Abstract
Chickpea is mostly grown on stored soil moisture, and deep/profuse rooting has been hypothesized for almost three decades to be critical for improving chickpea tolerance to terminal drought. However, temporal patterns of water use that leave water available for reproduction and grain filling could be equally critical. Therefore, variation in water use pattern and root depth/density were measured, and their relationships to yield tested under fully irrigated and terminal drought stress, using lysimeters that provided soil volumes equivalent to field conditions. Twenty chickpea genotypes having similar plant phenology but contrasting for a field-derived terminal drought-tolerance index based on yield were used. The pattern of water extraction clearly discriminated tolerant and sensitive genotypes. Tolerant genotypes had a lower water uptake and a lower index of stomatal conductance at the vegetative stage than sensitive ones, while tolerant genotypes extracted more water than sensitive genotypes after flowering. The magnitude of the variation in root growth components (depth, length density, RLD, dry weight, RDW) did not distinguish tolerant from sensitive genotypes. The seed yield was not significantly correlated with the root length density (RLD) in any soil layers, whereas seed yield was both negatively related to water uptake between 23–38 DAS, and positively related to water uptake between 48–61 DAS. Under these conditions of terminal drought, the most critical component of tolerance in chickpea was the conservative use of water early in the cropping cycle, explained partly by a lower canopy conductance, which resulted in more water available in the soil profile during reproduction leading to higher reproductive success.
Keywords: Chickpea, drought, lysimeter, roots, terminal drought, water use pattern, yield
Introduction
Drought is the most important abiotic stress in chickpea worldwide. Terminal drought can reduce seed yields by 58–95% compared with irrigated plants and reductions in pod production and grain filling are key factors impacting final seed yield (Leport et al., 1999, 2006). Breeding for root traits has been the major target in the last 20 years to improve drought tolerance in chickpea (Saxena and Johansen, 1990; Saxena et al., 1993). Extensive root development has been proposed as the main drought avoidance trait to contribute to seed yield under terminal drought conditions (Ludlow and Muchow, 1990; Subbarao et al., 1995; Turner et al., 2001; Kashiwagi et al., 2005) as it was assumed that root density per se would help in the greater extraction of available soil water. While this has tremendous relevance, it is also important to consider that root systems are important as long as they allow water extraction at critical times for the plant. Besides, assessing roots is time-consuming and the methods that are currently in use only provide ‘static’ data. Root need to be considered in a more ‘dynamic’ fashion and therefore water uptake/use and its temporal pattern, rather than roots, may be a better indicator of their exact role (Vadez et al., 2007a, 2008). Consequently, root assessment, particularly during the vegetative stage, may not reflect differences in water uptake at key stages like reproduction and grain-filling and, therefore, whether roots relate to water extraction is worthy of investigation.
The pattern of water extraction/use is crucial for crops grown with a limited amount of water in the soil profile because crop reproductive success depends largely on a sustained water use into the reproductive growth stage (Merah, 2001; Kato et al., 2008). Indeed, water shortage during flower and pod production has a dramatic negative impact on final seed yield (Leport et al., 2006; Fang et al., 2010). So accurate estimation of water availability across the cropping cycle is an important tool for assessing crop performance, particularly in the post-rainy cropping systems where water supply is limited. In these systems, the amount of water available during the reproductive stage depends, for one part, on the way water was used by the plant earlier in the cropping cycle, i.e. on the capability of the plant to limit water use at the early stages to allow a significant amount of water to remain for the reproduction/pod-filling stage. A recent work shows that lower canopy conductance in terminal drought tolerant near-isogenic lines of pearl millet saves water under non-stressed conditions, allowing plants to have water available to fill up grains (Kholova et al., 2010a, b). To achieve this, water may also be extracted rather slowly by a smaller root system or a by a smaller shoot system with a reduced water demand (Pantuwan et al., 2002). Indeed, it was recently shown by simulation modelling that an increased growth rate of the root system of soybean, which would lead to the faster depletion of soil moisture, would have a negative effect on soybean yield under water stress (Sinclair et al., 2010). There is no such information available in chickpea.
Having water available for the reproduction and grain-filling period may also depend on having rooting systems that are capable of extracting large amounts of water, especially at depth. Recent data indicate that the progress in maize hybrids under drought conditions in the past 20 years was mostly explained by rooting differences (Robertson et al., 1993; Hammer et al., 2009). Similarly, crop simulation modelling indicates that increasing root depth would improve the grain yield of sorghum and maize in a large proportion of cases (Sinclair and Muchow, 2001). Differences have also been found in the soil extraction profile in lentils (Silim et al., 1993). On the other hand, increases in yield in maize have also been associated with less vigorous roots (Bruce et al., 2002). Chickpea genotypes with a higher root length density at depth also had a smaller advantage under mild stress (Kashiwagi et al., 2006). So roots clearly have a role to play in drought adaptation but further clarification of their role will probably require precise water extraction data. Obtaining such precise measurements of water extraction in the field is difficult and prone to errors. Recently, a method has been developed, which uses lysimeters, i.e. long and large PVC tubes filled with natural soil and mimicking a real soil profile, from the standpoint of volume of soil available and aerial space available for plants (Vadez et al., 2008; Ratnakumar et al., 2009). It is used here to compare whether different genotypes of chickpea, highly contrasting for terminal drought tolerance (Krishnamurthy et al., 2010) have differences in their capacity to extract water from a soil profile, and whether root length density correlates with water extraction.
In summary, the overall hypothesis was that tolerant genotypes may use less water from the soil profile at the vegetative stage and would then have more water available for uptake during the pod development/filling stage than the sensitive lines. Specifically the study aimed at (i) testing the lysimetric method for a yield-based evaluation of chickpeas; (ii) comparing the pattern of water use in contrasting chickpea lines and its relationship to canopy conductance differences; (iii) measuring rooting traits and testing their relationships with water extraction; and (iv) testing and comparing the relationship between water extraction pattern, rooting traits, and yield components.
Material and methods
Plant material
Twenty genotypes (12 tolerant and 8 sensitive) with the same phenological characteristics in previous field trials, and having the maximum contrast in yield and the drought-tolerance index under terminal drought stress in the field were selected (Table 1). These genotypes were selected after testing the ICRISAT mini-core collection under terminal drought and fully irrigated conditions in the field for three years (Krishnamurthy et al., 2010). Although Annigeri, ICC 4958 and ICCV 10 are known to be slightly early flowering lines, they were included in the trial because of their long record of drought tolerance testing and because ICCV 10 is currently a very popular variety.
Table 1.
Variation of number of days to maturity (DM) and drought tolerance index (DTI) across the studied chickpea genotypes (sensitive in bold; tolerant in normal font). Values are means of three years field experiment (Krishnamurthy et al., 2010).
| Geno | DM | DTI | Geno | DM | DTI | Geno | DM | DTI | Geno | DM | DTI |
| 7323 | 100.6 | -0.44 | 1052 | 94.5 | -0.52 | 12947 | 96.2 | 0.82 | 3325 | 89.9 | 0.69 |
| 7184 | 96.3 | -0.94 | 4182 | 94.3 | -0.33 | 8950 | 90.7 | 0.32 | 14778 | 92.0 | 0.94 |
| 4814 | 92.1 | -0.54 | 2507 | 92.0 | -0.38 | 2263 | 90.3 | 0.29 | 14799 | 89.9 | 0.63 |
| 3776 | 92.4 | -0.67 | ICCV10 | * | * | 14815 | 92.2 | 0.23 | 4958 | 81.9 | 0.30 |
| 8058 | 95.6 | -0.77 | 16524 | 90.4 | 0.37 | 867 | 87.6 | 0.75 | Annigeri | 84.0 | 0.16 |
*: not available
Soil filling and growth conditions of the lysimeters
The plants were grown in lysimeters, consisting of PVC cylinders (20 cm diameter, 120 cm height) filled with a Vertisol at ICRISAT, Patancheru (17°30' N; 78°16' E; altitude 549 m). The temperature and relative humidity of the air were collected from a temperature and relative humidity recorder (Gemini Tinytag Ultra 2 TGU-4500 Datalogger), which was located in the crop canopy (see Supplementary Fig. S1 at JXB online). A PVC end plate was placed on top of four screws at the bottom of the cylinders, 3 cm from the very bottom, to prevent the soil from seeping through. The endplate did not fit the cylinder tightly and allowed water drainage. To allow a rigorous control of the bulk density of the soil profile, the Vertisol used to fill the tubes, which was collected on the ICRISAT farm, was sieved into particles smaller than 1 cm. The cylinders were filled with 42 kg of dry soil, leaving the top 15 cm empty, and irrigated to more than field capacity before being allowed to drain. A top-up using dry soil was done to ensure that all cylinders would be filled to the same level, about 5 cm from the brim. This top-up varied little between the cylinders so the bulk density was similar in all tubes, at a value of approximately 1.2. Weighing of the cylinders indicated that all saturated cylinders weighed between 58 kg and 59 kg. The soil that was used to fill up the lysimeters had been fertilized with di-ammonium phosphate at a rate of 100 mg kg−1 soil.
Space arrangement of the lysimeters and weighing
The experimental design was a complete randomized block design with treatment as the main factor (three blocks) and genotype as a sub-factor replicated five times within each block. In each block, planted in adjacent but separated trenches, the lysimeters were arranged next to one another and therefore the chickpea crop was planted at a density of approximately 25 plants m−2, a plant population close to the field planting (row-to-row distance of 30 cm and plant-to-plant spacing of 10 cm). This was an important characteristic of the lysimetric approach to be able accurately to assess the water extraction pattern of a crop cultivated in conditions that are quite similar to the field. The three trenches were 1.2 m deep and 1.8 m wide, and separated from the adjacent trench by a 20 cm concrete wall.
The top of the cylinders was equipped with a metal collar and rings that allowed the cylinder to be lifted. Weighing of the cylinders was done by lifting the cylinders with a block-chained pulley, and an S-type load cell (Mettler-Toledo, Geneva, Switzerland) was inserted between the rings of the cylinder and the pulley. The scale, of 100 kg capacity, allowed repeated measurements and gave an accuracy of 10 g on each weighing.
Sowing and crop management
Four seeds of each genotype were sown in the soil on 2 November 2009. The cylinders were then irrigated with 500 ml of water immediately after sowing and twice on alternate days with 250 ml until the seedlings emerged uniformly. The plants were thinned to two individuals per cylinder at 7 d after sowing (DAS) and then to a single plant per cylinder at 14 DAS. One block was assigned to a well-watered treatment (WW) and two blocks to a water-stressed treatment (WS). One of these blocks was kept until maturity while the second block of WS treatment was prepared for the purpose of root sampling at about 6 weeks after stress imposition. The WS treatment was imposed by cessation of watering from 23 DAS (25 November). WW plants were watered every 5 d to maintain the soil above 80% field capacity until maturity. Before initiating the weighing, the top of the cylinders were covered with a round and slit plastic sheet, on top of which 2 cm of low density polyethylene granules were laid. These layers prevented more than 90% of soil evaporation, so that successive weighing measured plant transpiration. The cylinders were weighed every 5 d from 23 DAS until 48 DAS, then once a week until 61 DAS, and then every 2 weeks until maturity. The transpiration data calculated from each pair of consecutive weighing were assigned to the date of the latest weighing so that, for simplicity, transpiration at 28 DAS meant the transpiration during the 23–28 DAS interval. Plant water uptake was estimated from the losses in weight of each cylinder. Dates to flowering and maturity were recorded for each genotype.
Root sampling at six weeks after stress imposition
At 61 DAS (about six weeks after the initiation of the water stress imposition), soon after cylinder weighing, plants of one WS block were cut at the soil surface. The soil in the cylinders was washed to collect the whole root system. After gently removing the soil particles, the roots were laid on a table without stretching to measure their maximum length as an estimate of rooting depth. The root system was then sliced in portions of 30 cm in order to measure the total root length at each of the 30 cm depths of the root system using image analysis software (WinRhizo, Regent Instruments INC., Canada). Root length density in each 30 cm layer was obtained by dividing the root length by the volume of a 30 cm section of the cylinder, assuming roots had colonized the entire volume at each depth.
Estimation of canopy conductance
The index of stomatal conductance (Ig) was used as an indirect estimation of the absolute stomatal conductance (Jones, 1999), using canopy temperature measurements. A parallel report in chickpea showed that canopy temperature relates very closely at the vegetative stage with the canopy conductance (in g water transpired h−1 cm−2) (Zaman-Allah et al., 2011). Thermal images were obtained using an IR FlexCam S (Infrared solutions, USA) with a sensitivity of 0.09 °C and an accuracy of ±2%. The images were taken at the highest atmospheric VPD of the day at a height of 2.0 m above the canopy. The canopy image was separated from the background from a frequency distribution of the pixels, where only temperature fitting in a Gauss distribution were considered to be part of the canopy. This approach was previously tested successfully to separate the canopy image from the background (Zaman-Allah et al., 2011). The software, SmartView 2.1.0.10 (Fluke Thermography), was used for the analysis of the thermal images and the estimation of canopy temperatures following the prior report by Zaman-Allah et al. (2011). From the canopy temperature, Ig was estimated as:
where Twet is the temperature of a wet surface and Tdry is the temperature of a non-transpiring surface. Twet was measured on green leaves after soaking them with water 5 min, Tdry is the temperature of dry leaves, and Tleaf is the leaf canopy temperature measured with the IR camera. These temperatures were measured at 32 DAS and 37 DAS in plants grown under water-stress conditions, using green and dried leaves from extra plants of all genotypes, which were pooled to make the measurements.
Final harvest
When the plants reached maturity, they were harvested at soil level and individually partitioned into pods, leaves, and stems. Pods and seeds were counted for each plant and the seed weight was then recorded. The percentage of seed abortion was calculated as the proportion of empty pods from the total number of pods.
Statistical analysis
Statistical analyses were performed using GenStat 10.1.0.72 by one-way ANOVA and t test. Differences between mean values of treatments were evaluated using least significant difference (LSD) at a 0.05 significance level.
Results
Phenology and yield components
ICC 4958, Annigeri, and ICCV 10 flowered quite earlier (37, 39, and 42 DAS, respectively) than the remaining genotypes where the flowering time was up to 58 DAS for ICC 7323 (Table 2). With the lysimetric system, the number of days to maturity was quite similar to what was reported in the field (Krishnamurthy et al., 2010) for all the genotypes (Tables 1, 2). All genotypes together, there was a negative relationship between seed yield and flowering time (R2=0.42, P <0.01), but this relationship was weak and non-significant when these three genotypes were excluded (R2=0.16, data not shown). Compared with the well-watered plants, the water-stressed plants matured earlier by at least 3 d (Table 2). Pod production per plant showed consistent differences among the genotypes, irrespective of the treatment (Table 2). Under well-watered conditions, the pod number, seed number or seed weight did not clearly discriminate the tolerant from the sensitive genotypes, although sensitive genotypes tended to have a lower pod and seed number. Under drought stress, the seed weight of sensitive genotypes was below 4 g plant−1, except ICC 2507. By contrast, the seed weight under drought stress of tolerant genotypes was above 5.5 g plant−1 for all genotypes except ICC 12947 (Table 2). On average, the seed yield of tolerant lines, excluding ICC 12947, was 6.70 g plant−1, whereas the average seed weight of sensitive lines, excluding ICC 2507, was 3.42 g plant−1, about 50% less. The most pod-productive genotypes under water stress were ICCV 10, ICC 8950, ICC 14778, ICC 867, and ICC 3325 (Table 2). A similar trend was recorded for seed number and pod number between the tolerant and susceptible genotypes, which were about 45% less.
Table 2.
Phenology and yield components of chickpea genotypes contrasting for terminal drought tolerance index (DTI) (sensitive in bold; tolerant in normal font) grown in 1.2 m length PVC tubes under well watered and water stress conditions during the reproductive stage. The data are means of five replicated plants, grown individually in each PVC tube.
| Flowering time (DAS) | Maturity (DAS) |
Pod number plant-1 |
Seed number plant-1 |
Seed weight plant-1 |
|||||
| Genotypes | WW | WS | WW | WS | WW | WS | WW | WS | |
| ICC7323 | 58 | 98 | 94 | 31.00 | 14.40 | 29.00 | 12.20 | 5.08 | 2.35 |
| ICC7184 | 56 | 96 | 91 | 73.00 | 37.20 | 69.50 | 32.60 | 8.52 | 3.70 |
| ICC4814 | 53 | 95 | 91 | 39.33 | 30.50 | 36.67 | 25.75 | 5.08 | 4.00 |
| ICC3776 | 52 | 91 | 88 | 52.75 | 23.00 | 50.50 | 22.25 | 6.90 | 3.65 |
| ICC8058 | 46 | 93 | 89 | 22.75 | 18.00 | 20.50 | 14.67 | 4.22 | 3.40 |
| ICC1052 | 53 | 94 | 92 | 48.75 | 23.80 | 46.00 | 21.40 | 5.85 | 3.21 |
| ICC4182 | 55 | 97 | 95 | 42.33 | 31.50 | 39.33 | 26.00 | 5.38 | 3.65 |
| ICC2507 | 45 | 92 | 90 | 48.67 | 40.00 | 46.67 | 35.80 | 6.19 | 5.17 |
| ICCV 10 | 42 | 92 | 89 | 57.75 | 48.75 | 53.75 | 44.25 | 8.23 | 7.48 |
| ICC16524 | 51 | 90 | 90 | 80.25 | 39.50 | 76.75 | 37.00 | 11.73 | 6.00 |
| ICC12947 | 55 | 94 | 89 | 29.75 | 24.67 | 28.00 | 22.67 | 4.94 | 3.81 |
| ICC8950 | 50 | 91 | 88 | 64.00 | 49.50 | 60.00 | 45.50 | 7.28 | 6.57 |
| ICC2263 | 51 | 93 | 90 | 45.67 | 48.25 | 38.33 | 44.25 | 7.03 | 6.63 |
| ICC14815 | 55 | 94 | 91 | 41.00 | 37.00 | 37.25 | 33.75 | 6.19 | 5.91 |
| ICC867 | 46 | 88 | 87 | 77.67 | 47.40 | 74.33 | 42.00 | 11.01 | 6.36 |
| ICC 3325 | 50 | 92 | 89 | 70.25 | 45.25 | 65.50 | 43.25 | 10.18 | 6.49 |
| ICC14778 | 53 | 94 | 89 | 42.75 | 55.25 | 40.50 | 51.75 | 5.39 | 7.44 |
| ICC14799 | 49 | 91 | 87 | 54.75 | 40.60 | 50.75 | 36.80 | 8.11 | 5.52 |
| ICC 4958 | 37 | 85 | 84 | 39.00 | 37.00 | 36.00 | 33.75 | 9.74 | 7.94 |
| Annigeri | 39 | 86 | 83 | 51.00 | 41.75 | 47.67 | 38.00 | 9.72 | 7.32 |
| LSD (5%) | - | - | - | 13.59 | 11.20 | 13.20 | 11.56 | 1.87 | 1.56 |
| Mean tolerant | 54.03 | 42.68 | 50.42 | 39.13 | 8.03 | 6.35 | |||
| Mean sensitive | 44.27 | 25.48 | 41.64 | 22.12 | 5.86 | 3.42 | |||
There was very close correspondence between the classification for terminal drought tolerance/sensitivity made in the lysimeters and in the three years of yield data from the field (Krishnamurthy et al., 2010) that led to the selection of these 20 genotypes. In addition, the mean yield per plant in the lysimeters, extrapolated to a yield per hectare using a 25 plant m−2 density gave a yield of about 1800 kg ha−1 and 1330 kg ha−1 under WW and WS conditions, close to the 2000 kg ha−1 and 1200 kg ha−1 reported across the three years in Krishnamurthy et al., (2010).
Pattern of soil water use
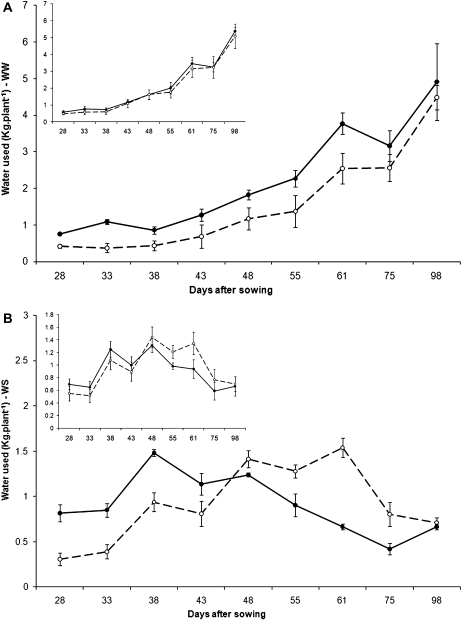
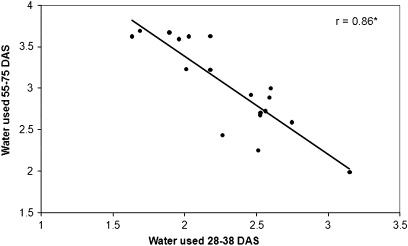
The pattern of soil water use by plants varied among the genotypes and across the 10 last weeks of the cropping cycle particularly in the WS treatment. In well-watered plants, the average water uptake curve of the tolerant genotypes tended to be below the average curve for the sensitive lines (Table 3; inset in Fig. 1A). In fact, tolerant ICC 867 had lower water uptake than sensitive ICC 8058 (Fig. 1A). Similarly, tolerant ICC 14799 and, to some extent, ICCV 10, ICC 8950, ICC 3325, and ICC 2263, were the genotypes that showed the lowest amount of water used, especially before 55 DAS, compared with most of the remaining genotypes that tended to use a higher amount of water (Table 3). In water-stressed plants, the pattern of water use was different from that of well-watered plants (Fig. 1B). At the vegetative stage, i.e. 28 DAS and to some extent 33 and 38 DAS, there was a clear trend of higher water uptake across susceptible genotypes compared with the tolerant ones (Table 4; inset of Fig 1B). By contrast, from 48 DAS onward until 75 DAS, the tolerant genotypes as a group had a higher water uptake than the sensitive group, especially at 48 and 75 DAS (inset of Fig. 1B). Figure 1B shows an example of tolerant ICC 867 with a lower water uptake at the vegetative stage than the sensitive ICC 8058, and a reverse situation after flowering (Fig. 1B). Overall, in the WS treatment, water extracted at the vegetative stage strongly and negatively correlated with water extracted during the reproductive phase (r=0.86, P <0.01) (Fig. 2).
Table 3.
Water extraction (kg H2O.plant-1) of chickpea genotypes (sensitive in bold; tolerant in normal font) in well watered conditions at different dates (days after sowing). Values at 28 DAS are water extraction in the 23-28 DAS period.
| Days after sowing |
||||||||||||
| Genotypes | 28 | 33 | 38 | 43 | 48 | 55 | 61 | 75 | 98 | 23-38 | 48-75 | Total |
| ICC 7323 | 0.630 | 0.817 | 0.888 | 1.298 | 1.975 | 2.600 | 4.055 | 3.160 | 5.535 | 2.335 | 9.815 | 20.96 |
| ICC 7184 | 0.510 | 0.610 | 0.645 | 1.063 | 1.445 | 1.630 | 3.370 | 3.590 | 5.403 | 1.765 | 8.590 | 18.27 |
| ICC 4814 | 0.595 | 0.800 | 0.678 | 1.110 | 1.660 | 1.955 | 3.385 | 2.835 | 5.510 | 2.073 | 8.175 | 18.53 |
| ICC 3776 | 0.555 | 0.647 | 0.595 | 1.003 | 1.068 | 1.655 | 2.840 | 3.080 | 5.398 | 1.798 | 7.575 | 16.84 |
| ICC 8058 | 0.755 | 1.085 | 0.860 | 1.270 | 1.828 | 2.270 | 3.770 | 3.160 | 4.910 | 2.700 | 9.200 | 19.91 |
| ICC 1052 | 0.490 | 0.660 | 0.662 | 1.250 | 1.658 | 1.920 | 3.425 | 3.565 | 5.503 | 1.813 | 8.910 | 19.13 |
| ICC 4182 | 0.543 | 0.695 | 0.795 | 1.198 | 1.708 | 1.995 | 3.400 | 3.363 | 5.420 | 2.033 | 8.758 | 19.12 |
| ICC 2507 | 0.550 | 0.693 | 0.753 | 1.195 | 1.725 | 2.105 | 3.365 | 3.320 | 5.403 | 1.995 | 8.790 | 19.11 |
| ICCV 10 | 0.440 | 0.507 | 0.670 | 1.220 | 1.728 | 1.960 | 3.010 | 2.590 | 3.513 | 1.618 | 7.560 | 15.64 |
| ICC 16524 | 0.653 | 0.685 | 0.698 | 1.230 | 1.953 | 2.245 | 4.200 | 4.720 | 5.993 | 2.035 | 11.165 | 22.38 |
| ICC 12947 | 0.492 | 0.610 | 0.620 | 1.195 | 1.723 | 1.595 | 2.845 | 3.250 | 5.488 | 1.723 | 7.690 | 17.82 |
| ICC 8950 | 0.445 | 0.578 | 0.513 | 1.130 | 1.763 | 1.855 | 3.160 | 2.795 | 4.930 | 1.535 | 7.810 | 17.17 |
| ICC 2263 | 0.472 | 0.648 | 0.683 | 1.228 | 1.725 | 2.085 | 3.125 | 3.170 | 5.375 | 1.803 | 8.380 | 18.51 |
| ICC 14815 | 0.518 | 0.643 | 0.655 | 1.250 | 1.710 | 1.955 | 3.590 | 3.790 | 5.438 | 1.815 | 9.335 | 19.55 |
| ICC 867 | 0.418 | 0.373 | 0.440 | 0.685 | 1.170 | 1.370 | 2.540 | 2.560 | 4.483 | 1.230 | 6.470 | 14.04 |
| ICC 3325 | 0.518 | 0.585 | 0.590 | 1.065 | 1.610 | 1.765 | 3.375 | 3.505 | 5.858 | 1.693 | 8.645 | 18.87 |
| ICC 14778 | 0.528 | 0.765 | 0.772 | 1.310 | 1.793 | 1.815 | 3.295 | 3.080 | 4.725 | 2.065 | 8.190 | 18.08 |
| ICC 14799 | 0.382 | 0.345 | 0.328 | 0.607 | 1.063 | 1.055 | 2.425 | 2.890 | 5.000 | 1.055 | 6.370 | 14.10 |
| ICC4958 | 0.713 | 0.995 | 1.035 | 1.615 | 2.565 | 2.430 | 3.205 | 2.905 | 4.213 | 2.743 | 8.540 | 19.68 |
| Annigeri | 0.637 | 0.780 | 0.785 | 1.273 | 1.648 | 2.095 | 3.310 | 3.055 | 4.900 | 2.203 | 8.460 | 18.48 |
| LSD (5%) | 0.150 | 0.238 | 0.240 | 0.359 | 0.599 | 0.718 | 0.976 | 1.242 | 1.427 | 0.541 | - | 4.04 |
Fig. 1.
Variation in water uptake profile in two chickpea genotypes contrasting for the drought stress index (DTI) under terminal drought stress (sensitive ICC 8058, closed symbols and solid lines; tolerant ICC 867, open symbols and dashed lines) and grown under (A) well watered and (B) water-stressed conditions. Water stress imposition was initiated by giving a final irrigation at 23 DAS. Plants were grown in 1.2 m lengh PVC cylinders. The water extraction values are those for the period finishing at the date when the data are plotted. For instance, water extracted at 28 DAS corresponds to that in the period between 23 and 28 DAS. Insets in (A) and (B) show the water uptake profile for the group of tolerant and sensitive entries under each water regime. Data for the sensitive group are the mean of water use data across all sensitive lines except ICC 2507 (closed symbols and solid lines). Data for the tolerant group are the mean of water use data across all sensitive lines except Annigeri and ICC 4958 (open symbols and dashed lines).
Table 4.
Water extraction (kg H2O.plant-1) of chickpea genotypes (sensitive in bold; tolerant in normal font) in water stress conditions at different dates (days after sowing). The water stress imposition (last irrigation) was initiated at 23 DAS. Values at 28 DAS are water extraction in the 23-28 DAS period.
| Days after sowing |
||||||||||||
| Genotypes | 28 | 33 | 38 | 43 | 48 | 55 | 61 | 75 | 98 | 23-38 | 48-75 | Total |
| ICC 7323 | 0.685 | 0.655 | 1.185 | 0.970 | 1.255 | 0.955 | 0.995 | 0.728 | 0.878 | 2.525 | 2.678 | 8.31 |
| ICC 7184 | 0.740 | 0.660 | 1.345 | 1.063 | 1.358 | 0.960 | 0.965 | 0.665 | 0.630 | 2.745 | 2.590 | 8.39 |
| ICC 4814 | 0.560 | 0.547 | 1.155 | 1.048 | 1.360 | 0.987 | 0.965 | 0.482 | 0.430 | 2.263 | 2.435 | 7.53 |
| ICC 3776 | 0.693 | 0.610 | 1.225 | 1.115 | 1.530 | 1.060 | 1.015 | 0.628 | 0.738 | 2.528 | 2.703 | 8.61 |
| ICC 8058 | 0.815 | 0.850 | 1.483 | 1.138 | 1.240 | 0.903 | 0.665 | 0.420 | 0.663 | 3.148 | 1.988 | 8.18 |
| ICC 1052 | 0.750 | 0.587 | 1.123 | 0.778 | 1.213 | 1.030 | 1.130 | 0.760 | 0.795 | 2.460 | 2.920 | 8.17 |
| ICC 4182 | 0.638 | 0.630 | 1.243 | 0.868 | 1.230 | 0.972 | 0.840 | 0.438 | 0.520 | 2.510 | 2.250 | 7.38 |
| ICC 2507 | 0.502 | 0.385 | 1.005 | 0.823 | 1.515 | 1.253 | 1.345 | 1.073 | 1.057 | 1.893 | 3.670 | 8.96 |
| ICCV 10 | 0.447 | 0.385 | 0.855 | 0.738 | 1.360 | 1.373 | 1.465 | 0.855 | 0.558 | 1.688 | 3.693 | 8.04 |
| ICC 16524 | 0.638 | 0.713 | 1.248 | 1.060 | 1.478 | 1.003 | 1.215 | 0.780 | 0.893 | 2.598 | 2.998 | 9.03 |
| ICC 12947 | 0.528 | 0.485 | 0.947 | 0.645 | 1.143 | 1.223 | 1.635 | 0.735 | 0.590 | 1.960 | 3.593 | 7.93 |
| ICC 8950 | 0.713 | 0.605 | 1.245 | 0.970 | 1.305 | 1.118 | 1.120 | 0.487 | 0.530 | 2.563 | 2.725 | 8.09 |
| ICC 2263 | 0.557 | 0.455 | 1.015 | 0.760 | 1.370 | 1.250 | 1.425 | 0.948 | 0.690 | 2.028 | 3.623 | 8.47 |
| ICC 14815 | 0.488 | 0.432 | 1.090 | 0.920 | 1.558 | 1.268 | 1.335 | 0.628 | 0.725 | 2.010 | 3.230 | 8.44 |
| ICC 867 | 0.305 | 0.390 | 0.938 | 0.810 | 1.413 | 1.278 | 1.540 | 0.805 | 0.712 | 1.633 | 3.623 | 8.19 |
| ICC 3325 | 0.663 | 0.593 | 1.333 | 1.103 | 1.475 | 1.138 | 1.105 | 0.645 | 0.700 | 2.588 | 2.888 | 8.76 |
| ICC 14778 | 0.560 | 0.548 | 1.070 | 0.980 | 1.685 | 1.190 | 1.390 | 1.045 | 0.780 | 2.178 | 3.625 | 9.25 |
| ICC 14799 | 0.618 | 0.520 | 1.040 | 0.987 | 1.645 | 1.238 | 1.220 | 0.762 | 0.828 | 2.178 | 3.220 | 8.86 |
| ICC4958 | 0.672 | 0.925 | 1.218 | 1.058 | 1.283 | 0.990 | 0.750 | 0.565 | 0.555 | 2.815 | 2.305 | 8.02 |
| Annigeri | 0.535 | 0.655 | 1.068 | 0.938 | 1.285 | 0.803 | 0.695 | 0.690 | 0.810 | 2.258 | 2.188 | 7.48 |
| LSD (5%) | 0.198 | 0.220 | 0.275 | 0.311 | 0.297 | 0.235 | 0.394 | 0.369 | 0.241 | 0.567 | 0.816 | 0.77 |
Fig. 2.
Relationship between water used (kg plant−1) between 23 and 38 DAS and between 48 and 75 DAS of chickpea genotypes grown in 1.2 m lengh PVC tubes under water stress during the reproductive stage. Data are the means of five replicated plants for each genotype and treatment.
The total water uptake under WW conditions varied between genotypes, with ICC 7323 and ICC 16524 having the highest water uptake (20.96 and 22.38 kg plant−1, respectively), whereas ICC 867, ICC 14799, and ICCV 10 had the lowest (below 16 kg plant−1). Yet, there was no trend discriminating tolerant and sensitive genotypes on the basis of their total water uptake under WW conditions (Table 3). Under WS conditions, there were also genotypic variations in total water uptake. For example, sensitive ICC 4814 and ICC 4182 had the lowest water uptake whereas tolerant ICC 16524 and ICC 14778, and sensitive ICC 3776 and ICC 2507 had the largest water uptake. However, under these conditions there was also no trend discriminating between the tolerant and the sensitive lines on the basis of the total water extracted (Table 4).
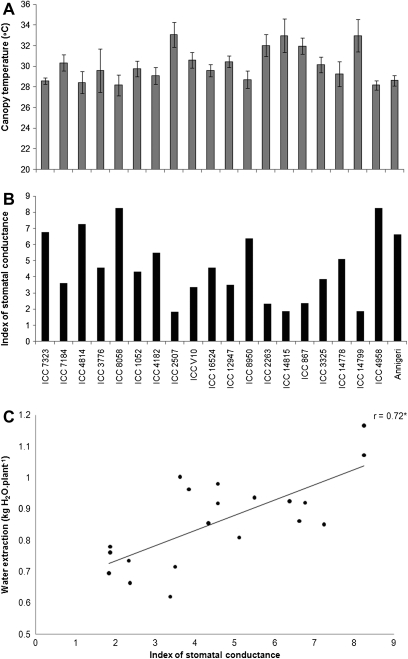
Canopy conductance
Canopy temperature and the index of stomatal conductance were measured at 32 and 37 DAS using infrared thermography and showed significant variation among the genotypes (Fig. 3A, B). These measurements were made at a time when the transpiration of the water-stressed plants had not declined significantly compared with the well-watered control, therefore presumably in the absence of stress. Compared with canopy temperature, the index of stomatal conductance provided a somewhat better discrimination between the tolerant and sensitive genotypes. Except for early flowering Annigeri and ICC 4958, and tolerant ICC 8950, there was a trend for a higher index of stomatal conductance in the susceptible genotypes (below 30 °C, except ICC 7184) compared with the group of tolerant genotypes where around 50% showed a relatively hotter canopy (above 32 °C) (Fig. 3). A recent report indicates a close relationship between either canopy temperature, Ig, and canopy conductance measured gravimetrically (Zaman-Allah et al., 2011). The average amount of water extracted between 28–33 DAS and 33–38 DAS was plotted against the mean index of stomatal conductance estimated from the canopy temperature taken at 32 and 37 DAS. Results showed that water extracted during the period 28–38 DAS was indeed significantly and positively related to the index of stomatal conductance (Fig. 3C), indicating that genotypes having a high canopy conductance also extracted more water overall.
Fig. 3.
Variation of canopy temperature (°C) (A) and index of stomatal conductance (Ig) (B) in chickpea genotypes contrasting for terminal drought tolerance index (DTI) grown in 1.2 m lengh PVC tubes under water stress. Values are means ±SE of data collected at 32 and 37 DAS. Relationship between water extraction (kg H2O plant−1) and the index of stomatal conductance (Ig) (C) measured using infrared thermography in chickpea genotypes contrasting for terminal drought tolerance index (DTI) grown in 1.2 m lengh PVC tubes under water stress. Water extraction values are the means of those for the period 28–33 DAS and 33–38 DAS and Ig values are means of data collected at 32 and 37 DAS. Genotypes are sorted out in order of increasing DTI on the x-axis, within group of sensitivity and tolerance.
Root development and relationships with water extraction
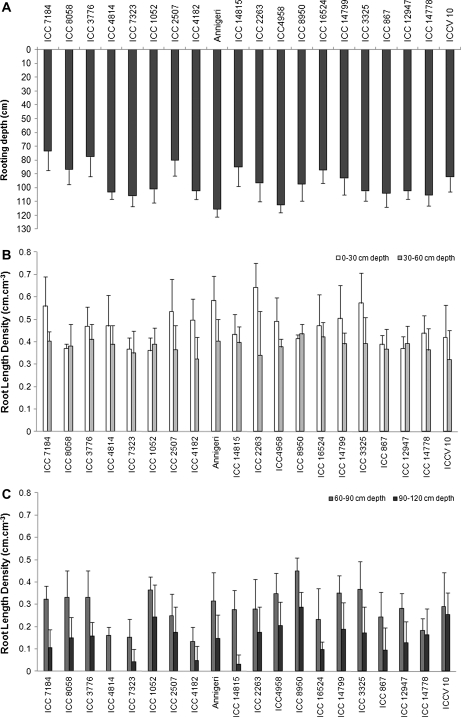
Rooting depth did not show large differences between the 20 genotypes studied in the set of plants that was harvested at 61 DAS, (i.e. 38 d after stress imposition) (Fig. 4A). Nevertheless, the group of tolerant genotypes tended to have, on average, a deeper root system than the susceptible ones (100 cm versus 89 cm). Thus, sensitive ICC 7323, ICC 4814, ICC 4182 and to a lesser extent ICC 7184 presented the shallowest root system while ICC V 10 and ICC 8950 exhibited the deepest root system among all genotypes. Yet, at that stage, tolerant ICC 16524 and ICC 14815 also had shallow root depth while sensitive ICC 3776, ICC 8058, and ICC 1052 had deep roots (Fig. 4A).
Fig. 4.
Variation of (A) rooting depth and (B, C) root length density at different layers of soil profile in chickpea genotypes contrasting for terminal drought tolerance index (DTI) after 6 weeks of water stress imposition by withholding irrigation. Data are the means (±SE) of five replicated plants for each genotype and treatment. Genotypes are placed in order of increasing DTI on the x-axis, within group of sensitivity and tolerance.
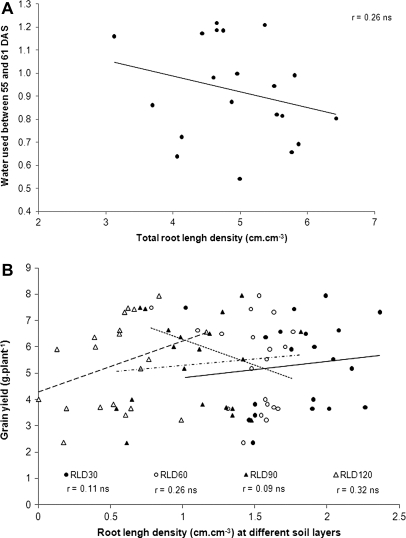
Root length density varied substantially among the genotypes across the soil depth and the variation was largest in the 90–120 cm soil layer (Fig. 4B, C). The maximum RLDs were recorded at the 0–30 cm soil profile with all genotypes being above 0.35 cm cm−3 of soil, particularly ICC 7184, ICC 4182, ICC 2507, ICC 2263, ICC 3325, and Annigeri (Fig. 4B). At the 30–60 cm soil depth, RLD was comparable for all the genotypes. The genotypes showed significant RLD differences at the 60–90 cm soil layer, with sensitive ICC 7323, ICC 4814, and ICC 4182 and tolerant ICC 14778 showing a less profuse root system with RLD below 0.2 cm cm−3 of soil (Fig. 4C). In the 90–120 cm soil layer, sensitive ICC 7323, ICC 4182, and ICC 4814 and tolerant ICC 16524, ICC 12947, ICC 14815, and ICC 867 presented the lowest values of RLD (Fig. 4C). Overall, at 61 DAS, there was no clear discrimination between tolerant and susceptible genotypes regarding root length density at any of the layers investigated.
Root length density was assessed at 61 DAS, about 6 weeks after stress imposition, and showed a non-significant relationship with the total water extracted in these plants until then (Fig. 5A). A similar analysis was carried out by taking each soil depth layer individually. There was also no significant relationship between root length density and water extraction at any of the layers (data not shown).
Fig. 5.
Relationship (A) between water uptake at the time of root harvest (61 DAS) and RLD and (B) between grain yield and root length density (RLD) at different depths in chickpea genotypes grown in 1.2 m lengh PVC tubes under well-watered (WW) and water-stress (WS) conditions during the reproductive stage. Data are the means of five replicated plants for each genotype and treatment.
Relationship between root growth, patterns of water extraction, and yield components
Root length density at different depth was not significantly related to the seed yield or any of the yield components such as pod number or seed number (Table 5; Fig. 5B). The percentage of pod abortion was also not significantly related to the RLD (data not shown).
Table 5.
Correlation matrix of yield components against root development parameters and patterns of water uptake.
| CORRELATION MATRIX |
||||||||||||
| RLD 90 | RLD 120 | 28 DASa | 33 DAS | 38 DAS | 43 DAS | 48 DAS | 55 DAS | 61 DAS | 75 DAS | 98 DAS | TWUb | |
| Pod Number | -0.001 | 0.27 | -0.49* | -0.51* | -0.38 | -0.16 | 0.53* | 0.64** | 0.53* | 0.46 | -0.04 | 0.37 |
| Seed Number | 0.03 | 0.30 | -0.47* | -0.49* | -0.37 | -0.14 | 0.56* | 0.65** | 0.45* | 0.48* | -0.002 | 0.43 |
| Seed Weight | 0.10 | 0.32 | -0.53* | -0.51* | -0.42 | -0.16 | 0.56* | 0.72** | 0.59* | 0.46 | -0.003 | 0.42 |
| % Seed Abortion | -0.33 | -0.42 | 0.16 | 0.32 | 0.26 | 0.02 | -0.53* | -0.49* | -0.54* | -0.44 | -0.26 | -0.62* |
DAS: days after sowing
TWU: total water uptake. (*) significant at 5% and (**) at 1%
NB: ICC4958 and Annigeri were excluded from the correlation analysis regarding water uptake but not for RLD because they are relatively early compared to the other genotypes.
To assess whether the yield difference between the tolerant and the susceptible genotypes was due to reproductive or grain-filling failure, the 100-seed weight reduction between well-watered and water-stressed plants was calculated (100-seed weight WS/100-seed weight WW), as in Vadez et al. (2007b). Similar ratios were calculated for the reduction in seed number and seed yield. The regression between these variables, using the reduction in seed yield as the dependent variable, showed that the seed yield decrease was highly significantly related to a reduction in seed number (R2=0.79). By contrast, the reduction in seed yield, relatively more important in the sensitive lines, was not significantly related to a decrease in seed filling (see Supplementary Fig. S2 at JXB online).
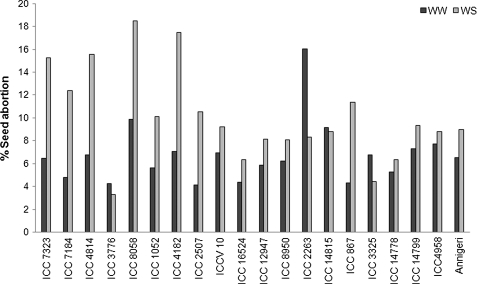
The percentage of pod abortion under water stress was significantly higher in the sensitive group except ICC 3776 (Fig. 6). Compared with water-stressed plants, well-watered plants showed less pod abortion and the amplitude of variation among all genotypes was low, except with ICC 2263 (Fig. 6). In addition, water uptake during the period 48–61 DAS was found to be significantly and negatively correlated with the percentage of pod abortion (Table 5) (r=0.54, P <0.05).
Fig. 6.
Variation of percentage seed abortion in chickpea genotypes contrasting for terminal drought tolerance index (DTI) grown in 1.2 m lengh PVC tubes under water stress (WS) during the reproductive stage. Data are the means of five replicated plants for each genotype and treatment. Genotypes are placed in order of increasing DTI on the x-axis, within group of sensitivity and tolerance.
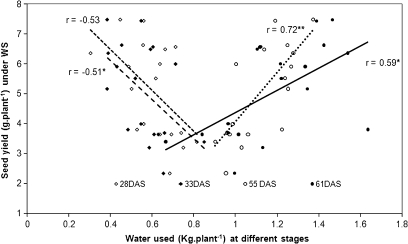
A correlation analysis between the yield components and the water-uptake profile was carried out to assess at what stage water extraction had a particular importance for chickpea yield (Table 5). There was no significant correlation between yield or yield components and the total water uptake between 23 DAS and the final harvest except the negative correlation for the percentage of pod abortion. However, pod and seed number and seed weight were significantly and positively correlated with the amount of water taken at 55 and 61 DAS (water extraction of the 48–55 DAS and 55–61 DAS periods, respectively) while being negatively correlated with the water uptake at 28, 33, and 48 DAS (Table 5 and Fig. 7). Figure 7 also showed that water extraction differences of only 400 g plant−1, i.e. 4 mm of water on a per hectare basis, led to seed yield differences over 100%. Significant positive correlation was found between the seed yield and the cumulative water uptake during the period 48–61 DAS (data not shown, r=0.66, P <0.05). The data show also that the genotypic variation in water extracted in that critical period was about 1.0 l of water plant−1, corresponding to about 25 mm of water when extrapolated to a field situation. By contrast, the yield was negatively correlated with the cumulative water uptake much earlier (23–38 DAS) (data not shown, r= –0.50, P <0.05).
Fig. 7.
Relationship between seed yield and the pattern of water use at different dates in chickpea genotypes grown in 1.2 m lengh PVC tubes under water stress (WS) during the reproductive stage. Data are the means of five replicated plants for each genotype and treatment.
Yield potential is known to contribute to the yield under water stress in several crops including chickpea (Vadez et al., 2007b). To assess the extent of its contribution to the yield performance under water stress, seed yield under WS was plotted against seed yield under WW conditions. A significant linear relationship was found between the two (r=0.56, P <0.05) (see Supplementary Fig. S3 at JXB online). Therefore, the seed yield performance under WS is partly explained by the yield potential but residual yield variations are not explained by the yield potential. Those residuals represent the part of variation in yield under WS that is not explained by yield potential. After computing these residuals, they were regressed, as dependant variables, against water uptake at 55 DAS and 55–61 DAS (water extraction period 48–55 DAS and 48–61 DAS, respectively) that were the most related to the seed yield (see Supplementary Fig. S3 at JXB online). A significant relationship was found between the amount of water that was taken at 55–61 DAS and to a higher extent at 55 DAS (Fig. 7), therefore leading to the same conclusion made above on the seed yield under stress, and its close relationship to the pattern of water extraction.
Discussion
Our lysimetric assessment confirmed the seed yield contrast that was previously found in these genotypes in the field, except for one exception in each group. There was no trend separating tolerant and sensitive genotypes on the basis of total water use, rooting depth, and root length density at the reproductive stage. Clearly, the advantage of tolerant genotypes was in their different profile of water extraction. Indeed, the lysimeter assessment revealed that tolerant genotypes had a lower water uptake pattern than sensitive genotypes during the vegetative stages and until 43 DAS. This was generally related to lower indices of stomatal conductance in the tolerant rather than the sensitive lines. By contrast, tolerant lines extracted more water from the soil profile than the sensitive lines from 48 until 75 DAS, corresponding to the reproductive and grain-filling period. In fact, water extracted at the vegetative stage negatively and very strongly correlated with water extracted during grain filling (Fig. 2). A correlation analysis revealed then that water extracted between 48 and 61 DAS were the most closely and positively related to seed yield, whereas water extracted between 23 and 33 DAS were the most closely and negatively related to seed yield (Table 5). Root length density at different depths was either not or poorly related to seed yield (Fig. 5B). These data indicate overall that terminal drought tolerance in chickpea depends mostly on a more conservative use of water earlier in the cropping cycle, which is partly related to lower canopy conductance, but not to differences in deep or profuse rooting.
Lysimetric confirmation of tolerance and the lack of discrimination based on rooting traits at early reproductive stage
This study shows the validity of the lysimetric system to carry out yield-based evaluation of germplasm. Indeed, the ranking of the genotypes was quite similar to the ranking in the field (Krishnamurthy et al., 2010), which summed up three years of data across more than 220 genotypes. In addition, the seed yield obtained in the tubes, extrapolated to their planting density, was very close to those previously obtained in the field. The strategy to breed for terminal drought tolerance in chickpea has so far focused on a deep and profuse root system (Saxena and Johansen, 1990; Saxena et al., 1993; Johansen et al, 1994; Krishnamurthy et al 1998; Kashiwagi et al., 2005, 2006). Nonetheless, the root system is important as long as it allows water extraction at critical times for the plant, as it has recently been shown in groundnut (Ratnakumar et al., 2009). Differences in root development were reported to play a significant role in determining water uptake in several crops including chickpea (Krishnamurthy et al., 1998; Kashiwagi et al., 2005) with considerable impact on the final yield. The present work has shown that, during reproductive growth, differences in rooting depth as well as RLD and RDW were small among genotypes highly contrasting for terminal drought tolerance (Fig. 4A–C) and that they did not discriminate the tolerant from the sensitive group of genotypes. This is clear evidence that, during the reproductive stage, the availability of water in the soil profile, more that an extensive root system, is a key determinant of the final yield.
The profile of water use relates to stomatal conductance and discriminates genotypes for tolerance to water stress
This study has shown that there is a clear discrimination for water uptake profile between the tolerant and susceptible genotypes under water stress (Table 2; Fig. 1B). Up to 38 DAS, a stage where the transpiration of WS plants was still similar to that of the WW control, the tolerant genotypes had a lower water use, which was significantly related to a lower stomatal conductance compared with the sensitive ones, thereby maintaining water in the soil profile for a longer period of time with a subsequent significant amount of water being available for the pod-filling stage. The genotypic variation in the extent and timing of water extraction was reported earlier in chickpea with some genotypes depleting the least moisture at 6 weeks after sowing in an alluvial sandy loam field (Nagarajrao et al., 1980). The lower conductance of tolerant genotypes explaining about 50% of their lower water extraction at the vegetative stage under non-stress conditions agrees with a recent report (Zaman-Allah et al., 2011), also showing lower early vigour in some of the tolerant lines. These differences in the water use pattern at the vegetative stage require more investigation as to a possible hydraulic limitation at the vegetative stage in tolerant lines. These results are indeed quite similar to recent findings on contrasting genotypes of pearl millet (Kholova et al., 2010b). The variation in the timing of water extraction could be attributed to differences in the balance between rooting and canopy development and/or conductance. From 48 DAS, as the water stress developed, the tolerant genotypes were found to extract more water than the susceptible genotypes (Fig. 1B) which would have contributed, to a large extent, to the reproductive success and also a proper grain filling. This was also found in a recent report by Zaman-Allah et al. (2011).
Water extracted at key time is critical
While root growth is a key trait for plants to cope with drought prone environments, root systems are important as long as they extract water at the critical stages for the plant, in agreement with previous suggestions (Vadez et al., 2007a; McIntyre et al., 1995). This study provided evidence that the plant water use profile during the cropping cycle had a critical impact on the seed yield (Fig. 7). Sustained water use and transpiration during the grain-filling period was indeed reported to be crucial for seed yield (Merah, 2001; Kato et al., 2008). Here, water extracted at the vegetative stage strongly and negatively correlated to water extracted during the reproductive phase (r=0.86*). Under terminal stress, the differential water extraction profile during the cropping cycle could have resulted in water-stress symptoms, based on transpiration differences with the well-watered control, being different. However, no major differences were found between the tolerant and the sensitive group in how rapidly transpiration started to decline. Clearly, transpiration started declining significantly between 38 and 43 DAS in all genotypes and there the relative transpiration was not very different among genotypes, regardless of tolerance / sensitivity (data not shown). A critical finding in the present work is that the seed yield was positively correlated with the amount of water taken during the period 48–75 DAS (r=0.64*) while being negatively correlated with the amount of water used earlier at 23-38 DAS (Fig. 7). This explained the higher reproductive success of the tolerant genotypes.
It was indeed a higher reproductive success, due to a better water uptake during reproduction, rather than the capacity to fill up grain (see Supplementary Fig. S2 at JXB online), that discriminated tolerant from sensitive entries, as earlier suggested for salt stress (Vadez et al., 2007b). The development of water deficit during the reproductive stage plays an important role in determining the number of flowers and pods that produced a seed (Turner, 2003). Pod abortion is known to be important in determining seed yield of chickpea when exposed to terminal drought (Leport et al., 2006), but flower production and abortion are, as well, important factors reducing seed yield (Fang et al., 2010). The reduction of flower production due to water stress may reach up to 60% in chickpea (Fang et al., 2010). In the present study, the susceptible genotypes showed a fairly higher percentage of seed abortion (10–18% except ICC 3776) (Fig. 6). The quite low percentage of seed abortion in tolerant genotypes was, in fact, related to the higher water uptake during the flowering period. Indeed, it was found that the percentage of seed abortion was negatively correlated (r=0.54*) with water uptake during the period 55–75 DAS.
Rooting depth and root length density did not show a clear discrimination between tolerant and sensitive genotypes (Fig. 4), and did not relate to the variation of yield performance of the tolerant genotypes under terminal stress conditions. It is concluded that these traits, though important for soil water extraction, are less critical than an adequate temporal pattern of water extraction. This contrasts with most previous work carried out in chickpea which have assumed that root trait, assayed at the vegetative stage (35 DAS) (Kashiwagi et al., 2006), would be the key parameter of adaptation to terminal drought in chickpea.
Conclusion
The present work has shown that there is a clear discrimination for water uptake profile between the tolerant and susceptible genotypes while there was no trend separating the two on the basis of rooting depth or root length density during the reproductive stage. Clearly, the advantage of tolerant genotypes came from a conservative use of water at the vegetative stage, before there is any water stress (similar transpiration in WW and WS). Water saved at the vegetative stage was explained, in part, by a lower canopy conductance in the tolerant genotypes, and this led to higher reproductive success under stress. Therefore, the temporal pattern of water uptake by roots, more than root growth, is critical for an understanding of water management and adaptation to terminal drought.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Fig. S1. The environmental conditions during the experimental period. Supplementary Fig. S2. The relative seed yield (yield DS/yield WW) was highly significantly related to the relative seed number (seed number DS/seed number WW), but not to the relative 100-seed weight (100-seed weight DS / 100-seed weight WW), which indicates that the decrease in seed yield was probably related to a reproduction failure (less number of seeds under DS) but not to a seed-filling failure (same seed size under DS). Supplementary Fig. S3. A significant relationship between the seed yield under DS and that under WW conditions.
Acknowledgments
The authors grately acknowledge support from the Government of Japan and from the Bill and Melinda Gates Foundation (Tropical Legume Project—Phase 2) through the Generation Challenge Program for the funds they provided to ICRISAT to support this work. The lysimetric facility was developed by a grant (Center of Excellence in Genomics) from the Department of Biotechnology (DBT) from the Government of India to ICRISAT.
Glossary
Abbreviations
- RLD
Root length density
- WU
water uptake
- DAS
days after sowing
References
- Bruce WB, Edmeades GO, Barker TC. Molecular and physiological approaches to maize improvement for drought tolerance. Journal of Experimental Botany. 2002;53:13–25. [PubMed] [Google Scholar]
- Fang X, Turner NC, Guijun Yan, Fengmin Li, Siddique KHM. Flower numbers, pod production, pollen viability, and pistil function are reduced and flower and pod abortion increased in chickpea (Cicer arietinum L.) under terminal drought. Journal of Experimental Botany. 2010;61:335–345. doi: 10.1093/jxb/erp307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer GK, Dong Z, McLean G, Doherty A, Messina C, Schussler J, Zinselmeier C, Paszkiewicz S, Cooper M. Can changes in canopy and/or root system architecture explain historical maize yield trends in the US Corn Belt? Crop Science. 2009;49:299–312. [Google Scholar]
- Johansen C, Krishnamurthy L, Saxena NP, Sethi SC. Genotypic variation in moisture responseof chickpea grown under line-source sprinklers in a semi-arid tropical environment. Field Crops Research. 1994;37:103–112. [Google Scholar]
- Jones HG. Use of infrared thermometry for estimation of stomatal conductance as a possible aid to irrigation scheduling. Agricultural and Forest Meteorology. 1999;95:139–149. [Google Scholar]
- Kashiwagi J, Krishnamurthy L, Crouch JH, Serraj R. Variability of root characteristics and their contributions to seed yield in chickpea (Cicer arietinum L.) under terminal drought stress. Field Crops Research. 2006;95:171–181. [Google Scholar]
- Kashiwagi J, Krishnamurthy L, Upadhyaya HD, Krishna H, Chandra S, Vadez V, Serraj R. Genetic variability of drought-avoidance root traits in the mini-core germplasm collection of chickpea (Cicer arietinum L.) Euphytica. 2005;146:213–222. [Google Scholar]
- Kato Y, Kamoshita A, Yamagishi J. Preflowering abortion reduces spikelet number in upland rice (Oryza sativa L.) under water stress. Crop Science. 2008;48:2389–2395. [Google Scholar]
- Kholova J, Hash CT, Kakkera A, Kocova M, Vadez V. Constitutive water-conserving mechanisms are correlated with the terminal drought tolerance of pearl millet [ Pennisetum glaucum (L.) R. Br.] Journal of Experimental Botany. 2010a;61:369–377. doi: 10.1093/jxb/erp314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholova J, Hash CT, Kumar PL, Yadav RS, Kocova M, Vadez V. Terminal drought-tolerant pearl millet [Pennisetum glaucum (L.) R. Br.] have high leaf ABA and limit transpiration at high vapour pressure deficit. Journal of Experimental Botany. 2010b;61:1431–1440. doi: 10.1093/jxb/erq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy L, Ito O, Johansen C, Saxena NP. Length to weight ratio of chickpea roots under progressively receding soil moisture conditions in a Vertisol. Field Crops Research. 1998;58:177–185. [Google Scholar]
- Krishnamurthy L, Kashiwagi J, Gaur PM, Upadhyaya HD, Vadez V. Sources of tolerance to terminal drought in the chickpea (Cicer arietinum L.) minicore germplasm. Field Crops Research. 2010;119:322–330. [Google Scholar]
- Leport L, Turner NC, Davies SL, Siddique KHM. Variation in pod production and abortion among chickpea cultivars under terminal drought. European Journal of Agronomy. 2006;24:236–246. [Google Scholar]
- Leport L, Turner NC, French RJ, Barr MD, Duda R, Davies SL, Tennant D, Siddique KHM. Physiological responses of chickpea genotypes to terminal drought in a Mediterranean-type environment. European Journal of Agronomy. 1999;11:279–291. [Google Scholar]
- Ludlow MM, Muchow RC. A critical evaluation of traits for improving crop yields in water limited environments. Advances in Agronomy. 1990;43:107–153. [Google Scholar]
- McIntyre BD, Riha SJ, Flower DJ. Water uptake by pearl millet in a semi-arid environment. Field Crops Research. 1995;43:67–76. [Google Scholar]
- Merah O. Potential importance of water status traits for durum wheat improvement under Mediterranean conditions. Journal of Agricultural Sciences. 2001;137:139–145. [Google Scholar]
- Nagarajrao Y, Mallick S, Gurcharan Singh. Moisture depletion and root growth of different varieties of chickpea under rainfed conditions. Indian Journal of Agronomy. 1980;25:289–293. [Google Scholar]
- Pantuwan G, Fukai S, Cooper M, Rajatasereekul S, O'Toole JC. Yield response of rice (Oryza sativa L.) genotypes to drought under rainfed lowland. 3. Plant factors contributing to drought resistance. Field Crops Research. 2002;73:181–200. [Google Scholar]
- Ratnakumar P, Vadez V, Nigam SN, Krishnamurthy L. Assessment of transpiration efficiency in peanut (Arachis hypogaea L.) under drought using a lysimetric system. Plant Biology (Stuttgart) 2009;11(Supplement 1,):124–130. doi: 10.1111/j.1438-8677.2009.00260.x. [DOI] [PubMed] [Google Scholar]
- Robertson MJ, Fukai S, Ludlow MM, Hammer GL. Water extraction by grain sorghum in a sub-humid environment. I. Analysis of the water extraction pattern. Field Crops Research. 1993;33:81–97. [Google Scholar]
- Saxena NP, Krishnamurthy L, Johansen C. Registration of a drought-resistant chickpea germplasm. Crop Science. 1993;33:1424. [Google Scholar]
- Saxena NP, Johansen C. Chickpea ideotypes for genetic enhancement of yield and yield stability in South Asia. In: van Rheenen HA, Saxena MC, editors. Chickpea in the nineties. 1990. Proceedings of the Second International Workshop on Chickpea Improvement, 4–8 December 1989. Patancheru 502324, AP, India: ICRISAT Center, 81–85. [Google Scholar]
- Silim SN, Saxena MC, Erskine W. Adaptation of lentil to the Mediteranean environment. II. Response to moisture supply. Experimental Agriculture. 1993;29:21–28. [Google Scholar]
- Sinclair TR, Muchow RC. System analysis of plant traits to increase grain yield on limited water supplies. Agronomy Journal. 2001;93:263–270. [Google Scholar]
- Sinclair TR, Messina CD, Beatty A, Samples M. Assessment across the United States of the benefits of altered soybean drought traits. Agronomy Journal. 2010;102:475–482. [Google Scholar]
- Subbarao GV, Johansen C, Slinkard AE, Rao RCN, Saxena NP, Chauhan YS. Strategies for improving drought resistance in grain legumes. Critical Reviews in Plant Science. 1995;14:469–523. [Google Scholar]
- Turner NC. Adaptation to drought: lessons from studies with chickpea. Indian Journal of Plant Physiology. 2003 (Special issue) 11–17. [Google Scholar]
- Turner NC, Wright GC, Siddique KHM. Adaptation of grain legumes (pulses) to water-limited environments. Advances in Agronomy. 2001;71:193–231. [Google Scholar]
- Vadez V, Krishnamurthy L, Kashiwagi JW, et al. Exploiting the functionality of root systems for dry, saline, and nutrient deficient environments in a changing climate. The Journal of Semi-Arid Tropical Agricultural Research. 2007 a;4 (Special Symposium edition) http://www.icrisat.org/journal/specialproject.htm. [Google Scholar]
- Vadez V, Krishnamurthy L, Serraj R, Gaur PM, Upadhyaya HD, Hoisington DA, Varshney RK, Turner NC, Siddique KHM. Large variation in salinity tolerance in chickpea is explained by differences in sensitivity at the reproductive stage. Field Crops Research. 2007 b;104:123–129. [Google Scholar]
- Vadez V, Rao S, Kholova J, Krishnamurthy L, Kashiwagi J, Ratnakumar P, Sharma KK, Bhatnagar-Mathur P, Basu PS. Root research for drought tolerance in legumes: quo vadis? Journal of Food Legumes. 2008;21:77–85. [Google Scholar]
- Zaman-Allah M, Jenkinson DM, Vadez V. Chickpea genotypes contrasting for seed yield under terminal drought stress in the field differ for traits related to the control of water use. Functional Plant Biology. 2011;38:270–281. doi: 10.1071/FP10244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.