Abstract
Recent reports challenge the widely accepted idea that drought may offer protection against ozone (O3) damage in plants. However, little is known about the impact of drought on the magnitude of O3 tolerance in winter wheat species. Two winter wheat species with contrasting sensitivity to O3 (O3 tolerant, primitive wheat, T. turgidum ssp. durum; O3 sensitive, modern wheat, T. aestivum L. cv. Xiaoyan 22) were exposed to O3 (83ppb O3, 7h d−1) and/or drought (42% soil water capacity) from flowering to grain maturity to assess drought-induced modulation of O3 tolerance. Plant responses to stress treatments were assessed by determining in vivo biochemical parameters, gas exchange, chlorophyll a fluorescence, and grain yield. The primitive wheat demonstrated higher O3 tolerance than the modern species, with the latter exhibiting higher drought tolerance than the former. This suggested that there was no cross-tolerance of the two stresses when applied separately in these species/cultivars of winter wheat. The primitive wheat lost O3 tolerance, while the modern species showed improved tolerance to O3 under combined drought and O3 exposure. This indicated the existence of differential behaviour of the two wheat species between a single stress and the combination of the two stresses. The observed O3 tolerance in the two wheat species was related to their magnitude of drought tolerance under a combination of drought and O3 exposure. The results clearly demonstrate that O3 tolerance of a drought-sensitive winter wheat species can be completely lost under combined drought and O3 exposure.
Keywords: A–Ci curve parameters, chlorophyll a fluorescence, drought, gas exchange, ozone tolerance, stomatal conductance, winter wheat, yield
Introduction
Ozone (O3) episodes and incidences of drought often occur together during the reproductive stage of crops during the summer months over widespread areas of the world (Mittler, 2006; Ainsworth et al., 2008; Mittler and Blumwald, 2010). Although drought alone suppresses crop growth and yield, it is widely accepted that drought may reduce O3 injury in crop plants and forest species through drought-induced suppression of stomatal conductance (Tingey and Hogsett, 1985; Pearson and Mansfield, 1993; Karlsson et al., 1995; Reichenauer et al., 1998; Khan and Soja, 2003). However, the discovery of ethylene-dependent reductions in stomatal sensitivity to abscisic acid (ABA) under O3 stress indicates that stomatal conductance under soil water deficit is greater under elevated O3, and plants continue to lose water, despite the potential for dehydration and increased O3 flux (Wilkinson and Davies, 2010). This result conflicts with predictions that drought may offer some protection against O3 damage by inducing stomatal closure and restricting O3 uptake (Wittig et al., 2009; Wilkinson and Davies, 2010). Recent meta-analyses have demonstrated contrasting effects of elevated O3 on stomatal responses and growth under drought and well-watered conditions (Wittig et al., 2007, 2009). It is therefore suggested that more attention should be given to the interactions between O3 and other concurrently changing climatic variables such as drought, to assess the impacts of current and future climates on crop plants and natural vegetation accurately (Fuhrer, 2003; Wittig et al., 2009; Wilkinson and Davies, 2010).
Wheat is normally sensitive to O3, as documented by reductions in photosynthesis, growth, and yield (Mulholland et al., 1997; Heagle et al., 2000; McKee and Long, 2001; Biswas et al., 2008a). There are also considerable intra- and interspecific variations in O3 sensitivity in winter wheat (Biswas et al., 2008a, b) that can be greatly modified by other environmental variables (Biswas et al., 2009). It has been suggested that the high O3 tolerance of specific genotypes observed under favourable conditions could be reduced or even lost under changing climatic conditions (Fuhrer, 2003). While there have been a few studies to determine wheat responses to drought and O3 stresses in terms of growth and yield (Khan and Soja, 2003), little attention has been paid to the impact of a combination of drought and O3 on the photosynthetic processes and stomatal function of the flag leaves which provide the major contribution of assimilate to grain yield. In addition, there have been no studies of drought-induced modulation of O3 tolerance in winter wheats with differential sensitivity to O3. It is important to examine whether any cross-tolerance exists in winter wheat between O3 and drought stress, so that an appropriate breeding effort can be made to avoid yield reductions in the changing climatic conditions anticipated in the future (Mittler, 2006; Mittler and Blumwald, 2010).
Crop sensitivity to environmental stresses is typically assessed by the decline in growth and/or grain yield. Chlorophyll a fluorescence and gas exchange provide useful non-destructive tools for in vivo stress detection and are widely used to examine the effects of environmental stresses on photosynthesis (Guidi et al., 1997; Maxwell and Johnson, 2000). In addition, in vivo biochemical parameters provide more insights into photosynthetic processes and the mechanisms of O3/drought tolerance. Since the stomata are an important determinant for O3/drought effects on plant, the stomatal response to varying internal CO2 concentrations and light intensity could allow the examination of stomatal functioning in wheat exposed to drought and O3. Therefore, integration of in vivo biochemical parameters with gas exchange, chlorophyll a fluorescence, and grain yield data might improve the present understanding of the mechanisms underlying wheat responses to combined drought and O3 exposure.
Although understanding of cross-tolerance and the mechanisms responsible is crucial to predict agricultural yield under natural field conditions (Sabehat et al., 1998; Rizhsky et al., 2002; Tausz et al., 2007), little is known about whether any cross-tolerance exists in winter wheat species between drought and O3 stress. Since plants induce similar defence mechanisms to avoid oxidative stress resulting from both O3 and drought (Tausz et al., 2007), it was hypothesized first that O3-tolerant wheat species may have greater tolerance to drought. Secondly, it was hypothesized that drought might offer greater protection against O3 damage in O3-tolerant species than in O3-sensitive winter wheat species under combined drought and O3 exposure. One O3-tolerant and one O3-sensitive winter wheat species were therefore utilized to test these hypotheses. Plant responses to drought and/or O3 have been regarded as being determined by stomatal regulation and photosynthesis following simultaneous measurements of gas exchange and chlorophyll fluorescence, in vivo biochemical parameters, and yield components. The present results may be valuable in understanding crop adaptation to environmental stresses and prediction of food security under changing climatic conditions such as increased drought and O3 exposure.
Materials and methods
Plant establishment and treatments
An O3-sensitive modern winter wheat (Triticum aestivum L. cv. Xiaoyan 22) and an O3-tolarant primitive wheat (T. turgidum ssp. durum) were selected to assess drought-induced modulation of O3 tolerance. The study was carried out at the experimental station at the Institute of Botany of the Chinese Academy of Sciences. The O3 sensitivity of these two winter wheat species has been extensively evaluated in controlled greenhouse experiments reported elsewhere (Biswas et al., 2008a, b, 2009). Twenty germinated seeds were sown in each of 24 pots (15.0l) filled with clay loam soil for both species. Organic C, total N, total P, and total K in the soil were 1.24%, 0.045%, 296mg kg−1, and 14.7g kg−1, respectively. Seedlings were thinned to leave 10 plants per pot after stand establishment. The plants were initially grown outdoors in a greenhouse (October–April) under continental climate conditions (i.e. cold and dry winter) for natural vernalization. They experienced three snowfalls during the winter months at their vegetative stage. Irrigation was applied twice a week to maintain the soil near field capacity and avoid drought stress during plant growth outdoors in the greenhouse. At the jointing stage, all plants were top-dressed with N as urea at the rate of 15.0g N m−2. Pesticide was also applied at the rate of 10% of Imidachloroprid WP (Huayang Tech Co., Ltd, Shandong, China) once to control insect pests at the jointing stage. The weather was typical for early summer in Beijing during the post-flowering stage of wheat, with mean daily air temperature varying from 15°C (night) to 35°C (day) and a maximum photosynthetic photon flux density (PPFD) of ∼2000μmol m−2 s−1 at midday. Seasonal temperature varied from 3°C to 14°C, and seasonal relative humidity varied from 29% to 98%.
All 48 pots (24 pots per species) were moved to four open top chambers (OTCs; 1.2m in diameter and 1.6m in height) in a temperature-controlled double-glazed greenhouse at the flag leaf stage of wheat before initiating the water stress and O3 fumigation treatments. The OTCs were placed inside the greenhouse to avoid natural precipitation. The plants were allowed to acclimate for 1 week in the OTCs to adapt to the chamber environment before imposing the treatments. During this period, all plants received charcoal-filtered (CF) air (<5ppb O3). The maximum PPFD in the chambers was ∼1236μmol m−2 s−1. The temperature in the OTCs fluctuated from 17°C (night) to 33°C (day) and relative humidity ranged from 65% to 88% during the experiment runs.
The O3 and water stress treatments were initiated when 50% of the plants had begun to flower. O3 was generated by electrical discharge using ambient oxygen (Balaguer et al., 1995) and an O3 generator (CF-KG1, Beijing Sumsun Hi-Tech., Co. Ltd, China) and this was bubbled through distilled water before entering the two elevated O3 chambers to remove harmful compounds other than O3 (Balaguer et al., 1995). Manual mass flow controllers were used to regulate the flow of O3-enriched air to the OTCs. O3 concentrations in the OTCs were continuously monitored at ∼10cm above the plant canopy using an analyser (APOA-360, Horiba, Ltd, Japan), which was cross-calibrated before starting the O3 treatment. O3 concentration within the treatment chambers averaged 83±7ppb for 7h d−1 (10:00–17:00 h) over 3 weeks from 50% flowering to physiological maturity. The elevated O3 and CF air control treatments were assigned randomly to the chambers in each of the two blocks and were replicated twice. Plants in each chamber were subjected to two levels of soil water. For each species, 12 pots were irrigated to maintain soil water content (SWC) at 88±6% and the remaining 12 pots received a reduced supply of water to exert drought stress (SWC 42±4%). Soil water capacity was determined as the ratio of actual water content to maximum capillary capacity, and water content was adjusted gravimetrically on alternate days during water stress treatment (Khan and Soja, 2003). Plants receiving two levels of soil water, and O3 treatments were switched between chambers on alternate days to minimize the effects of environmental heterogeneity and variation between chambers on plant responses, with the location of the plants within the chambers being randomized on each occasion.
Simultaneous measurement of gas exchange and chlorophyll a fluorescence
Three plants of each species receiving the drought stress and well-irrigated treatments were selected from three different pots from each of the four OTCs (elevated O3 and CF air, replicated twice) on each sampling date for simultaneous measurements of gas exchange and chlorophyll fluorescence. These measurements were repeated three times at 7d intervals during the post-flowering stage of wheat species for all treatments. The measurement was initiated with the most recently fully expanded flag leaves of the main stem using a portable Gas Exchange Fluorescence System (GFS-3000, Heinz Walz, Germany) connected to a PC fitted with data acquisition software (GFS-Win). All measurements were kept consistent on the main stem flag leaves to minimize age-related heterogeneity of leaf tissue between the plants on each sampling date. The plant used was not used again for further measurements if any leaf injury resulted from the leaf chamber. The system was zeroed prior to each set of measurements. Relative humidity was maintained at 70% and leaf temperature in the leaf chamber was set at 25°C. The flow rate was set at 600mol s−1 and CO2 concentration in the leaf chamber maintained at 400ppm. The flag leaf was illuminated with a PPFD of 1500μmol m−2 s−1 using the internal chamber light. The A–Ci and A–PPFD response curves were recorded automatically for the same flag leaves using the A–Ci and A–PPFD response curve programs. The area of each individual flag leaf was calculated and entered into the automatic curve program prior to inserting the leaf into the leaf chamber. For A–Ci curves, the steady-state rate of net photosynthesis under saturating irradiance (Asat) was determined at external CO2 concentrations of 400, 300, 200, 100, 50, 400, 400, 600, and 800ppm. The duration of each step of the A–Ci response curves was 4min and data were automatically recorded six times to check stability of data.
On the other hand, the A–PPFD response curve was programmed to determine both gas exchange and chlorophyll fluorescence parameters. A–PPFD response curves were recorded at PPFDs of 1800, 1500, 1000, 500, 300, 150, 80, 50, 20, and 0 μmol m−2 s−1 at the leaf surface. At each PPFD, CO2 assimilation, stomatal conductance, steady-state fluorescence, and maximum and minimum fluorescence were recorded simultaneously. During A–PPFD response curve measurement, the CO2 concentration was maintained at 700ppm.
The duration of each step of the A–PPFD response curves was 3min and data were automatically recorded six times to check the stability of the data. After recording basic fluorescence parameters, the actual yield of photosystem II (PSII; Fv'/Fm') and the electron transport rate (ETR) were calculated according to Equation 1 and 2, respectively, as follows:
| (1) |
| (2) |
where Fm' and Fo' are the maximum and minimum fluorescence, respectively, at each light level. The factor of 0.5 was derived because transport of one electron requires the absorption of two quanta, while the factor of 0.84 was estimated based on the assumption that the proportion of incident quanta absorbed by the leaf was ∼84% (Meyer et al., 1997).
The data obtained for each flag leaf were analysed using a mechanistic A–Ci curve analysis program (Photosynthesis Assistant, Version 1.1, Dundee Scientific, UK), to obtain the maximum rate of carboxylation by Rubisco (Vcmax), the PAR-saturated rate of electron transport (Jmax), carboxylation efficiency (CE), and respiratory processes for day and night (R). The program followed the model proposed by Farquhar et al. (1980). On the other hand, data obtained as part of the gas exchange measurements included the area-based light-saturated net photosynthetic rate (Asat), stomatal conductance (gs), intercellular CO2 concentration (Ci), transpiration rate (E), and leaf-level photosynthetic water use efficiency, or instantaneous transpiration efficiency (ITE), which was calculated as assimilation/transpiration.
Grain yield, yield attributes, and harvest index
Three plants per pot for each species and each treatment combination (n=18) were harvested for determination of grain yield and yield attributes. Grains were removed from each ear by hand and the number of grains per ear was counted. Yield per ear, yield per plant, and 1000-grain weight were determined for sun-dried seeds. The harvest index (HI) was calculated as the ratio of grain dry mass to total above-ground dry mass per plant.
Statistical analysis
The experimental design consisted of two blocks, each containing one elevated O3 and one CF air chamber and each chamber contained plants experiencing two levels of soil moisture with 30 plants per replicate. Statistical analyses of data were performed using analysis of variance (ANOVA) in the general linear model procedure of the SPSS package (version 13, SPSS, Chicago, IL, USA). The main effects (species, O3, and drought) and their interactions were analysed using three-way ANOVA on the measured variables. Linear regression coefficients for the A–PPFD response curve and their significance (P-value) were also performed using the curve estimation program of SPSS. Differences between treatments were considered significant if P <0.05.
Results
Gas exchange
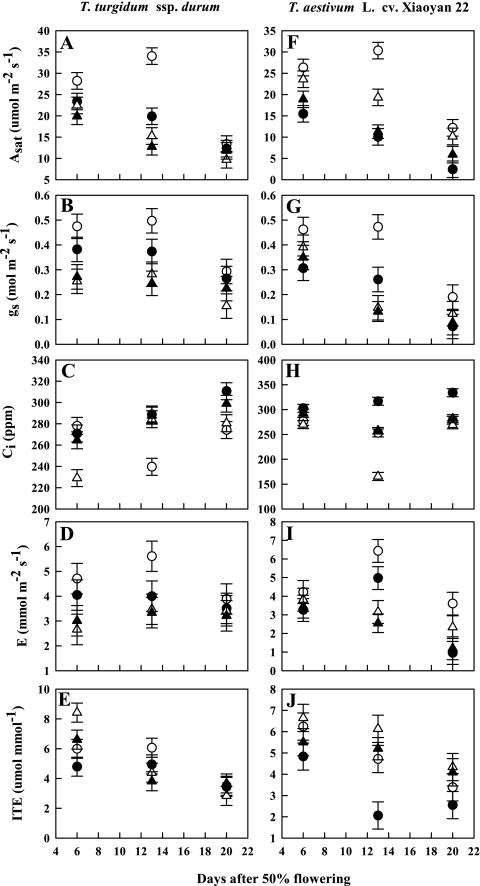
O3 significantly (P <0.05) decreased Asat, gs, E, and ITE, but increased Ci in both wheat species (Table 1). Water stress drastically (P <0.001) reduced Asat, gs, and Ci, but increased ITE. Overall, there were considerable interspecific variations (P <0.05) for Asat, gs, and E (Table 1). The primitive wheat displayed higher Asat, gs, and E values than modern species (data not shown). A significant (P <0.001) gradual decrease in gas exchange characteristics was also noted for both species. The interaction between water stress and species was significant (P <0.05) for Asat, Ci, and ITE. For instance, drought reduced Asat and ITE by 34% and 2%, respectively, in the primitive wheat, whereas it decreased Asat by 21% and increased ITE by 22% in the modern species. The interaction between O3 and species was significant (P <0.05) for Asat, Ci, gs, E, and ITE. The primitive species showed O3-induced reductions in gs and Asat of 18% and 22%, respectively, compared with 47% and 63% in the modern species. Elevated O3 increased Ci in the primitive and modern species by 10% and 18%, respectively. The interaction between water stress and O3 was significant (P <0.05) for Asat and gs. The primitive wheat exhibited an O3-induced reduction in Asat in drought-stressed plants of 37%, whereas the modern wheat demonstrated a reduction of 29%. Similarly, elevated O3 reduced gs in water-stressed primitive wheat plants by 26%, while the corresponding reduction in the modern wheat was 22%.
Table 1.
Analysis of variance of the effects of O3, drought, species, growth stage, and their interactions on photosynthetic gas exchange and A–Ci curve parameters of a primitive wheat, T. turgidum ssp. durum, and a modern wheat, T. aestivum L. cv. Xiaoyan 22
P-values were calculated using the general linear model with the main effects of O3, drought, species, growth stage, and their interactions. Two wheat species were well irrigated (88±6% SWC) and exposed to CF air (5±0ppb O3, 7 h d−1) or elevated O3 (83±7ppb O3 7 h d−1), or subjected to drought stress (42±4% SWC) and exposed to CF air or elevated O3 for 3 weeks during the post-flowering stage in open top chambers.
| Parameters | Ozone (O3) | Drought (ws) | Species (sp) | Stage (st) | O3×sp | ws×sp | O3×ws | O3×ws×sp | O3×ws×sp×st |
| Asat | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 | 0.003 | 0.000 | 0.477 | 0.277 |
| gs | 0.012 | 0.000 | 0.019 | 0.000 | 0.045 | 0.412 | 0.026 | 0.375 | 0.776 |
| Ci | 0.000 | 0.000 | 0.777 | 0.000 | 0.006 | 0.000 | 0.624 | 0.554 | 0.000 |
| E | 0.009 | 0.000 | 0.044 | 0.000 | 0.057 | 0.714 | 0.172 | 0.572 | 0.822 |
| ITE | 0.003 | 0.004 | 0.534 | 0.000 | 0.411 | 0.017 | 0.200 | 0.393 | 0.837 |
| Vcmax | 0.000 | 0.010 | 0.000 | 0.000 | 0.001 | 0.093 | 0.004 | 0.874 | 0.244 |
| Jmax | 0.000 | 0.586 | 0.001 | 0.000 | 0.000 | 0.207 | 0.208 | 0.229 | 0.290 |
| R | 0.000 | 0.435 | 0.008 | 0.000 | 0.202 | 0.006 | 0.277 | 0.096 | 0.942 |
| CE | 0.000 | 0.089 | 0.019 | 0.000 | 0.009 | 0.144 | 0.005 | 0.108 | 0.433 |
In vivo photosynthetic biochemical properties
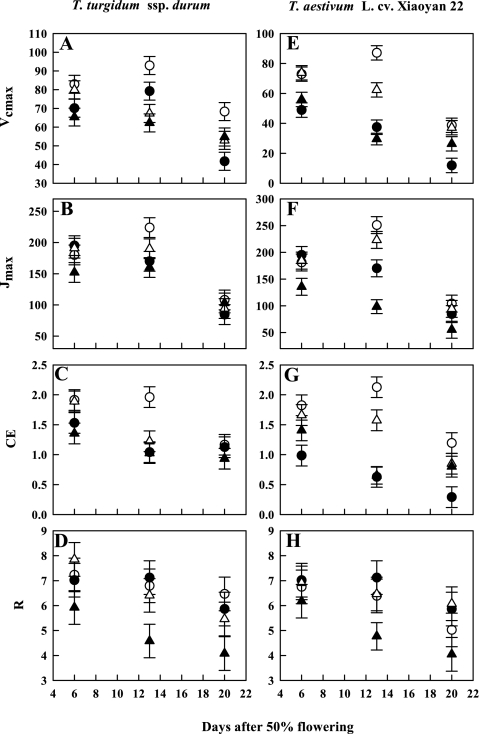
Elevated O3 decreased in vivo photosynthetic biochemical variables including Vcmax, Jmax, R, and CE (P <0.001; Fig. 2) in both wheat species. Reduced water supply also considerably (P <0.1) decreased Vcmax and CE, but had no effect on Jmax and R. Sampling date had a profound effect (P <0.001) on in vivo photosynthetic biochemical traits, with gradual reductions in Vcmax, Jmax, R, and CE being noted in both species. The primitive wheat had significantly higher Vcmax, Jmax, R, and CE values than the modern species. The interaction between O3 and species was significant (P <0.01) for Vcmax, Jmax, R, and CE. For instance, O3-induced reductions in Vcmax, Jmax, and CE in the primitive wheat were 23, 13, and 24%, while the corresponding reductions in the modern wheat were 53, 59, and 64%. The interaction between drought and species was significant (P <0.1) only for Vcmax and R. Water stress reduced Vcmax and R in the primitive species by 18% and 4%, respectively, but decreased Vcmax by 10% and increased R by 8% in the modern wheat. The water stress×O3 interaction was also significant (P <0.01) for Vcmax and CE. The primitive species showed an O3-induced reduction in Vcmax and CE in drought-stressed plants of 3% and 12%, respectively, whereas the modern wheat showed an O3-induced increase in Vcmax and CE in drought-stressed plants of 14% and 32%, respectively.
Fig. 2.
Impact of elevated O3 and/or drought stress on photosynthetic biochemical properties (in vivo) of the flag leaves of a primitive wheat, T. turgidum ssp. durum, and a modern wheat, T. aestivum L. cv. Xiaoyan 22. Both species were exposed to stress treatments for 3 weeks during the post-flowering stage in open top chambers. Treatments were well irrigated and exposed to CF air (open circles) or elevated O3 (filled circles), or drought stressed and exposed to CF air (open triangles) or elevated O3 (filled triangles). Vcmax, Jmax, CE, and R indicate the maximum rate of carboxylation by Rubisco, the PAR-saturated rate of electron transport, carboxylation efficiency, and respiratory processes for day and night, respectively. Error bars indicate the SEM. n=6.
Fig. 1.
Impact of elevated O3 and/or drought stress on gas exchange characteristics of the flag leaves of a primitive wheat, T. turgidum ssp. durum, and a modern wheat, T. aestivum L. cv. Xiaoyan 22. Both species were exposed to stress treatments for 3 weeks during the post-flowering stage in open top chambers. Treatments were well irrigated (88±6% SWC) and exposed to CF air (5±0ppb O3, open circles) or elevated O3 (83±7ppb O3, filled circles), or drought stressed (42±4% SWC) and exposed to CF air (open triangles) or elevated O3 (filled triangles). Error bars indicate the SEM. n=6.
Photosynthesis and stomatal conductance at varying internal CO2 pressures
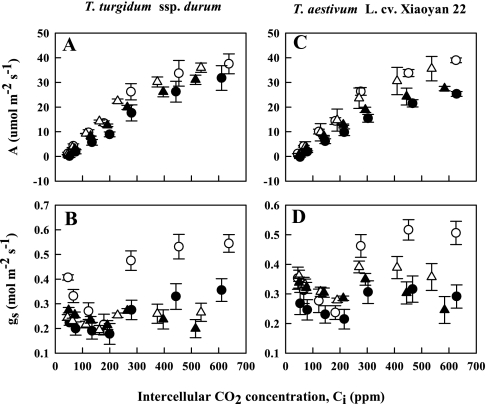
The photosynthetic rates (A) of both wheat species increased with increasing internal Ci for all treatment combinations (Fig. 3). The primitive wheat showed a smaller O3-induced reduction in A relative to control plants than the modern species at higher internal Ci. Initially gs was relatively high at very low Ci values in both species regardless of treatment, but gradually decreased with increasing Ci up to 200ppm, and afterwards it increased again at high Ci values. The well-watered plants of both wheat species showed a gradual increase in gs from 300ppm to 600ppm in the CF air treatment. However, the well-watered plants of the primitive wheat exposed to elevated O3 showed a higher increase in gs than that of the modern wheat as Ci increased from 300ppm to 600ppm. The drought-stressed plants of the primitive wheat maintained lower gs values than those of the modern wheat under CF air or elevated O3 at high Ci values.
Fig. 3.
Photosynthetic rate (A) and stomatal conductance (gs) in the flag leaves of a primitive wheat, T. turgidum ssp. durum, and a modern wheat, T. aestivum L. cv. Xiaoyan 22, as affected by intercellular CO2 concentrations, Ci (ppm), on the 13th day after initiation of stress treatments. Both species were exposed to drought and/or O3 for 3 weeks during the post-flowering stage in open top chambers. Treatments were well irrigated and exposed to CF air (open circles) or elevated O3 (filled circles), or drought stressed and exposed to CF air (open triangles) or elevated O3 (filled triangles). Error bars indicate the single SEM. n=6.
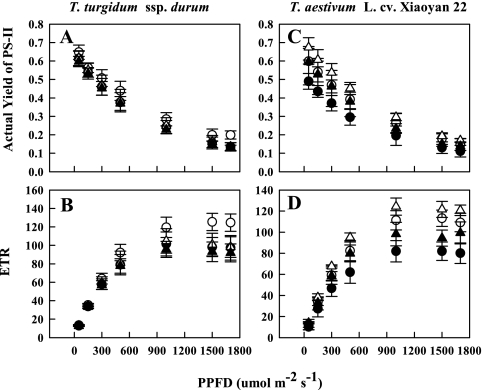
Response of photosynthesis, stomatal conductance, actual yield, and electron transport rate to PPFD
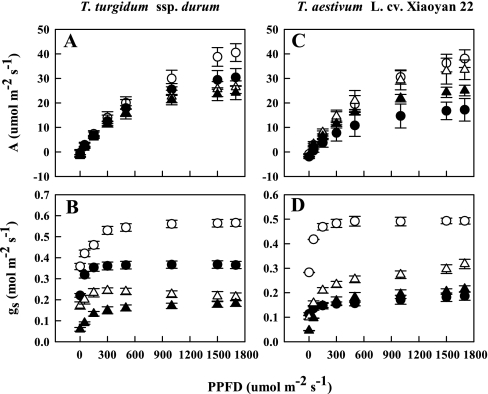
The photosynthesis rate (A) increased with increasing PPFD in both species regardless of treatment (Fig. 4). There were no detectable differences among treatments at low PPFD, but considerable differences were apparent at high PPFD in both species (Table 2). The initial slope of the A–PPFD response curve for O3-treated plants of the primitive wheat (b=0.037, P=0.001) was greater than that for the modern wheat (b=0.025, P <0.05). Similarly, the initial slope of the A–PPFD response curve in water-stressed plants of the modern wheat (b=0.044, P <0.05) was greater than that for the primitive wheat (b=0.035, P <0.05). However, the gradient of the A–PPFD response curve in both species remained similar under combined drought and O3 stress.
Fig. 4.
Photosynthetic rate (A) and stomatal conductance (gs) in the flag leaves of a primitive wheat, T. turgidum ssp. durum, and a modern wheat, T. aestivum L. cv. Xiaoyan 22, as affected by photosynthetic photon flux densities (PPFDs) on the 13th day after initiation of stress treatments. Both species were exposed to drought and/or O3 for 3 weeks during the post-flowering stage in open top chambers. Treatments were well irrigated and exposed to CF air (open circles) or elevated O3 (filled circles), or drought stressed and exposed to CF air (open triangles) or elevated O3 (filled triangles). Error bars indicate the SEM. n=6.
Table 2.
Slope (b), intercept (c), R2, and P-value of the linear part of the response of flag leaf photosynthesis, stomatal conductance, actual yield of PSII, and electron transport rate to PPFD of a primitive wheat, T. turgidum ssp. durum, and a modern wheat, T. aestivum L. cv. Xiaoyan 22, on the 13th day after initiation of drought and/or O3 treatments
Both species were well irrigated (88±6% SWC) and exposed to CF air (5±0ppb O3, 7 h d−1) or elevated O3 (83±7ppb O3 7 h d−1), or subjected to drought stress (42±4% SWC) and exposed to CF air or elevated O3 for 3 weeks during the post-flowering stage in open top chambers.
| Parameter | T. turgidum ssp. durum | T. aestivum cv. Xiaoyan 22 | ||||
| y=bx+c | R2 | P-value | y=bx+c | R2 | P-value | |
| (a) Photosynthesis | ||||||
| CF air | y=0.040x+0.67 | 0.987 | 0.001 | y=0.040x+0.32 | 0.994 | 0.001 |
| O3 | y=0.037x+0.42 | 0.980 | 0.001 | y=0.025x–0.85 | 0.982 | 0.003 |
| Drought | y=0.035x–0.27 | 0.974 | 0.002 | y=0.044x+0.23 | 0.986 | 0.002 |
| O3+drought | y=0.034x–0.07 | 0.970 | 0.002 | y=0.034x+0.16 | 0.983 | 0.003 |
| (b) Stomatal conductance | ||||||
| CF air | y=1.187x+36 | 0.998 | 0.010 | y=1.137x+314 | 0.903 | 0.283 |
| O3 | y=0.808x+24 | 0.802 | 0.294 | y=0.212x+118 | 0.933 | 0.235 |
| Drought | y=0.391x+18 | 0.980 | 0.089 | y=0.703x+109 | 0.952 | 0.140 |
| O3+drought | y=0.487x+61 | 0.993 | 0.053 | y=0.652x+53 | 0.982 | 0.123 |
| (c) Actual yield | ||||||
| CF air | y= –4×10–4x+0.65 | 0.958 | 0.021 | y= –5×10–4x+0.61 | 0.996 | 0.004 |
| O3 | y= –5×10–4x+0.63 | 0.984 | 0.008 | y= –4×10–4x+0.51 | 0.997 | 0.003 |
| Drought | y= –5×10–4x+0.63 | 0.990 | 0.005 | y= –5×10–4x+0.69 | 0.996 | 0.004 |
| O3+drought | y= –5×10–4x+0.61 | 0.99 | 0.005 | y= –5×10–4x+0.61 | 0.995 | 0.005 |
| (d) ETR | ||||||
| CF air | y=0.174x+7.69 | 0.995 | 0.005 | y=0.154x+8.61 | 0.991 | 0.009 |
| O3 | y=0.145x+9.56 | 0.989 | 0.011 | y=0.114x+8.21 | 0.985 | 0.015 |
| Drought | y=0.149x+ 9.56 | 0.989 | 0.011 | y=0.178x+9.10 | 0.992 | 0.008 |
| O3+drought | y=0.143x+ 9.40 | 0.989 | 0.001 | y=0.149x+8.72 | 0.990 | 0.010 |
In general, gs increased with increasing PPFD regardless of species and treatment (Fig. 4). There were considerable treatment effects on stomatal function in both species (Table 2). The initial gradient of the relationship between gs and PPFD was much higher in ozonated plants of the primitive wheat (b=0.808, P=0.294) than in those of the modern wheat (b=0.212, P=0.235). The initial slope of the gs–PPFD response curve in the plants of the primitive species (b=0.391, P <0.1) was lower than in those of the modern wheat (b=0.703, P=0.140) under drought. Similarly, the gradient of the gs–PPFD response curve in the plants of the primitive wheat (b=0.487, P <0.1) was lower than in those of the modern wheat (b=0.625, P=0.123) under combined drought and O3 exposure.
The actual yield of PSII and ETR decreased and increased, respectively, with increasing PPFD regardless of species and treatment (Fig. 5). There were no detectable treatment differences in the initial gradient of the relationship between actual yield of PSII and PPFD in both species. However, there were considerable treatment differences in the initial slope of the ETR–PPFD response curve in both species. The primitive wheat (b=0.145, P <0.05) showed a higher gradient of the ETR–PPFD response curve than the modern wheat (b=0.114, P <0.05) under elevated O3. The modern wheat (b=0.178, P <0.01) displayed a higher gradient of the ETR–PPFD response curve than the primitive wheat (b=0.149, P <0.05) under drought. However, both wheat species showed an almost similar initial slope of the ETR–PPFD response curve under combined drought and O3 exposure.
Fig. 5.
Actual yield of PSII and electron transport rate (ETR) in the flag leaves of a primitive wheat, T. turgidum ssp. durum, and a modern wheat, T. aestivum L. cv. Xiaoyan 22, as affected by photosynthetic photon flux densities (PPFDs) on the 13th day of initiation of stress treatments. Both species were exposed to drought and/or O3 for 3 weeks during the post-flowering stage in open top chambers. Treatments were well irrigated and exposed to CF air (open circles) or elevated O3 (filled circles), or drought stressed and exposed to CF air (open triangles) or elevated O3 (filled triangles). Error bars indicate the SEM. n=6.
Yield and yield attributes
Elevated O3 decreased (P <0.05) 1000-grain weight, yield per ear, yield per plant, and HI, but had no impact on ears per plant and seeds per ear (Table 3). Water stress significantly (P <0.05) reduced 1000-grain weight, yield per ear, and yield per plant, but had no effect on ears per plant, seeds per ear, and HI (Table 2). Overall, there were noteworthy differences (P <0.05) between the two species for the grain yield and yield attributes investigated (Table 3). The modern wheat had a greater number of seeds per ear, 1000-grain weight, yield per ear, yield per plant, and HI than the primitive wheat. The number of ears per plant was higher in primitive wheat than in the modern wheat. The species×water stress interaction was significant (P <0.05) for 1000-grain weight as the primitive wheat showed a smaller 1000-grain weight than the modern wheat under drought. The species×O3 interaction was significant (P <0.01) for seeds per ear, seed yield per ear, and HI because the primitive wheat exhibited higher levels of seeds per ear, seed yield per ear, and HI than the modern wheat at elevated O3. A strong interaction (P=0.001) between water stress and O3 was detected for HI. The species×water stress×O3 interaction was not significant for any yield parameter except 1000-grain weight and HI (P <0.05).
Table 3.
Yield and yield attributes of a primitive wheat, T. turgidum ssp. durum, and a modern wheat, T. aestivum L. cv. Xiaoyan 22 Both species were well-irrigated (88±6% SWC) and exposed to CF air (5±0 ppb O3, 7 h d−1) or elevated O3 (83±7 ppb O3 7 h d−1), or subjected to drought stress (42±4% SWC) and exposed to CF air or elevated O3 for 3 weeks during the post-flowering stage in open top chambers. Mean (±SEM). n=18. Similar letters indicate a non-significant difference at P <0.05.
| Wheat species | Water level (% SWC) | O3 concentation (ppb) | Ears per plant (n) | Seeds per ear (n) | 1000-grain weight (g) | Yield per ear (g) | Yield per plant (g) | HI | |
| 88±6 | 5±0 | 3.00±0.15 a,b | 18.73±1.39 | 23.83±1.32 a | 0.46±0.05 | 1.34±0.12 a | 0.37±0.01 | ||
| 88±6 | 83±7 | 2.61±0.14 b,c | 18.85±1.31 | 21.98±1.24 a,b | 0.43±0.05 | 1.11±0.11 a,b | 0.35±0.01 | ||
| T. turgidum ssp. durum | 42±4 | 5±0 | 3.18±0.14 a | 16.21±1.35 | 19.25±1.28 b,c | 0.33±0.05 | 0.95±0.12 b | 0.33 ±0.01 | |
| 42±4 | 83±7 | 2.44±0.15 c | 18.78±1.39 | 17.04±1.32 c | 0.36±0.05 | 0.84±0.12 b | 0.33±0.01 | ||
| 88±6 | 5±0 | 2.44±0.20 | 26.61±1.85 | 27.24±1.76 a | 0.73±0.07 a | 1.67±0.16 a | 0.48±0.02 a | ||
| T. aestivum L. cv. Xiaoyan 22 | 88±6 | 83±7 | 2.50±0.21 | 21.33±1.97 | 18.23±1.86 b | 0.41±0.07 b | 1.04±0.17 b | 0.33±0.02 c | |
| 42±4 | 5±0 | 2.22±0.20 | 23.65±1.85 | 23.17±1.76 a,b | 0.56 ±0.07 a,b | 1.28±0.16 a.b | 0.43±0.02 a,b | ||
| 42±4 | 83±7 | 2.55±0.18 | 20.27±1.68 | 22.98±1.59 a,b | 0.46±0.06 b | 1.14±0.14 b | 0.40±0.02 b | ||
| Source of variation (ANOVA) | |||||||||
| Species (sp) | 0.002 | 0.000 | 0.031 | 0.000 | 0.028 | 0.000 | |||
| Water stress (ws) | 0.720 | 0.316 | 0.044 | 0.050 | 0.017 | 0.383 | |||
| Ozone (O3) | 0.125 | 0.389 | 0.003 | 0.011 | 0.006 | 0.000 | |||
| sp×ws | 0.711 | 0.456 | 0.021 | 0.611 | 0.337 | 0.083 | |||
| sp×O3 | 0.002 | 0.004 | 0.238 | 0.008 | 0.279 | 0.002 | |||
| ws×O3 | 0.865 | 0.168 | 0.054 | 0.084 | 0.128 | 0.001 | |||
| sp×ws×O3 | 0.205 | 0.579 | 0.036 | 0.306 | 0.343 | 0.045 | |||
Discussion
Physiological and yield response of winter wheat species to O3
The winter wheat species examined displayed significant reductions in Asat, gs,Vcmax, Jmax, R, and CE in response to O3, in general agreement with reports for spring wheat (Farage and Long, 1999; Cardoso-Vilhena et al., 2004). However, the results indicate that the O3-induced decrease in Asat might be due to both enzymatic and stomatal limitation as evidenced by the O3-induced reduction in gs and increase in Ci accompanied by decreases in Vcmax, CE, and R. The observed reduction in Asat and considerable decline in Vcmax indicated that these effects might be attributable to a decrease in the quantity of active Rubisco (Farage and Long, 1999; Cardoso-Vilhena et al., 2004). The photosynthetic rate under light- and CO2-saturating conditions may be significantly reduced by elevated O3, as indicated by the reduction in Jmax. This suggests that O3 restrained the capacity for regeneration of the primary CO2 acceptor, ribulose bisphosphate (RuBP), which depends on adequate synthesis of ATP and NADPH and hence on the rate of electron transport (Farage and Long, 1999).
There were significant differences (P=0.001) in the impacts of elevated O3 on photosynthesis between the modern and primitive species. The primitive species demonstrated higher Asat accompanied by lower Ci and higher gs and ITE values than the modern species at elevated O3. Although O3 uptake was greater in the primitive wheat than in the modern species, the former demonstrated greater levels of Rubisco and RuBP regeneration than the latter. Moreover, gs–PPFD curve analysis indicated that the primitive species had faster stomatal control (higher slope) than the modern species (lower slope) under elevated O3. The results obtained here suggest that higher mesophyll cell activity against O3-induced oxidative stress, but not O3 exclusion through stomatal closure, contributed to the observed higher O3 tolerance of the primitive species.
O3 significantly (P <0.05) decreased 1000-grain weight, yield per ear, yield per plant, and HI, in general agreement with previous studies for both spring and winter wheat (Pleijel et al., 2000; Khan and Soja, 2003). The observed reductions in grain yield in both species were entirely attributable to reductions in seed weight, rather than the number of seeds per ear. As found in the present study, reduction in 1000-grain weight also explained most of the reduction in grain yield following exposure of wheat to O3 in OTCs in previous studies (Ojanpera et al., 1998; Pleijel et al., 2000). The primitive wheat exhibited greater carbon partitioning to the grain, as demonstrated by its higher HI resulting from its greater ear yield, seeds per ear, and ears per plant relative to the modern species under elevated O3. These results demonstrating the differential sensitivity to O3 of these wheat species as determined by flag leaf photosynthesis and grain yield are fully consistent with their differential O3 sensitivity as determined by growth, photosynthetic, and anti-oxidative activities during the vegetative stage observed in previous investigations (Biswas et al., 2008a, b).
Physiological and yield response to drought
Both species showed significant (P <0.001) reductions in Asat and gs in response to drought. Water stress also decreased Vcmax and CE, but did not decrease Jmax and R significantly, in general agreement with a previous report for wheat (Martin and Ruiz-Torres, 1992). The decline of Vcmax in plants exposed to water stress has also been found by other authors (Wilson et al., 2000; Xu and Baldocchi, 2003) and suggests a predominant role for biochemical limitation during drought. However, it was found that drought-induced loss in Asat in winter wheat species might be attributed to a combination of reduced carboxylation efficiency and a decrease in Ci resulting from stomatal closure.
There were considerable differences (P <0.05) in drought tolerance between wheat species as documented by higher Asat and ITE values in the modern wheat than in the primitive wheat. There was also interspecific difference in the mechanism of drought-induced loss in Asat. For example, drought reduced Asat in the primitive wheat initially due to low Ci resulting from higher stomatal closure and later on due to biochemical limitation as Ci increased gradually over the drought period. In contrast, drought decreased Asat in the modern wheat mostly due to low Ci throughout the drought period. Higher drought tolerance in the modern wheat can be further demonstrated by the higher slopes for the response of A, gs, and ETR to PPFD than that in the primitive wheat. The results agree with the previous study of Xiong et al. (2006), who demonstrated that drought resistance was significantly greater in hexaploid spring wheat than in the tetraploid or diploid wheat. This might conceivably be because winter wheat cultivars in China, as elsewhere, were selected for improved resistance to drought, low temperatures, pests, and diseases (Jiang et al., 2003; Biswas et al., 2008a).
Water stress reduced (P <0.05) 1000-grain weight, yield per ear, and yield per plant in winter wheat, but did not significantly decrease the HI. The results are consistent with a previous report in which a winter wheat cultivar was exposed to drought stress (Khan and Soja, 2003). In the present study, considerable interspecific variation was noted in the negative impact of drought on 1000-grain weight and HI. While the primitive wheat displayed significant reductions in mean seed weight and HI, the modern wheat showed slight increases in these variables in response to drought stress. The higher yield stability of the modern winter wheat cultivar under drought found in the present study has also been documented in spring wheat (Xiong et al., 2006). The higher drought resistance of the modern wheat in terms of physiology and yield might be due to selection of a cultivar with improved drought tolerance (Jiang et al., 2003; Biswas et al., 2008a).
Differential drought-induced modulation of O3 tolerance under combination of drought and O3 exposure
It is widely accepted that water stress may reduce the effects of O3 by reducing stomatal opening and limiting O3 uptake (Tingey and Hogsett, 1985; Pearson and Mansfield, 1993; Karlsson et al., 1995; Reichenauer et al., 1998; Khan and Soja, 2003; McLaughlin et al., 2007). As a result, one of the main aims of the present study was to test whether drought offers greater protection against O3 damage in O3-tolerant species compared with O3-sensitive wheat. However, unlike expected, a different result was obtained when two wheat species were exposed to combined drought and O3 exposure. The primitive wheat tolerant to O3 showed greater O3-induced reduction in Asat due to higher loss of Rubisco and carboxylation efficiency in drought-stressed plants than that in the modern species sensitive to O3. The gs–PPFD curve analysis also suggests that there was higher O3-induced negative impact on guard cells, as indicated by slower stomatal control (lower slope) in drought-stressed plants of the primitive wheat than of the modern wheat (higher slope). The findings of differential drought-induced O3 tolerance in winter wheat species agree with the view that the response of a plant to a combination of two different abiotic stresses is unique and cannot be directly extrapolated from the response of plants to each of the different stresses applied individually (Mittler, 2006).
The differential drought-induced modulation of O3 effects on the photosynthetic processes in the flag leaves of the two winter wheat species was reflected by yield and yield attributes. The findings imply that drought reduced negative impacts of O3 on the photosynthetic processes and yield in the modern wheat, but not in the primitive species. Although it has been reported that drought protects against O3-induced yield reduction in one cultivar of winter wheat (Khan and Soja, 2003), it is clear from the present investigation that this was not the case for the primitive wheat species, which was tolerant to O3. The primitive wheat demonstrated a higher sensitivity to drought and lost its tolerance to O3 under a combination of drought and O3 exposure. In contrast, the modern species tolerant to drought displayed increased O3 tolerance under combined drought and O3 exposure in terms of gas exchange, chlorophyll a fluorescence, in vivo biochemical properties, and yield. These results suggest that the observed O3 tolerance in the two wheat species was related to their magnitude of drought tolerance under combination of drought and O3 exposure.
In conclusion, O3 significantly decreased Asat, gs, E, and ETR, but increased Ci in both winter wheat species. O3 also decreased Vcmax, Jmax, CE, and R. Drought decreased Asat, gs, and Ci, but increased ITE. Drought also decreased Vcmax and CE, but not Jmax and R. O3 reduced Asat through both biochemical and stomatal limitation in both species. Drought decreased Asat in the primitive wheat mostly due to biochemical limitation, while in the modern wheat it was mainly due to stomatal limitation. There were significant interspecific differences in winter wheat species in response to O3 or drought. The primitive wheat demonstrated higher O3 tolerance than the modern species, with the latter exhibiting higher drought tolerance than the former. A faster stomatal control was detected in O3-stressed plants of the primitive species than in those of the modern wheat. However, stomatal control became slower in the primitive wheat than in the modern species when O3 stress was combined with drought. Overall, the primitive species lost O3 tolerance, while the modern wheat exhibited improved tolerance to O3, suggesting that sensitivity to drought determined the magnitude of O3 tolerance in wheat species under combination of drought and O3 exposure. The findings demonstrate that the O3 tolerance of a drought-sensitive winter wheat species can be completely lost under combined drought and O3 exposure.
Acknowledgments
The authors wish to thank the anonymous reviewers for their valuable comments and suggestions on an early version of the manuscript. This study was funded by the Innovative Group Grant of Natural Science Foundation of China (No. 30521002).
References
- Ainsworth EA, Rogers A, Leakey ADB. Targets for crop biotechnology in a future high-CO2 and high-O3 world. Plant Physiology. 2008;147:13–19. doi: 10.1104/pp.108.117101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaguer L, Barnes JD, Panicucci A, Borland AM. Production and utilization of assimilate in wheat (Triticum aestivum L.) leaves exposed to O3 and/or CO2. New Phytologist. 1995;129:557–568. [Google Scholar]
- Biswas DK, Xu H, Li YG, Liu MZ, Chen YH, Sun JZ, Jiang GM. Assessing the genetic relatedness of higher ozone sensitivity of modern wheat to its wild and cultivated progenitors/relatives. Journal of Experimental Botany. 2008b;59:951–963. doi: 10.1093/jxb/ern022. [DOI] [PubMed] [Google Scholar]
- Biswas DK, Xu H, Li YG, Sun JZ, Wang XZ, Han XG, Jiang GM. Genotypic differences in leaf biochemical, physiological and growth responses to ozone in 20 winter wheat cultivars released over the past 60 years. Global Change Biology. 2008a;14:46–59. [Google Scholar]
- Biswas DK, Xu H, Yang JC, et al. Impacts of methods and sites of plant breeding on ozone sensitivity in winter wheat cultivars. Agriculture, Ecosystems and Environment. 2009;134:168–177. [Google Scholar]
- Cardoso-Vilhena J, Balaguer L, Eamus D, Ollerenshaw J, Barnes J. Mechanisms underlying the amelioration of O3-induced damage by elevated atmospheric concentrations of CO2. Journal of Experimental Botany. 2004;55:771–781. doi: 10.1093/jxb/erh080. [DOI] [PubMed] [Google Scholar]
- Farage PK, Long SP. The effects of O3 fumigation during leaf development on photosynthesis of wheat and pea: an in vivo analysis. Photosynthesis Research. 1999;59:1–7. [Google Scholar]
- Farquhar GD, Caemmerer von S, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149:78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- Fuhrer J. Agroecosystem responses to combination of elevated CO2, ozone, and global climate change. Agriculture, Ecosystems and Environment. 2003;97:1–20. [Google Scholar]
- Guidi L, Nali C, Ciompi S, Lorenzini G, Soldatini GF. The use of chlorophyll fluorescence and leaf gas exchange as methods for studying the different responses to ozone of two bean cultivars. Journal of Experimental Botany. 1997;48:173–179. [Google Scholar]
- Heagle AS, Miller JE, Pursley WA. Growth and yield response of winter wheat to mixtures of ozone and carbon dioxide. Crop Science. 2000;40:1656–1664. [Google Scholar]
- Jiang GM, Sun JZ, Liu HQ, Qu CM, Wang KJ, Guo RJ, Bai KZ, Gao LM, Kuang TY. Changes in the rate of photosynthesis accompanying the yield increase in wheat cultivars released in the past 50 years. Journal of Plant Research. 2003;116:347–354. doi: 10.1007/s10265-003-0115-5. [DOI] [PubMed] [Google Scholar]
- Karlsson PE, Medin E-L, Wickström H, Selldén G, Wallin G, Ottosson S, Skärby L. Ozone and drought stress: interactive effects on the growth and physiology of Norway spruce (Picea abies (L.) Karst.) Water, Air and Soil Pollution. 1995;85:1325–1330. [Google Scholar]
- Khan S, Soja G. Yield responses of wheat to ozone exposure as modified by drought-induced differences in ozone uptake. Water, Air and Soil Pollution. 2003;147:299–315. [Google Scholar]
- Martin B, Ruiz-Torres NA. Effects of water-deficit stress on photosynthesis, its components and component limitations, and on water use efficiency in wheat (Triticum aestivum L.) Plant Physiology. 1992;100:733–739. doi: 10.1104/pp.100.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence: a practical guide. Journal of Experimental Botany. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- McKee IF, Long SP. Plant growth regulators control ozone damage to wheat yield. New Phytologist. 2001;152:41–51. doi: 10.1046/j.0028-646x.2001.00207.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin SB, Nosal M, Wullschleger SD, Sun G. Interactive effects of ozone and climate on tree growth and water use in a southern Appalachian forest in the USA. New Phytologist. 2007;174:109–124. doi: 10.1111/j.1469-8137.2007.02018.x. [DOI] [PubMed] [Google Scholar]
- Meyer U, Kollner B, Willenbrink J, Krause GHM. Physiological changes on agricultural crops induced by different ambient ozone exposure regimes I. Effects on photosynthesis and assimilate allocation in spring wheat. New Phytologist. 1997;136:645–652. doi: 10.1046/j.1469-8137.1997.00777.x. [DOI] [PubMed] [Google Scholar]
- Mittler R. Abiotic stress, the field environment and stress combination. Trends in Plant Science. 2006;11:15–19. doi: 10.1016/j.tplants.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Mittler R, Blumwald E. Genetic engineering for modern agriculture: challenges and perspectives. Annual Review of Plant Biology. 2010;61:1–20. doi: 10.1146/annurev-arplant-042809-112116. [DOI] [PubMed] [Google Scholar]
- Mulholland BJ, Craigon J, Black CR, Colls JJ, Atherton J, Landon G. Effects of elevated carbon dioxide and ozone on the growth and yield of spring wheat (Triticum aestivum L.) Journal of Experimental Botany. 1997;48:113–122. [Google Scholar]
- Ojanperä K, Patsikka E, Ylaranta T. Effects of low ozone exposure of spring wheat on net CO2 uptake, Rubisco, leaf senescence and grain filling. New Phytologist. 1998;138:451–460. [Google Scholar]
- Pearson M, Mansfield TA. Interacting effects of ozone and water stress on the stomatal resistance of beech (Fagus sylvatica L.) New Phytologist. 1993;123:351–358. doi: 10.1111/j.1469-8137.1994.tb04249.x. [DOI] [PubMed] [Google Scholar]
- Pleijel H, Gelang J, Sild E, Danielsson H, Younis S, Per-Erik Karlsson PE, Wallin G, Skärby L, Selldén G. Effects of elevated carbon dioxide, ozone and water availability on spring wheat growth and yield. Physiologia Plantarum. 2000;108:61–70. [Google Scholar]
- Reichenauer TG, Goodman BA, Kostecki P, Soja G. Ozone sensitivity in Triticum durum and T. aestivum with respect to leaf injury and free radical content. Physiologia Plantarum. 1998;104:681–686. [Google Scholar]
- Rizhsky L, Liang HJ, Mittler R. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiology. 2002;130:1–9. doi: 10.1104/pp.006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabehat A, Weiss D, Lurie S. Heat-shock proteins and cross-tolerance in plants. Physiologia Plantarum. 1998;103:437–441. [Google Scholar]
- Tausz M, Grulke NE, Wieser G. Defense and avoidance of ozone under global change. Environmental Pollution. 2007;147:525–531. doi: 10.1016/j.envpol.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Tingey DT, Hogsett WE. Water stress reduces ozone injury via a stomatal mechanism. Plant Physiology. 1985;77:944–957. doi: 10.1104/pp.77.4.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Davies W. Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant, Cell and Environment. 2010;33:510–525. doi: 10.1111/j.1365-3040.2009.02052.x. [DOI] [PubMed] [Google Scholar]
- Wilson KB, Baldocchi DD, Hanson PJ. Quantifying stomatal and non-stomatal limitations to carbon assimilation resulting from leaf aging and drought in mature deciduous tree species. Tree Physiology. 2000;20:787–797. doi: 10.1093/treephys/20.12.787. [DOI] [PubMed] [Google Scholar]
- Wittig VE, Ainsworth EA, Long SP. To what extent do current and projected increases in surface ozone affect photosynthesis and stomatal conductance of trees? A meta-analytic review of the last three decades of experiments. Plant, Cell and Environment. 2007;30:1150–1162. doi: 10.1111/j.1365-3040.2007.01717.x. [DOI] [PubMed] [Google Scholar]
- Wittig VE, Ainsworth EA, Naidu SL, Karnosky DF, Long SP. Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: a quantitative meta-analysis. Global Change Biology. 2009;15:396–424. [Google Scholar]
- Xiong YC, Li FM, Zhang T. Performance of wheat crops with different chromosome ploidy: root-sourced signals, drought tolerance, and yield performance. Planta. 2006;224:710–718. doi: 10.1007/s00425-006-0252-x. [DOI] [PubMed] [Google Scholar]
- Xu L, Baldocchi DD. Seasonal trends in photosynthetic parameters and stomatal conductance of blue oak (Quercus douglasii) under prolonged summer drought and high temperature. Tree Physiology. 2003;23:865–877. doi: 10.1093/treephys/23.13.865. [DOI] [PubMed] [Google Scholar]