The mitochondrial import receptor Tom70 and outer membrane protein Mim1 regulate recognition and insertion of the multispan protein Ugo1.
Abstract
The mitochondrial outer membrane (MOM) harbors several multispan proteins that execute various functions. Despite their importance, the mechanisms by which these proteins are recognized and inserted into the outer membrane remain largely unclear. In this paper, we address this issue using yeast mitochondria and the multispan protein Ugo1. Using a specific insertion assay and analysis by native gel electrophoresis, we show that the import receptor Tom70, but not its partner Tom20, is involved in the initial recognition of the Ugo1 precursor. Surprisingly, the import pore formed by the translocase of the outer membrane complex appears not to be required for the insertion process. Conversely, the multifunctional outer membrane protein mitochondrial import 1 (Mim1) plays a central role in mediating the insertion of Ugo1. Collectively, these results suggest that Ugo1 is inserted into the MOM by a novel pathway in which Tom70 and Mim1 contribute to the efficiency and selectivity of the process.
Introduction
The mitochondrial outer membrane (MOM) contains a diverse set of proteins with various functions (Burri et al., 2006; Schmitt et al., 2006; Zahedi et al., 2006). All of these proteins, like the vast majority of mitochondrial proteins, are nuclear encoded, synthesized in the cytosol, and imported into the organelle (Neupert and Herrmann, 2007; Chacinska et al., 2009; Endo and Yamano, 2009; Walther and Rapaport, 2009). Multispan proteins comprise a distinct class of MOM proteins and are integrated into the lipid bilayer via multiple transmembrane segments (TMSs). Some of them, like Fzo1 in yeast (Mfn1/2 in mammals), cross the membrane twice, exposing N- and C-terminal domains toward the cytosol (Fritz et al., 2001; Rojo et al., 2002). Additional multispan MOM proteins with three or more TMSs are Ugo1 and OM14 in yeast and members of the Bcl-2 family and human peripheral benzodiazepine receptor (PBR) in higher eukaryotes (Burri et al., 2006; Coonrod et al., 2007; Otera et al., 2007; Hoppins et al., 2009; Chipuk et al., 2010). Previous research using mutants of Ugo1 and Mfn2 revealed that the domain that includes the predicted TMS harbors the information necessary for mitochondrial targeting, although additional targeting signals in other regions of the protein could not be excluded (Rojo et al., 2002; Coonrod et al., 2007). Experiments with Mfn2 indicate similarities between polytopic and tail-anchored (TA) proteins in terms of motifs and mechanisms responsible for their insertion into MOM (Rojo et al., 2002).
The idea that import pathways of TA and multispan proteins overlap (at least partially) is supported by import competition assays in which import of PBR was strongly inhibited by an excess amount of the TA protein Bak (Otera et al., 2007). However, in contrast to the biogenesis of TA proteins in which import receptors are probably not essential for the process (Setoguchi et al., 2006; Kemper et al., 2008), such receptors appear to play a role in the membrane integration of multispan proteins. Fzo1 requires protease-sensitive import receptors for its insertion into the MOM (Rapaport et al., 1998), and later investigations revealed that import of PBR and Mfn2 requires interaction with Tom70 but is independent of other translocase of the outer membrane (TOM) components (Otera et al., 2007; Yamano et al., 2008).
Despite the aforementioned progress, the mechanisms by which newly synthesized multispan proteins are recognized at the organelle surface and inserted into the MOM are still largely unresolved. It is especially unclear whether dedicated membrane insertion machinery for such proteins exists. To address these questions, we studied the membrane integration of the model multispan protein Ugo1. Our results suggest a novel insertion pathway in which the mitochondrial import receptor Tom70 and the outer membrane protein mitochondrial import 1 (Mim1) regulate recognition and insertion of Ugo1.
Results and discussion
A specific assay to monitor the in vitro insertion of Ugo1
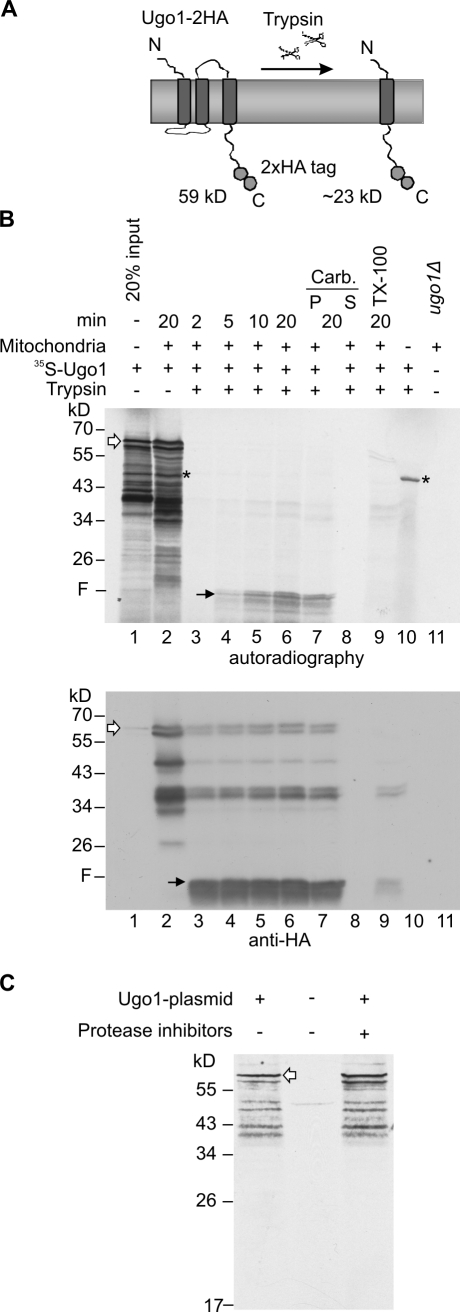
A long-lasting problem in analyzing the integration of multispan proteins is the lack of a reliable assay for correct membrane integration. To overcome this problem, we developed a proteolytic assay based on previous experiments suggesting Ugo1 to have at least three TMSs (Coonrod et al., 2007; Hoppins et al., 2009). These earlier observations and our current results indicate that the addition of trypsin to mitochondria containing C-terminally HA-tagged Ugo1 resulted in the formation of a 23-kD C-terminal fragment probably as a result of a proteolytic cleavage site between putative TMS 2 and 3 (Fig. 1 A). Of note, C-terminally HA-tagged Ugo1 is fully functional and thus has a nativelike topology (Hoppins et al., 2009).
Figure 1.
A novel assay to study in vitro import of Ugo1. (A) A schematic representation of 2HA-tagged Ugo1 (Ugo1-2HA) and Ugo1-2HA C-terminal 23-kD fragment protected from trypsin activity. The scissors represent the protease trypsin. (B) A proteolytic fragment of 23 kD is formed upon the correct insertion of Ugo1-2HA. Radiolabeled Ugo1-2HA was incubated for the indicated time periods with mitochondria isolated from ugo1Δ cells expressing plasmid-encoded Ugo1-2HA. Mitochondria were further incubated without (lane 2) or with trypsin (lanes 3–6) and pelleted. An additional sample was analyzed after import and trypsinization by carbonate extraction (Carb.), and pellet (P) and supernatant (S) fractions were loaded (lanes 7 and 8). In one sample, trypsin treatment was performed only after the mitochondria were solubilized with Triton X-100 (lane 9). As a control, 100% of input radiolabeled protein was treated with trypsin in the absence of mitochondria (lane 10). The proteolytic fragment (F) is indicated by black arrows, and full-length Ugo1 is indicated by white arrows. A nonspecific band resulting from preexisting mRNA in the reticulocyte lysate is indicated by the asterisks. In lane 11, mitochondria from the ugo1Δ strain harboring the empty plasmid were loaded as a control for the specificity of the HA antibody. All samples were analyzed by SDS-PAGE followed by autoradiography (top) and then immunodecorated with HA antibody (bottom). (C) The transcription–translation-coupled system was incubated with or without a plasmid encoding Ugo1-HA. In one sample, a commercial protease inhibitor cocktail was added. Samples were analyzed by SDS-PAGE and autoradiography. Full-length Ugo1 is indicated by a white arrow.
To allow for a comparison with the endogenous protein and to check whether the observed proteolytic fragment indeed represents a proper membrane insertion, we isolated mitochondria from ugo1Δ cells expressing plasmid-encoded Ugo1-HA. Next, we incubated these mitochondria with radiolabeled precursors of Ugo1-HA and, upon completion of the import reaction, performed the trypsin treatment. As expected, we observed a time-dependent formation of the anticipated C-terminal fragment in trypsin-treated mitochondria (Fig. 1 B, lanes 4–6, marked with F). This fragment behaved as a membrane-embedded polypeptide, as it remained in the membrane fraction of an alkaline extraction (Fig. 1 B, lanes 7 and 8). Furthermore, it disappeared upon solubilization of the organelle with detergent and was strictly dependent on the presence of mitochondria (Fig. 1 B, lanes 9 and 10). Thus, formation of the tryptic fragment requires an intact MOM and is not caused by aggregation. Immunodecoration of the same membrane with an anti-HA antibody demonstrated that the newly inserted Ugo1 molecules behave identically to preexisting endogenous Ugo1-HA (Fig. 1 B, bottom, lanes 3–9). Of note, the radiolabeled material contains several bands with smaller size that probably represent translation initiation on internal methionine residues (Fig. 1 B, lanes 1–2). We disfavor the possibility that these additional species result from proteolytic digestion of the protein because they were also observed upon synthesis of the protein in the presence of a mixture of protease inhibitors (Fig. 1 C). Collectively, the proteolytic treatment of newly synthesized molecules of Ugo1 provides a specific assay for membrane integration.
Import of Ugo1 depends on Tom70 but not on Tom20
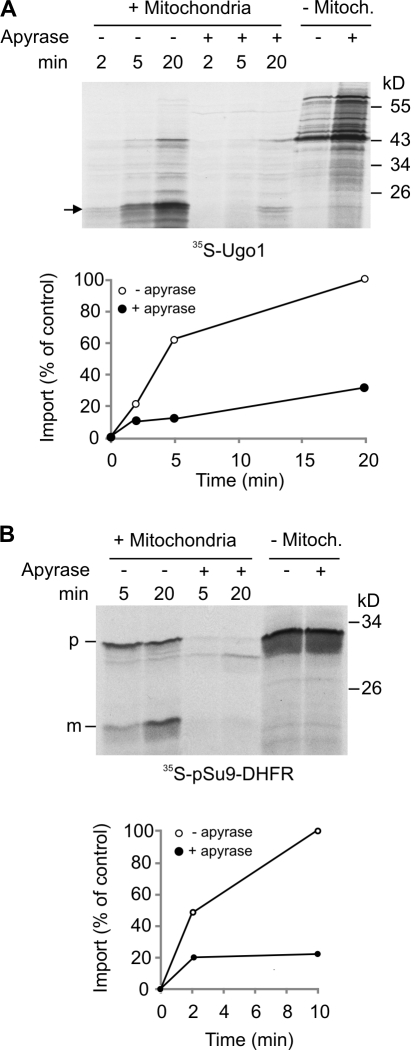
Upon their synthesis in the cytosol, precursors of multispan proteins should be protected from aggregation. This task can be executed by cytosolic chaperones of the Hsp70 and Hsp90 families (Young et al., 2003). Accordingly, an overall dependency on ATP was reported for the import of various mitochondrial membrane proteins like Tom40, PBR, and the ATP–ADP carrier (AAC; Rapaport and Neupert, 1999; Wiedemann et al., 2001; Otera et al., 2007). We examined whether the import of Ugo1 shares this feature. When apyrase, which hydrolyzes ATP, was added to the import reaction, a dramatic reduction in the import of both Ugo1 and the control matrix–destined precursor pSu9–dihydrofolate reductase (DHFR) was observed (Fig. 2, A and B). We excluded the possibility that the apyrase sample contained protease contamination, as no proteolytic fragments were observed when only apyrase was added to the radiolabeled proteins (Fig. 2, A and B, last two lanes). As there are currently no indications that mitochondrial matrix chaperones are involved in the biogenesis of MOM proteins, we assume that the effect of apyrase on matrix ATP is irrelevant for the reduction in Ugo1 membrane integration. We propose that the ATP is required for the release of the hydrophobic multispan precursor proteins from the cytosolic chaperones to which they are associated.
Figure 2.
Import of Ugo1 is strongly compromised by removal of ATP. (A and B) Radiolabeled Ugo1 (A) or pSu9-DHFR (B) was incubated in import buffer with mitochondria for the indicated time periods in the presence or absence of apyrase. As a control, samples without mitochondria (− Mitoch.) were incubated in the presence or absence of apyrase and analyzed directly by SDS-PAGE. At the end of the import reactions, mitochondria were treated with either trypsin (Ugo1 import reactions) or proteinase K (PK; pSu9-DHFR import reactions) and reisolated. Imported proteins were analyzed by SDS-PAGE and autoradiography. The insertion of Ugo1 was quantified by analyzing the formation of the 23-kD fragment (indicated by an arrow), whereas for pSu9-DHFR, the protease-protected mature form (m) was quantified. The amount of proteins imported into untreated mitochondria for the longest time period was set to 100%. An experiment representative of three independent repeats is presented. p, precursor.
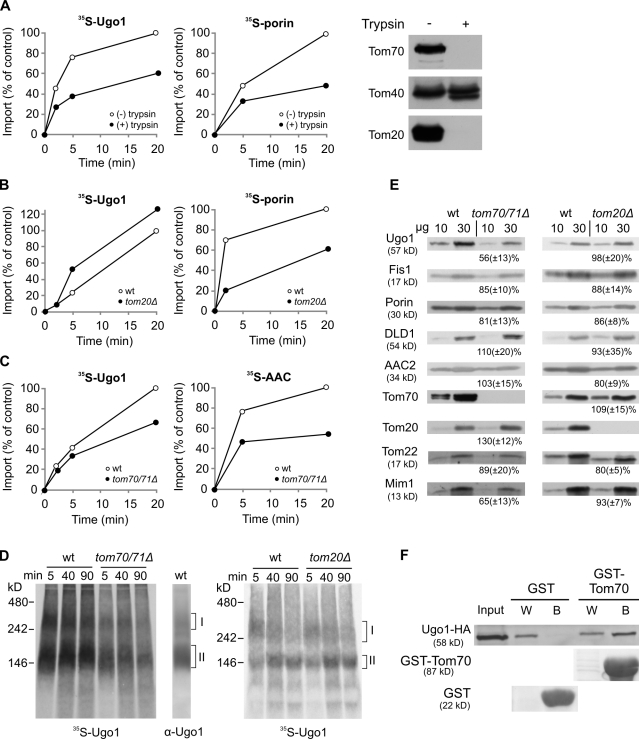
The import of multispan proteins into mitochondria requires their recognition by the organelle. For example, the recognition of β-barrel proteins is mediated mainly by the import receptor Tom20 (Rapaport and Neupert, 1999; Krimmer et al., 2001; Yamano et al., 2008), whereas Tom70 preferentially recognizes precursors of the inner membrane carrier-like proteins and mammalian multispan outer membrane proteins like PBR (Schlossmann et al., 1994; Brix et al., 1999; Wiedemann et al., 2001; Young et al., 2003; Otera et al., 2007). We examined whether pretreatment of mitochondria with trypsin, which removes any exposed parts of surface receptors, can affect the insertion of Ugo1. A substantial reduction in the membrane integration of Ugo1 was observed upon such a treatment, similar to the expected effect on the membrane integration of porin (Fig. 3 A; Krimmer et al., 2001).
Figure 3.
Ugo1 requires the import receptor Tom70 for its import and assembly. (A) Mitochondria were left intact or pretreated with trypsin followed by reisolation of the organelles. Aliquots of both trypsin-treated and intact mitochondria were removed, and the trypsin activity was monitored by immunodecoration with antibodies against Tom components (right). Next, radiolabeled Ugo1 and porin were incubated with the trypsin-treated or intact mitochondria for the indicated time periods. At the end of the import reactions, samples containing Ugo1 were treated again with trypsin, whereas to those harboring porin, PK was added. Proteins were analyzed by SDS-PAGE and autoradiography. The insertion of Ugo1 was quantified by analyzing the formation of the 23-kD fragment, whereas for porin, the PK-protected molecules were quantified. The amount of precursor proteins imported into intact mitochondria for 20 min was set to 100%. (B) Radiolabeled precursors were imported into mitochondria isolated from either wild-type (wt) or tom20Δ strains. Imported proteins were analyzed as described in A. (C) Radiolabeled precursors of Ugo1 and AAC were imported into mitochondria isolated from either wild-type or tom70Δtom71Δ strains. Imported proteins were analyzed by SDS-PAGE and radiography. The insertion of Ugo1 was quantified as described in A, whereas the PK-protected molecules of AAC were quantified. (A–C) An experiment representative of three independent repeats is presented. (D) Radiolabeled precursor of Ugo1 was imported into mitochondria isolated from tom70Δtom71Δ, tom20Δ, or their corresponding wild-type strains. After import, the mitochondria were analyzed by BN-PAGE. For comparison, wild-type mitochondria were analyzed by BN-PAGE and immunodecoration with an antibody against Ugo1. Ugo1-containing complexes are indicated on the right (I and II). (E) Mitochondria were isolated from tom70Δtom71Δ, tom20Δ, or their corresponding wild-type strains. Mitochondrial proteins (10 and 30 µg) were analyzed by SDS-PAGE and immunodecoration with antibodies against the indicated proteins. The intensity of the bands in three independent experiments was quantified, and the amount of proteins in mutant mitochondria is expressed as the mean (±SD) percentage of their level in the wild-type organelle. (F) The cytosolic domain of Tom70 can recognize newly synthesized Ugo1 molecules. Chemical amounts of Ugo1-HA (input) were mixed with either GST or GST fused to the cytosolic domain of Tom70 (GST-Tom70) bound to glutathione beads. The beads were washed, after which bound material was eluted with sample buffer. Aliquots of the input (10%), wash (W; 2%), and bound material (B; 100%) were analyzed by SDS-PAGE and either Ponceau staining (GST and GST-Tom70) or immunodecoration against HA tag.
To identify the involved receptors, we analyzed the import into mitochondria isolated from strains lacking either Tom20 or Tom70/71. In contrast to the dramatic effect on the import of porin, the absence of Tom20 did not cause any reduction in the membrane insertion of Ugo1 (Fig. 3 B). When we monitored the import into tom70Δtom71Δ mitochondria, we observed that the insertion of Ugo1 into these organelles was clearly compromised (although less than that of the prototype Tom70 substrate AAC; Fig. 3 C). To verify the importance of Tom70, we analyzed the in vitro import reaction by blue native (BN)–PAGE. Of note, endogenous and newly synthesized radiolabeled Ugo1 molecules were found in two oligomeric species with apparent molecular masses of ∼300 and 150 kD (Fig. 3 D, indicated as oligomer I and II, respectively). The bottom band represents a homodimer of Ugo1 (Hoppins et al., 2009), whereas the composition of the top one is unknown. In support of the aforementioned results, the formation of the Ugo1-containing oligomers was hampered in mitochondria lacking Tom70/71 but was unaffected by the absence of Tom20 (Fig. 3 D).
To further investigate the dependency on import receptors, we monitored the steady-state levels of Ugo1 in mitochondria lacking either Tom70/71 or Tom20. The endogenous levels of Ugo1 were indeed reduced in tom70/71Δ organelles but not in tom20Δ mitochondria (Fig. 3 E). Importantly, it appears that, in vivo, Tom70 plays a more crucial role in the biogenesis of Ugo1 than in that of AAC. We and others did not observe a reduction in the steady-state levels of AAC in tom70/71Δ mitochondria (Fig. 3 E; Bömer et al., 1996). Of note, the steady-state levels of Mim1, which is crucial for the membrane integration of Ugo1 (Fig. 4), are moderately reduced in mitochondria lacking Tom70 (Fig. 3 E). This observation raises the possibility that the hampered biogenesis of Ugo1 in tom70Δ cells is actually a result of reduced levels of Mim1. However, we regard this scenario as unlikely because removal of the exposed receptor domains by trypsin dramatically reduced the insertion of Ugo1 (Fig. 3 A), whereas deletion of the exposed N terminus of Mim1 does not result in a clear phenotype (Popov-Celeketić et al., 2008; Lueder and Lithgow, 2009) or alteration of the steady-state levels of Ugo1 (Fig. S1).
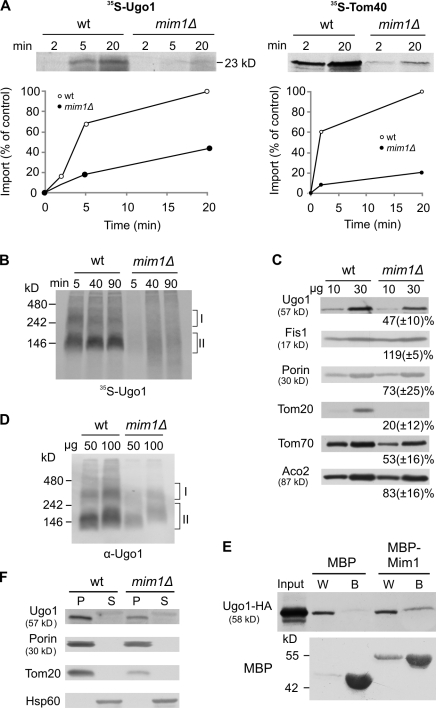
Figure 4.
Mim1 is playing an important role in the membrane integration of Ugo1. (A) Radiolabeled Ugo1 and Tom40 were imported into mitochondria isolated from either wild-type (wt) or mim1Δ strains. The insertion of Ugo1 was analyzed as described in Fig. 3 A, whereas for Tom40, the PK-protected molecules were quantified. An experiment representative of three independent repeats is presented. (B) Radiolabeled precursor of Ugo1 was imported for the indicated time periods into mitochondria isolated from either wild-type or mim1Δ strains. After import, mitochondria were analyzed by BN-PAGE and autoradiography. Ugo1-containing complexes are indicated (I and II). (C) Mitochondria isolated from either wild-type or mim1Δ strains were analyzed by SDS-PAGE and immunodecoration with antibodies against the indicated mitochondrial proteins. The intensities of the bands were quantified as described in Fig. 3 E. (D) The indicated amounts of mitochondria isolated from either wild-type or mim1Δ strains were analyzed by BN-PAGE and immunodecoration with antibody against Ugo1. Ugo1-containing complexes are indicated. (E) Chemical amounts of Ugo1-HA (input) were mixed with either MBP or MBP-Mim1 bound to maltose beads. The beads were washed, and then bound material was eluted with sample buffer. Aliquots of the input (10%), wash (W; 2%), and bound material (B; 80%) were analyzed by SDS-PAGE and immunodecoration with antibodies against HA tag and MBP. (F) Mitochondria isolated from either wild-type or mim1Δ strains were subjected to carbonate extraction. The pellet (P) and the supernatant (S) fractions were analyzed by SDS-PAGE and immunodecoration with antibodies against the indicated proteins.
To substantiate a direct involvement of Tom70, we incubated newly synthesized Ugo1 molecules with either the recombinant cytosolic domain of Tom70 fused to GST or with GST alone and observed specific interactions only with the former construct (Fig. 3 F). Hence, the binding assay demonstrates the ability of Tom70 to directly recognize a precursor form of Ugo1. Collectively, the results of four different assays suggest that Tom70 plays an important role in the import of Ugo1. The receptor is not absolutely essential for the import of Ugo1 but rather accelerates and enhances this process as with other substrates of Tom70 (Steger et al., 1990; Ramage et al., 1993). In its absence, these tasks are probably partially performed by the other import receptor Tom20. Accordingly, Tom20 is actually found in higher amounts when Tom70 is deleted (Fig. 3 E).
We suggest that cytosolic chaperones help in delivering the multispan precursor proteins in an import-competent form to Tom70 that in turn provides the first recognition on the organelle surface. Supporting this notion is the involvement of Tom70 in the insertion of multispan MOM proteins in mammalian cells (Otera et al., 2007) and its function as a docking element for chaperone-associated precursor proteins of the inner membrane carrier proteins (Young et al., 2003).
Membrane integration of Ugo1 does not require the TOM complex pore, elements residing in the intermembrane space (IMS), and the topogenesis of MOM β-barrel protein (TOB) complex
Because β-barrel proteins are translocated through the import pore of the TOM complex before their insertion into the MOM (Pfanner et al., 2004; Ryan, 2004; Paschen et al., 2005), we asked whether multispan proteins follow a similar pathway. To this end, an excess of recombinant matrix-destined precursor (pSu9(1–69)-DHFR), which can compete with import of other TOM-dependent precursors, was added to the import reaction of Ugo1. This treatment did not affect the membrane integration of Ugo1 but, as expected, caused a strong reduction in the import of porin (Fig. S2 A). Thus, it appears that the TOM import pore is not required for the biogenesis of Ugo1.
Upon their translocation across the MOM, β-barrel proteins are engaged by the small Tim chaperones residing in the IMS (Hoppins and Nargang, 2004; Wiedemann et al., 2004; Habib et al., 2005). Interestingly, Otera et al. (2007) reported that mammalian multispan proteins also require elements in the IMS for their biogenesis. Hence, we asked whether swelling of mitochondria, which results in ruptured MOM and loss of most of IMS proteins, can affect the biogenesis of Ugo1. This treatment did not influence the membrane integration of Ugo1 under short incubation periods and had only a slight effect after incubation for 90 min. In sharp contrast, rupturing the MOM resulted in severely compromised assembly of the β-barrel protein Tom40 (Fig. S2 B). Of note, immunodecoration with antibodies against Tim10 and Tim13 showed that negligible amounts of these proteins are still associated with the swollen mitochondria. To exclude the possibility that these residual amounts are sufficient to support an efficient biogenesis of Ugo1, we analyzed the biogenesis of Ugo1 in a strain deleted for TIM8/TIM13. The double deletion did not interfere with the in vitro import of Ugo1 nor did it cause any reduction in the steady-state levels of the protein (Fig. S2, C and D). Similarly, mitochondria isolated from a strain harboring a temperature-sensitive allele of TIM10 were not compromised in their capacity to import in vitro newly synthesized Ugo1 molecules. In contrast, they were severely affected in their ability to import the known Tim10 substrate AAC (Fig. S2 E). Collectively, elements of the IMS do not appear to be essential for the biogenesis of Ugo1.
The TOB/SAM (sorting and assembly machinery) complex was initially reported to be a dedicated machinery for the membrane insertion of β-barrel proteins (Gentle et al., 2004; Pfanner et al., 2004; Paschen et al., 2005). However, later studies proposed its involvement also in the biogenesis of small helical Tom components (Stojanovski et al., 2007; Thornton et al., 2010). Hence, we investigated the biogenesis of Ugo1 in mitochondria lacking Mas37, a subunit that mediates the release of substrate proteins from the TOB complex (Wiedemann et al., 2003; Chan and Lithgow, 2008; Dukanovic et al., 2009). Deletion of MAS37 caused, as expected, a clear reduction in the in vitro import of the β-barrel protein Tom40 and a minor one in steady-state levels of Tom40 and porin (Fig. S3, A and B). This deletion neither resulted in reduced in vitro membrane integration of Ugo1 nor compromised its steady-state levels (Fig. S3, A and B). Collectively, we conclude that the multispan protein Ugo1 does not require the TOB complex.
The MOM protein Mim1 is crucial for the biogenesis of Ugo1
We next asked which membrane-embedded protein can mediate membrane integration of Ugo1. Based on its known functions, Mim1 is a good candidate to accomplish this role. The protein is important for the biogenesis of the TOM complex and is required for membrane integration of Tom helical components (Ishikawa et al., 2004; Waizenegger et al., 2005; Becker et al., 2008; Hulett et al., 2008; Popov-Celeketić et al., 2008; Lueder and Lithgow, 2009; Stefan Dimmer and Rapaport, 2010; Thornton et al., 2010).
When mitochondria lacking Mim1 were used, a strong reduction in the import of newly synthesized Ugo1 (and of Tom40 as a control) was observed by both the proteolytic assay and BN-PAGE (Fig. 4, A and B). Accordingly, the steady-state levels of Ugo1 and the amounts of endogenous Ugo1-containing oligomers were greatly reduced in mim1Δ mitochondria (Fig. 4, C and D). As the steady-state levels of Tom20, Tom70, and Tom40 are also reduced in mim1Δ mitochondria (Fig. 4 C; Ishikawa et al., 2004; Waizenegger et al., 2005), a theoretical scenario could be that the compromised biogenesis of Ugo1 is actually a result of reduced levels of these Tom components. However, this possibility is unlikely because deletion of Tom20 itself or blocking the Tom40 import pore does not have any influence on the biogenesis of Ugo1, and deletion of Tom70/71 causes a less severe phenotype. Next, we tested whether Mim1 can bind Ugo1 precursor molecules and observed that maltose-binding protein (MBP)–Mim1 but not MBP alone could interact with Ugo1 (Fig. 4 E). These results support a direct role of Mim1 in the membrane integration of Ugo1. The findings of a parallel study (see Becker et al. in this issue) underscore the importance of Mim1 in the biogenesis of multispanning outer membrane proteins and its capacity to directly bind these proteins during their membrane integration process. Of note, the residual amount of Ugo1 molecules in MOM lacking Mim1 behaved as membrane-embedded proteins that cannot be extracted by alkaline solution (Fig. 4 F). Thus, it might well be that an additional, yet to be identified, element also contributes to the membrane integration of Ugo1.
The newly discovered function of Mim1 in the integration of multispan proteins raises the question of how Mim1 performs this role. Based on our previous observation that Mim1 can form homooligomers (Popov-Celeketić et al., 2008), we speculate that multiple molecules of Mim1 form a distinct site in the MOM that can provide an entry platform for the transmembrane helices of single-span and multispan proteins.
Conclusions
We propose that the integration of Ugo1 into the MOM occurs via a novel pathway. This pathway involves initial docking of chaperone-associated Ugo1 to the import receptor Tom70. Ugo1 precursor is then inserted into the membrane in a process that is facilitated by the membrane-embedded protein Mim1.
Materials and methods
Yeast strains and growth media
Standard genetic techniques were used for growth and manipulation of yeast strains. Unless stated otherwise, the wild-type strains YPH499 and W303 were used. For construction of ugo1Δ and tom20Δ mutant strains in W303 background, the UGO1 and TOM20 genes were deleted by PCR-mediated gene replacement with kanMX4 and HIS3-MX6 cassette, respectively. The mas37Δ (Habib et al., 2005) and tim8Δ/tim13Δ (Paschen et al., 2000) strains were previously described. mim1Δ, mim1Δ+MIM1ΔN, and mim1Δ+MIM1−FL strains were constructed as reported by Popov-Celeketić et al. (2008). The tom70Δ/tom71Δ double deletion (Kondo-Okamoto et al., 2008) and TIM10-1 (Koehler et al., 1998) strains were gifts from K. Okamoto (Osaka University, Osaka, Japan) and C. Koehler (University of California, Los Angeles, Los Angeles, CA), respectively. Transformation of yeast was performed according to the lithium-acetate method. Yeast cells were grown under aerobic conditions in yeast peptone dextrose, YPGal (1% yeast extract, 2% bactopeptone, and 2% galactose), Lac, synthetic dextrose–Trp, or synthetic dextrose–Ura media.
Recombinant DNA techniques
To obtain a C-terminally HA-tagged Ugo1, a sequence encoding 2× HA was PCR amplified from the pFA6a-3HA-KanMX4 vector and inserted into the target vector pYX113 using SalI and XhoI restriction sites. UGO1 without its stop codon was amplified via PCR from genomic DNA isolated from the YPH499 strain and introduced into the modified vector using EcoRI and SalI restriction sites. For cell-free experiments, this construct (pYX113 UGO1-2HA) was used as a template for PCR amplification of UGO1-2HA. PCR product obtained in this way was inserted into pGEM4 vector by use of the SmaI and XbaI restriction sites. The cytosolic domain of Tom70 (Δ amino acid residues 1–34) was amplified by PCR and introduced into the pGEX4T vector using the BamHI and HindIII restriction sites.
Biochemical procedures
Mitochondria were isolated from yeast cells by differential centrifugation as previously described (Daum et al., 1982). For swelling experiments, isolated mitochondria were incubated with a hypotonic buffer (20 mM Hepes, pH 7.2) for 30 min on ice. Then, it was supplemented with urea to a final concentration of 1 M and incubated on ice for a further 5 min. Swollen mitochondria were reisolated by centrifugation and resuspended in import buffer. Chemical amounts of Ugo1-HA were produced in wheat germ lysate according to the manufacturer´s instructions (RTS 100 Wheat Germ CECF kit; 5Prime). The recombinant proteins GST, GST-Tom70 (cytosolic domain), MBP, and MBP-Mim1 were expressed in Escherichia coli BL21 cells as soluble proteins. Purification of recombinant proteins was performed by affinity chromatography according to the manufacturer´s instructions using either glutathione beads (Macherey-Nagel) or maltose-coupled beads (New England Biolabs, Inc.).
Protein samples were analyzed by SDS-PAGE and blotting to nitrocellulose membranes followed by visualization through autoradiography. Alternatively, incubation with antibodies was performed according to standard procedures, and visualization was performed via the ECL method. The antibody against Ugo1 was a gift from S. Hoppins and J. Nunnari (University of California, Davis, Davis, CA). Intensity of the observed bands was quantified with automatic imaging data analysis software (raytest GmbH). Unless stated otherwise, each presented experiment represents at least three repetitions.
In vitro protein import
Import experiments with radiolabeled precursor proteins and isolated mitochondria were performed in an import buffer containing 250 mM sucrose, 0.25 mg/ml BSA, 80 mM KCl, 5 mM MgCl2, 10 mM MOPS-KOH, 2 mM NADH, and 2 mM ATP, pH 7.2. Radiolabeled precursor proteins were synthesized in rabbit reticulocyte lysate in the presence of [35S]methionine. In some cases, the import reaction was treated with 0.3 U/µl apyrase. Trypsin treatment of mitochondria was performed by adding 50 µg/ml trypsin for 25 min on ice. Trypsin was then inhibited by adding 1.5 mg/ml soybean trypsin inhibitor for 10 min on ice. For blocking the TOM complex import pore, the recombinant precursor protein pSu9-DHFR was added to 30 µg of isolated mitochondria immediately before the import reaction. In the carbonate extraction reaction, mitochondria were dissolved in 0.1 M Na2CO3. After 30 min on ice, the sample was centrifuged (75,000 g for 30 min at 2°C), and pellet and supernatant were analyzed.
BN-PAGE
Mitochondria were lysed in 40 µl digitonin-containing buffer (1–1.5% digitonin, 20 mM Tris-HCl, 0.1 mM EDTA, 50 mM NaCl, 10% glycerol, and 1 mM PMSF, pH 7.4). After incubation for 15 min at 4°C and a clarifying spin (30,000 g for 15 min at 2°C), 5 µl of sample buffer (5% [weight/volume] Coomassie brilliant blue G-250, 100 mM Bis-Tris, and 500 mM 6-aminocaproic acid, pH 7.0) was added, and the mixture was analyzed by electrophoresis in a 6–13% gradient BN gel (Schägger et al., 1994). Gels were blotted to polyvinylidene fluoride membranes, and proteins were further analyzed by autoradiography or immunodecoration.
Online supplemental material
Fig. S1 presents data supporting the proposal that the N-terminal domain of Mim1 is not required for optimal biogenesis of Ugo1. Fig. S2 shows that the biogenesis of Ugo1 does not require the TOM import pore or elements in the IMS. Fig. S3 includes results of experiments demonstrating that the insertion of Ugo1 is independent of the TOB complex. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201102041/DC1.
Acknowledgments
We thank K. Rehn and E. Kracker for technical support, H. Kato and E. Merklinger for helpful discussions, C. Koehler, K. Tokatlidis, and K. Okamoto for yeast strains, and S. Hoppins and J. Nunnari for constructs and antibodies.
This work was supported by a Deutsche Forschungsgemeinschaft grant (RA 1048/4-1 to D. Rapaport) and a postdoctoral fellowship from the Carl Zeiss Stiftung (to K.S. Dimmer).
Footnotes
Abbreviations used in this paper:
- AAC
- ATP–ADP carrier
- BN
- blue native
- DHFR
- dihydrofolate reductase
- IMS
- intermembrane space
- MBP
- maltose-binding protein
- MOM
- mitochondrial outer membrane
- PBR
- peripheral benzodiazepine receptor
- PK
- proteinase K
- TA
- tail anchored
- TMS
- transmembrane segment
- TOM
- translocase of the outer membrane
References
- Becker T., Pfannschmidt S., Guiard B., Stojanovski D., Milenkovic D., Kutik S., Pfanner N., Meisinger C., Wiedemann N. 2008. Biogenesis of the mitochondrial TOM complex: Mim1 promotes insertion and assembly of signal-anchored receptors. J. Biol. Chem. 283:120–127 10.1074/jbc.M706997200 [DOI] [PubMed] [Google Scholar]
- Becker T., Wenz L.-S., Krüger V., Lehmann W., Müller J.M., Goroncy L., Zufall N., Lithgow T., Guiard B., Chacinska A., et al. 2011. The mitochondrial import protein Mim1 promotes biogenesis of multispanning outer membrane proteins. J. Cell Biol. 194:387–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bömer U., Pfanner N., Dietmeier K. 1996. Identification of a third yeast mitochondrial Tom protein with tetratrico peptide repeats. FEBS Lett. 382:153–158 10.1016/0014-5793(96)00156-1 [DOI] [PubMed] [Google Scholar]
- Brix J., Rüdiger S., Bukau B., Schneider-Mergener J., Pfanner N. 1999. Distribution of binding sequences for the mitochondrial import receptors Tom20, Tom22, and Tom70 in a presequence-carrying preprotein and a non-cleavable preprotein. J. Biol. Chem. 274:16522–16530 10.1074/jbc.274.23.16522 [DOI] [PubMed] [Google Scholar]
- Burri L., Vascotto K., Gentle I.E., Chan N.C., Beilharz T., Stapleton D.I., Ramage L., Lithgow T. 2006. Integral membrane proteins in the mitochondrial outer membrane of Saccharomyces cerevisiae. FEBS J. 273:1507–1515 10.1111/j.1742-4658.2006.05171.x [DOI] [PubMed] [Google Scholar]
- Chacinska A., Koehler C.M., Milenkovic D., Lithgow T., Pfanner N. 2009. Importing mitochondrial proteins: machineries and mechanisms. Cell. 138:628–644 10.1016/j.cell.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan N.C., Lithgow T. 2008. The peripheral membrane subunits of the SAM complex function codependently in mitochondrial outer membrane biogenesis. Mol. Biol. Cell. 19:126–136 10.1091/mbc.E07-08-0796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk J.E., Moldoveanu T., Llambi F., Parsons M.J., Green D.R. 2010. The BCL-2 family reunion. Mol. Cell. 37:299–310 10.1016/.molcel.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonrod E.M., Karren M.A., Shaw J.M. 2007. Ugo1p is a multipass transmembrane protein with a single carrier domain required for mitochondrial fusion. Traffic. 8:500–511 10.1111/j.1600-0854.2007.00550.x [DOI] [PubMed] [Google Scholar]
- Daum G., Gasser S.M., Schatz G. 1982. Import of proteins into mitochondria. Energy-dependent, two-step processing of the intermembrane space enzyme cytochrome b2 by isolated yeast mitochondria. J. Biol. Chem. 257:13075–13080 [PubMed] [Google Scholar]
- Dukanovic J., Dimmer K.S., Bonnefoy N., Krumpe K., Rapaport D. 2009. Genetic and functional interactions between the mitochondrial outer membrane proteins Tom6 and Sam37. Mol. Cell. Biol. 29:5975–5988 10.1128/MCB.00069-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T., Yamano K. 2009. Multiple pathways for mitochondrial protein traffic. Biol. Chem. 390:723–730 10.1515/BC.2009.087 [DOI] [PubMed] [Google Scholar]
- Fritz S., Rapaport D., Klanner E., Neupert W., Westermann B. 2001. Connection of the mitochondrial outer and inner membranes by Fzo1 is critical for organellar fusion. J. Cell Biol. 152:683–692 10.1083/jcb.152.4.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentle I., Gabriel K., Beech P., Waller R., Lithgow T. 2004. The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J. Cell Biol. 164:19–24 10.1083/jcb.200310092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib S.J., Waizenegger T., Lech M., Neupert W., Rapaport D. 2005. Assembly of the TOB complex of mitochondria. J. Biol. Chem. 280:6434–6440 10.1074/jbc.M411510200 [DOI] [PubMed] [Google Scholar]
- Hoppins S.C., Nargang F.E. 2004. The Tim8-Tim13 complex of Neurospora crassa functions in the assembly of proteins into both mitochondrial membranes. J. Biol. Chem. 279:12396–12405 10.1074/jbc.M313037200 [DOI] [PubMed] [Google Scholar]
- Hoppins S., Horner J., Song C., McCaffery J.M., Nunnari J. 2009. Mitochondrial outer and inner membrane fusion requires a modified carrier protein. J. Cell Biol. 184:569–581 10.1083/jcb.200809099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulett J.M., Lueder F., Chan N.C., Perry A.J., Wolynec P., Likić V.A., Gooley P.R., Lithgow T. 2008. The transmembrane segment of Tom20 is recognized by Mim1 for docking to the mitochondrial TOM complex. J. Mol. Biol. 376:694–704 10.1016/j.jmb.2007.12.021 [DOI] [PubMed] [Google Scholar]
- Ishikawa D., Yamamoto H., Tamura Y., Moritoh K., Endo T. 2004. Two novel proteins in the mitochondrial outer membrane mediate β-barrel protein assembly. J. Cell Biol. 166:621–627 10.1083/jcb.200405138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper C., Habib S.J., Engl G., Heckmeyer P., Dimmer K.S., Rapaport D. 2008. Integration of tail-anchored proteins into the mitochondrial outer membrane does not require any known import components. J. Cell Sci. 121:1990–1998 10.1242/jcs.024034 [DOI] [PubMed] [Google Scholar]
- Koehler C.M., Jarosch E., Tokatlidis K., Schmid K., Schweyen R.J., Schatz G. 1998. Import of mitochondrial carriers mediated by essential proteins of the intermembrane space. Science. 279:369–373 10.1126/science.279.5349.369 [DOI] [PubMed] [Google Scholar]
- Kondo-Okamoto N., Shaw J.M., Okamoto K. 2008. Tetratricopeptide repeat proteins Tom70 and Tom71 mediate yeast mitochondrial morphogenesis. EMBO Rep. 9:63–69 10.1038/sj.embor.7401113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimmer T., Rapaport D., Ryan M.T., Meisinger C., Kassenbrock C.K., Blachly-Dyson E., Forte M., Douglas M.G., Neupert W., Nargang F.E., Pfanner N. 2001. Biogenesis of porin of the outer mitochondrial membrane involves an import pathway via receptors and the general import pore of the TOM complex. J. Cell Biol. 152:289–300 10.1083/jcb.152.2.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueder F., Lithgow T. 2009. The three domains of the mitochondrial outer membrane protein Mim1 have discrete functions in assembly of the TOM complex. FEBS Lett. 583:1475–1480 10.1016/j.febslet.2009.03.064 [DOI] [PubMed] [Google Scholar]
- Neupert W., Herrmann J.M. 2007. Translocation of proteins into mitochondria. Annu. Rev. Biochem. 76:723–749 10.1146/annurev.biochem.76.052705.163409 [DOI] [PubMed] [Google Scholar]
- Otera H., Taira Y., Horie C., Suzuki Y., Suzuki H., Setoguchi K., Kato H., Oka T., Mihara K. 2007. A novel insertion pathway of mitochondrial outer membrane proteins with multiple transmembrane segments. J. Cell Biol. 179:1355–1363 10.1083/jcb.200702143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen S.A., Rothbauer U., Káldi K., Bauer M.F., Neupert W., Brunner M. 2000. The role of the TIM8-13 complex in the import of Tim23 into mitochondria. EMBO J. 19:6392–6400 10.1093/emboj/19.23.6392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen S.A., Neupert W., Rapaport D. 2005. Biogenesis of β-barrel membrane proteins of mitochondria. Trends Biochem. Sci. 30:575–582 10.1016/j.tibs.2005.08.009 [DOI] [PubMed] [Google Scholar]
- Pfanner N., Wiedemann N., Meisinger C., Lithgow T. 2004. Assembling the mitochondrial outer membrane. Nat. Struct. Mol. Biol. 11:1044–1048 10.1038/nsmb852 [DOI] [PubMed] [Google Scholar]
- Popov-Celeketić J., Waizenegger T., Rapaport D. 2008. Mim1 functions in an oligomeric form to facilitate the integration of Tom20 into the mitochondrial outer membrane. J. Mol. Biol. 376:671–680 10.1016/j.jmb.2007.12.006 [DOI] [PubMed] [Google Scholar]
- Ramage L., Junne T., Hahne K., Lithgow T., Schatz G. 1993. Functional cooperation of mitochondrial protein import receptors in yeast. EMBO J. 12:4115–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport D., Neupert W. 1999. Biogenesis of Tom40, core component of the TOM complex of mitochondria. J. Cell Biol. 146:321–331 10.1083/jcb.146.2.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport D., Brunner M., Neupert W., Westermann B. 1998. Fzo1p is a mitochondrial outer membrane protein essential for the biogenesis of functional mitochondria in Saccharomyces cerevisiae. J. Biol. Chem. 273:20150–20155 10.1074/jbc.273.32.20150 [DOI] [PubMed] [Google Scholar]
- Rojo M., Legros F., Chateau D., Lombès A. 2002. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J. Cell Sci. 115:1663–1674 [DOI] [PubMed] [Google Scholar]
- Ryan M.T. 2004. Chaperones: inserting beta barrels into membranes. Curr. Biol. 14:R207–R209 10.1016/j.cub.2004.02.024 [DOI] [PubMed] [Google Scholar]
- Schägger H., Cramer W.A., von Jagow G. 1994. Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal. Biochem. 217:220–230 10.1006/abio.1994.1112 [DOI] [PubMed] [Google Scholar]
- Schlossmann J., Dietmeier K., Pfanner N., Neupert W. 1994. Specific recognition of mitochondrial preproteins by the cytosolic domain of the import receptor MOM72. J. Biol. Chem. 269:11893–11901 [PubMed] [Google Scholar]
- Schmitt S., Prokisch H., Schlunck T., Camp D.G., II, Ahting U., Waizenegger T., Scharfe C., Meitinger T., Imhof A., Neupert W., et al. 2006. Proteome analysis of mitochondrial outer membrane from Neurospora crassa. Proteomics. 6:72–80 10.1002/pmic.200402084 [DOI] [PubMed] [Google Scholar]
- Setoguchi K., Otera H., Mihara K. 2006. Cytosolic factor- and TOM-independent import of C-tail-anchored mitochondrial outer membrane proteins. EMBO J. 25:5635–5647 10.1038/sj.emboj.7601438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan Dimmer K., Rapaport D. 2010. The enigmatic role of Mim1 in mitochondrial biogenesis. Eur. J. Cell Biol. 89:212–215 10.1016/j.ejcb.2009.11.002 [DOI] [PubMed] [Google Scholar]
- Steger H.F., Söllner T., Kiebler M., Dietmeier K.A., Pfaller R., Trülzsch K.S., Tropschug M., Neupert W., Pfanner N. 1990. Import of ADP/ATP carrier into mitochondria: two receptors act in parallel. J. Cell Biol. 111:2353–2363 10.1083/jcb.111.6.2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovski D., Guiard B., Kozjak-Pavlovic V., Pfanner N., Meisinger C. 2007. Alternative function for the mitochondrial SAM complex in biogenesis of α-helical TOM proteins. J. Cell Biol. 179:881–893 10.1083/jcb.200706043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton N., Stroud D.A., Milenkovic D., Guiard B., Pfanner N., Becker T. 2010. Two modular forms of the mitochondrial sorting and assembly machinery are involved in biogenesis of α-helical outer membrane proteins. J. Mol. Biol. 396:540–549 10.1016/j.jmb.2009.12.026 [DOI] [PubMed] [Google Scholar]
- Waizenegger T., Schmitt S., Zivkovic J., Neupert W., Rapaport D. 2005. Mim1, a protein required for the assembly of the TOM complex of mitochondria. EMBO Rep. 6:57–62 10.1038/sj.embor.7400318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther D.M., Rapaport D. 2009. Biogenesis of mitochondrial outer membrane proteins. Biochim. Biophys. Acta. 1793:42–51 10.1016/j.bbamcr.2008.04.013 [DOI] [PubMed] [Google Scholar]
- Wiedemann N., Pfanner N., Ryan M.T. 2001. The three modules of ADP/ATP carrier cooperate in receptor recruitment and translocation into mitochondria. EMBO J. 20:951–960 10.1093/emboj/20.5.951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann N., Kozjak V., Chacinska A., Schönfisch B., Rospert S., Ryan M.T., Pfanner N., Meisinger C. 2003. Machinery for protein sorting and assembly in the mitochondrial outer membrane. Nature. 424:565–571 10.1038/nature01753 [DOI] [PubMed] [Google Scholar]
- Wiedemann N., Truscott K.N., Pfannschmidt S., Guiard B., Meisinger C., Pfanner N. 2004. Biogenesis of the protein import channel Tom40 of the mitochondrial outer membrane: intermembrane space components are involved in an early stage of the assembly pathway. J. Biol. Chem. 279:18188–18194 10.1074/jbc.M400050200 [DOI] [PubMed] [Google Scholar]
- Yamano K., Yatsukawa Y., Esaki M., Hobbs A.E., Jensen R.E., Endo T. 2008. Tom20 and Tom22 share the common signal recognition pathway in mitochondrial protein import. J. Biol. Chem. 283:3799–3807 10.1074/jbc.M708339200 [DOI] [PubMed] [Google Scholar]
- Young J.C., Hoogenraad N.J., Hartl F.-U. 2003. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell. 112:41–50 10.1016/S0092-8674(02)01250-3 [DOI] [PubMed] [Google Scholar]
- Zahedi R.P., Sickmann A., Boehm A.M., Winkler C., Zufall N., Schönfisch B., Guiard B., Pfanner N., Meisinger C. 2006. Proteomic analysis of the yeast mitochondrial outer membrane reveals accumulation of a subclass of preproteins. Mol. Biol. Cell. 17:1436–1450 10.1091/mbc.E05-08-0740 [DOI] [PMC free article] [PubMed] [Google Scholar]