical Analysis
Pre-lesion skill over the last 5 testing sessions before the lesion by the preferred and non-preferred (ipsilesional) hands were compared using two-tailed t-tests to assess whether the two hands attained similar skill-levels before the lesion. Post-lesion recovery of ipsilesional hand motor performance by each monkey was assessed from changes in performance scores and skill-levels. The first 5 post-lesion tests were used to establish whether the monkeys showed clear decrements in motor function after the lesion. The best 5 consecutive post-lesion tests were used as an index of recovery. Ratios of these post-lesion skill measures to the skill during the last 5 pre-lesion trials were used to evaluate effects of the lesion on skill. In general, the monkeys performed with similar skill levels with each hand after the initial training process.
The level of recovery of fine motor skill was assessed by taking the ratio of the highest skill attained in 5 consecutive post-lesion testing sessions (until the 2nd surgery was performed to inject neurotracers) to the skill level in the final 5 pre-lesion testing sessions. Skill was defined as the mean performance score divided by the standard deviation (S.D.) of performance scores over 25 trials in 5 consecutive test sessions (Pizzimenti, et al., 2007). Thus, the recovery skill ratio ranged from 0 (if there were no post-lesion attempts at the task, indicating no recovery of reach/grasp) to 1 (if the highest pre-lesion skill equaled post-lesion skill) or even higher values if the highest post-lesion skill exceeded pre-lesion skill. We used a skill ratio rather than a difference score to evaluate recovery because performance was assessed within subjects rather than in an absolute manner. Multiple and simple linear regression analyses were used to determine whether changes in skill depended on gray and white matter lesion volumes. Specifically, a multiple linear regression was performed with skill change (ratio of post-lesion to pre-lesion skill) as the dependent variable and gray and white matter lesion volumes as independent (predictor) variables. This was followed by simple linear regression analyses to assess whether one of the independent variables or total lesion volume was the primary contributor to explain post-lesion skill changes.
We examined our hypothesis concerning a possible relationship between skill recovery and strength of preference by comparing recovery of reach and manipulation skill in monkeys with weak versus strong hand preference. This approach was taken instead of a regression approach because the handedness indexes were less than 25 in 7 monkeys (i.e., weak hand preference) and greater than 75 in 3 monkeys (i.e., strong hand preference). There were no monkeys with intermediate hand preferences (i.e., HI of 25-75). Due to the small number of subjects with strong hand preference, we did not apply statistical tests but simply made qualitative comparisons.
Results
Pre-lesion skill was not consistently better with the preferred hand than with the non-preferred hand. Two-tailed paired t-tests comparing manipulation skills of the two hands showed no differences in the mDB (best well, 2nd well) tasks (p > 0.2) or in the mMAP curved rod task (p > 0.05). Differences in manipulation skill between the two hands varied considerably among monkeys and was not correlated with handedness index in the mDB or mMAP tasks (p > 0.2). However, in some cases one hand exhibited clearly higher pre-lesion skill than the other, but in a task-dependent manner. For example, SDM45 (low handedness index of 21.3) had similar manipulation skill with the two hands in the mDB best well task but exhibited clearly better manipulation skill in the mDB 2nd well task (69% higher than the contralesional hand) and mMAP task (75% higher than the contralesional hand). In contrast, SDM46 (high handedness index of 96) was clearly better with the preferred hand in the mDB tasks (40-50% higher skill in the best well and 2nd well tasks), but not in the mMAP curved rod task (only 2.8% better skill with the preferred hand). Overall, subjects with high hand preference performed with high skill levels with the non-preferred hand before the lesion on some tasks.
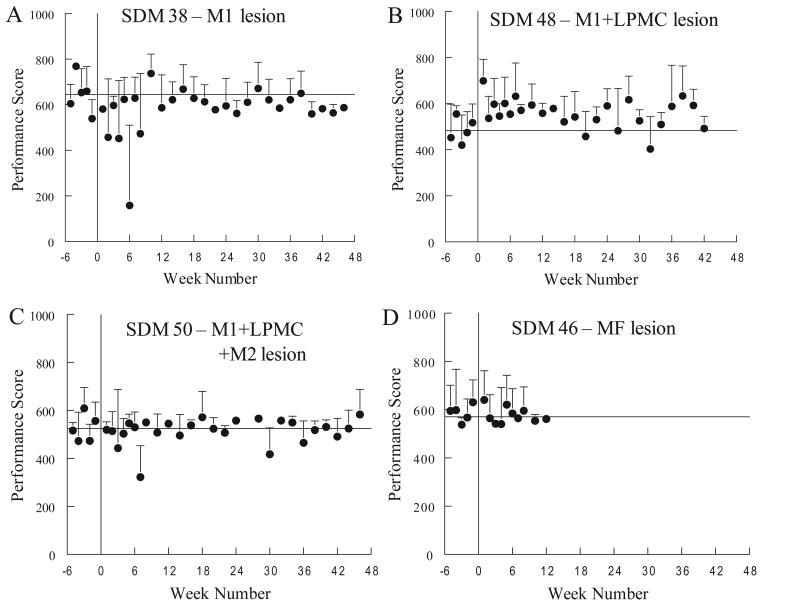
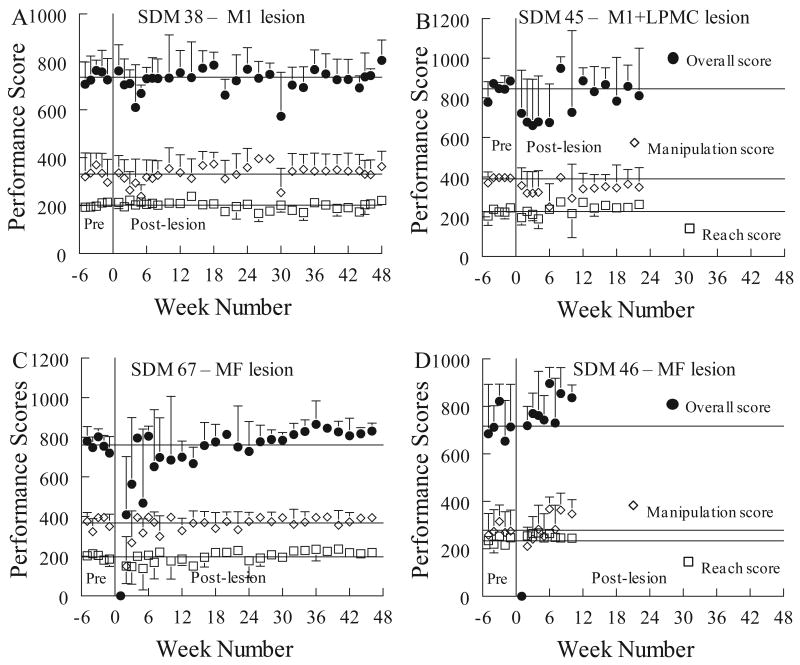
Observations of cage behavior in all monkeys during the first few days after the lesion showed good use of the ipsilesional hand for reaching, grasping and postural support, in contrast to the contralesional hand (Darling, et al., 2009). All monkeys but the two with multifocal lesions (SDM67, SDM46) had 5 successful acquisitions in each of the mMAP curved rod and mDB (best well and 2nd well) tasks in the first motor tests one week after the lesion. On the mMAP curved rod task SDM67 had 3 successful acquisitions (of 5 trials) and SDM46 had 5 successful acquisitions with similar performance scores to those on the final pre-lesion tests (Fig. 5D). In contrast, both of these monkeys had no attempts in the mDB task at the first post-lesion test on their best well and 2nd well (e.g., Fig. 4C, D). Incidentally, SDM46 did have some successful acquisitions on well E, but SDM67 did not. Thus, SDM46 had good use of the ipsilesional hand at one week post-lesion despite having by far the largest lesion.
Figure 5.
Pre- and post-lesion mean performance scores on the mMAP curved rod task by 4 monkeys. Each plotted point shows the mean performance score by a single monkey in a single testing session. Error bars are 1 S.D.
Figure 4.
Pre- and post-lesion mean performance scores on the mDB test by 4 monkeys. Each plotted point shows the mean overall performance score, manipulation performance score or reach performance score for a single monkey in a single testing session on the best well (with highest pre-lesion skill). Error bars are 1 standard deviation (S.D.).
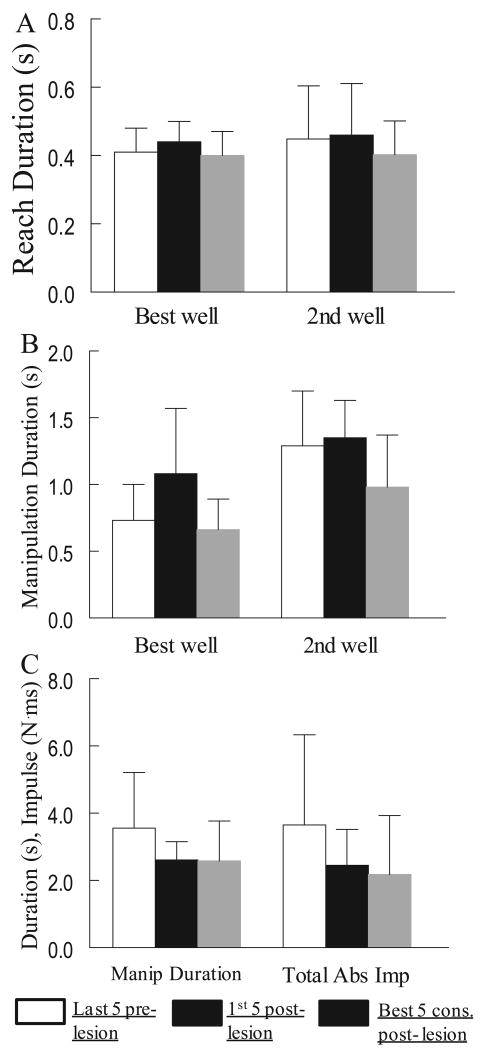
Overall, there were relatively minor effects of the lesion on ipsilesional hand motor performance on the mMAP curved rod task in the first 5 weeks post-lesion. Average mMAP performance scores over the first 5 post-lesion tests averaged 97% ± 18% (S.D.) of average scores over the last 5 pre-lesion tests (e.g., Fig. 5). Average total absolute impulse and duration both decreased by an average of about 20% ± 25%, but with considerable variability among monkeys (Fig. 6C). Average performance scores did not increase despite improvements in duration and impulse because there were more trials in which the carrot chip was dropped and occasionally there were unsuccessful trials. Notably, trial-to-trial and session-to-session variability of mMAP performance scores usually increased in the first 5 weeks following the lesion (e.g., Fig. 5A), such that skill on the mMAP was reduced by greater than 20% in six monkeys during this period. However, two monkeys exhibited increased skill by 20% or more in the first 5 weeks (Table 1).
Figure 6.
Bar graphs showing mean reach duration (A) and manipulation duration (B) for the mDB best well and 2nd well tasks and mean manipulation duration and total absolute impulse (C) in the mMAP task for the last 5 pre-lesion tests, first 5 post-lesion tests and best 5 consecutive post-lesion tests. Each bar shows the mean for 10 monkeys. Error bars are 1 S.D.
Table 1.
Ratios of post-lesion skill (mean/S.D. of performance scores) to pre-lesion skill for the first 5 post-lesion weeks and the best 5 consecutive post-lesion weeks on the mDB (best well) and mMAP curved rod tasks.
| Monkey | 1st 5 Post-lesion weeks | Best 5 post-lesion weeks | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| mDB Reach1 | mDB Manip2 | mMAP3 | mDB Reach | mDB Manip | mMAP | |||||
| 1st well | 2nd well | 1st well | 2nd well | 1st well | 2nd well | 1st well | 2nd well | |||
| SDM38 | 0.62 | 0.36 | 0.84 | 0.68 | 0.54 | 0.85 | 1.01 | 1.18 | 2.29 | 1.72 |
|
| ||||||||||
| SDM64 | 0.88 | 2.07 | 1.21 | 1.34 | 0.79 | 1.29 | 3.22 | 1.32 | 2.39 | 1.09 |
|
| ||||||||||
| SDM48 | 0.43 | 1.98 | 0.54 | 1.50 | 1.24 | 2.21 | 5.72 | 1.28 | 1.56 | 1.85 |
|
| ||||||||||
| SDM55 | 1.05 | 0.96 | 0.41 | 0.55 | 0.23 | 1.87 | 1.57 | 1.01 | 1.37 | 1.11 |
|
| ||||||||||
| SDM45 | 0.74 | 1.09 | 0.19 | 1.24 | 1.07 | 1.22 | 1.09 | 0.30 | 1.24 | 1.44 |
|
| ||||||||||
| SDM70 | 1.08 | 1.56 | 0.77 | 1.04 | 1.72 | 1.63 | 1.59 | 1.32 | 1.56 | 2.27 |
|
| ||||||||||
| SDM50 | 0.38 | 0.65 | 0.67 | 0.80 | 0.77 | 0.72 | 9.06 | 0.99 | 3.55 | 1.71 |
|
| ||||||||||
| SDM56 | 0.63 | 1.13 | 1.69 | 1.16 | 0.41 | 0.69 | 1.25 | 2.65 | 1.21 | 7.75 |
|
| ||||||||||
| SDM67 | 0.17 | 0.18 | 0.18 | 0.37 | 0.33 | 1.48 | 1.57 | 1.31 | 2.81 | 1.55 |
|
| ||||||||||
| SDM46 | 0.21 | 0.88 | 0.44 | 1.06 | 0.99 | 2.09 | 5.72 | 1.09 | 2.22 | 1.64 |
|
| ||||||||||
| Mean | 0.62 | 1.09 | 0.69 | 0.98 | 0.81 | 1.41 | 3.18 | 1.25 | 2.02 | 2.21 |
|
| ||||||||||
| S.D. | 0.32 | 0.63 | 0.47 | 0.36 | 0.46 | 0.55 | 2.75 | 0.58 | 0.77 | 1.97 |
Reach skill ratio
Manipulation (Manip) skill ratio
Best well (well with highest overall pre-lesion skill)
Performance with the ipsilesional hand over the first five post-lesion weeks on the mDB task was similar to the mMAP task, but with greater variability among monkeys (Fig. 4, Table 1). Overall performance scores during the first five post-lesion weeks averaged 87% ± 13% (best well) and 95% ± 16% (2nd well) of mean scores in the last 5 pre-lesion tests. Notably, reach and manipulation skill both decreased in most monkeys in the best well task with some monkeys showing large decreases due to a lack of attempts primarily in the first post-lesion test (SDM46, SDM67) while other showed small improvements (e.g., SDM64, SDM70) (Table 1). In contrast, reach durations were nearly unaffected by the lesion (Fig. 6A). However, the effects of the lesion on grasping from the smaller 2nd well were more variable. Some monkeys improved (e.g., SDM64, SDM48), while others had clear decreases in skill (e.g., SDM38, SDM55, SDM67) (Table 1). The decreases in skill were primarily due to increases in trial-to-trial (within a session) and session-to-session variability of performance scores in the first 5 post-lesion weeks (e.g., Fig. 4) as well as increased average manipulation duration (Fig. 6B) which reflected more lost contacts with the food pellet during manipulation. Remarkably, skill did not change similarly for the reach and manipulation movements for a particular well and could also be quite different for different wells and on the mMAP task (Table 1). For example, SDM55 showed little effects of the lesion on reach and manipulation skill for the mDB best well task (Table 1 – skill ratios near 1.0), but showed clear decreases in skill after the lesion on the mDB 2nd well and mMAP tasks (skill ratios of 0.55 and 0.23 respectively). Moreover, changes in skill on the mMAP (which requires manipulation of a larger food object using forearm and digit motions and depends on exerted forces as well as duration) during the first 5 post-lesion weeks was similar to manipulation skill on the mDB tasks in some monkeys (e.g., SDM38, SDM45) but quite different in others (e.g., SDM55, SDM67) (Table 1).
There were increases in mean mMAP and/or mDB performance scores and skill after the first 5 post-lesion weeks in all monkeys (Fig. 4,5,7,8 Table 1). In the curved rod mMAP task during the 5 consecutive tests with highest post-lesion skill, performance scores improved to about 10% ± 11% better than pre-lesion scores. These increased performance scores resulted from reductions in mean duration and impulse averaging 25% and 35% respectively relative to pre-lesion (Fig. 6C). Highest post-lesion skill on the mMAP improved in all monkeys relative to the last 5 pre-lesion tests (Table 1, t9 = 3.37, p < 0.05). Most monkeys improved mMAP skill by 10% - 85%. SDM56 and SDM70 had very large improvements in skill (Table 1 – post/pre-lesion skill ratio of 7.76 and 2.27) which were due to reductions in mean and variability of both duration and impulse. A major contributor to the large increase in skill in SDM56 was that there were no trials in which the carrot chip was dropped during the best 5 consecutive post-lesion tests whereas there were a few such trials during the last 5 pre-lesion tests. On the mDB (best well) task, reach skill increased by more than 10% in 7 of 10 monkeys (Table 1; t9 = 1.51, p < 0.1) and manipulation skill increased by more than 10% in 6 monkeys (Table 1; t9 = 0.39, p > 0.3). Consistent with the small changes in skill, reach and manipulation durations for the best well task in the best 5 consecutive post-lesion testing sessions were only slightly decreased relative to pre-lesion values (Fig. 6A,B). In contrast, when grasping from the smaller 2nd well reach skill increased by more than 10% in 9 monkeys (Table 1; t9 = 2.93, p < 0.05) and manipulation skill increased by more than 10% in all monkeys (Table 1; t9 = 3.23, p < 0.01). Both reach and manipulation durations were shorter than pre-lesion in most monkeys for the 2nd well task (Fig. 6A,B).
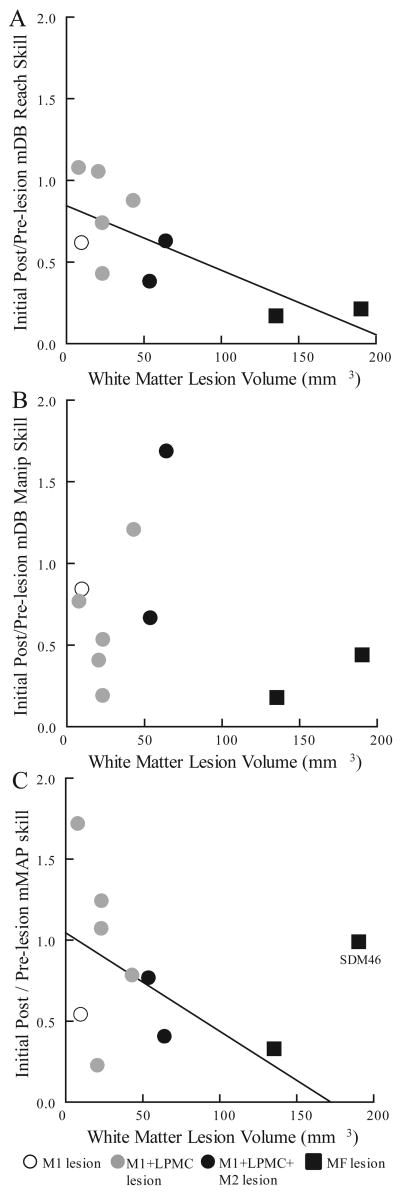
Figure 7.
Scatterplots of changes in skill during first 5 post-lesion weeks relative to last 5 pre-lesion weeks versus white matter lesion volume in the mDB best well task (A,B) and mMAP task (C). Each plotted point is data from a single monkey. The plotted lines are the least squares fits including SDM46 in A but excluding SDM46 in C.
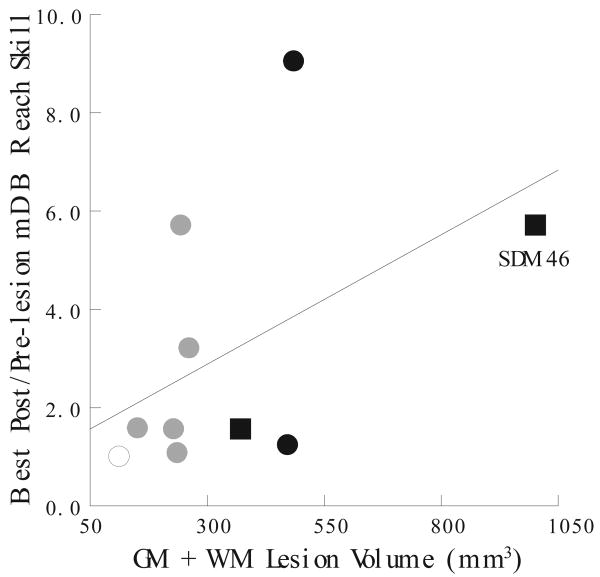
Figure 8.
Scatterplot of changes in reach skill during the best 5 post-lesion weeks relative to the last 5 pre-lesion weeks versus total (gray matter + white matter) lesion volume in the mDB 2nd well task. Each plotted point is data from a single monkey. The plotted line is the least squares fit.
The post-lesion week in which highest skill occurred was highly variable among monkeys. In the mDB 2nd well task, highest post-lesion skill was observed as early as the first post-lesion week (SDM45) and as late as the 28th post-lesion week (SDM50), with a mean for all monkeys of 9.6 weeks (SD = 9.5 weeks). In the mMAP curved rod task, highest skill was observed as early as 4 weeks post-lesion (SDM45) and as late as 40 weeks post-lesion (SDM38), with a mean for all monkeys of 12.9 weeks (SD = 13.3 weeks). Average skill levels then decreased in both tasks, but remained above pre-lesion skill by, on average, 31.5% in the mDB 2nd well task and 31.7% in the mMAP curved rod task.
Early post-lesion changes in reach and manipulation skill relative to pre-lesion skill showed some dependence on white matter lesion volumes. The ratios of reach (mDB task) and manipulation (mMAP and mDB tasks) skill in the first 5 post-lesion weeks to the last 5 pre-lesion weeks were generally negatively correlated with white matter lesion volume indicating that motor performance decreased more with larger lesions (Table 2, Fig. 7). These inverse relationships occurred because most monkeys with small lesions had either increases or small decreases in skill during the first 5 post-lesion weeks whereas monkeys with large lesions usually had large decreases in reach and manipulation skill during this time (Fig. 7). Notably SDM46, the monkey with the largest lesion, had little change in manipulation skill on the mMAP and mDB (2nd well) tasks, but did exhibit decreased reach and manipulation skill on the mDB best well task (Table 1). Only the relationship between changes in reach skill and white matter lesion volume was strong both with and without SDM46 included in the regression analyses (Table 2, R2 > 0.4).
Table 2.
Coefficients of determination for relationships between hand motor skill relative to pre-lesion during the first 5 post-lesion weeks and lesion volume with (All) subjects and without (wo) SDM46.
| Regress. Model |
mDB (best well) |
mDB (2nd well) |
mMAP (curved rod) |
||
|---|---|---|---|---|---|
| Reach All(woSDM46) |
Manip All(woSDM46) |
Reach All(woSDM46) |
Manip All(woSDM46) |
All(woSDM46) | |
| MLR1 | 0.54* (0.49*) | 0.13 (0.20) | 0.2 (0.21) | 0.25 (0.26) | 0.10 (0.26) |
|
| |||||
| SLR-GM2 | 0.31 (0.16) | 0.00 (0.12) | 0.02 (0.01) | 0.01 (0.00) | 0.00 (0.08) |
|
| |||||
| SLR-WM3 | 0.54* (0.48*) | 0.04 (0.01) | 0.13 (0.01) | 0.04 (0.18) | 0.04 (0.25) |
|
| |||||
| SLR-GM+WM4 | 0.38* (0.29) | 0.00 (0.07) | 0.04 (0.04) | 0.00 (0.01) | 0.00 (0.15) |
multiple linear regression (MLR) with white matter (WM) and gray matter (GM) lesion volumes as independent variables
simple linear regression (SLR) with gray matter lesion volume as the independent variable
simple linear regression with white matter lesion volume as the independent variable
simple linear regression with total lesion volume (WM+GM) as the independent variable
p < 0.05
The ratio of post-lesion skill during the best 5 consecutive post-lesion weeks to pre-lesion skill showed no clear dependence on total lesion volume (Table 3). Only the improvements in reaching skill on the mDB 2nd well task showed some indication of a relationship with gray and white matter lesion volume in the multiple and simple linear regression analyses (Table 3). Surprisingly, the ratio of highest post-lesion reach skill to pre-lesion skill tended to increase with total lesion volume for the 2nd well task (Fig. 8, Table 3, p < 0.1), indicating that recovery of ipsilesional reaching is generally better for larger lesion volumes. Recovery of manipulation skill was not correlated with lesion volume in either the mDB or mMAP tasks (Table 3).
Table 3.
Coefficients of determination for relationships between hand motor skill relative to pre-lesion during the best 5 consecutive post-lesion weeks and lesion volume with (All) and without (wo) SDM46.
| Regress. Model |
mDB (best well) |
mDB (2nd well) |
mMAP (curved rod) |
||
|---|---|---|---|---|---|
| Reach All(woSDM46) |
Manip All(woSDM46) |
Reach All(woSDM46) |
Manip All(woSDM46) |
All(woSDM46) | |
| MLR1 | 0.06 (0.23) | 0.01 (0.18) | 0.37 (0.35) | 0.14 (0.22) | 0.07 (0.30) |
|
| |||||
| SLR-GM2 | 0.03 (0.23) | 0.01 (0.17) | 0.05 (0.30) | 0.05 (0.08) | 0.03 (0.29) |
|
| |||||
| SLR-WM3 | 0.06 (0.03) | 0.01 (0.08) | 0.07 (0.00) | 0.13 (0.21) | 0.00 (0.03) |
|
| |||||
| SLR-GM+WM4 | 0.04 (0.2) | 0.01 (0.18) | 0.25 (0.22) | 0.07 (0.14) | 0.02 (0.25) |
Abbreviations: same as in Table 2.
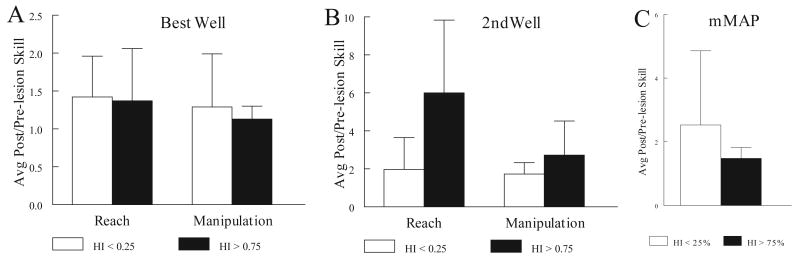
Changes in reach and manipulation skill also showed some dependence on strength of hand preference as indicated by the handedness index (HI), especially on the mDB 2nd well task. On the mDB best well task, the ratio of highest post-lesion reach and manipulation skill to pre-lesion skill were similar for the seven monkeys with weak hand preference (HI < 25) and for the three monkeys with strong hand preference (HI > 75) (Fig. 9A, C)). Similar results were obtained in the case of mMAP skill (Fig. 9C), although the monkeys with strong hand preference tended to have lower skill improvements in this task. However, in the mDB 2nd well task, which had lower pre-lesion skill levels due to the smaller well diameter, monkeys with stronger hand preference made clearly larger improvements in reach and manipulation skill than the monkeys with weak hand preference (Fig. 9B). Surprisingly, highest post-lesion/pre-lesion reach skill ratio was substantially lower in the 2nd well task than in the best well task for monkeys with a weak hand preference, but was similar in the two tasks for monkeys with strong hand preference (Fig. 9B). Manipulation skill ratios were clearly lower in the 2nd well task for both groups.
Figure 9.
Comparison of skill changes during the best 5 post-lesion weeks in monkeys with weak hand preference (HI < 25%) and strong hand preference (HI > 75%). Each bar represents the mean skill change for 7 monkeys (HI < 25% group) – open bars) or 3 monkeys (HI > 75% group – filled bars).
There was no evidence that monkeys receiving more pre-lesion experience (training + testing sessions) with the mMAP and mDB tasks showed less ipsilesional skill improvement during post-lesion recovery. Specifically, there were no statistically significant relationships between number of pre-lesion training + testing sessions and recovery of skill in any of the tasks (R2 < 0.1, p > 0.05). Thus, the skill improvement observed in most monkeys post-lesion was not simply related to the amount of pre-lesion practice/training. There was also no evidence of a strong positive relationship between recovery of motor skill of the ipsilesional and contralesional hands. Post/pre-lesion skill ratios for the mMAP and mDB tasks by the ipsilesional hand were not significantly correlated with the ratios for the contralesional hand (R2 < 0.17, p > 0.05). Indeed, a monkey that showed very poor recovery of the contralesional hand (SDM46 – no successful acquisitions in the mMAP curved rod or mDB tasks) performed at higher than pre-lesion skill with the ipsilesional hand while other monkeys that recovered to similar or higher than pre-lesion skill with the contralesional hand had a poorer recovery with the ipsilesional hand than SDM46 (e.g., SDM55 – see Table 2 of Darling et al. 2009).
Qualitative analyses of video recordings of the monkeys performing the mMAP (curved rod) and mDB (best well, 2nd well) tasks revealed that after the lesion, most monkeys used the same or very similar ipsilesional reach and grasp strategy as before the lesion. The pre and post-lesion strategies used by the ipsilesional hand in the mMAP curved rod task were also similar to those described previously for the contralesional hand (Darling, et al., 2009). Some monkeys used precision or key-type grasp of the carrot chip, sometimes with the 3rd digit under the index and then lifted the carrot chip while manipulating hand orientation to orient it over the curved rod while others spun the carrot chip to help move it up and off the rod. After the lesion, most monkeys manipulated the carrot chip more rapidly and with lower force application as described above, but with the same basic strategy as before the lesion. Analysis of force traces provided further confirmation that strategies remained similar in this task. For example, the monkey with the largest lesion (SDM46) exhibited quite similar force patterns and similar force magnitudes during slow and fast manipulations of the carrot chip in the curved rod mMAP task before and after the lesion (e.g., Fig. 10).
Figure 10.
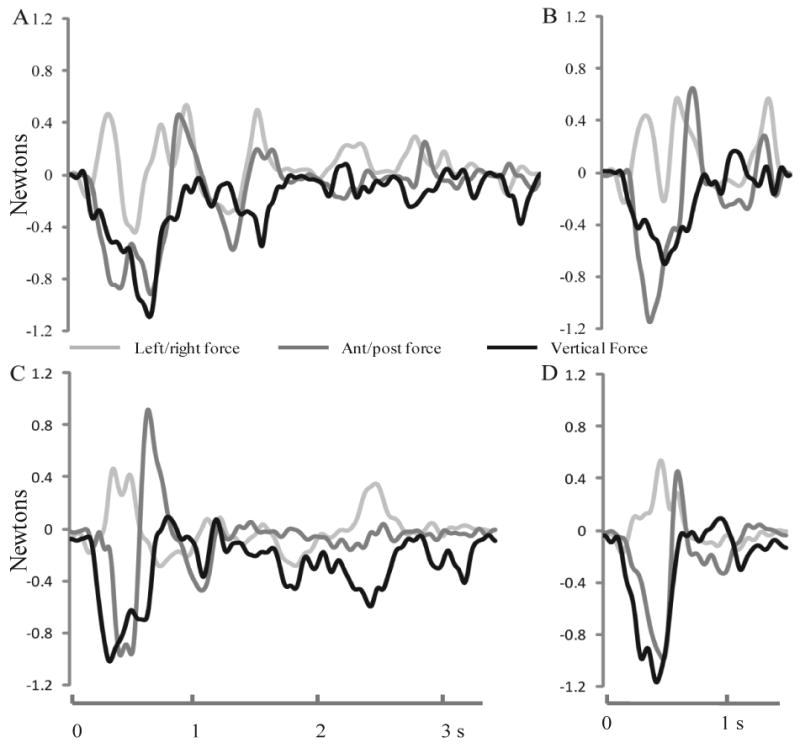
Force traces during slow (A,C) and fast (B,D) performances on the curved rod mMAP task before the lesion (top – A,B) and after the lesion (bottom – C,D) by SDM46. Each plotted line represents force exerted on the curved rod in one of the three cardinal directions.
In the mDB task the usual strategy was to reach to the food pellet and land with the hand pronated and wrist slightly extended, touching the pellet first with the index finger and then pulling the pellet towards the thumb to grasp it either with a precision grasp at the thumb tip or more proximal on the thumb (e.g., at the interphalangeal crease). While manipulating the pellet, the 3rd, 4th and 5th digits usually rested in a relaxed flexed posture on the Plexiglas plate next to the well holding the food pellet. As described previously (Darling, et al., 2009), some monkeys successfully used a single digit strategy in this task on some trials with each hand, while others used a strategy in which they first contacted the food pellet with the thumb and then dragged it towards the index. After the lesion, most monkeys used the same strategy with the ipsilesional hand and improved performance scores were mostly due to reductions in reach and manipulation duration as described above. However, some changes in strategy with the ipsilesional hand were observed after the lesion. For example, SDM38 placed only the index finger into the best well (D) rather than both index and thumb. Another change observed was that SDM46 used different hand/digit postures during manipulation of the food pellet after the lesion. Specifically, before the lesion the 3rd, 4th and 5th digits were in a relaxed flexed posture touching the Plexiglas plate, but post-lesion these digits were maintained in a claw-like posture with hyperextension at the metarcarpophalangeal joints and flexion at the interphalangeal joints and the palm and the tips of the fingers pressed against the Plexiglas plate.
Discussion
Advancing our understanding of the clinical consequences and recovery potential in patients with brain injury is critical for improving post-injury treatments and rehabilitative strategies. The present study shows convincingly that after an isolated unilateral lesion to cortical motor areas, skill in reaching, grasping and manipulating small objects with the ipsilesional upper limb can improve to higher than pre-lesion skill levels with a relatively small amount of continued practice after the injury. All ten monkeys showed higher reaching skill on either or both of the mDB best well or 2nd well tasks and higher manipulation skill on both the mMAP task and the mDB 2nd well task. The majority of the monkeys also exhibited higher manipulation skill on the mDB best well task. An important issue to consider is that the monkeys were not trained to the highest possible skill levels pre-lesion, thereby leaving room to improve speed/accuracy of reaching and skill of dexterous manipulation after the lesion. Moreover, the task was practiced under conditions of very high motivation to perform the task quickly and efficiently. Previous work showed that some of these monkeys also recovered to perform at higher than pre-lesion skill levels in the hand contralesional to the lesion (Darling, et al., 2009). Overall, these findings suggest that the primate central nervous system has a remarkable ability to reorganize after unilateral isolated frontal cortex injury to permit recovery of skill in reaching and manipulation in both hands, as we demonstrated recently for corticospinal mechanisms underlying recovery of contralesional hand motor function (McNeal, et al., 2010).
The higher manipulation skill exhibited by the ipsilesional hand after the lesion cannot be attributed to poor performance with the ipsilesional hand before the lesion or to major impairment of the dominant hand causing use of only the ipsilesional hand in the post-lesion phase. In general, the monkeys attained high skill levels before the lesion with both hands in the mDB and mMAP tasks. There were no statistical differences in pre-lesion skill levels of the two hands. Thus, improved skill of the ipsilesional hand represented clear improvement over high pre-lesion skill levels, in some cases to higher than pre-lesion skill achieved by the preferred (contralesional) hand. Moreover, all monkeys except SDM46 showed good recovery of the contralesional hand and, except for SDM56, showed use of the contralesional hand in a task in which the monkey could choose to use either hand (Darling, et al., 2010). Thus, the excellent post-lesion performance of the ipsilesional hand was not due to poor recovery of the contralesional hand leading to sole use, and thereby improved skill, of the ipsilesional hand. However, due to the lack of more extensive pre-lesion training, it was not possible to determine whether the ipsilesional hand attained exceptional skill after the lesion or simply continued to improve performance due to the continued practice.
Our findings of improved skill in the ipsilesional hand are consistent with a recent study which showed that some monkeys improve fine motor function to higher than pre-lesion levels after small ibotenic acid lesions to hand/digit areas of sensorimotor cortex (Kaeser, et al., 2010). It should be noted, however, that mean contact time with the pellet (similar to our measure of manipulation duration) did not improve relative to pre-lesion in this previous report. This suggested that the improved performance was primarily due to developing a more systematic strategy in terms of the order of grasping food from multiple wells (decreasing the time to transport the hand to each well) rather than an improvement in grasping skill when reaching to a single food target as in the present work. Also, this previous work only considered duration in the measure of skill whereas we considered a number of temporal and kinematic variables as well as trial-to-trial variability of performance in our measure of skill. We did observe post-lesion reductions in manipulation duration (and reductions in variability of manipulation duration in the mMAP and mDB tasks) in most monkeys, which more clearly demonstrate increased grasp/manipulation skill. However, these improvements are likely related to the lack of extensive pre-lesion daily training on the motor tasks in the present work in comparison to the work of Kaeser and colleagues (Kaeser, et al., 2010). That is, the lower pre-lesion training in our tasks left more room for improvement toward optimal performance. In addition, our skill measure considered variability in performance and other aspects of skill (accuracy of reach, number of lost pellet-digit contacts) whereas Kaeser and colleagues considered only mean duration of performance.
Our hypothesis that there would be an initial decrement in ipsilesional hand skill after the lesion followed by recovery to pre-lesion levels or higher was partially supported. Some monkeys showed clear decrements in skill in the first 5 post-lesion weeks and then recovered to better than pre-lesion levels (Table 2). However, other monkeys showed similar or higher skill during the first 5 post-lesion weeks than during the last 5 pre-lesion weeks and recovered to even higher skill levels with more post-lesion practice (Fig. 4, 7, Table 2). The increase in skill starting from the first post-lesion week in some monkeys may reflect reduced trans-callosal inhibition and the nearly exclusive use of the ipsilesional hand for motor tasks during the first post-lesion week. Indeed, one might expect an early post-lesion increase in ipsilesional hand motor skill for these reasons, followed by a gradual reduction in skill as the damaged hemisphere recovers to re-exert some trans-callosal inhibition while permitting greater use of the contra-lesional hand. The high variability among monkeys in the post-lesion week at which highest skill occurred and the finding that most monkeys retained higher than pre-lesion skill argue against this hypothesis. Moreover, the decrement in ipsilesional skill in the early post-lesion period in some of our monkeys contradicts this hypothesis and is consistent with the findings of a recent study which showed that some monkeys exhibited a decrease in performance for a period following the lesion (Kaeser, et al., 2010). Such transient deficits may be due to initial global effects of brain injury on brain function due to edema, inflammation, and diaschisis as well. However, such global effects would be expected to be largest in the monkeys with the largest lesions (e.g., SDM46, 50, 56), but all these monkeys had successful acquisitions on all attempts in the mMAP curved rod task at 1 week post-lesion and only SDM46 (with the largest multifocal lesion) did not have similar success on the mDB (best well) task at this time. All monkeys with smaller lesions (with the exception of SDM67 with a multifocal lesion) were successful on both tasks at one week post-lesion. Indeed, the 4 monkeys with M1+LPMC lesions all had successful acquisitions on all attempts for the mDB best and 2nd well tasks and mMAP curved rod tasks, often with similar or better performance scores than on pre-lesion tests. Thus, the global effects on brain function probably had little effect beyond one week post-lesion.
There was also some support for the hypothesis that monkeys with larger lesions would show greater ipsilesional decrements in skill initially after the lesion (Fig. 7). The strongest evidence for this hypothesis was in reach skill which decreased initially after the lesion in most monkeys, with larger decreases in monkeys with larger white matter lesions. However, there may be a floor effect as the 4 monkeys with the largest lesions had similar reach skill decrements despite large variations in white matter lesion volume (i.e., 50 to 200 mm3 - Fig. 7A). Thus, if there is damage to the three major cortical motor areas (M1, LPMC and M2) in one hemisphere, additional white matter damage as in SDM67 and SDM46 probably does not produce larger decrements in ipsilesional reaching skill. Ipsilesional manipulation skill decreases showed less evidence of a floor effect, especially since SDM46 showed almost no decrement in manipulation skill except for a lack of attempts in the mDB task at the first post-lesion test one week following the lesion, after which performance scores on the 2nd well were much better than the last 5 pre-lesion tests.
In our study, M1 was removed in all animal experiments and increased lesion size involved the selective removal of additional premotor arm regions. Thus, larger lesions were associated with progressive increase in damage to premotor areas, which may give rise to more uncrossed corticospinal tract fibers than M1 (Dum and Strick, 1996, McNeal, et al., 2010), and thereby contribute more to ipsilateral upper limb movement control. Thus, the greater initial ipsilesional hand motor impairments observed with larger lesions may be due primarily to greater involvement of motor areas involved in controlling the ipsilateral upper limb (Callaert, et al., Solodkin, et al., 2001). However, previous work that has examined the effects of large permanent lesions of M2 (that also may have involved the caudal part of the pre-SMA) and/or LPMC without M1 injury and have reported only short-duration deficits in unimanual movements by both upper limbs in macaques (Brinkman, 1981, Brinkman, 1984). Earlier studies also reported that unilateral lesions limited to premotor areas of macaques and humans did not cause paresis of either upper limb (Penfield and Welch, 1951, Travis, 1955), although Travis observed mild bilateral hypertonia at the shoulders that persisted for up to 6 months (Travis, 1955). Moreover, some work has suggested that all the major cortical motor areas participate in ipsilateral, unimanual and bimanual motor actions (for a review – see (Carson, 2005)). From an anatomical standpoint, primary motor cortex also has extensive ipsilateral and bilateral connections in the cervical spinal cord that could permit control of ipsilateral arm movement (Rosenzweig, et al., 2009). Indeed, one of our cases with a lesion limited to M1 exhibited some post-lesion deficits in hand motor skill in the first 5 post-lesion weeks (Table 1). Thus, it is possible that the greater initial deficits associated with larger lesions found in our study are a consequence of the combined loss of M1 and premotor areas.
The hypothesis that ipsilesional hand motor function would recover to pre-lesion levels or higher with continued practice of the tasks was clearly supported as all monkeys showed higher manipulation skill in at least one of the tasks and most animals exhibited higher reach skill on the mDB task. As stated above, we attribute these improvements to the continued practice with high motivation to perform the task quickly and efficiently, along with potential changes in contralesional motor cortex function as discussed below. However, the hypothesis that post-lesion skill changes would be inversely correlated with lesion volume was not supported (Table 3). These findings are in contrast to a recent report that long-term changes in ipsilesional fine motor performance were inversely correlated with lesion volume to M1 and S1 (Kaeser, et al., 2010). However, as mentioned above, this previously reported finding was primarily related to improvements in sequence strategy (i.e., the order in which pellets were picked up sequentially from a larger number of wells rather than manipulation skill).
One potential mechanism underlying the attainment of higher ipsilesional skill is reduction of trans-callosal interhemispheric inhibition (TCI) from the damaged hemisphere (Ferbert, et al., 1992, Liepert, et al., 2000, Netz, et al., 1995). This reduction in TCI would be expected to increase excitability of the undamaged hemisphere, which primarily influences movements of the ipsilesional upper limb in macaque monkeys and higher primates. In humans, it has recently been shown that stronger TCI of the motor cortex is exerted by the dominant hemisphere, especially for intrinsic hand muscles (Duque, et al., 2007). Since in our study the motor cortex contralateral to the preferred hand was damaged, removal of TCI from the undamaged hemisphere may promote enhanced control over muscles of the ipsilesional hand. Thus, improved ipsilesional hand motor performance in the present work may be due to better control of hand/digit muscles exerted by the undamaged motor cortex due to its higher excitability. We tested this hypothesis by examining whether monkeys with strong pre-lesion hand preference would exhibit greater ipsilesional hand skill improvement after the lesion because there may be a greater reduction in TCI. Three monkeys with strong hand preferences had greater post-lesion skill improvements than 7 monkeys with weak hand preferences in the difficult mDB 2nd well task but there were no differences between monkeys with strong versus weak hand preference in the mDB best well and mMAP curved rod tasks. This conclusion must be viewed as preliminary, however, due to the small number of monkeys with high hand preference. Also, reduced TCI is unlikely to be the only mechanism underlying the increase in ipsilesional hand motor skill.
It is important to note that transcallosal fibers can also be excitatory to the opposite hemisphere. For example, some studies have reported “motor irradiation” or activation of homologous muscles of the other hand when performing various types of contractions in healthy adults (Aranyi and Rosler, 2002, Shinohara, et al., 2003). Thus, after unilateral motor cortex damage the contralesional hemisphere loses some excitation as well as inhibition due to (Carson, 2005). However, the balance of transcallosal output from motor cortex appears inhibitory, especially to homologous muscles that are active in a task (Hinder, et al., 2010). Moreover, it has been shown that inhibitory rTMS to the motor cortex of one hemisphere can improve motor performance of the ipsilateral digits, presumably by reducing transcallosal inhibition to the motor cortex contralateral to the performing hand (Kobayashi, et al., 2004). The reduced TCI appears to be a viable mechanism to explain improved ipsilesional hand function following unilateral motor cortex damage. However, further experiments designed to examine the cellular mechanisms involved in transcallosal interactions are required to unravel the precise contribution of transcallosal inhibition in the non-human primate model.
A second potential mechanism supporting ipsilesional recovery is corticospinal axon sprouting to reinnervate spinal neurons controlling the ipsilesional hand that were partially denervated by the lesion (i.e., from the loss of uncrossed corticospinal tract fibers from lesioned M1 and LPMC). Such reinnervation could potentially arise from either uncrossed (ipsilateral) corticospinal projections originating from the spared motor areas in the damaged hemisphere, or from crossed (contralateral) corticospinal projections originating from motor cortex in the undamaged hemisphere. The former mechanism is at least in part, unlikely under the current lesion scenario given that our previous work has shown that uncrossed corticospinal projections originating from spared M2 in the damaged hemisphere do not proliferate in terms of terminal bouton density, although the crossed corticospinal projection does selectively increase connectivity to spinal neurons primarily controlling the contralesional hand (McNeal, et al., 2010). However, much of the contralateral sprouting from M2 was in lamina VII which in part, contains commissural neurons that are exclusively excitatory and target spinal premotor interneurons on the opposite side (Bannatyne, et al., 2009, Jankowska, et al., 2009). Thus, it is possible that this contralateral projection may indirectly contribute to improved motor performance in the ipsilesional hand through a spinal commissural circuit. Unmasking of existing ineffective synapses on neurons controlling ipsilesional hand movement is also a potential mechanism for recovery of ipsilesional hand function (Chen, et al., 2002). As mentioned above, it is also possible that crossed (contralateral) corticospinal projections originating from motor cortex in the undamaged hemisphere reorganize in some fashion to affect ipsilesional hand motor function, but this subject will have to be addressed in future experiments specifically designed to examine this issue. Reorganization of connections from subcortical nuclei such as the red nucleus and reticular formation onto spinal motor neurons or interneurons represent additional possible explanations. For example, previous work has shown that the distribution of red nucleus excitatory and inhibitory outputs during ICMS on wrist and extrinsic digit muscles following pyramidal tract lesions are substantially different from controls (Belhaj-Saif and Cheney, 2000). Specifically, controls exhibited much stronger facilitation to upper limb extensors than flexors in red nucleus output but after a pyramidal tract lesion there was reduced facilitation to extensors and increased facilitation to flexors resulting in more balanced excitation associated with recovery of fine hand motor performance.
It is also possible that nearly exclusive use of the ipsilesional hand and digits for fine motor tasks during the first few weeks after the lesion drives neuroplastic responses that support enhanced control of muscles in the ipsilesional limb. Previous work in rats showed that induced ischemic stroke causes enhanced skill of ipsilesional limb reaching that is associated with increases in the total number of motor cortical layer V synapses per neuron in the undamaged hemisphere. Moreover, they observed increases in the types of synapses that are linked to enhanced synaptic efficacy (i.e., perforated and multisynaptic boutons) (Luke, et al., 2004). Indeed, reach training with the ipsilesional limb actually caused poorer recovery of the contralesional limb in rats (Allred and Jones, 2007). However, there was no indication in the 10 monkeys studied in the present work that ipsilesional fine motor testing and/or hand use in the cage had detrimental effects on recovery of the contralesional hand, which improved to better than pre-lesion skill in some monkeys (Darling, et al., 2009). However, recovery of reach and manipulation skill in the ipsilesional hand was poorly correlated with recovery of skill in the contralesional hand in the monkeys studied here (R2 < 0.17, p > 0.05). This finding contrasts with recent reports of a high positive correlation in recovery of fine motor performance in the ipsilesional and contralesional hands in macaques (Kaeser, et al., 2010) and the negative effects of ipsilesional forepaw training in rats (Allred and Jones, 2007). However, the monkeys in the present work received post-lesion testing at most once per week post-lesion whereas the previous studies used daily training/testing in rats and monkeys. Thus, the poor correlation between ipsilesional and contralesional fine motor recovery observed here may be related to the lack of daily training/testing sessions. That is, daily training may reinforce learned nonuse and poor recovery of the contralesional limb if confined to the ipsilesional hand (Allred and Jones, 2007) or promote recovery (and enhanced performance) of both hands in a correlated manner if both hands are trained (Kaeser, et al., 2010).
In conclusion, the present work shows clearly that unilateral damage to the three major motor areas of the frontal lobe, and also to cingulate/prefrontal regions in one monkey, does not cause long-term impairments in skilled use of the ipsilesional upper extremity. Indeed, despite relatively large brain lesions affecting all the major cortical motor areas, a relatively small amount of continued practice of fine hand motor tasks (i.e., weekly or every other week) causes continued improvement of skill beyond the highest levels observed before the lesion. Specific improvements found in our study included decreased reach and manipulation duration and decreased force exertion during manipulation of food targets by the ipsilesional hand. These findings, together with the non-human primate observations of Kaeser and colleagues (Kaeser, et al., 2010), contrast with the long-lasting impairments in control of ipsilesional upper limb reaching movements and gripping by the index and thumb observed in humans following unilateral stroke. (Haaland, et al., 2009, Schaefer, et al., 2007, Schaefer, et al., 2009, Seo, et al., 2009, Seo, et al., 2010). Notably, the ipsilesional reaching deficits in humans are correlated with the level of paresis of the contralesional limb and differ according to which cerebral hemisphere is damaged (Haaland, et al., 2009, Schaefer, et al., 2007, Schaefer, et al., 2009). It is possible that the lack of strong hand preference and, presumably, lack of cerebral dominance in macaque monkeys allows for much better recovery of the ipsilesional hand than in humans. That is, even in monkeys that exhibited a strong hand preference in our study using the dexterity board, it is likely that hemispheric dominance and hand preference are weaker than suggested by the handedness index because previous work has shown that hand preference in macaques can vary in different tasks (Lehman, 1980, Lehman, 1989). It is also likely that more substantial subcortical injury, which is typical in stroke patients and commonly involves subcortical gray matter structures, contributes to the more persistent ipsilesional upper limb impairments noted in the patient studies. Although speculative at the present time, our findings indicate that patients with more localized cortical injury that is limited to the pericentral region or caudal frontal region, may attain remarkable levels of ipsilesional arm/hand motor recovery given appropriate therapeutic intervention. Finally, as we have shown for the contralesional hand (Darling et al., 2009), neurosurgical resection of frontal motor cortex for the treatment of peri-Rolandic tumors, epileptogenic foci, or A-V malformations that concomitantly involves subjacent white matter is likely to be more devastating to recovery of ipsilesional hand movements than resection that is limited to gray mater.
Research Highlights.
lesions of frontal lobe motor areas in Rhesus monkeys produce acute deficits in control of ipsilesional dexterous hand movements
ipsilesional hand movement deficits do not persist beyond a few weeks post-lesion
recovery allows the ipsilesional hand to perform better than in pre-lesion fine motor tests
reduced transcallosal inhibition from the damaged motor cortex likely contributes to the improved ipsilesional fine motor skill
Acknowledgments
Supported by National Institutes of Health grants NS 046367 and The South Dakota Spinal Cord Injury and Traumatic Brain Injury Research Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al-Senawi D, Cooke JD. Matching of Movements Made Independently by the Two Arms in Normal Humans. Journal of Motor Behavior. 1985;17:321–334. doi: 10.1080/00222895.1985.10735352. [DOI] [PubMed] [Google Scholar]
- 2.Allred RP, Jones TA. Maladaptive effects of learning with the less-affected forelimb after focal cortical infarcts in rats. Exp Neurol. 2007 doi: 10.1016/j.expneurol.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aranyi Z, Rosler KM. Effort-induced mirror movementsA study of transcallosal inhibition in humans. Exp Brain Res. 2002;145:76–82. doi: 10.1007/s00221-002-1101-1. [DOI] [PubMed] [Google Scholar]
- 4.Bannatyne BA, Liu TT, Hammar I, Stecina K, Jankowska E, Maxwell DJ. Excitatory and inhibitory intermediate zone interneurons in pathways from feline group I and II afferents: differences in axonal projections and input. J Physiol. 2009;587:379–399. doi: 10.1113/jphysiol.2008.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbas H, Pandya DN. Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol. 1987;256:211–228. doi: 10.1002/cne.902560203. [DOI] [PubMed] [Google Scholar]
- 6.Belhaj-Saif A, Cheney PD. Plasticity in the distribution of the red nucleus output to forearm muscles after unilateral lesions of the pyramidal tract. J Neurophysiol. 2000;83:3147–3153. doi: 10.1152/jn.2000.83.5.3147. [DOI] [PubMed] [Google Scholar]
- 7.Black P, Markowitz RS, Cianci SN. Recovery of motor function after lesions in motor cortex of monkey. Ciba Found Symp. 1975:65–83. doi: 10.1002/9780470720165.ch5. [DOI] [PubMed] [Google Scholar]
- 8.Brinkman C. Lesions in supplementary motor area interfere with a monkey's performance of a bimanual coordination task. Neurosci Lett. 1981;27:267–270. doi: 10.1016/0304-3940(81)90441-9. [DOI] [PubMed] [Google Scholar]
- 9.Brinkman C. Supplementary motor area of the monkey's cerebral cortex: short- and long-term deficits after unilateral ablation and the effects of subsequent callosal section. J Neurosci. 1984;4:918–929. doi: 10.1523/JNEUROSCI.04-04-00918.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinkman C, Porter R. Supplementary motor area in the monkey: activity of neurons during performance of a learned motor task. J Neurophysiol. 1979;42:681–709. doi: 10.1152/jn.1979.42.3.681. [DOI] [PubMed] [Google Scholar]
- 11.Bury SD, Jones TA. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the “unaffected” forelimb and training-induced dendritic structural plasticity in the motor cortex. J Neurosci. 2002;22:8597–8606. doi: 10.1523/JNEUROSCI.22-19-08597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callaert DV, Vercauteren K, Peeters R, Tam F, Graham S, Swinnen SP, Sunaert S, Wenderoth N. Hemispheric asymmetries of motor versus nonmotor processes during (visuo)motor control. Hum Brain Mapp. 2010 doi: 10.1002/hbm.21110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S. Growth-associated gene expression after stroke: evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol. 2005;193:291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Carson RG. Neural pathways mediating bilateral interactions between the upper limbs. Brain Res Brain Res Rev. 2005;49:641–662. doi: 10.1016/j.brainresrev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111:761–773. doi: 10.1016/s0306-4522(02)00025-8. [DOI] [PubMed] [Google Scholar]
- 16.Cisek P, Crammond DJ, Kalaska JF. Neural activity in primary motor and dorsal premotor cortex in reaching tasks with the contralateral versus ipsilateral arm. J Neurophysiol. 2003;89:922–942. doi: 10.1152/jn.00607.2002. [DOI] [PubMed] [Google Scholar]
- 17.Colebatch JG, Gandevia SC. The distribution of muscular weakness in upper motor neuron lesions affecting the arm. Brain. 1989;112(Pt 3):749–763. doi: 10.1093/brain/112.3.749. [DOI] [PubMed] [Google Scholar]
- 18.Darling WG, Peterson CR, Herrick JL, McNeal DW, Stilwell-Morecraft KS, Morecraft RJ. Measurement of coordination of object manipulation in non-human primates. Journal of Neuroscience Methods. 2006;154:38–44. doi: 10.1016/j.jneumeth.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Darling WG, Pizzimenti MA, Rotella DL, Hynes SM, Ge J, Stilwell-Morecraft KS, Vanadurongvan T, McNeal DW, Solon-Cline KM, Morecraft RJ. Minimal forced use without constraint stimulates spontaneous use of the impaired upper extremity following motor cortex injury. Exp Brain Res. 2010;202:529–542. doi: 10.1007/s00221-010-2157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darling WG, Pizzimenti MA, Rotella DL, Peterson CR, Hynes SM, Ge J, Solon K, McNeal DW, Stilwell-Morecraft KS, Morecraft RJ. Volumetric effects of motor cortex injury on recovery of dexterous movements. Exp Neurol. 2009;220:90–108. doi: 10.1016/j.expneurol.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davare M, Duque J, Vandermeeren Y, Thonnard JL, Olivier E. Role of the ipsilateral primary motor cortex in controlling the timing of hand muscle recruitment. Cereb Cortex. 2007;17:353–362. doi: 10.1093/cercor/bhj152. [DOI] [PubMed] [Google Scholar]
- 22.Denny-Brown D, Botterell EH. The motor functions of agranular frontal cortex. Research Publications - Association for Research in Nervous and Mental Disease. 1948;27:235–345. [PubMed] [Google Scholar]
- 23.Desrosiers J, Bourbonnais D, Bravo G, Roy PM, Guay M. Performance of the ‘unaffected’ upper extremity of elderly stroke patients. Stroke. 1996;27:1564–1570. doi: 10.1161/01.str.27.9.1564. [DOI] [PubMed] [Google Scholar]
- 24.Donchin O, Gribova A, Steinberg O, Bergman H, Vaadia E. Primary motor cortex is involved in bimanual coordination. Nature. 1998;395:274–278. doi: 10.1038/26220. [DOI] [PubMed] [Google Scholar]
- 25.Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. Journal of Neuroscience. 1996;16:6513–6525. doi: 10.1523/JNEUROSCI.16-20-06513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duque J, Murase N, Celnik P, Hummel F, Harris-Love M, Mazzocchio R, Olivier E, Cohen LG. Intermanual differences in movement-related interhemispheric inhibition. J Cogn Neurosci. 2007;19:204–213. doi: 10.1162/jocn.2007.19.2.204. [DOI] [PubMed] [Google Scholar]
- 27.Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganguly K, Secundo L, Ranade G, Orsborn A, Chang EF, Dimitrov DF, Wallis JD, Barbaro NM, Knight RT, Carmena JM. Cortical Representation of Ipsilateral Arm Movements in Monkey and Man. J Neurosci. 2009;29:12948–12956. doi: 10.1523/JNEUROSCI.2471-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao H, Liu Y, Lu S, Xiang B, Wang C. A reversible middle cerebral artery occlusion model using intraluminal balloon technique in monkeys. J Stroke Cerebrovasc Dis. 2006;15:202–208. doi: 10.1016/j.jstrokecerebrovasdis.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez CL, Gharbawie OA, Williams PT, Kleim JA, Kolb B, Whishaw IQ. Evidence for bilateral control of skilled movements: ipsilateral skilled forelimb reaching deficits and functional recovery in rats follow motor cortex and lateral frontal cortex lesions. Eur J Neurosci. 2004;20:3442–3452. doi: 10.1111/j.1460-9568.2004.03751.x. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez CL, Kolb B. A comparison of different models of stroke on behaviour and brain morphology. European Journal of Neuroscience. 2003;18:1950–1962. doi: 10.1046/j.1460-9568.2003.02928.x. [DOI] [PubMed] [Google Scholar]
- 32.Haaland KY, Schaefer SY, Knight RT, Adair J, Magalhaes A, Sadek J, Sainburg RL. Ipsilesional trajectory control is related to contralesional arm paralysis after left hemisphere damage. Exp Brain Res. 2009;196:195–204. doi: 10.1007/s00221-009-1836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hermsdorfer J, Ulrich S, Marquardt C, Goldenberg G, Mai N. Prehension with the ipsilesional hand after unilateral brain damage. Cortex. 1999;35:139–161. doi: 10.1016/s0010-9452(08)70791-3. [DOI] [PubMed] [Google Scholar]
- 34.Hinder MR, Schmidt MW, Garry MI, Summers JJ. Unilateral contractions modulate interhemispheric inhibition most strongly and most adaptively in the homologous muscle of the contralateral limb. Exp Brain Res. 2010;205:423–433. doi: 10.1007/s00221-010-2379-z. [DOI] [PubMed] [Google Scholar]
- 35.Jankowska E, Bannatyne BA, Stecina K, Hammar I, Cabaj A, Maxwell DJ. Commissural interneurons with input from group I and II muscle afferents in feline lumbar segments: neurotransmitters, projections and target cells. J Physiol. 2009;587:401–418. doi: 10.1113/jphysiol.2008.159236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones RD, Donaldson IM, Parkin PJ. Impairment and recovery of ipsilateral sensory-motor function following unilateral cerebral infarction. Brain. 1989;112(Pt 1):113–132. doi: 10.1093/brain/112.1.113. [DOI] [PubMed] [Google Scholar]
- 37.Kaeser M, Wyss AF, Bashir S, Hamadjida A, Liu Y, Bloch J, Brunet JF, Belhaj-Saif A, Rouiller EM. Effects of Unilateral Motor Cortex Lesion on Ipsilesional Hand's Reach and Grasp Performance in Monkeys: Relationship With Recovery in the Contralesional Hand. J Neurophysiol. 2010;103:1630–1645. doi: 10.1152/jn.00459.2009. [DOI] [PubMed] [Google Scholar]
- 38.Kermadi I, Liu Y, Tempini A, Rouiller EM. Effects of reversible inactivation of the supplementary motor area (SMA) on unimanual grasp and bimanual pull and grasp performance in monkeys. Somatosens Mot Res. 1997;14:268–280. doi: 10.1080/08990229770980. [DOI] [PubMed] [Google Scholar]
- 39.Kim SH, Pohl PS, Luchies CW, Stylianou AP, Won Y. Ipsilateral deficits of targeted movements after stroke. Arch Phys Med Rehabil. 2003;84:719–724. doi: 10.1016/s0003-9993(02)04973-0. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi M, Hutchinson S, Theoret H, Schlaug G, Pascual-Leone A. Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology. 2004;62:91–98. doi: 10.1212/wnl.62.1.91. [DOI] [PubMed] [Google Scholar]
- 41.Laufer Y, Gattenio L, Parnas E, Sinai D, Sorek Y, Dickstein R. Time-related changes in motor performance of the upper extremity ipsilateral to the side of the lesion in stroke survivors. Neurorehabil Neural Repair. 2001;15:167–172. doi: 10.1177/154596830101500303. [DOI] [PubMed] [Google Scholar]
- 42.Lehman RA. The handedness of rhesus monkeys. III. Consistency within and across activities. Cortex. 1980;16:197–204. doi: 10.1016/s0010-9452(80)80055-4. [DOI] [PubMed] [Google Scholar]
- 43.Lehman RA. Hand preferences of rhesus monkeys on differing tasks. Neuropsychologia. 1989;27:1193–1196. doi: 10.1016/0028-3932(89)90102-4. [DOI] [PubMed] [Google Scholar]
- 44.Lewis GN, Perreault EJ. Side of lesion influences interhemispheric inhibition in subjects with post-stroke hemiparesis. Clin Neurophysiol. 2007;118:2656–2663. doi: 10.1016/j.clinph.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liepert J, Hamzei F, Weiller C. Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle & Nerve. 2000;23:1761–1763. doi: 10.1002/1097-4598(200011)23:11<1761::aid-mus14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Rouiller EM. Mechanisms of recovery of dexterity following unilateral lesion of the sensorimotor cortex in adult monkeys. Exp Brain Res. 1999;128:149–159. doi: 10.1007/s002210050830. [DOI] [PubMed] [Google Scholar]
- 47.Luke LM, Allred RP, Jones TA. Unilateral ischemic sensorimotor cortical damage induces contralesional synaptogenesis and enhances skilled reaching with the ipsilateral forelimb in adult male rats. Synapse. 2004;54:187–199. doi: 10.1002/syn.20080. [DOI] [PubMed] [Google Scholar]
- 48.Marque P, Felez A, Puel M, Demonet JF, Guiraud-Chaumeil B, Roques CF, Chollet F. Impairment and recovery of left motor function in patients with right hemiplegia. J Neurol Neurosurg Psychiatry. 1997;62:77–81. doi: 10.1136/jnnp.62.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marshall JW, Ridley RM, Baker HF, Hall LD, Carpenter TA, Wood NI. Serial MRI, functional recovery, and long-term infarct maturation in a non-human primate model of stroke. Brain Res Bull. 2003;61:577–585. doi: 10.1016/s0361-9230(03)00214-4. [DOI] [PubMed] [Google Scholar]
- 50.McNeal DW, Darling WG, Ge J, Stilwell-Morecraft KS, Solon KM, Hynes SM, Pizzimenti MA, Rotella DL, Vanadurongvan T, Morecraft RJ. Selective long-term reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury. J Comp Neurol. 2010;518:586–621. doi: 10.1002/cne.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morecraft RJ, Cipolloni PB, Stilwell-Morecraft KS, Gedney MT, Pandya DN. Cytoarchitecture and cortical connections of the posterior cingulate and adjacent somatosensory fields in the rhesus monkey. Journal of Comparative Neurology. 2004;469:37–69. doi: 10.1002/cne.10980. [DOI] [PubMed] [Google Scholar]
- 52.Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol. 1992;323:341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- 53.Morecraft RJ, Herrick JL, Stilwell-Morecraft KS, Louie JL, Schroeder CM, Ottenbacher JG, Schoolfield MW. Localization of arm representation in the corona radiata and internal capsule in the non-human primate. Brain. 2002;125:176–198. doi: 10.1093/brain/awf011. [DOI] [PubMed] [Google Scholar]
- 54.Morecraft RJ, Louie JL, Herrick JL, Stilwell-Morecraft KS. Cortical innervation of the facial nucleus in the non-human primate: a new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain. 2001;124:176–208. doi: 10.1093/brain/124.1.176. [DOI] [PubMed] [Google Scholar]
- 55.Morecraft RJ, McNeal DW, Stilwell-Morecraft KS, Gedney M, Ge J, Schroeder CM, Van Hoesen GW. Amygdala interconnections with the cingulate motor cortex in the rhesus monkey. J Comp Neurol. 2007;500:134–165. doi: 10.1002/cne.21165. [DOI] [PubMed] [Google Scholar]
- 56.Nagamoto-Combs K, McNeal DW, Morecraft RJ, Combs CK. Prolonged microgliosis in the rhesus monkey central nervous system after traumatic brain injury. J Neurotrauma. 2007;24:1719–1742. doi: 10.1089/neu.2007.0377. [DOI] [PubMed] [Google Scholar]
- 57.Nagamoto-Combs K, Morecraft RJ, Darling WG, Combs CK. Long-term gliosis and molecular changes in the cervical spinal cord of the rhesus monkey after traumatic brain injury. J Neurotrauma. 2010;27:565–585. doi: 10.1089/neu.2009.0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Netz J, Ziemann U, Homberg V. Hemispheric asymmetry of transcallosal inhibition in man. Experimental Brain Research. 1995;104:527–533. doi: 10.1007/BF00231987. [DOI] [PubMed] [Google Scholar]
- 59.Noskin O, Krakauer JW, Lazar RM, Festa JR, Handy C, O'Brien KA, Marshall RS. Ipsilateral motor dysfunction from unilateral stroke: implications for the functional neuroanatomy of hemiparesis. J Neurol Neurosurg Psychiatry. 2008;79:401–406. doi: 10.1136/jnnp.2007.118463. [DOI] [PubMed] [Google Scholar]
- 60.Nudo R, Jenkins W, Merzenich M, Prejean T, Grenda R. Neurophysiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. J Neurosci. 1992;12:2918–2947. doi: 10.1523/JNEUROSCI.12-08-02918.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 62.O'Bryant A, Bernier B, Jones TA. Abnormalities in skilled reaching movements are improved by peripheral anesthetization of the less-affected forelimb after sensorimotor cortical infarcts in rats. Behav Brain Res. 2007;177:298–307. doi: 10.1016/j.bbr.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Penfield W, Welch K. The supplementary motor area of the cerebral cortex; a clinical and experimental study. AMA Arch Neurol Psychiatry. 1951;66:289–317. doi: 10.1001/archneurpsyc.1951.02320090038004. [DOI] [PubMed] [Google Scholar]
- 64.Pizzimenti MA, Darling WG, Rotella DL, McNeal DW, Herrick JL, Ge J, Stilwell-Morecraft KS, Morecraft RJ. Measurement of reaching kinematics and prehensile dexterity in nonhuman primates. J Neurophysiol. 2007;98:1015–1029. doi: 10.1152/jn.00354.2007. [DOI] [PubMed] [Google Scholar]
- 65.Pohl PS, Filion DL, Kim SH. Effects of practice and unpredictable distractors on planning and executing aiming after stroke. Neurorehabil Neural Repair. 2003;17:93–100. doi: 10.1177/0888439003017002003. [DOI] [PubMed] [Google Scholar]
- 66.Roitberg B, Khan N, Tuccar E, Kompoliti K, Chu Y, Alperin N, Kordower JH, Emborg ME. Chronic ischemic stroke model in cynomolgus monkeys: behavioral, neuroimaging and anatomical study. Neurol Res. 2003;25:68–78. doi: 10.1179/016164103101200950. [DOI] [PubMed] [Google Scholar]
- 67.Rosenzweig ES, Brock JH, Culbertson MD, Lu P, Moseanko R, Edgerton VR, Havton LA, Tuszynski MH. Extensive spinal decussation and bilateral termination of cervical corticospinal projections in rhesus monkeys. J Comp Neurol. 2009;513:151–163. doi: 10.1002/cne.21940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schaefer SY, Haaland KY, Sainburg RL. Ipsilesional motor deficits following stroke reflect hemispheric specializations for movement control. Brain. 2007;130:2146–2158. doi: 10.1093/brain/awm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schaefer SY, Haaland KY, Sainburg RL. Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia. 2009;47:2953–2966. doi: 10.1016/j.neuropsychologia.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmahmann JD, Pandya DN. Fiber pathways of the brain. Oxford University Press; New York: 2006. [Google Scholar]
- 71.Seo NJ, Rymer WZ, Kamper DG. Delays in Grip Initiation and Termination in Persons With Stroke: Effects of Arm Support and Active Muscle Stretch Exercise. J Neurophysiol. 2009;101:3108–3115. doi: 10.1152/jn.91108.2008. [DOI] [PubMed] [Google Scholar]
- 72.Seo NJ, Rymer WZ, Kamper DG. Altered digit force direction during pinch grip following stroke. Exp Brain Res. 2010;202:891–901. doi: 10.1007/s00221-010-2193-7. [DOI] [PubMed] [Google Scholar]
- 73.Shinohara M, Keenan KG, Enoka RM. Contralateral activity in a homologous hand muscle during voluntary contractions is greater in old adults. J Appl Physiol. 2003;94:966–974. doi: 10.1152/japplphysiol.00836.2002. [DOI] [PubMed] [Google Scholar]
- 74.Solodkin A, Hlustik P, Noll DC, Small SL. Lateralization of motor circuits and handedness during finger movements. Eur J Neurol. 2001;8:425–434. doi: 10.1046/j.1468-1331.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- 75.Travis AM. Neurological deficiencies after ablation of the precentral motor area in Macaca mulatta. Brain. 1955;78:155–173. doi: 10.1093/brain/78.2.155. [DOI] [PubMed] [Google Scholar]
- 76.Travis AM. Neurological deficiencies following supplementary motor area lesions in Macaca mulatta. Brain. 1955;78:174–198. doi: 10.1093/brain/78.2.174. [DOI] [PubMed] [Google Scholar]
- 77.Wetter S, Poole JL, Haaland KY. Functional implications of ipsilesional motor deficits after unilateral stroke. Arch Phys Med Rehabil. 2005;86:776–781. doi: 10.1016/j.apmr.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 78.Yarosh CA, Hoffman DS, Strick PL. Deficits in movements of the wrist ipsilateral to a stroke in hemiparetic subjects. J Neurophysiol. 2004;92:3276–3285. doi: 10.1152/jn.00549.2004. [DOI] [PubMed] [Google Scholar]
- 79.Yelnik A, Bonan I, Debray M, Lo E, Gelbert F, Bussel B. Changes in the execution of a complex manual task after ipsilateral ischemic cerebral hemispheric stroke. Arch Phys Med Rehabil. 1996;77:806–810. doi: 10.1016/s0003-9993(96)90261-0. [DOI] [PubMed] [Google Scholar]