Abstract
Background
Toll-like receptor 3 (TLR3) recognizes double-stranded RNA (dsRNA) and induces inflammation. In this study we attempted to ascertain if there are endogenous host molecules controlling the production of cytokines and chemokines. Two candidates, ribosomal protein L19 and L22, were analyzed to determine if they influence cytokine production followed by TLR3 activation. In this study we report that L19 acts upon production of IP-10 or IL-8 differently in glioblastoma cells.
Methods
L19 or L22 was transfected into HEK293-TLR3, A549 or A172 cells. After treatment with several inhibitors of NF-kB, PI3K, p38 or ERK, production of IL-8 or IP-10 was measured by ELISA. siRNA was introduced to suppress expression of L19. After Vesicular stomatitis virus infection, viral multiplication was measured by western blot.
Results
L19 increased ERK activation to produce IL-8. In A172 cells, in which TLR3 is expressed at endosomes, L19 inhibited interferon regulatory factor 3 (IRF3) activation and IP-10 production to facilitate viral multiplication, whereas L19 inhibited viral multiplication in A549 cells bearing TLR3 on their cell membrane.
Conclusion
Our results suggest that L19 regulates TLR3 signaling, which is cell type specific and may be involved in pathogenesis of autoimmune diseases and chronic inflammatory diseases.
Keywords: RPL19, RPL22, TLR3
INTRODUCTION
Toll-like receptor 3 (TLR3), the typical anti-viral pattern recognition receptor (PRR), recognizes dsRNA and activates nuclear factor-kB (NF-kB), interferon regulatory factor 3 (IRF3), and AP-1. TLR3 generally mediates anti-viral immunity, however, in some infections, cytokines or chemokines produced by TLR3 change the host immunological niche to facilitate viral replication. Therefore, there is some debate about the role of TLR3 in viral infection. For example, TLR3 binds to dsRNA of the West Nile virus (WNV), which is produced during viral replication. When TLR3 deficient mice are infected with WNV, the virus multiply and encephalomyelitis does not severely progress. Pathological findings were less than control mice, in which the blood-brain barrier is broken after viral infection and TNF-α or IL-β is produced via TLR3 activation (1). The lung is another organ where TLR3 is abundantly expressed. Invasion of the influenza A virus increases TLR3 expression and leads to acute pneumonitis (2). In TLR3 knock-out mice, the survival time is longer than in wild type mice, although considerable amount of virus is detected in the lung. These two reports showed that lack of TLR3 inhibits an unfavorable immune response to the host by modulating activation of the innate immune response or CD8+T cells. Therefore, although the TLR3 immune response inhibits viral multiplication, it may also induce harmful and pathological inflammation (3,4). TLR3 signaling controls pathological phenomena by decreasing mucus secretion in respiratory syncytial virus (RSV) infection rather than acting directly on inhibiting viral multiplication. When TLR3 deficient mice are infected with RSV, T helper 2 (TH2) cytokines are induced and mucus secretion increases, which is the representative finding in RSV infection.Therefore, these diverse TLR3 immune reactions depend on virus type, the amount of virus, infection route, target cells and infection time (5,6).
In respect to pathogens, several viral components are known to regulate innate immunity. NS1 of the influenza A virus (7), E3 of the vaccinia virus (8), and VP39 of the ebola virus (9) bind to viral dsRNA and interfere the binding with TLR3. NS3-4A (10) of the hepatitis C virus inhibits TLR3 signaling by degrading Toll-interleukin 1 receptor domain-containing adapter inducing interferon-β (TRIF).
The ribosome consists of a small 40S subunit and a large 60S subunit. These subunits are composed of 4 RNA species and approximately 80 structurally distinct proteins. RPL19 is found in the large ribosomal subunit (60S) of eukaryotes and archaea. RPL19 consists of two small globular domains connected by an extended segment. RPL19 is located towards the surface of the large subunit, with one exposed end involved in forming the inter-subunit bridge with the small subunit. The other exposed end is involved in forming the translocon binding site, along with L22, L23, L24, L29, and L31e subunits (11,12).
EBER (Epstein-Barr virus encoded small RNA)-1 produced in cells infected with EBV (Epstein-Barr virus) is a 167 bp non-translated RNA and has a stable stem-loop. EBER interacts with PKR (RNA dependent protein kinase) to inactivate PKR and further inhibits IFN-induced apoptosis of host cells. EBER binds to L22 through stem-loop III and IV. L22 and PKR compete with each other to bind to the same region of EBER-1. When EBV infection occurs, L22 binds to EBER which blocks PKR activation and maintains the host anti-viral immune response (13,14).
In this study we attempted to determine if there are endogenous host molecules controlling the production of cytokines and chemokines. Two candidates, ribosomal protein L19 and L22, were analyzed to determine if they influence cytokine production followed by TLR3 activation.
MATERIALS AND METHODS
Reagents
The p38 MAPK inhibitor SB203580, JNK inhibitors (I, II), PI3K inhibitors (LY294002, Wortmannin), NF-κB inhibitor BAY11-7082 and the MEK1/2 inhibitor UO126 were obtained from Calbiochem (San Diego, CA., USA) and each dissolved in dimethyl sulfoxide to achieve a stock concentration of 20 mM. For western blot analysis, anti-IκB-α or anti-phospho-IκB-α, anti-IRF3 or anti-phospho-IRF3, anti-ERK or anti-phospho-ERK, anti-phospho-JNK, anti-phospho-p38, anti-phospho-AKT, anti-phospho-PTEN, anti-phospho-PDK1, and anti-RIG-I antibodies were all purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-V5 and anti-VSV G antibody were purchased from Invitrogen. For secondary antibodies, goat anti-mouse IgG and goat anti-rabbit IgG (Jackson Immuno Research, Baltimore, MD, USA) were used.
Cell culture
HEK293-TLR3 cell lines were purchased from InvivoGen and were then maintained and subcultured in 10% fetal bovine serum (FBS; Gibco BRL, Grand Island, NY, USA) -Dulbecco's modified Eagle's medium (DMEM; Gibco BRL, Grand Island, NY, USA) at 37℃ in 5% CO2. The cell culture medium contained 10µg/ml of blasticidin (InvivoGen, San Diego, CA, USA) as selection antibiotics. The human glioblastoma cell line A172 was obtained from ATCC (CRL-1620) and maintained in minimal essential medium (MEM, Gibco BRL, Grand Island, NY, USA) supplemented with 10% FBS and penicillin and streptomycin (Gibco BRL, Grand Island, NY, USA) at 37℃ in 5% CO2. A549 cells originated from lung epithelial carcinoma (CCL-185™, ATCC, Manassas, VA, USA) and were cultured in MEM containing 10% FBS, penicillin and streptomycin.
Transient transfection and luciferase reporter gene assay
Luciferase reporter gene assay was performed with p133-Luc (IL-8 promoter, kindly provided by Professor Naofumi Mukaida, Kanazawa University, Japan) and pISRE-Luc plasmid (Stratagene, West Cedar Creek, TX, USA). HEK293- TLR3 or A172 cells were grown in 24-well plates with 10% FBS-DMEM at 37℃ in 5% CO2. Cells were cotransfected with 0.05 mg/ml p133-Luc, pISRE-Luc plasmids, and pCMV-β-galactosidase vectors (Promega, Madison, WI, USA) using FuGENE 6 Reagent (Roche, Indianapolis, IN, USA). After 24 hrs, cells were stimulated with poly(I:C) (10µg/ml) (InvivoGen, San Diego, CA, USA) for 6 hrs, and cell extracts were prepared. Luciferase activity was measured using a Luciferase Reporter Assay System (Promega, Madison, WI, USA) and β-galactosidase activity was measured using O-nitrophenyl-β-D-galactopyranoside (Sigma-Aldrich, St. Louis, MI, USA) as the substrate. Luciferase activity was normalized for transfection efficiency with β-galactosidase activity.
ELISA
A172 cells transiently transfected with L19 or a control vector (pcDNA3.1/V5) were treated with poly(I:C) (25µg/ml). After the indicated treatment with poly(I:C), culture medium was collected to measure IP-10 or IL-8 production. IP-10 or IL-8 in the culture supernatant was quantified using commercial specific ELISA kits (BD Biosciences, Franklin Lakes, New Jersey, USA) according to the manufacturer's instructions. The experiments were performed in triplicate.
Western blot analysis
For transient transfection, HEK293 or A172 cells (~1×106) were transfected with L19 plasmids for 24 hrs. The transfected cells were lysed in 300µl of lysis buffer [20 mMTris, pH 7.5, 150 mM NaCl, 1% Triton, 1mM ethylenediaminetetraacetic acid (EDTA), 10µg/ml aprotinin, 10µg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride].
Total protein from either HEK293 or A172 cells transfected with L19 or control plasmids were separated with 10~12% SDS-PAGE and transferred to nitrocellulose membranes. The blots were blocked in PBS-0.1%Tween 20 (PBST) containing 5% non-fat milk and incubated with appropriate primary antibodies at a dilution of 1:1,000. After three washes in PBST, peroxidase-conjugated secondary antibodies were incubated at a dilution of 1:5,000, washed three times with PBST and developed with an ECL system (GE Healthcare, Buckinghamshire, UK).
RNAi assay
Stealth™siRNAs were obtained from Invitrogen. Invitrogen supplied the sequences of the L19 siRNA and Stealth™RNAi negative control duplexes. Stealth™siRNA is chemically modified dsRNA that was developed to overcome the limitation of traditional siRNA. Stealth™RNAi negative control duplex does not induce the interferon-mediated stress response pathway. The targeted sequence of L19 Stealth™siRNA was 5'-CUU GGA UAA AGU CUU GAU GAU CUCC-3'. HEK293/L19 cells transfected with the indicated siRNAs were then stimulated with poly(I:C) (20µg/ml) and activation of IRF3, and NF-κB, and protein levels of L19 were measured by western blot.
Vesicular stomatitis virus infection
Vesicular stomatitis virus (VSV, Indiana serotype, Mudd-Summmers strain) was propagated in Vero cells. African green monkey kidney epithelial Vero cells were maintained in DMEM supplemented with 10% FBS. The viral titer was monitored by a plaque assay using Vero cells. A549 cells were transfected with either V5-tagged L19 genes, L22, or mock vectors. Cells were grown to 80~90% confluence for 24 hrs and then infected with 1 MOI of VSV for 6 hrs in serum free medium.
RESULTS
Effect of L19 on induction of IP-10 and IL-8 followed by poly(I:C) administration
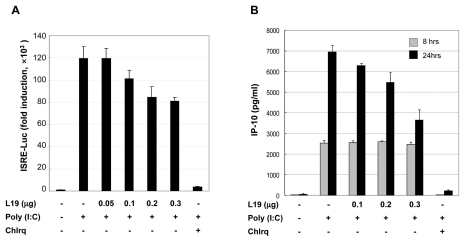
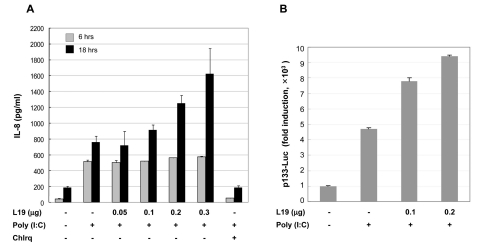
To assess the effect of L19 on the production of IRF3 dependent chemokines and IP-10, ISRE promoter activity, and NF-κB or ERK dependent IL-8 production was evaluated. First, HEK293 cells bearing TLR3 (HEK293/TLR3) were transfected with L19. Then 25µg/ml of poly(I:C) was administrated for 6 hrs with or without chloroquine. As a result, ISRE activity decreased according to the transfected amount of L19 (Fig. 1A). A172 cells, glioblastoma cells expressing TLR3, were transfected with L19 and treated with poly(I:C). After the indicated time, culture supernatants were harvested and the amount of IP-10 and IL-8 produced was measured by ELISA. Production of IP-10 or IL-8 changed in a L19 dose-dependent manner at only later time points (24 and 16 hrs after poly(I:C) treatment, respectively) but not at early time points (8 and 6 hrs after poly(I:C) treatment, respectively) (Figs. 1B and 2A). IL-8 promoter activity increased dependently with the L19 dose (Fig. 2B). These results suggest that TLR3 signaling is influenced by L19.
Figure 1.
Effect of L19 on poly(I:C)-induced activation of ISRE promoter and IP-10 production. (A) HEK293-TLR3 cells were transfected with empty vectors or expression vectors for L19 together with ISRE (IRF3 promoter) reporter plasmids. 18 hrs after transfection, cells were treated with poly(I:C) (10µg/ml) for 6 hrs in the presence of 25µM chloroquine (inhibitor of endosome acidification) or no inhibitor. After poly(I:C) treatment luciferase assays were performed. Luciferase activity is normalized to β-galactosidase activity; results are means±s.d. from three separate transfections. (B) A172 cells were transfected with empty vectors or V5-tagged L19 plasmid. 18 hrs after transfection, cells were stimulated with poly(I:C) (25µg/ml) in the presence or absence 25µM chloroquine for 8 or 24 hrs and thensupernatants were measured by ELISA for IP-10 production. Values represent the mean±S.E. of triplicate samples.
Figure 2.
Effect of L19 on poly(I:C)-induced activation of IL-8 promoter and IL-8 production. (A) A172 cells were transfected with empty vectors or expression vectors for L19 together with p133-Luc (IL-8 promoter) reporter plasmids. 18 hrs after transfection, cells were treated with poly(I:C) (25µg/ml) for 6 hrs. After poly(I:C) treatment luciferase assays were performed. Luciferase activity is normalized to β-galactosidase activity; results are means±s.d. from three separate transfections. (B) A172 cells were transfected with empty vectors or V5-tagged L19 plasmid. 18 hrs after transfection, cells were stimulated with poly(I:C) (25µg/ml) in the presence or absence 25µM chloroquine (inhibitor of endosome acidification) for 6 or 24 hrs and then supernatants were measured by ELISA for IL-8. Values represent the mean±S.E. of triplicate samples.
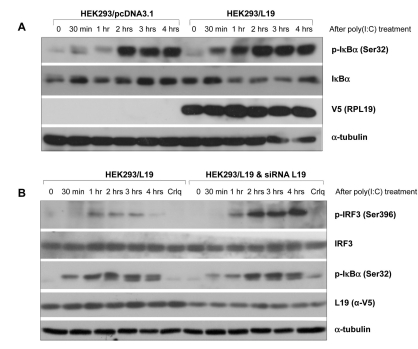
Effect of L19 on phosphorylation of NF-κB or IRF-3
Using cells over-expressing or lacking L19, immunoblotting of IκBα and IRF3 was performed. In HEK293 cells over-expressing L19, IκBα was phosphorylated 30 min after poly(I:C) stimulation, which started early when compared to the control vector (Fig. 3A). When the expression of L19 was inhibited by siRNA, IRF3 activation increased, however activation of IκBα decreased slightly (Fig. 3B). These results suggest that inhibition of IP-10 produciton by L19, elicits strong activation of the TLR3-TRIF-NF-kB pathway to compensate decreased IRF3 activation and further decreased production of IP-10. A similar phenomenon was reported in a prior study demonstrating that the TRIF-dependent pathway is activated when the MyD88 dependent pathway is inhibited.
Figure 3.
Effect of L19 on activation of IRF3 or IκBα in TLR3 signaling. (A) HEK293 cells grown in 60mm plates were transfected with control (pcDNA3.1) or V5-tagged L19. 48 hrs after transfection, cells were stimulated with 20µg/ml poly(I:C) for the indicated time. Phosphorylated IκBα (p-IκBα) and total IκBα were determined by western blotting using anti-p-IκBα (Ser32) and anti-IκBα antibodies. The expression level of L19 in the cell lysates was analyzed with anti-V5 antibody. To confirm equal loading, membranes were re-probed with anti-α-tubulin antibody. (B) siRNA oligo targeting L19 or negative control were transfected into L19 expressing HEK293 (HEK293-L19) cells to silence the expression of L19. 48 hrs after transfection, cells were stimulated without or with 20µg/ml of poly (I:C) for the indicated time. Total cell lysates analyzed by immunoblotting with anti-phospho-IRF3 (Ser396) or anti-IRF-3 antibody, anti-p-IκBα (Ser32) or anti-IκBα antibodies. The efficiency of L19 RNAi was confirmed by immunoblotting with anti-V5 (L19) antibody.
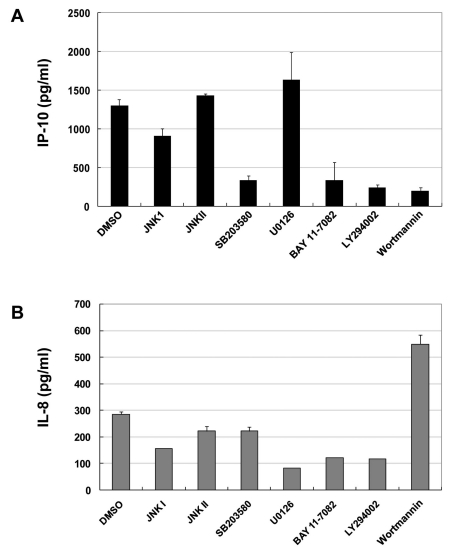
Identification of signaling molecules or transcription factors participating in production of IP-10 or IL-8
Several inhibitors including JNKI, JNKII, SB203580, U0126, BAY11-7082, LY294002, and wortmannin were used to further detail the important pathways to produce IP-10 and IL-8. Inhibitors were pretreated and poly(I:C) was administrated. ELISA revealed that IP-10 was decreased by either the p38 inhibitor (SB203580), PI3K inhibitor (LY294002, wortmannin), or NF-κB inhibitor (BAY11-7082) (Fig. 4A). IL-8 production was inhibited most effectively by treatment with ERK inhibitor (U0126).Additionally, NF-kB inhibitor (BAY11-7082) and PI3K inhibitor (LY294002) blocked the signaling to produce IL-8 (Fig. 4A). On the contrary, PI3K inhibitor (wortmannin) induced IL-8 production (Fig. 4B).
Figure 4.
PI3 kinase plays differential roles in the production of TLR3-mediated IP-10 and IL-8. A172 cells were incubated with medium alone or with poly(I:C) (10µg/ml) for 18 hrs in the absence or presence of various pharmacological inhibitors at the indicated concentrations: JNK I or II, SB203580, U0126, BAY11-7082, LY294002 and Wortmannin. Each inhibitor was added 45 min before poly(I:C) stimulation. After treatment with poly(I:C), supernatants were measured by ELISA for IP-10 production (A) and IL-8 production (B). Values represent the mean±S.E. of triplicate samples.
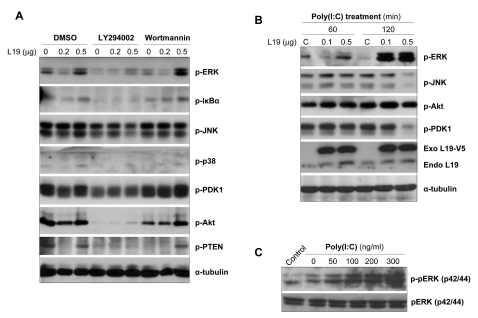
Effect of L19 on ERK phosphorylation
As shown above, L19 has an inhibitory effect on IP-10 production but an stimulatory effect on IL-8 production in A172 cells. We then attempted to identify the molecules responsible for IL-8 induction in cooperation with L19. A172 cells were transfected with L19 and then pretreated with LY294002 and wortmannin. Activation of ERK, IKBα, JNK, p38, PDK1, Akt, and PTEN were then assessed after poly(I:C) stimulation. Results showed that LY294002 inhibited activation of all the tested signaling molecules, whereas the decreased phosphorylation of ERK, NF-κB, PDK1, and PTEN by wortmannin was recovered by transfection with L19 in a dose-dependent manner (Fig. 5A).We also assessed the phosphorylation of ERK, JNK, Akt, and PDK from 1-12 hrs after poly(I:C) stimulation. Phosphorylation of ERK increased with transfection of L19 in a dose-dependent manner at 2 hrs (Fig. 5B) and at 12 hrs (Fig. 5C) after poly(I:C) treatment. However, activation of JNK and PDK decreased with transfection of L19 in a dose-dependent manner at 2 hrs after poly(I:C) treatment and phosphorylation of Akt increased at 1 hr after poly(I:C) treatment (Fig. 5B). These results suggest that L19 increases the activation of ERK, however decreases the activation of JNK and PDK.
Figure 5.
Effects of PI3K inhibitors together with L19 on TLR-mediated activation of intracellular signaling molecules. (A) A172 cells were transfected with empty vectors or V5-tagged L19 plasmid. 18 hrs after transfection, cells were stimulated with poly(I:C) (25µg/ml) in the presence or absence LY294002 (LY, 20µM) and wortmannin (Wort, 5µM) for 20 hrs and then total cell lysates analyzed by immunoblotting with anti-phospho-ERK, -IκBα, -JNK, -p38, -PDK1, -Akt, -PTEN antibodies. To confirm equal loading, membranes were re-probed with anti-α-tubulin antibody. (B) A172 cells were transfected with empty vectors or V5-tagged L19 plasmid. 20 hrs after transfection, cells were stimulated with poly(I:C) (25µg/ml) for 60 or 120 min (B) and 12 hrs (C) and then total cell lysates analyzed by immunoblotting with anti-phospho-ERK, -JNK, -PDK1, -Akt antibodies. The expression level of exogenous or endogenous L19 in the cell lysates were analyzed with anti-L19 monoclonal antibody. To confirm equal loading, membranes were re-probed with anti-α-tubulin antibody.
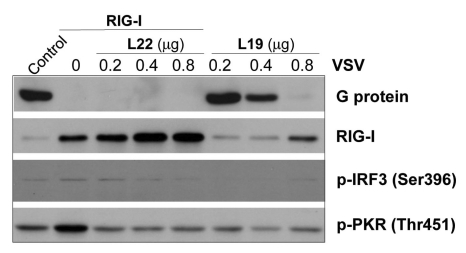
Effect of L19 on anti-viral response in association with L22
Next we demonstrated the effect of L19 on VSV infection using A549 cells originating from lung epithelial cells, where the major expression site of TLR3 is at the cell membrane. RIG expression was also inhibited by introducing shRNA into these cells. VSV multiplication was then evaluated by measuring levels of viral G protein 24 hrs after initial viral infection. When RIG-1 and L22 were transfected together, viral multiplication was inhibited and RIG-1 activation was induced (Fig. 6).
Figure 6.
Effect of L19 together with L22 in anti-viral immunity. A549 cells treated with RIG-I siRNAs were transfected with L19 or L22. 24 hrs after transfection, cells were infected with 1MOI VSV for 6 hrs and then extracts from VSV-infected cells were analyzed by western blotting with antibodies against G protein (VSV), RIG-I, phospho-IRF3 (Ser396) and phospho-PKR (Thr451).
When L19 were transfected in A549 cells, viral multiplication was inhibited (Fig. 6). Especially L19 induced RIG-1 expression and activation of IRF3 (Fig. 6). This finding is contrary to the results acquired with A172 cells and suggests that the effect of L19 varies with different cell types and the localization site of TLR3.
DISCUSSION
Recently the influence of TLR members has been reported in various inflammatory and immune disorders such as sepsis, asthma, atherosclerosis, Alzheimer's disease, and rheumatoid arthritis (15,16). With certain genetic predispositions, TLR signaling leads to overt symptoms in various autoimmune diseases, for example, systemic lupus erythematosus, multiple sclerosis, and inflammatory bowel disease (17). Therefore, TLR molecules and related signaling have become potential therapeutic targets and are under evaluation.
Here we report that L19 modulates TLR3 signaling induced by poly(I:C). In A172 cells, a glioblastoma cell line, over-expression of L19 inhibits production of IP-10 and increases production of IL-8. Generally TLR3 activation following viral infection increases production of TNF-α, IL-1β, IL-6, IP-10, and IL-8 to eliminate pathogens (18). However, this response is cell type specific. For example, TLR3 activation increased the production of TNF-α, RANTES, and MIP-1α in murine fetalskin-derived cultured mast cells (FSMC), whereas it induced production of IL-6 and IL-12, not chemokines, in microglial cells (19). In another experiment, respiratory syncytial virus (RSV) was infected into two different cells, MRC-5 (human lung fibroblasts) and A549 (lung epithelial cells) cells, respectively. In this particular study, chemokines such as IP-10, CCL5 and IL-8 were induced and the expression of TLR3 increased in A549 cells. However, TLR3 knock-down using RNAi decreased production of IP-10 and CCL5, whereas IL-8 was either not affected or rather increased in MRC5 cells (20). All these reports suggest that signaling induced by TLR3 differs with cell types.
TLR3 signaling induces activation of IKK, ERK, and JNK and further produces TNF-α, IL-1β, IL-6, and IL-8. The pharmacological inhibitor of JNK, SP600125, blocked production of IP-10 and IL-8. The ERK inhibitor study suggests ERK is responsible for inhibiting IL-8, but not IP-10 (21,22). PI3K is necessary for full activation of IRF3, an important transcription factor involved in the production of IFN-β and IP-10, whereas it inhibits activation of GSK-3β and ERK. During TLR3 signaling PI3K contributes to IP-10 production by activating IRF3, however it inactivates GSK-3β and ERK to decrease IL-8 production (23). All of these studies demonstrate that the end products of TLR3 signaling are affected by major signaling pathways and that each responds in a different manner.
First, to identify which signaling molecules were responsible for IP-10 and IL-8 production in association with L19, inhibitors were used against JNKI, JNKII, EKR, p38, PI3K, and NF-kB in A172 cells. As a result, we found that through activation of both NF-κB and ERK, L19 further enhanced the increase in production of IL-8 by poly(I:C). When L19 was over-expressed, the phosphorylation of IκB-α and ERK increased with L19 in a dose dependent manner. Moreover, knock-down of L19 lead to decreased phosphorylation of IκB-α at an early stage after poly(I:C) treatment. The finding that IRF3-dependent IP-10 production was inhibited by either LY294002 or wortmannin suggests that the PI3K pathway is affected by L19 involvement. Furthermore, phosphorylation of IRF3 increased with knock-down of L19. These results suggest that introduction of L19 into cells inhibits the TLR3-TRIF-PI3K-IRF3 pathway and the production of IP-10. At the same time, L19 induces activation of the TLR3-TRIF-NF-κB and ERK pathway and production of IL-8. Although LY294002 and wortmannin belong to the same family of PI3K inhibitors, the underlying mechanism of action is different. Wortmannin inhibits myosin light chain kinases and PI3K-related protein kinases, whereas LY294002 is the effective inhibitor for casein kinase2 (24). This explains our discordant result showing that TLR3-mediated cytokine production increases with wortmannin treatment, but decreases with LY294002.
Next, A549 cells, lung epithelial cells, were infected with VSV. Introduction of L19 inhibited VSV replication. Another ribosomal protein, L22, also blocked VSV multiplication. These results are contrary to those found with A172 cells, in which L19 induced inactivation of the TLR3-TRIF-PI3K-IRF3 pathway and decreased the production of IP-10. These findings demonstrate that the effect of L19 on TLR3 signaling is cell type-specific and dependent upon the localization of TLR3 in cells.
Therefore, with these results we suggest that L19 could have a pivotal role in viral multiplication dependent upon cell type and may be involved in the pathogenesis of autoimmune diseases and chronic inflammatory diseases.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (Grant No: 531-2008-1-E00028 and 532-2008-1-E00013) and also by the Brain Korea 21 Project for Medical Science, Yonsei University.
Footnotes
The author have no financial conflict of interest.
References
- 1.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 2.Diamond MS, Klein RS. West Nile virus: crossing the blood-brain barrier. Nat Med. 2004;10:1294–1295. doi: 10.1038/nm1204-1294. [DOI] [PubMed] [Google Scholar]
- 3.Le Goffic R, Balloy V, Lagranderie M, Alexopoulou L, Escriou N, Flavell R, Chignard M, Si-Tahar M. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2:e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, Si-Tahar M. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem. 2005;280:5571–5580. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- 5.Rudd BD, Smit JJ, Flavell RA, Alexopoulou L, Schaller MA, Gruber A, Berlin AA, Lukacs NW. Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J Immunol. 2006;176:1937–1942. doi: 10.4049/jimmunol.176.3.1937. [DOI] [PubMed] [Google Scholar]
- 6.Thomas KW, Monick MM, Staber JM, Yarovinsky T, Carter AB, Hunninghake GW. Respiratory syncytial virus inhibits apoptosis and induces NF-kappa B activity through a phosphatidylinositol 3-kinase-dependent pathway. J Biol Chem. 2002;277:492–501. doi: 10.1074/jbc.M108107200. [DOI] [PubMed] [Google Scholar]
- 7.Basu D, Walkiewicz MP, Frieman M, Baric RS, Auble DT, Engel DA. Novel influenza virus NS1 antagonists block replication and restore innate immune function. J Virol. 2009;83:1881–1891. doi: 10.1128/JVI.01805-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng L, Dai P, Parikh T, Cao H, Bhoj V, Sun Q, Chen Z, Merghoub T, Houghton A, Shuman S. Vaccinia virus subverts a mitochondrial antiviral signaling protein-dependent innate immune response in keratinocytes through its double-stranded RNA binding protein, E3. J Virol. 2008;82:10735–10746. doi: 10.1128/JVI.01305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung DW, Ginder ND, Fulton DB, Nix J, Basler CF, Honzatko RB, Amarasinghe GK. Structure of the Ebola VP35 interferon inhibitory domain. Proc Natl Acad Sci U S A. 2009;106:411–416. doi: 10.1073/pnas.0807854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maisnier-Patin S, Paulander W, Pennhag A, Andersson DI. Compensatory evolution reveals functional interactions between ribosomal proteins S12, L14 and L19. J Mol Biol. 2007;366:207–215. doi: 10.1016/j.jmb.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 11.Bee A, Ke Y, Forootan S, Lin K, Beesley C, Forrest SE, Foster CS. Ribosomal protein l19 is a prognostic marker for human prostate cancer. Clin Cancer Res. 2006;12(7 Pt 1):2061–2065. doi: 10.1158/1078-0432.CCR-05-2445. [DOI] [PubMed] [Google Scholar]
- 12.Fok V, Mitton-Fry RM, Grech A, Steitz JA. Multiple domains of EBER 1, an Epstein-Barr virus noncoding RNA, recruit human ribosomal protein L22. RNA. 2006;12:872–882. doi: 10.1261/rna.2339606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elia A, Vyas J, Laing KG, Clemens MJ. Ribosomal protein L22 inhibits regulation of cellular activities by the Epstein-Barr virus small RNA EBER-1. Eur J Biochem. 2004;271:1895–1905. doi: 10.1111/j.1432-1033.2004.04099.x. [DOI] [PubMed] [Google Scholar]
- 14.Baril M, Racine ME, Penin F, Lamarre D. MAVS dimer is a crucial signaling component of innate immunity and the target of hepatitis C virus NS3/4A protease. J Virol. 2009;83:1299–1311. doi: 10.1128/JVI.01659-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finberg RW, Kurt-Jones EA. Viruses and Toll-like receptors. Microbes Infect. 2004;6:1356–1360. doi: 10.1016/j.micinf.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Bowie AG, Haga IR. The role of Toll-like receptors in the host response to viruses. Mol Immunol. 2005;42:859–867. doi: 10.1016/j.molimm.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Schröder NW, Schumann RR. Single nucleotide polymorphisms of Toll-like receptors and susceptibility to infectious disease. Lancet Infect Dis. 2005;5:156–164. doi: 10.1016/S1473-3099(05)01308-3. [DOI] [PubMed] [Google Scholar]
- 18.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 19.Matsushima H, Yamada N, Matsue H, Shimada S. TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-derived mast cells. J Immunol. 2004;173:531–541. doi: 10.4049/jimmunol.173.1.531. [DOI] [PubMed] [Google Scholar]
- 20.Rudd BD, Burstein E, Duckett CS, Li X, Lukacs NW. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J Virol. 2005;79:3350–3357. doi: 10.1128/JVI.79.6.3350-3357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park C, Lee S, Cho IH, Lee HK, Kim D, Choi SY, Oh SB, Park K, Kim JS, Lee SJ. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia. 2006;53:248–256. doi: 10.1002/glia.20278. [DOI] [PubMed] [Google Scholar]
- 22.Kutsch O, Oh J, Nath A, Benveniste EN. Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 tat in astrocytes. J Virol. 2000;74:9214–9221. doi: 10.1128/jvi.74.19.9214-9221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 24.Hazeki K, Nigorikawa K, Hazeki O. Role of phosphoinositide 3-kinase in innate immunity. Biol Pharm Bull. 2007;30:1617–1623. doi: 10.1248/bpb.30.1617. [DOI] [PubMed] [Google Scholar]