Abstract
Harpin proteins are well known as eliciters that induce multiple responses in plants, such as systemic acquired resistance, hypersensitive response, enhancement of growth, resistance to the green peach aphid, and tolerance to drought. Overexpression of Harpin-encoding genes enhances plant resistance to diseases in tobacco, rice, rape, and cotton; however, it is not yet known whether the expression of Harpin-encoding genes in vivo improves plant tolerance to abiotic stresses. The results of this study showed that overexpression of a Harpin-encoding gene hrf1 in rice increased drought tolerance through abscisic acid (ABA) signalling. hrf1- overexpression induces an increase in ABA content and promotes stomatal closure in rice. The hrf1 transgenic rice lines exhibited a significant increase in water retention ability, levels of free proline and soluble sugars, tolerance to oxidative stress, reactive oxygen species-scavenging ability, and expression levels of four stress-related genes, OsLEA3-1, OsP5CS, Mn-SOD, and NM_001074345, under drought stress. The study confirmed that hrf1 conferred enhanced tolerance to drought stress on transgenic crops. These results suggest that Harpins may offer new opportunities for generating drought resistance in other crops.
Keywords: Abscisic acid, drought tolerance, Harpin, hrf1, transgenic rice
Introduction
Drought is the most devastating factor that menaces food production and food security, especially in areas with inadequate agricultural water resources. The estimated annual loss from the national economy caused by drought alone exceeds US$25 billion in China (Deng, 1999). Global warming and increasingly frequent occurrence of drought exacerbate the situation (Chaves and Oliveira, 2004; Lobell and Field, 2007; Zhang, 2007; Farooq et al., 2009). Warming since 1981 has resulted in annual combined losses of three crops, wheat, maize, and barley, representing roughly 40 Mt or US$5 billion per year, as of 2002 (Lobell and Field, 2007). Globally, rice is the most important crop in terms of the number of people dependent on it as a direct source of calories (Long and Ort, 2010). It has been estimated that rice production consumes about half of the total water consumed in China. Drought stress is still the most important constraint in rice production, mostly due to annual variation in the rainfall patterns and uneven distribution of rainfall in the rice-growing season (Zhang, 2007). Consequently, with the global shortage of water, enhancing the drought tolerance of crops has a potentially huge impact on annual productivity, in addition to improved water management practices (Zhang, 2007; Long and Ort, 2010).
Harpin proteins, which are secreted through the type III protein secretion system of Gram-negative plant pathogenic bacteria, generally affect virulence in host plants, induce hypersensitive cell death in non-host plants, and elicit multiple plant responses (Wei et al., 1992; Alfano and Collmer, 2004; Oh and Beer, 2007). Exogenous applications of Harpins induce systemic acquired resistance (SAR) in plants by the activation of defence pathways mediated by salicylic acid, jasmonic acid, or ethylene (Shao et al., 2008). Transformation of Harpin-encoding genes improves disease resistance in tobacco, rice, rape, and cotton (Jang et al., 2006; Sohn et al., 2007; Shao et al., 2008; Huo et al., 2010; Miao et al. 2010). HrpNEa, a Harpin protein secreted by Erwinia amylovora, induces drought tolerance in Arabidopsis by activating the ABI2-dependent ABA signalling (Dong et al., 2005; Zhang et al., 2007; Ren et al., 2008). So far it is still unknown whether genetic transformation of Harpin-encoding genes improves plant tolerance to drought. Shao et al. (2008) have reported that expression of hrf1, a Harpin-encoding gene, in rice conferred durable non-specific resistance to Magnaporthe grisea. Here, the results demonstrated that transgenic rice plants overexpressing hrf1 showed enhanced tolerance to drought through ABA signalling, and these findings were supported by physiological results, including the promotion of stomatal closure, decreased rate of water loss, enhanced higher relative water content, the significantly increased levels of free Pro and soluble sugars, the improved ROS-scavenging efficiency, and increased expression levels of four stress-related genes.
Materials and methods
Plant growth and treatments
The homozygous seeds of transgenic line NJH12 (T3) and the wild type R109 were germinated at 28 °C after being sterilized with 0.01% Prochloraz, and then grown in nutrient solution in the greenhouse with a 14 h light/10 h dark cycle. Three-leaf stage seedlings were subjected to drought stress. For expression analysis of stress-related genes, drought stress was applied by exposing intact plants in the air without water supply and plant leaves were sampled at 0 h and 12 h after treatment.
Analysis of transgenic plants for drought tolerance
For drought tolerance testing of transgenic rice at the seedling stage, the plantlets of NJH12 (T3) and R109 that germinated on plates at 28 °C were transferred into barrels. To minimize the experimental error, each barrel was filled with the same weight of soil and supplied with the same volume of water. To induce drought stress, five-leaf stage NJH12 and R109 plants were not watered for 11 d followed by 12 d of watering. The numbers of plants that survived or continued to grow were then scored (Xiang et al., 2007).
Measurement of water loss rate and relative water content
To measure the water loss rate under dehydration conditions, the five-leaf stage seedlings were detached from roots and exposed to air at room temperature (∼25 °C). The plants were weighed at 0, 0.5, 2, 4, 6, 8, 12, and 24 h after being cut off. The plants were finally oven dried for 48 h at 80 °C to a constant dry weight (DW). The water loss rates were calculated by the formula: water loss rate (%)=(FW–desiccated weight)/FW×100. Relative water contents were measured according to the formula: RWC (%)=(desiccated weight–DW)/(FW–DW)×100 (Mao et al., 2010).
Scanning electron microscopy analysis of rice stomata
Samples were fixed and prepared for scanning electron microscopy analysis by a modification of the method of Jang et al. (2006). Leaves of four-leaf stage plants treated with 20% polyethylene glycol (PEG)-6000 for 0, 12, 24, and 48 h were detached and immediately fixed by 4% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) at 4 °C for 5 h, followed by washing twice with 0.1 M phosphate buffer for 10 min. Then the samples were fixed by 1% osmium tetroxide in 0.1 M phosphate buffer (pH 7.4) at 4 °C for 2 h, washed twice with 0.1 M phosphate buffer for 10 min, immersed in 50, 60, 70, 80, 90, and 100% ethanol for 10 min each, and then dried using a critical point dryer (EMITECH-K850, HITACHI, Japan). After sticking the samples onto the sample stages and conducting treatment, the stomatal pictures were obtained using a scanning electron microscope (FEI Quanta 200, The Netherlands).
Measurement of ABA content
For each treatment, a 2.5 g leaf sample collected from the four-leaf stage seedlings treated with 20% PEG for 0, 0.5, 1, 2, 3, 4, 5, and 6 d was ground with liquid nitrogen and homogenized with 3 ml of ice-cold solution containing 10% trichloroacetic acid. Leaf homogenates were maintained overnight at –20 °C, followed by centrifugation (8000 g, 4 °C, 1 h). The supernatants were discarded, and the precipitates were homogenized with 3 ml of acetone followed by centrifugation (8000 g, 4 °C, 15 min). The supernatants were again discarded, and the precipitates were dried at 40 °C in a vacuum, homogenized with lysate solution (1.35 g of urea, 0.1 g of CHAPS, 2.5 ml of double-distilled water), and then incubated for 30 min, followed by centrifugation (8000 g, 4 °C, 15 min). ABA contents in the aliquots of the supernatants were measured using a Plant Abscisic ELISA Kit (R&D, USA) according to the manufacturer's protocol.
Measurement of free Pro and soluble sugar contents
Seedlings at the four-leaf stage were used for biochemical analysis. NJH12 and R109 plants were transferred from the basal nutrient solution to nutrient solution containing 20% PEG-6000. After 5 d, the content of Pro in NJH12 and R109 lines with or without 20% PEG treatment were determined by the sulphosalicylic acid method (Troll and Lindsley, 1955). Total soluble sugars in leaves were determined by the modified phenol–sulphuric acid method (Dubois et al., 1956).
Measurement of monodehydroascorbate (MDA) content
Measurement of the MDA content was determined according to the methods of Hou et al. (2009). A 0.5 g aliquot of frozen leaves of rice seedlings treated or not with 20% PEG for 5 d was homogenized in 5 ml of ice-cold solution containing 10% trichloroacetic acid. The homogenates were then centrifuged at 4000 g for 10 min. The aliquots of supernatants were boiled with 5 ml of 0.67% 2-thiobarbituric acid for 30 min, and then measured at 450, 532, and 600 nm. The MDA content was calculated by the formula: MDA content=6.45×(A532–A600)–0.56×A450.
Measurements of peroxidase (POD) and superoxide dismutase (SOD) activities
The leaves of four-leaf-stage seedlings treated or not with 20% PEG for 5 d were used for enzyme activity assay. A 0.5 g aliquot of frozen leaves was homogenized in 5 ml of ice-cold solution containing 100 mM TRIS-HCl (pH 7.0), 20% glycerol, and 1% polyvinylpyrrolidone (PVP). The homogenates were then centrifuged at 10000 g for 30 min. The aliquots of supernatants were used for the analysis of POD and SOD activity. POD activity was measured by the H2O2-dependent oxidation of benzidine at 530 nm, in a reaction mixture containing 2 ml of 0.2 Macetate buffer (pH 4.8), 0.2 ml of 3% H2O2, 0.2 ml of 0.04 M benzidine, and 0.1 ml of extracted protein (Abeles and Biles, 1991). SOD activity was determined according to the methods described previously (Hodges and Forney, 2000; Hou et al., 2009). The reaction mixture consists of 50 mM potassium phosphate (pH 7.8), 13 mM methionine, 0.01 mM EDTA, 0.002 mM riboflavin, and 0.075 mM nitroblue tetrazolium. SOD activity was determined by monitoring the inhibition of the rate of reduction of nitroblue tetrazolium in the reaction mixture and the control without protein extract at 560 nm.
Quantitative real-time RT-PCR analysis
Total RNA from drought-treated and untreated rice seedlings was extracted using the Trizol reagent (TaKaRa, Dalian) according to the manufacturer's protocol. The DNase-treated RNA was reverse-transcribed using Prime Script™ reverse transcriptase (TaKaRa). Real-time RT-PCR was performed on the Applied Biosystems 7500 Real Time PCR System using SYBR Premix Ex Taq™ (TaKaRa). The PCR thermal cycle conditions were as follows: denaturing at 95 °C for 30 s and 40 cycles of 95 °C, 5 s; 60 °C, 34 s. The rice gene eEF-1a (GenBank accession no. AK061464; Kikuchi et al., 2003) was used as the internal reference gene for calculating relative transcript levels. The primers for real-time PCR analysis of stress-related genes were as follows (in parentheses): OsLea3 (5′-AATGATTTCCCTTTGGGTC-3′ and 5′-CATCAGTACACATCACCCA-3′), OsP5CS (5′- GTGGAGGAGGAGAGGCTG-3′ and5′-TGCCCAAAGCCAATCTTC-3′), Mn-SOD (5′-CTGGAATAACCTCAAGCCTATC-3′ and 5′- ACAAGTGCCTCAAATGAAC-3′), and NM_001074345 (5′-GGAGGTGGTTCAGTTCAAATGG-3′ and 5′-TTTCCCTTGAGATACTCCTTTG-3′).
Results
Overexpression of hrf1 significantly improves drought tolerance in rice
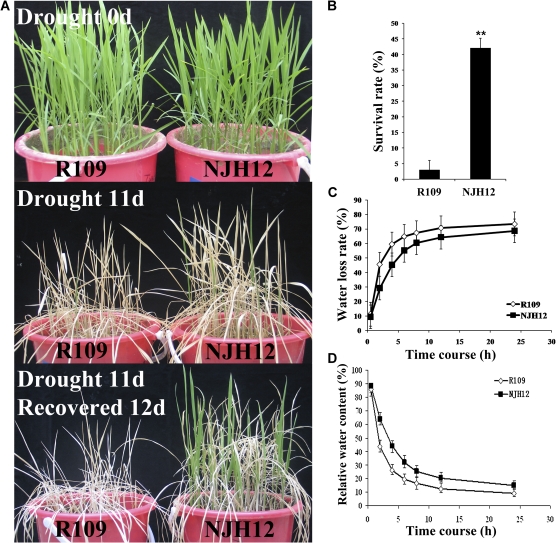
The homozygous T3 NJH12 line is a transgenic rice line generated by transforming rice cultivar R109 with hrf1-containing pBMH9 using Agrobacterium-mediated transformation (Shao et al., 2008). To examine whether the overexpression of the hrf1 gene conferred resistance to drought stress to NJH12, NJH12 rice plants with five true leaves were used for drought tolerance testing (Fig. 1A, B). NJH12 plants grew better than R109 plants under drought conditions. After 11 d of drought treatment and then being left to recover for 12 d, most (∼97%) R109 plants were dead, while >40% of NJH12 plants remained vigorous, suggesting that overexpression of hrf1 significantly improved drought tolerance in transgenic rice (t-test, P <0.01).
Fig. 1.
hrf1-overexpressing transgenic rice plants showed improved drought tolerance. (A) Growth performance of NJH12 and R109 seedling in the barrels (left, R109; right, NJH12 plants). Five-leaf-stage plants were not watered for 11 d (middle panel) and then left to recover for 12 d (bottom panel). The experiment was repeated three times. (B) Survival rates of NJH12 and R109 after being stressed for 11 d and then left to recover for 12 d. Values are means ±SD (n=30). **P <0.01 (t-test). (C) Comparison of water loss rates for detached plants at the five-leaf stage between NJH12 and R109 (n=15). (D) Comparison of relative water contents of detached plants at the five-leaf stage between NJH12 and R109 (n=15).
hrf1-overexpressing transgenic rice plants acquired stronger water retention ability
Water availability dominates the yields of global crops including rice (Boyer, 1982). Plants with high water retention ability could stay green to maintain the crop canopy and survive the low water potential of a drought (Long and Ort, 2010). To elucidate the physiological mechanism of drought tolerance in the hrf1-overexpressing plants, the water retention ability of R109 and NJH12 plants was investigated (Fig. 1C, D). The five-leaf stage seedlings under normal conditions were detached from roots, exposed to air at room temperature (∼25 °C), and weighed at different time points after being cut off. The results suggested that under dehydration stress the NJH12 plants lost significantly less water and maintained higher relative water content than R109 (t-test, P <0.01).
Stomatal movement changed in the transgenic plants due to ABA accumulation
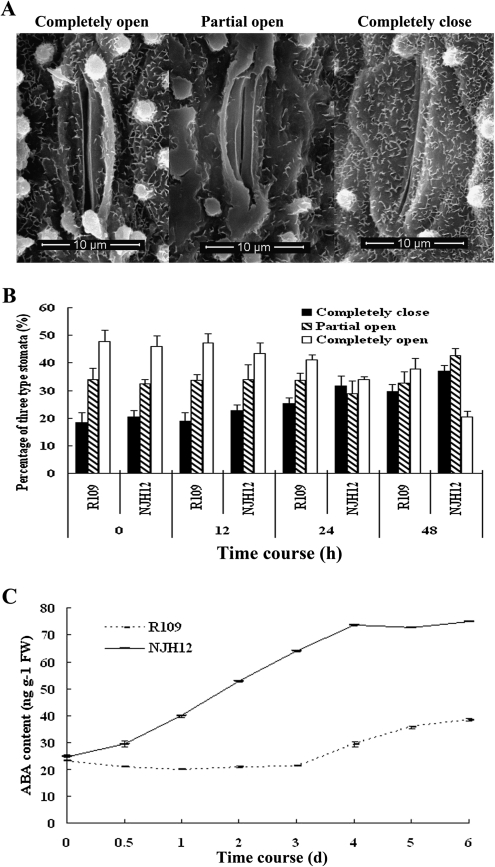
HrpNEa can promote stomatal closure by ABI2-dependent ABA signalling (Dong et al., 2005). Here, the stomatal status of NJH12 and R109 plants was examined. After being treated with 20% PEG-6000 for 24 h, NJH12 and R109 plants showed a different open and closed stomata ratio. After 48 h, 43% of stomata were completely closed in NJH12, but only 30% were completely closed in R109; while only 20% of stomata were completely open in NJH12, but 38% were completely open in R109 (Fig. 2A, B). The percentage of partially open stomata was similar in NJH12 and R109 plants (34–37%). These results showed that stomatal movement was greatly affected in NJH12, suggesting that the enhanced drought tolerance displayed by NJH12 might be primarily due to increased stomatal closure, which prevented water loss.
Fig. 2.
ABA accumulation induces stomatal closing in transgenic plants. (A) Scanning electron microscopy images of three levels of stomatal opening. Bar, 10 μm. (B) The percentage of three levels of stomatal opening in NJH12 and R109 plants (n=100 stomata for every treatment). (C) Quantitative measurement of ABA in the seedling leaves of NJH12 and R109 plants (n=3).
Since the phytohormone ABA can induce stomatal closure, the endogenous ABA content was measured in both NJH12 and R109 plants. After treatment with 20% PEG, R109 plants exhibited a slightly lower ABA content in the first 3 d, and ABA content began to increase slowly on the fourth day. However, the ABA content of NJH12 plants became higher quickly, reached the maximum on the third day, and this lasted for 2 d. After drought stress for 6 d, NJH12 plants accumulated an ∼2-fold higher content of ABA than R109 plants (Fig. 2C). Therefore, the increased stomatal closure of NJH12 might be the result of earlier and faster accumulation of ABA.
Overexpression of hrf1 increases free Pro and soluble sugar contents under drought stress in rice
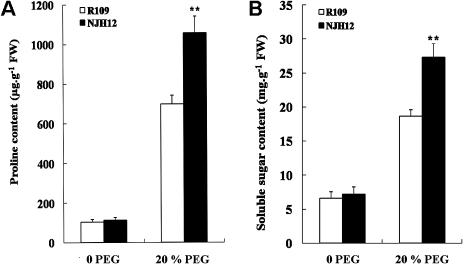
Free Pro and soluble sugar accumulation is a well-known response to drought stress in plants. These metabolites have been suggested to stabilize subcellular structures and facilitate cell recovery from stress. Overproduction of Pro and soluble sugar in plants may lead to increased tolerance against abiotic stresses (Igarashi et al., 1997; Mani et al., 2002; Hien et al., 2003; Penna, 2003; Chaves and Oliveira, 2004). To investigate the physiological basis for the improved drought tolerance of transgenic rice, the contents of free Pro and soluble sugar in NJH12 and R109 plants were measured under normal growth and stress conditions (Fig. 3A, B). No significant difference in Pro and soluble sugar contents in the leaves was detected between the NJH12 and R109 plants grown under normal growth conditions. However, after 5 d of drought stress, NJH12 plants accumulated an ∼9-fold higher content of Pro (Fig. 3A) and a 4-fold higher content of soluble sugars (Fig. 3B) than the NJH12 plants that were not drought stressed. The drought stress-induced increases of Pro and soluble sugar contents in the transgenic plants were significantly higher (t-test, P <0.01) than those in wild-type plants, in which drought stress induced only ∼6-fold increases of Pro and 3-fold increases of soluble sugars, respectively. The data indicated that hrf1 overexpression might regulate the accumulation of free Pro and soluble sugars in rice seedlings under drought stress.
Fig. 3.
The contents of free proline (A) and soluble sugars (B) in transgenic plants and the wild-type plants treated with 20% PEG for 5 d. Values are means ±SD (n=5). **P <0.01 (t-test).
The hrf1 gene enhances the ROS-scavenging ability and the tolerance to oxidative stress
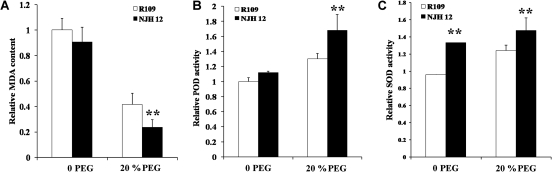
Many stresses, including drought stress, disrupt the cellular homeostasis of cells and enhance the production of ROS. Overaccumulation of ROS results in decreased cell viability and even cell death, while scavenging ROS will avoid or alleviate the effects of stresses on plant metabolism. PODs and SODs are important parts of the ROS-scavenging mechanisms of plants (Mittler, 2002; Xiong and Zhu, 2002; Apel and Hirt, 2004). MDA, a decomposition product of polyunsaturated fatty acid hydroperoxides, is a biomarker for lipid peroxidation, which is an effect of oxidative damage. The data showed that under normal growth conditions, the MDA content in NJH12 plants was lower than that in R109, but the difference was not significant. After drought stress, the MDA contents of both NJH12 and R109 plants declined; however, the MDA content of NJH12 was significantly lower than that of R109 plants (Fig. 4A; t-test, P <0.01). In contrast to the decreased MDA content in NJH12 plants, overexpression of hrf1 significantly increased the levels of POD and SOD under drought treatment (Fig. 4B, C; t-test, P <0.01). Interestingly, the SOD activity of NJH12 plants was significantly higher than that of R109 plants with and without drought stress (Fig. 4C; t-test, P <0.01). These results suggested that the increased drought tolerance of NJH12 plants was partially due to reduction of oxidative damage to cells caused by the enhanced ROS-scavenging activity.
Fig. 4.
hrf1 enhances ROS-scavenging ability in transgenic plants under drought stress conditions. (A) Relative MDA level in NJH12 (compared with R109) before and after drought stress for 5 d. Three independent experiments were performed and similar results were obtained. Values are means ±SD (n=5). **P <0.01 (t-test). (B, C) The POD and SOD activities of NJH12 and R109 under drought and unstressed conditions. Values are means ±SD (n=5). **P <0.01 (t-test).
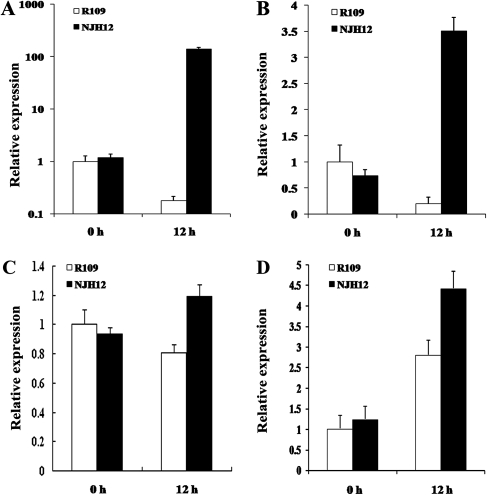
Expression of stress-related genes in hrf1 transgenic rice plants
To better understand the mechanisms of drought tolerance conferred by overexpressing hrf1, the expression of several stress-related genes in NJH12 and R109 plants was investigated. As shown in Fig. 5A–C, the expression levels of a late embryogenesis abundant (LEA) gene (OsLEA3-1, GenBank accession no. Z68090.1; Moons et al., 1997), a pyrroline-5-carboxylate synthetase gene (OsP5CS; GenBank accession no. D49714.1; Igarashi et al., 1997), and a manganese superoxide dismutase gene (Mn-SOD; GenBank accession no. L34038.1; Pan and Chen, unpublished data) were strongly induced in NJH12 plants under drought stress, compared with R109. Under normal growth conditions, the expression levels of both OsLEA3-1 and Mn-SOD were not significantly different in R109 and NJH12 and the expression level of OsP5CS in R109 was higher than that in NJH12. In Fig. 5D, the expression levels of NM_001074345, homologous to Lsi1 that is responsible for transport of silicon (Si) from the external solution to root cells (Ma et al., 2006; Ma and Yamaji, 2008), were higher in NJH12 than that in R109 with or without drought stress.
Fig. 5.
Expression analysis of two stress-responsive genes in NJH12 and R109 under drought for 0 h and 12 h. (A) OsLEA3-1. (B) OsP5CS. (C) Mn-SOD. (D) NM_001074345. Error bars are standard deviations of three technical repeats.
Discussion
Harpin proteins are well known as eliciters that induce multiple responses in plants, such as SAR, the hypersensitive response (HR), enhancing growth, drought tolerance, disease resistance, and insect defence (Wei et al., 1992; Alfano and Collmer, 2004; Dong et al., 2004, 2005; Oh and Beer, 2007; Ren et al., 2008; Liu et al, 2010). Overexpression of Harpin-encoding genes enhances plant resistance to diseases in tobacco, rice, rape, and cotton (Jang et al., 2006; Sohn et al., 2007; Shao et al., 2008; Huo et al., 2010; Miao et al., 2010). The data presented here are the first to document that ectopic expression of a Harpin-encoding gene hrf1 improved plant tolerance to drought.
Mechanisms that underlie the effects of Harpins in plants and the functions of the proteins as effectors of pathogenic bacteria during infection of plants have been of great interest (Dong et al., 2005). Different functions of Harpins in plants may depend on distinct signalling pathways (Dong et al., 2004, 2005; Shao et al., 2008; Miao et al., 2010). In Arabidopsis plants grown under drought stress, HrpNEa activated ABI2-dependent ABA signalling to promote stomatal closure and other adaptive responses, reducing the severity of drought effects on plants (Dong et al., 2005; Liu et al., 2010). ABA acted alone in response to HrpNEa depending on the immediate requirement for plant growth toward drought stress (Zhang et al., 2007). Extrinsic applications of HrpNEa distinctly increased the expression of AtMYB44 and induced drought tolerance and stomatal closure in wild-type Arabidopsis following treatment with PEG (Liu et al., 2010). The overexpression of AtMYB44 in Arabidopsis, which caused a more rapid ABA-induced stomatal closure response and a slower rate of water loss, enhanced drought and salt tolerance compared with wild-type and atmyb44 knockout plants (Jung et al., 2008). AtMYB44-overexpressing plants suppressed jasmonate-mediated responses and enhanced ABA-mediated responses (Jung et al., 2010). In the present study, under drought stress, the ABA content of NJH12 became higher quickly, but the ABA content of R109 became slightly lower in the first 3 d (Fig. 2C), and that was the reason for the down-regulated expression of OsLEA3-1 and OsP5CS in R109 under drought stress (Fig. 5A, B). The earlier and faster increase of ABA levels in NJH12 promoted earlier and faster stomatal closure (Fig. 2A, B), then increased the water retention ability of NJH12 leaves (Fig. 1C, D) and the expression levels of stress-related genes (Fig. 5), and finally enhanced the drought tolerance (Fig. 1A, B).
Even now, the site of action of Harpins in plants remains unknown. A previous study indicated that the plant cell wall was critical for the HR-inducing activity of HrpNEa and HrpNPss (Hoyos et al., 1996). The receptor(s) for Harpin could be extracellular in transgenic tobacco transformed with hrpZPsph (Tampakaki and Panopoulos, 2000). The HR phenotype of popA-overexpressing tobacco correlated with the presence of PopA at the plasma membrane, which was part of a system that aimed to attach the host cell plasma membrane and to allow molecules to cross this barrier (Racapé et al., 2005). The action site of HrpNEP was located in the plant cell walls (Sohn et al., 2007). Miao et al. (2010) speculated that the action sites of Harpins in plants vary depending on their origins. The Harpin protein encoded by the hrf1 gene, HarpinXoo, was detected as clustered particles mainly along the cell walls of transgenic cotton, indicating that the cotton cell wall could be important for the HR-inducing activity of HarpinXoo in transgenic cotton (Miao et al., 2010). In the present study, hrf1 enhanced drought tolerance when overexpressed in rice. The result is consistent with improved drought tolerance in plants resulting from exogenous application of Harpin (Dong et al., 2005). Furthermore, the effects of Harpins on stomatal movement are mediated by ABA signalling either by exogenous application of Harpins or by expression of Harpin-encoding genes in vivo. Based on the study results of Miao et al. (2010), it was speculated that the action sites of Harpin might be located in the plant cell wall, and that Harpin might be recognized by the same receptor(s). Oh and Beer (2007) found that two Harpin-interacting proteins, HIPM in apple and its orthologue in Arabidopsis (AtHIPM), associated with plasma membranes in clusters, suggesting that the specific position in plasma membranes for HIPM and AtHIPM is probably a lipid raft, as microdomains are enriched for many signalling molecules that regulate different signal transduction pathways. The data presented here implied that the receptor(s) of HarpinXoo might be different from Harpin and might localize in the cell wall. However, whether HarpinXoo exclusively localizes4 in the cell wall in rice and how HarpinXoo and its receptor(s) physically contact each other is unclear. Recently, several advances had led to the identification of ABA receptors and a new model for ABA action had been proposed and validated (Cutler et al., 2010; Hubbard et al., 2010), but how Harpin interacts with ABA and the location of their interaction need to be studied further.
Being sessile organisms, plants have evolved a series of mechanisms in response to abiotic stresses including high salinity, drought, and cold (Xiong and Zhu, 2002; Xiang et al., 2007; Farooq et al., 2009). In response to these stresses, many plants can accumulate compatible osmolytes to protect their subcellular structures from damage by adjusting the intracellular osmotic potentials (Boyer, 1982; Xiong and Zhu, 2002; Xiang et al., 2007; Farooq et al., 2009). Free Pro and soluble sugars are compatible osmolytes that are thought to function mainly in osmotic adjustment by lowering cellular osmotic potential to facilitate water absorption and restore intracellular salt concentrations (Xiong and Zhu, 2002; Chaves and Oliveira, 2004; Farooq et al., 2009). In this report, the improvement of water retention ability in the transgenic line NJH12 (Fig. 1C, D) might partially be a result of the increase of Pro and soluble sugar contents (Fig. 3A, B). Additionally, osmolytes have the ability to scavenge ROS by an unclear underlying mechanism (Mani et al., 2002; Xiong and Zhu, 2002), indicating that the higher levels of Pro and soluble sugars in NJH12 might play a role in ROS scavenging.
Plants actively produce ROS as signalling molecules to control processes such as programmed cell death (PCD), abiotic stress responses, pathogen defence, and systemic signalling (Mittler, 2002; Apel and Hirt, 2004). ROS are continuously produced in plants. The equilibrium between production and scavenging of ROS must be strictly controlled. However, abiotic stresses such as drought, low temperature, and salt stress often perturb the equilibrium, resulting in retarded growth, cell damage, or even death in plants. MDA is an important intermediate in ROS scavenging and often considered as a reflection of cellular membrane degradation or dysfunction; a high level of MDA induces PCD and is toxic to plant cells (Apel and Hirt, 2004; Hou et al., 2009). Increased levels of ROS-scavenging enzymes abrogate the plant's ability to build up sufficient ROS for inducing PCD (Mittler, 2002; Apel and Hirt, 2004; Chaves and Oliveira, 2004; Farooq et al., 2009; Hou et al., 2009). In NJH12 plants, the levels of POD and SOD, two key ROS eliminators, were increased under drought stress, while the MDA content in NJH12 was reduced (Fig. 4A–C), suggesting that the increased ROS-scavenging activity in NJH12 protected plant cells from damage by ROS induced by drought stress. Addionally, the expression level of Mn-SOD in NJH12 was higher than that in R109 under drought stress (Fig. 5C). These results confirmed that the increased drought tolerance of the NJH12 plants is partially attributed to the enhanced ROS-scavenging activity and tolerance to oxidative stress.
It is interesting that only the activity of SOD in the present study was constitutively increased in NJH12 plants under normal growth conditions (Fig. 4C), whereas the levels of Pro, soluble sugars, MDA, and POD activity were not significantly different between NJH12 plants and R109 plants under normal conditions (Figs 3A, B; 4A, B), despite the fact that the constitutive promoter was used to drive the transgene. hrf1 induced changes of Pro, soluble sugars, MDA, and POD only under drought stress, while SOD levels increased regardless of the presence of stress, suggesting that hrf1 directly up-regulated SOD expression. However, expression levels of Mn-SOD in NJH12 and R109 were not different under normal conditions (Fig. 5C), suggesting that the constitutive increase of SOD activity in NJH12 plants was induced by other types of SOD genes but not Mn-SOD.
LEA proteins are involved in plant stress resistance to drought, salt, and cold. OsLEA3-1, which belongs to the group 3 LEA family, is induced by drought, salt, and ABA, but not by cold stress. Overexpression of OsLEA3-1 in rice significantly enhanced drought resistance under field conditions (Xiao et al., 2007). P5CS, which catalyses the first two steps in Pro biosynthesis, is critical for the control of Pro biosynthesis and consequently the increase in osmotolerance. OsP5CS is induced by drought, salt, and ABA, and increased expression of OsP5CS increases Pro production and confers osmotolerance in transgenic plants (Xiang et al., 2007). The two target genes were both strongly induced in NJH12 by drought (Fig. 5A, B), suggesting that the drought tolerance induced by hrf1 is involved in the ABA signalling pathway. In addition, the increased expression of OsP5CS might be attributed to the improvement of Pro content in drought stress.
Although Harpin expression driven by the 35S promoter was considered constitutive, the expression levels of OsLEA3-1, OsP5CS, and Mn-SOD were not induced in NJH12 and R109 plants under normal conditions (Fig. 5A–C). These results are consistent with the levels of Pro, soluble sugars, MDA, and POD activity under normal conditions (Figs 3A, B; 4A, B), suggesting that the three stress-related genes are also only induced by hrf1 under drought stress.
Si is the second most abundant element in soil and a mineral substrate for most of the world's plant life. It is readily absorbed so that terrestrial plants contain appreciable concentrations ranging from a fraction of 1% of dry matter to ≥10% (Epstein, 1994). Its uptake and transport in rice, a typical Si-accumulator, are active processes (Epstein, 1994; Ma et al., 2006; Ma and Yamaji, 2008). The function of Si is to protect the plant from various biotic and abiotic stresses (Epstein, 1994; Ma and Yamaji, 2008). Si might decrease the transpiration rate and membrane permeability of rice under PEG-induced water deficit. Si application might also enhance drought resistance of plants (Farooq et al., 2009). The microarray analysis using the Affymetrix Rice Genome Genechip showed that the transcription level of NM_001074345 was increased significantly in NJH12 under normal conditions (unpublished data). The real-time RT-PCR results confirmed that the expression levels of NM_001074345 were higher in NJH12 than in R109 under normal or stressed conditions (Fig. 5D), which is consistent with the finding that the leaf Si concentration was significantly higher in NJH12 plants than in R109 at the tillering and final harvest stage in the field under normal conditions (Shao et al., 2008). These results suggested that the increase of Si content might partially enhance the drought tolerance of transgenic plants.
Previous studies have shown that constitutive overexpression of some stress-responsive genes, such as SNAC2 (Hu et al., 2008), OsDREB1A, OsDREB1B, AtDREB1A, and AtDREB1B (Shen et al., 2003; Ito et al., 2006), frequently resulted in growth retardation, which caused significant reduction of potential yield. However, hrf1-overexpressing plants (T0) and their T1–T7 progeny showed no obvious difference from the wild-type plants in all of the traits investigated. Besides, the result of quality analysis of NJH12 suggested that the quality of NJH12 was the same as that of wild-type R109, which is an important cultivar in production in south China (unpublished data). Such results provide another good feature for the usefulness of the hrf1 gene in improving drought resistance.
Drought tolerance is a complex trait that involves numerous aspects of developmental, physiological, biochemical, and molecular adjustments. These include, for example, changes in root growth, ABA content, osmotic adjustment, photosynthesis, and synthesis of protective proteins and antioxidants (Farooq et al., 2009). It is very difficult to understand clearly the regulatory pathways leading to these adjustments, so that the data obtained under laboratory or greenhouse conditions may not always be consistent with those obtained in the field (Zhang, 2007; Hu et al., 2008; Xiao et al., 2009). Hence, some genes, including hrf1 in the present study, demonstrated to be important for drought tolerance in laboratory tests, must be further confirmed in drought-stressed field environments.
The data clearly suggested that overexpression of the bacterial hrf1 gene in rice enhanced the drought tolerance mediated by ABA signalling. Further study on the functions of Harpin-encoding genes in different abiotic stress responses, such as salt and cold stresses, will greatly expand our understanding of the functions of Harpins and Harpin-encoding genes. Even though hrf1 as a Harpin-encoding gene has been tested for its effectiveness in improving drought tolerance in rice here, it is still necessary to investigate further whether some other Harpin-encoding genes are potentially useful for drought tolerance improvement in plants.
Acknowledgments
We thank Dr Shen Wenbiao for his help in measurement of SOD. We are also grateful to Ronald Chan for his constructive comments. This work was supported by the National High Technology Research and Development Programmes from the Ministry of Science and Technology of China (grant nos 2008AA10Z108 and 2007AA10Z188), and in part by the Major Program of National Transgenic Biology New Varieties Breeding from the Ministry of Agriculture of China (grant no. 2009ZX08001-005B).
References
- Abeles FB, Biles CL. Characterization of peroxidases in lignifying peach fruit endocarp. Plant Physiology. 1991;95:269–273. doi: 10.1104/pp.95.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano JR, Collmer A. Type III secretion system effector proteins: double agents in bacterial disease and plant defense. Annual Review of Phytopathology. 2004;42:385–414. doi: 10.1146/annurev.phyto.42.040103.110731. [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology. 2004;55:373–99. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Boyer JS. Plant productivity and environment. Science. 1982;218:443–448. doi: 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- Chaves MM, Oliveira MM. Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. Journal of Experimental Botany. 2004;55:2365–2384. doi: 10.1093/jxb/erh269. [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: emergence of a core signaling network. Annual Review of Plant Biology. 2010;2:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- Deng N. Formulating the outline of a national program of agricultural science and enhancing the revolution of agricultural science and technology development in China. Review of China Agricultural Science and Technology. 1999;2:3–8. [Google Scholar]
- Dong HP, Peng J, Bao Z, Meng X, Bonasera JM, Chen G, Beer SV, Dong H. Downstream divergence of the ethylene signaling pathway for harpin-stimulated Arabidopsis growth and insect defense. Plant Physiology. 2004;136:3628–3638. doi: 10.1104/pp.104.048900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HP, Yu H, Bao Zh Guo X, Peng J, Yao Z, Chen G, Qu S, Dong H. The ABI2-dependent abscisic acid signalling controls HrpN-induced drought tolerance in Arabidopsis. Planta. 2005;221:313–327. doi: 10.1007/s00425-004-1444-x. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 1956;28:350–356. [Google Scholar]
- Epstein E. The anomaly of silicon in plant biology. Proceedings of the National Academy of Sciences, USA. 1994;91:11–17. doi: 10.1073/pnas.91.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq M, Wahid A, Lee DJ, Ito O, Siddique KHM. Advances in drought resistance of rice. Critical Reviews in Plant Science. 2009;28:199–217. [Google Scholar]
- Hien DT, Jacobs M, Angenon G, Hermans C, Thu TT, Son LV, Roosens NH. Proline accumulation and Δ1-pyrroline-5-carboxylate synthetase gene properties in three rice cultivars differing in salinity and drought tolerance. Plant Science. 2003;165:1059–1068. [Google Scholar]
- Hodges DM, Forney CF. The effects of ethylene, depressed oxygen and elevated carbon dioxide on antioxidant profiles of senescing spinach leaves. Journal of Experimental Botany. 2000;51:645–655. doi: 10.1093/jexbot/51.344.645. [DOI] [PubMed] [Google Scholar]
- Hou X, Xie K, Yao J, Qi Z, Xiong L. A homolog of human ski-interacting protein in rice positively regulates cell viability and stress tolerance. Proceedings of the National Academy of Sciences, USA. 2009;106:6410–6415. doi: 10.1073/pnas.0901940106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyos AE, Stanley CM, He SY, Pike S, Pu XA, Novacky A. The interaction of harpinPss with plant cell walls. Molecular Plant-Microbe Interactions. 1996;9:608–616. [Google Scholar]
- Hu H, You J, Fang Y, Zhu X, Qi Z, Xiong L. Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Molecular Biology. 2008;67:169–181. doi: 10.1007/s11103-008-9309-5. [DOI] [PubMed] [Google Scholar]
- Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI. Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes and Development. 2010;24:1695–1708. doi: 10.1101/gad.1953910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo R, Wang Y, Ma L, Qiao J, Shao M, Gao X. Assessment of inheritance pattern and agronomic performance of transgenic rapeseed having harpinXooc-encoding hrf2 gene. Transgenic Research. 2010;19:841–847. doi: 10.1007/s11248-010-9365-x. [DOI] [PubMed] [Google Scholar]
- Igarashi Y, Yoshiba Y, Sanada Y, Wada K, Yamaguchi-Shinozaki K, Shinozaki K. Characterization of the gene for deltal-pyrroline-5-carboxylate synthetase and correlation between the expression of the gene and salt tolerance in Oryza sativa L. Plant Molecular Biology. 1997;33:857–865. doi: 10.1023/a:1005702408601. [DOI] [PubMed] [Google Scholar]
- Ito Y, Katsura K, Maruyama K, Taji T, Kobayashi M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant and Cell Physiology. 2006;47:141–153. doi: 10.1093/pcp/pci230. [DOI] [PubMed] [Google Scholar]
- Jang YS, Sohn SI, Wang MH. The hrpN gene of Erwinia amylovora stimulates tobacco growth and enhances resistance to Botrytis cinerea. Planta. 2006;223:449–456. doi: 10.1007/s00425-005-0100-4. [DOI] [PubMed] [Google Scholar]
- Jung C, Seo JS, Han SW, Koo YJ, Kim CH, Song SI, Nahm BH, Choi YD, Cheong JJ. Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic arabidopsis. Plant Physiology. 2008;146:623–635. doi: 10.1104/pp.107.110981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C, Shim JS, Seo JS, Lee HY, Kim CH, Choi YD, Cheong JJ. Non-specific phytohormonal induction of AtMYB44 and suppression of jasmonate-responsive gene activation in Arabidopsis thaliana. Molecules and Cells. 2010;29:71–76. doi: 10.1007/s10059-010-0009-z. [DOI] [PubMed] [Google Scholar]
- Kikuchi S, Satoh K, Nagata T, et al. Collection, mapping, and annotation of over 28,000 cDNA clones from japonica rice. Science. 2003;301:376–9. doi: 10.1126/science.1081288. [DOI] [PubMed] [Google Scholar]
- Liu R, Lü B, Wang X, Zhang C, Zhang S, Qian J, Chen L, Shi H, Dong H. Thirty-seven transcription factor genes differentially respond to a harpin protein and affect resistance to the green peach aphid in Arabidopsis. Journal of Bioscience. 2010;35:435–450. doi: 10.1007/s12038-010-0049-8. [DOI] [PubMed] [Google Scholar]
- Lobell DB, Field CB. Global scale climate–crop yield relationships and the impacts of recent warming. Environmental Research Letters. 2007;2:1–7. [Google Scholar]
- Long SP, Ort DR. More than taking the heat: crops and global change. Current Opinion in Plant Biology. 2010;13:1–8. doi: 10.1016/j.pbi.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M. A Si transporter in rice. Nature. 2006;440:688–691. doi: 10.1038/nature04590. [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N. Functions and transport of silicon in plants. Cellular and Molecular Life Sciences. 2008;65:3049–3057. doi: 10.1007/s00018-008-7580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S, Van De Cotte B, Van Montagu M, Verbruggen N. Altered levels of proline dehydrogenase cause hypersensitivity to proline and its analogs in Arabidopsis. Plant Physiology. 2002;128:73–83. [PMC free article] [PubMed] [Google Scholar]
- Mao X, Zhang H, Tian S, Chang X, Jing R. TaSnRK2.4, an SNF1-type serine/threonine protein kinase of wheat (Triticum aestivum L.), confers enhanced multistress tolerance in Arabidopsis. Journal of Experimental Botany. 2010;61:683–696. doi: 10.1093/jxb/erp331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao W, Wang X, Li M, Song C, Wang Y, Hu D, Wang J. Genetic transformation of cotton with a harpin-encoding gene hpaXoo confers an enhanced defense response against different pathogens through a priming mechanism. BMC Plant Biology. 2010;15:67. doi: 10.1186/1471-2229-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Moons A, De Keyser A, Van Montagu M. A group 3 LEA cDNA of rice, responsive to abscisic acid, but not to jasmonic acid, shows variety-specific differences in salt stress response. Gene. 1997;191:197–204. doi: 10.1016/s0378-1119(97)00059-0. [DOI] [PubMed] [Google Scholar]
- Oh CS, Beer SV. AtHIPM, an ortholog of the apple HrpN-interacting protein, is a negative regulator of plant growth and mediates the growth-enhancing effect of HrpN in Arabidopsis. Plant Physiology. 2007;145:426–436. doi: 10.1104/pp.107.103432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna S. Building stress tolerance through over-producing trehalose in transgenic plants. Trends in Plant Science. 2003;8:355–357. doi: 10.1016/S1360-1385(03)00159-6. [DOI] [PubMed] [Google Scholar]
- Racapé J, Belbahri L, Engelhardt S, et al. Ca2+-dependent lipid binding and membrane integration of PopA, a harpin-like elicitor of the hypersensitive response in tobacco. Molecular Microbiology. 2005;58:1406–1420. doi: 10.1111/j.1365-2958.2004.04910.x. [DOI] [PubMed] [Google Scholar]
- Ren X, Liu F, Bao Z, Zhang C, Wu X, Chen L, Liu R, Dong H. Root growth of Arabidopsis thaliana is regulated by ethylene and abscisic acid signaling interaction in response to HrpNEa, a bacterial protein of harpin group. Plant Molecular Biology Reports. 2008;26:225–240. [Google Scholar]
- Shao M, Wang J, Dean RA, Lin Y, Gao X, Hu S. Expression of a harpin-encoding gene in rice confers durable nonspecific resistance to Magnaporthe grisea. Plant Biotechnology Journal. 2008;6:73–81. doi: 10.1111/j.1467-7652.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- Shen YG, Zhang WK, He SJ, Zhang JS, Liu Q, Chen SY. An EREBP/AP2-type protein in Triticum aestivum was a DRE-binding transcription factor induced by cold, dehydration and ABA stress. Theoretical and Applied Genetics. 2003;106:923–930. doi: 10.1007/s00122-002-1131-x. [DOI] [PubMed] [Google Scholar]
- Sohn SI, Kim YH, Kim BR, Lee SY, Lim CK, Hur JH, Lee JY. Transgenic tobacco expressing the hrpNep gene from Erwinia pyrifoliae triggers defense responses against Botrytis cinerea. Molecules and Cells. 2007;24:232–239. [PubMed] [Google Scholar]
- Tampakaki AP, Panopoulos NJ. Elicitation of hypersensitive cell death by extracellularly targeted HrpZPsph produced in planta. Molecular Plant-Microbe Interactions. 2000;13:1366–1374. doi: 10.1094/MPMI.2000.13.12.1366. [DOI] [PubMed] [Google Scholar]
- Troll W, Lindsley J. A photometric method for the determination of proline. Journal of Biological Chemistry. 1955;215:655–660. [PubMed] [Google Scholar]
- Wei ZM, Laby RJ, Zumoff CH, Bauer DW, He SY, Collmer A, Beer SV. harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science. 1992;257:85–88. doi: 10.1126/science.1621099. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Huang Y, Xiong L. Characterization of stress-responsive CIPK genes in rice for stress tolerance improvement. Plant Physiology. 2007;144:1416–1428. doi: 10.1104/pp.107.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Chen X, Xiang C, Tang N, Zhang Q, Xiong L. Evaluation of seven function-known candidate genes for their effects on improving drought resistance of transgenic rice under field conditions. Molecular Plant. 2009;2:73–83. doi: 10.1093/mp/ssn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Huang Y, Tang N, Xiong L. Over-expression of a LEA gene in rice improves drought resistance under the Weld conditions. Theoretical and Applied Genetics. 2007;115:35–46. doi: 10.1007/s00122-007-0538-9. [DOI] [PubMed] [Google Scholar]
- Xiong L, Zhu JK. Molecular and genetic aspects of plant responses to osmotic stress. Plant, Cell and Environment. 2002;25:131–139. doi: 10.1046/j.1365-3040.2002.00782.x. [DOI] [PubMed] [Google Scholar]
- Zhang C, Qian J, Bao Z, Hong X, Dong H. The induction of abscisic-acid-mediated drought tolerance is independent of ethylene signaling in Arabidopsis plants responding to a harpin protein. Plant Molecular Biology Reports. 2007;25:98–114. [Google Scholar]
- Zhang Q. Strategies for developing Green Super Rice. Proceedings of the National Academy of Sciences, USA. 2007;104:16402–16409. doi: 10.1073/pnas.0708013104. [DOI] [PMC free article] [PubMed] [Google Scholar]