Abstract
Nodulin-26-like intrinsic proteins (NIPs) of the aquaporin family are involved in the transport of diverse solutes, but the mechanisms controlling the selectivity of transport substrates are poorly understood. The purpose of this study was to investigate how the aromatic/arginine (ar/R) selectivity filter influences the substrate selectivity of two NIP aquaporins; the silicic acid (Si) transporter OsLsi1 (OsNIP2;1) from rice and the boric acid (B) transporter AtNIP5;1 from Arabidopsis; both proteins are also permeable to arsenite. Native and site-directed mutagenized variants of the two genes were expressed in Xenopus oocytes and the transport activities for Si, B, arsenite, and water were assayed. Substitution of the amino acid at the ar/R second helix (H2) position of OsLsi1 did not affect the transport activities for Si, B, and arsenite, but that at the H5 position resulted in a total loss of Si and B transport activities and a partial loss of arsenite transport activity. Conversely, changes of the AtNIP5;1 ar/R selectivity filter and the NPA motifs to the OsLsi1 type did not result in a gain of Si transport activity. B transport activity was partially lost in the H5 mutant but unaffected in the H2 mutant of AtNIP5;1. In contrast, both the single and double mutations at the H2 and/or H5 positions of AtNIP5;1 did not affect arsenite transport activity. The results reveal that the residue at the H5 position of the ar/R filter of both OsLsi1 and AtNIP5;1 plays a key role in the permeability to Si and B, but there is a relatively low selectivity for arsenite.
Keywords: Arsenic, boron, NIP aquaporins, selectivity, silicon, substrate
Introduction
Aquaporins are channel proteins that mediate the transport of water and/or small neutral solutes (Maurel et al., 2008). There are 35 and 39 members, respectively, in the Arabidopsis thaliana and rice (Oryza sativa) genomes (Wallace and Roberts, 2004; Sakurai et al., 2005; Bansal and Sankararamakrishnan, 2007). On the basis of sequence homology and localization, plant aquaporins can be divided into four subfamilies: plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins (TIPs), small basic intrinsic proteins (SIPs), and nodulin-26-like intrinsic proteins (NIPs). Among them, NIPs are unique to plants, with nine and 10 members being present in A. thaliana and rice, respectively (Wallace and Roberts, 2004; Bansal and Sankararamakrishnan, 2007). NIPs are further subdivided into three subgroups (NIP I, II, and III) based on the sequence similarity of the aromatic/arginine (ar/R) constriction region (Wallace and Roberts, 2004; Mitani et al., 2008); these subgroups have different transport substrates. For example, NIP I proteins transport water, glycerol (Dean et al., 1999), and lactic acid (Choi and Roberts, 2007), while NIP II proteins are permeable to larger solutes, such as urea (Wallace and Roberts, 2005), formamide (Wallace and Roberts, 2005), and boric acid (Takano et al., 2006). The NIP III group appears to be unique to silicic acid-accumulating plant species and its members function as silicon influx transporters, although transport activities for other solutes such as undissociated arsenite and selenite have also been reported recently (Ma et al., 2006, 2008; Zhao et al., 2010a, c). However, the mechanisms responsible for the specificity of transport substrates between different NIP subgroups are unknown.
It has been proposed that the Asn-Pro-Ala (NPA) and ar/R regions of aquaporins exert a large influence on the substrate specificity (Wallace and Roberts, 2004; Forrest and Bhave, 2007). The NPA region is formed by the close apposition of the asparagines in the two NPA motifs at the center of the pore. These asparagine residues form hydrogen bond contacts with the transported molecules or other solute molecules and might play a role in the exclusion of protons (Tajkhorshid et al., 2002; Ilan et al., 2004; Forrest and Bhave, 2007). A second, narrower pore constriction is located close to the extracellular end of the pore, which is formed by four residues from helix 2 (H2), helix 5 (H5), and loop E (LE1 and LE2) (Fu et al., 2000; Sui et al., 2001; Harries et al., 2004). This region has been referred to as the ar/R region based on the presence of a conserved arginine residue in loop E and the high prevalence of aromatic residues at H2 (de Groot and Grubmuller, 2001). It has been proposed that the ar/R region serves as a selectivity filter for the transport of substrates, determines the rate of transport by acting as a size-exclusion barrier which sterically blocks the transport of bulkier substrates, and provides key hydrogen bonds and van der Waals contacts for the transported solutes and/or water molecules (Fu et al., 2000; Sui et al., 2001). For example, the selectivity filter residues of water-specific aquaporins tend to form a smaller (diameter of ∼2.8 Å) and more hydrophilic pore (Thomas et al., 2002; Wang et al., 2005), whereas those of glycerol transporters form a larger (3.4 Å) and more hydrophobic pore (Wu and Beitz, 2007). In a previous study on rat AQP1, wild-type AQP1 was impermeable to urea and glycerol, but ar/R substitution mutants of AQP1 showed permeability (Beitz et al., 2006). In plants, Wallace and Roberts (2005) showed that ar/R at the position of H2 is critical for the transport property in Arabidopsis NIP. Dynowski et al. (2008) showed that substituting residues in the constriction region of AtPIP2:1 with those found in the urea-transporting TIPs did not turn the AtPIP2;1 into a urea-permeable channel. However, the role of the ar/R selectivity filter of plant NIP in controlling substrate selectivity is still poorly understood. Since the substrates transported by NIP proteins also include toxic metalloids such as arsenite (Ma et al., 2008; Bienert et al., 2008; Isayenkov and Maathuis, 2008; Kamiya et al., 2009), it is important to understand the mechanism controlling substrate selectivity.
In the present study, site-direct mutagenesis analysis of the ar/R selectivity filter in two NIP proteins, OsLsi1 (OsNIP2;1; Ma et al., 2006) and AtNIP5;1 (Takano et al., 2006), which are known as transporters for silicic acid and boric acid, respectively, was performed and the transport activities for silicic acid, boric acid, arsenite, and water were tested. The aim was to identify key determinant in the ar/R selectivity filter of the two NIP proteins in controlling substrate selectivity.
Materials and methods
Site-directed mutagenesis
An N-terminal FLAG epitope tag (MDYKDDDDDK) with the OsLsi1 open reading frame (ORF) was inserted into the pXβG-ev1 Xenopus laevis oocyte expression vector according to the method described by Wallace and Roberts (2005). Site-directed mutants of OsLsi1 were generated on the pXβG-ev1 construct by PCR using the following synthetic oligonucleotide primers.
Common primers: T3, 5′-AATTAACCCTCACTAAAGGGAACA-3′; and T7, 5′-TAATACGACTCACTATAGGGCGA-3′. For ar/R modification of OsLsi1: G88A F, 5′-ACAGTCGATCGCCGCTGGCCTCATCGTGACGGTGA-3′; G88A R, 5′-TCACCGTCACGATGAGGCCAGCGGCGATCGACTGT-3′; S207I F, 5′-GTTTGCATTACGATCATCTTCGCAGGGGCAATTT-3′; and S207I R, 5′-AAATTGCCCCTGCGAAGATGATCGTAATGCAAAC-3′.
For the mutation of G88A, the first PCR was performed by using T3, G88AR, G88AF, and T7, respectively. FLAG-OsLsi1-pXβG-ev1 was a template for this first PCR. Then the first PCR products were purified and used as templates for the second PCR performed with the T3 and T7 promoters. Using the combination of these primers, five mutant types of OsLsi1 with the 5′ untranslated region (UTR) and 3′UTR of X. laevis β-globin were generated. Then these fragments were inserted into the pGEM-T easy vector (Promega) according to the manufacturer's protocol.
Site-directed mutagenesis was also performed on the boric acid transporter gene AtNIP5;1. AtNIP5;1 was first amplified by using the primers AtNIP5;1-F 5′-GAAGATCTATGGCTCCACCGG-3′ and AtNIP5;1-R 5′- GAAAGATCTTTAACGACGAAAGCT-3′, and cloned into FLAG-pXβG-ev1. AtNIP5;1 mutants were generated directly on the pXβG-ev1 construct by PCR using the following synthetic oligonucleotide primers and the common primers described above.
For ar/R modification of AtNIP5;1: A117G F, 5′-ATCGGTAACGCTGCATGCGGAGGACTCGCAGT-3′; A117G R, 5′-ACTGCGAGTCCTCCGCATGCAGCGTTACCGAT-3′; I236S F, 5′-ACGGTCATGCTCAATAGTCTAGTCGCAGGGCCA-3′; and I236S R, 5′-TGGCCCTGCGACTAGACTATTGAGCATGACCGT-3′. For NPA modification of AtNIP5;1: S139A F, 5′-CTCACCTAAACCCAGCACTGACCATAGCAT-3′; S139A R, 5′-ATGCTATGGTCAGTGCTGGGTTTAGGTGAG-3′; V250A F, 5′-GTGGATCTATGAATCCTGCGAGAACTCTAG-3′; and V250A F, 5′-CTAGAGTTCTCGCAGGATTCATAGATCCAC-3′.
Mutations in each amplified fragment were verified by DNA sequencing.
Oocyte preparation and microinjection
Oocytes at stage V or VI were extracted from adult female X. laevis frogs and then defolliculated by 0.1% collagenase B for 2 h with gentle mixing (Mitani et al., 2008). Capped cRNA was produced from XbaI- or BamHI-linearized pGEM-T easy vector containing the β-globin UTR, FLAG epitope tag, and OsLsi1 by in vitro transcription using the mMESSAGE mMACHINE kit (Ambion). The concentrations of synthesized cRNA were estimated by gel electrophoresis. A 50 nl (1 ng nl−1) aliquot of in vitro synthesized cRNA was injected into the selected oocytes using Nanoject II and incubated at 18 °C overnight in modified Barth's solution (MBS) (Mitani et al., 2008). As a negative control, oocytes were injected with 50 nl of distilled water. After incubation for 1 d, oocytes were used in the transport activity assays as described below.
Immmunochemical techniques for expression assay in oocytes
To determine the protein expression in oocytes, 20–30 oocytes were homogenized in 300 μl of extraction buffer [50 mM TRIS-HCl, 0.3 M sorbitol, 5 mM EDTA-Na2, 1 mM dithiothreitol (DTT) and protease inhibitor cocktail (Sigma)] at 4 °C. The lysates were centrifuged at 2000 g for 5 min, and the supernatants were centrifuged again at 100 000 g for 20 min. The pellet (containing the microsomal protein fraction) was resuspended with a small amount of the extraction buffer. A 15 μg aliquot of microsomal protein from each sample was resolved by SDS–PAGE and electrophoretically transferred onto polyvinylidene fluoride (PVDF) membranes. The blot was then incubated with a 1:1000 dilution of a primary antibody (mouse anti-FLAG M2 antibody; Stratagene) for 1 h at room temperature. The blots were washed, and then incubated with a 1:10 000 dilution of peroxidase-labelled anti-mouse antibody (GE Healthcare) for 1 h at room temperature. The chemiluminescent signals of the blots were determined using ECL plus (GE Healthcare).
Transport activity assays
The silicic acid transport activity of oocytes was assayed as described previously using 1 mM silicic acid spiked with 2 GBq mmol−1 radioactive 68Ge (Ma et al., 2006). Previous work has shown that 68Ge-labelled germanic acid is an excellent tracer of silicic acid during plant uptake (Ma et al., 2006). To determine the arsenite transport activity, oocytes (six oocytes per replicate) were exposed to MBS buffer containing 0.1 mM NaAsO2 for 30 min at 18 °C. The oocytes were then washed with ice-cold MBS and homogenized with 0.1 N HNO3. Arsenic concentration was measured by inductively coupled plasma mass spectrometry (ICP-MS). Three replicates were included for each assay.
The permeability of oocytes for water and boric acid was determined by swelling assay according to Preston et al. (1992) and Takano et al. (2006), respectively. After cRNA injection and initial incubation in the control MBS (200 mosmol kg−1), oocytes were transferred to a 5-fold diluted MBS (40 mosmol kg−1) for water permeability assay. Changes in the oocyte volume were measured at 20 s intervals with a charge-coupled device camera operated with Metaview software (Universal Imaging). Win Roof (Mitani Corporation) was used to determine the oocyte diameters, from which the volumes were calculated assuming spherical geometry. Osmotic water permeability (Pf) was calculated from the osmotic swelling data collected at 120 s using the following equation (Katsuhara et al., 2002):
where V0 is the initial oocyte volume (V0=9×10−4 cm3), V/V0 is the relative volume, S is the initial oocyte surface area (S=0.045 cm2), Vw is the molecular volume of water (Vw=18 cm mol−1), and osmin and osmout are the osmolarity in the oocyte (200 mosmol kg−1) and of the medium (40 mosmol kg−1), respectively (Katsuhara et al., 2002).
For the boric acid transport assay, oocytes were transferred to an isotonic solution containing 5-fold diluted MBS supplemented with boric acid to adjust the osmolarity to 200 mosmol kg−1 (Mitani et al., 2008). Changes in the oocyte volume were monitored as described above. Boric acid permeability was presented as oocyte volume change [d(V V0–1) dt−1] during 0–120 s.
Results
Comparison of the OsLsi1 and AtNIP5;1 ar/R selectivity filters
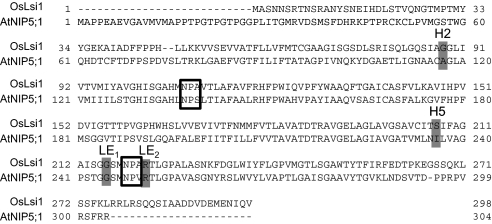
AtNIP5;1 and OsLsi1 belong to the NIP II and III subgroups, respectively. They differ in the ar/R selectivity filter in the H2 and H5 positions (Fig. 1; Mitani et al., 2008). The amino acids of the OsLsi1 ar/R filter consist of glycine, serine, glycine, and arginine (GSGR), compared with alanine, isoleucine, glycine, and arginine (AIGR) in AtNIP5;1.
Fig. 1.
Alignment of the sequences of OsLsi1 and AtNIP5;1. The residues corresponding to NPA motifs and ar/R regions are boxed or shaded grey, respectively.
Influence of the ar/R selectivity filter of OsLsi1 on transport substrate selectivity
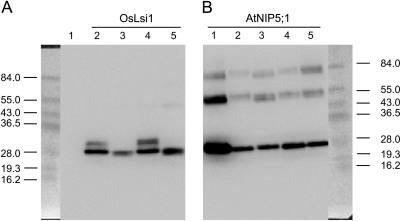
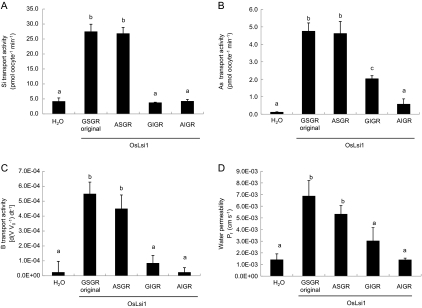
The selectivity filter was first mutated in the background of OsLsi1. When glycine at H2 was substituted by alanine (selectivity filter ASGR), western blot analysis showed a similar pattern of the mutated protein to that of the native OsLsi1 (selectivity filter GSGR); both had two bands near 30 kDa, with the lower band being stronger (Fig. 2A). The transport activities for both silicic acid and arsenite were unaffected by the G/A substitution at H2 of OsLsi1 (Fig. 3A, B). This site-directed mutation also did not affect the transport activities for boric acid or water significantly (Fig. 3C, D). In contrast, when serine at the H5 position was substituted by isoleucine (selectivity filter GIGR), only one band was detected in the western blot (Fig. 2A) and the transport activity for silicic acid was totally lost (Fig. 3A). The transport activities for boric acid and water were also lost by the S/I substitution at H5, as shown by the insignificant differences between the mutant and the H2O control (Fig. 3C, D). This substitution also resulted in a lower transport activity for arsenite; however, unlike the total loss of permeability for silicic acid, boric acid and water, ∼40% of the arsenite uptake ability was retained (Fig. 3B). These results suggest that the residue at the H5 position is critical for the transport of silicic acid, boric acid, and water, but less critical for arsenite. When both residues at the H2 and H5 positions were substituted (G/A and S/I, respectively), the transport activities for all of the four solutes tested were completely lost (Fig. 3). In contrast to the native protein, only one band of the H2/H5 mutated protein was detected (Fig. 2A).
Fig. 2.
Western blot analysis of native and mutated OsLsi1 and AtNIP5;1 proteins. Proteins were extracted from oocytes expressing OsLsi1, AtNIP5;1, and their mutants. A 15 μg aliquot of membrane fractions was resolved on a polyacrylamide gel and probed with anti-FLAG antibody. (A) Native OsLsi1 and its mutants: lane 1, water-injected negative control; lane 2, OsLsi1 original; lane 3, AIGR (G88A-S207I); lane 4, ASGR (G88A); lane 5, GIGR (S207I). (B) Native AtNIP5;1 and its mutants: lane 1, native AtNIP5;1; lane 2, GSGR (A117G-I236S); lane 3, GIGR (A117S); lane 4 ASGR (I236S); lane 5, NPA, NPA, GSGR (S139A-V250A, A117G-I236S).
Fig. 3.
Influence of ar/R selectivity filter substitution of OsLsi1 on the transport activity for silicic acid (A), arsenite (B), boric acid (C), and water (D). Transport activity was determined for the water-injected negative control (H2O), oocytes expressing native OsLsi1 (GSGR original), or mutated proteins G88A (ASGR), S207I (GIGR), and G88A-S207I (AIGR). Data are means ±SD (n=3) for (A) and (B), (n=5–8) for (C) and (D). Different letters above the columns indicate statistically significant differences at P <0.05 by Tukey's test.
Influence of the ar/R selectivity filter of AtNIP5;1 on transport substrate selectivity
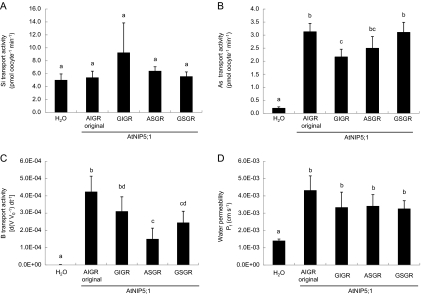
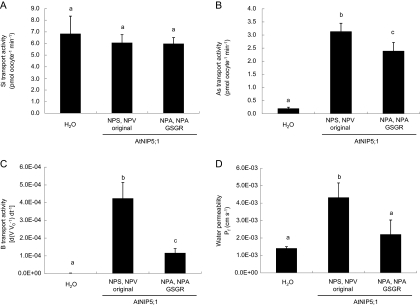
The ar/R selectivity filter was mutated in the background of AtNIP5;1. Western blot analysis showed similar patterns for all mutated and the native proteins of AtNIP5;1, with the strongest band being observed at the expected size (31 kDa; Fig. 2B). Native AtNIP5;1 is able to transport boric acid, water, and arsenite, but not silicic acid (Fig. 4). When either alanine at H2 or isoleucine at H5 was changed to the OsLsi1 type (i.e. A/G and I/S substitution, respectively), no transport activity for silicic acid was observed (Fig. 4A). Double mutations at both the H2 and H5 positions also did not result in any transport activity for silicic acid (Fig. 4A). In contrast, both single and double mutation at the H2 and/or H5 positions showed transport activity for arsenite (Fig. 4B), although site-directed mutation at the H2 position (A/G substitution) resulted in a slight but significant reduction in its transport activity (Fig. 4B). Mutation at the H5 position (I/S substitution) caused decreased boric acid transport activity, whereas that at H2 (A/G substitution) had no significant effect (Fig. 4C). The permeability of water was unaffected by site-directed mutation at both H2 and H5 positions (Fig. 4D).
Fig. 4.
Influence of ar/R selectivity filter substitution of AtNIP5;1 on the transport activity for silicic acid (A), arsenite (B), boric acid (C), and water (D). Transport activity was determined for the water-injected negative control (H2O), native AtNIP5;1 (AIGR original), A117G (GIGR), I236S (ASGR), and A117G-I236S (GSGR). Data are means ±SD (n=3) for (A) and (B), (n=5–6) for (C) and (D). Different letters above the columns indicate statistically significant differences at P <0.05 by Tukey's test.
Because the NPA motifs also differ between OsLsi1 and AtNIP5;1, both NPA motifs and the ar/R selectivity filter were further mutated from the AtNIP5;1 type to the OsLsi1 type. The mutated protein did not show any transport activity for silicic acid (Fig. 5A), but was able to transport arsenite similarly to the original AtNIP5;1 (Fig. 5B). Mutations of both NPA motifs and the selectivity filter also resulted in decreased transport activities for boric acid and water (Fig. 5C, D).
Fig. 5.
Influence of double substitution of NPA motifs and the ar/R selectivity filter of AtNIP5;1 on the transport activity for silicic acid (A), arsenite (B), boric acid (C), and water (D). Transport activity was determined for the water-injected negative control (H2O), native AtNIP5;1 (NPS, NPV, AIGR), or S139A-V250A and A117G-I236S (NPA, NPA, GSGR). Data are means ±SD (n=3) for (A) and (B), (n=5–6) for (C) and (D). Different letters above the columns indicate statistically significant differences at P <0.05 by Tukey's test.
Discussion
Proteins belonging to the NIP subfamily transport diverse substrates such as silicic acid (Ma et al., 2006), boric acid (Takano et al., 2006), lactic acid (Choi and Roberts, 2007), urea (Wallace et al., 2005), and formamide (Wallace et al., 2005), depending on individual proteins. The molecules transported differ in size and chemical properties; therefore, there must be mechanisms regulating the permeability of these molecules. Although the crystal structures of NIPs have not been determined, information from other plant aquaporins shows that there may be two constriction filters (the NPA and ar/R region) regulating solute permeability. Since all NIPs possess two NPA motifs, the present study focused on the role of ar/R regions in controlling the transport substrate selectivity. OsLsi1, a silicon influx transporter (Ma et al., 2006), and AtNIP5;1, a boric acid influx transporter (Takano et al., 2006), were selected for mutagenesis analysis. The ar/R region of OsLsi1 consists of four small-sized residues, GSGR, forming a larger constriction pore compared with other NIP subgroups; this characteristic allows relatively large molecules of silicic acid (4.38 Å) to permeate through (Wu and Beitz, 2007). The difference in the selectivity filter between OsLsi1 and AtNIP5;1 resides in H2 and H5. Substitution of glycine in H2 of OsLsi1 by alanine did not affect the transport activities for all solutes tested (Fig. 3), suggesting that the G/A substitution at the H2 position is not important for transport substrate selectivity. Wallace and Roberts (2005) mutated AtNIP6;1, which also belongs to the NIP II subgroup as AtNIP5;1. They found that the substitution of the tryptophan residue (NIP I type) by alanine at the H2 position resulted in enhanced water transport activity, a loss of urea transport activity, and no effect on the transport activities for glycerol and formamide. They suggested that the alanine substitution at the H2 position of the AtNIP6;1 ar/R filter is a critical determinant for transport substrate selectivity (Wallace and Roberts, 2005). Since the transport activity for urea and substitution of tryptophan by alanine were not tested in the present study and the mutation from tryptophan to alanine represents a bigger chemical and stoical change than that from glycine to alanine, a direct comparison with the study of Wallace and Roberts (2005) is difficult. However, ther present results suggest that the difference in the residue at the H2 position is not responsible for the difference in the transport substrates between the NIP III and NIP II subgroups.
In contrast, the point mutation from serine to isoleucine at the H5 position of OsLsi1 was found to affect strongly the transport activities for all of the four substrates tested in this study (Fig. 3). Mutation of both residues at the H2 and H5 positions also showed similar results (Fig. 3). Compared with serine, the residue size of isoleucine is considerably larger and therefore the S/I substitution at the H5 position may reduce the pore size at the ar/R region. In addition, serine is a polar amino acid, whereas isoleucine is non-polar. These differences may affect the folding and/or orientation of key channel residues. Another possibility for the loss of transport activity is that the S/I substitution at the H5 position may affect the post-translation process. Western blot analysis showed different patterns between the native and the H5-mutated proteins (Fig. 2A). Two bands were detected in the native and H2 mutated proteins, but, for unknown reasons, the upper band disappeared in the H5 mutated protein (Fig. 2A). Further work is required to address this aspect.
On the other hand, when both NPA motifs and the ar/R selectivity filter of AtNIP5;1 were changed to those of the OsLsi1 type, the mutated protein did not acquire transport activity for silicic acid (Figs 4A, 5A). This result suggests that the transport substrate selectivity is not simply controlled by NPA motifs and the ar/R selectivity filter. In fact, AtNIP6;1, a boric acid transporter, is predicted to have a pore size of 5 Å in diameter, large enough to accommodate approximately two water molecules (Wallace and Roberts, 2005). However, this protein showed extremely low water permeability (Wallace and Roberts, 2005; Tanaka et al., 2008). Also, OsLsi1 is not permeable to glycerol although the molecular size of glycerol is smaller than that of silicic acid (Ma et al., 2006). Similarly, Dynowski et al. (2008) showed that substituting residues in the constriction region of AtPIP2:1 with those found in the urea-transporting TIPs did not turn AtPIP2;1 into a urea-permeable channel. They found that the amino acid at position 55, which is not in the ar/R region, was important for AtPIP2;1 to become permeable to urea (Dynowski et al., 2008). These observations suggest that, in addition to pore size, other structural features are also involved in controlling the transport substrate specificity of the NIP subgroups. Further study is required to identify the residue(s) beyond those in the ar/R and NPA regions which are important for the permeability of silicic acid.
Overall, the permeability to silicic acid and boric acid appears to be strictly controlled. This is supported by the findings that the native proteins (OsLsi1 or AtNIP5;1) show the highest transport activity for either silicic acid or boric acid compared with any mutated proteins, except for A/G substitution in silicic acid transport (Figs 3A, 4C). In comparison, the selectivity for arsenite appears to be lower. Modification of the ar/R selectivity filter of AtNIP5;1 had only a small effect on the transport activity for arsenite (Fig. 4B). Also, mutations in the OsLsi1 selectivity filter at the H5 position resulted in a complete loss of silicic acid permeability, but only a partial loss of arsenite permeability (Fig. 3B). These differences indicate that arsenite permeability is less affected by the changes in the ar/R filter tested in this study than the permeability for silicic acid and boric acid. This observation is consistent with the arsenite permeability being widespread among different subgroups of NIP aquaporins possessing different types of ar/R filters (Zhao et al., 2009). Arsenic accumulation in rice grain is a serious problem for human health, and reducing the accumulation of arsenite in the grain is an important goal (Zhao et al., 2010b). Uptake of arsenite in rice is mediated by the silicic acid transporters OsLsi1 and OsLsi2; the latter is responsible for the efflux silicic acid and arsenite from exo- and endodermal cells toward the stele (Ma et al., 2008). The present results suggest that it may be difficult to control arsenite uptake by modifying OsLsi1 without affecting silicic acid uptake because of the lower selectivity for arsenite than for silicic acid. Alternatively, manipulation of other transporters for arsenite (e.g. OsLsi2) may provide more promising opportunities to achieve this goal.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan (no. 22119002 to JFM), a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Genomics for Agricultural Innovation IPG-0006 to JFM), a BBSRC grant (BB/H006303/1 to FJZ), and a Royal Society joint project to FJZ and JFM.
References
- Bansal A, Sankararamakrishnan R. Homology modeling of major intrinsic proteins in rice, maize and Arabidopsis: comparative analysis of transmembrane helix association and aromatic/arginine selectivity filters. BMC Structual Biology. 2007;7:27. doi: 10.1186/1472-6807-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitz E, Wu B, Holm LM, Schultz JE, Zeuthen T. Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons. Proceedings of the National Academy of Sciences, USA. 2006;103:269–274. doi: 10.1073/pnas.0507225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienert GP, Thorsen M, Schüssler MD, Nilsson HR, Wagner A, Tamás MJ, Jahn TP. A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biology. 2008;6:26. doi: 10.1186/1741-7007-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WG, Roberts DM. Arabidopsis NIP2;1: a major intrinsic protein transporter of lactic acid induced by anoxic stress. Journal of Biological Chemistry. 2007;282:24209–24218. doi: 10.1074/jbc.M700982200. [DOI] [PubMed] [Google Scholar]
- Dean RM, Rivers RL, Zeidel ML, Roberts DM. Purification and functional reconstitution of soybean nodulin 26. An aquaporin with water and glycerol transport properties. Biochemistry. 1999;38:347–353. doi: 10.1021/bi982110c. [DOI] [PubMed] [Google Scholar]
- de Groot BL, Grubmuller H. Water permeation across biological membranes: mechanism and dynamics of aquaporin-1 and GlpF. Science. 2001;294:2353–2357. doi: 10.1126/science.1066115. [DOI] [PubMed] [Google Scholar]
- Dynowski M, Mayer M, Moran O, Ludewig U. Molecular determinants of ammonia and urea conductance in plant aquaporin homologs. FEBS Letters. 2008;582:2458–2462. doi: 10.1016/j.febslet.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Forrest KL, Bhave M. Major intrinsic proteins (MIPs) in plants: a complex gene family with major impacts on plant phenotype. Functional Integrative Genomics. 2007;7:263–289. doi: 10.1007/s10142-007-0049-4. [DOI] [PubMed] [Google Scholar]
- Fu D, Libson A, Miercke LJ, Weitzman C, Nollert P, Krucinski J, Stroud RM. Structure of a glycerol-conducting channel and the basis for its selectivity. Science. 2000;290:481–486. doi: 10.1126/science.290.5491.481. [DOI] [PubMed] [Google Scholar]
- Harries WE, Akhavan D, Miercke LJ, Khademi S, Stroud RM. The channel architecture of aquaporin 0 at a 2.2 Å resolution. Proceedings of the National Academy of Sciences, USA. 2004;101:14045–14050. doi: 10.1073/pnas.0405274101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan B, Tajkhorshid E, Schulten K, Voth GA. The mechanism of proton exclusion in aquaporin channels. Proteins. 2004;55:223–228. doi: 10.1002/prot.20038. [DOI] [PubMed] [Google Scholar]
- Isayenkov SV, Maathuis FJM. The Arabidopsis thaliana aquaglyceroporin AtNIP7;1 is a pathway for arsenite uptake. FEBS Letters. 2008;582:1625–1628. doi: 10.1016/j.febslet.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Kamiya T, Tanaka M, Mitani N, Ma JF, Maeshima M, Fujiwara T. NIP1;1, an aquaporin homolog, determines the arsenite sensitivity of Arabidopsis thaliana. Journal of Biological Chemistry. 2009;284:2114–2120. doi: 10.1074/jbc.M806881200. [DOI] [PubMed] [Google Scholar]
- Katsuhara M, Akiyama Y, Koshio K, Shibasaka M, Kasamo K. Functional analysis of water channels in barley roots. Plant and Cell Physiology. 2002;43:885–893. doi: 10.1093/pcp/pcf102. [DOI] [PubMed] [Google Scholar]
- Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M. A silicon transporter in rice. Nature. 2006;440:688–691. doi: 10.1038/nature04590. [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proceedings of the National Academy of Sciences, USA. 2008;105:9931–9935. doi: 10.1073/pnas.0802361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Luu DT, Santoni V. Plant aquaporins: membrane channels with multiple integrated functions. Annual Review of Plant Biology. 2008;59:595–624. doi: 10.1146/annurev.arplant.59.032607.092734. [DOI] [PubMed] [Google Scholar]
- Mitani N, Yamaji N, Ma JF. Characterization of substrate specificity of a rice silicon transporter, Lsi1. Pflugers Archiv. 2008;456:679–686. doi: 10.1007/s00424-007-0408-y. [DOI] [PubMed] [Google Scholar]
- Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Sakurai J, Ishikawa F, Yamaguchi T, Uemura M, Maeshima M. Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant and Cell Physiology. 2005;46:1568–1577. doi: 10.1093/pcp/pci172. [DOI] [PubMed] [Google Scholar]
- Sui H, Han BG, Lee JK, Walian P, Jap BK. Structural basis of water-specific transport through the AQP1 water channel. Nature. 2001;414:872–878. doi: 10.1038/414872a. [DOI] [PubMed] [Google Scholar]
- Tajkhorshid E, Nollert P, Jensen MO, Miercke LJ, O'Connell J, Stroud RM, Schulten K. Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science. 2002;296:525–530. doi: 10.1126/science.1067778. [DOI] [PubMed] [Google Scholar]
- Takano J, Wada M, Ludewig U, Schaaf G, von Wirén N, Fujiwara T. The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. The Plant Cell. 2006;18:1498–1509. doi: 10.1105/tpc.106.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Wallace IS, Takano J, Roberts DM, Fujiwara T. NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. The Plant Cell. 2008;20:1860–1875. doi: 10.1105/tpc.108.058628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Bron P, Ranchy G, Duchesne L, Cavalier A, Rolland JP, Raguenes-Nicol C, Hubert JF, Haase W, Delamarche C. Aquaglyceroporins, one channel for two molecules. Biochimica et Biophysica Acta. 2002;1555:181–186. doi: 10.1016/s0005-2728(02)00275-x. [DOI] [PubMed] [Google Scholar]
- Wallace IS, Roberts DM. Homology modeling of representative subfamilies of Arabidopsis major intrinsic proteins. Classification based on the aromatic/arginine selectivity filter. Plant Physiology. 2004;135:1059–1068. doi: 10.1104/pp.103.033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace IS, Roberts DM. Distinct transport selectivity of two structural subclasses of the nodulin-like intrinsic protein family of plant aquaglyceroporin channels. Biochemistry. 2005;44:16826–16834. doi: 10.1021/bi0511888. [DOI] [PubMed] [Google Scholar]
- Wang Y, Schulten K, Tajkhorshid E. What makes an aquaporin a glycerol channel? A comparative study of AqpZ and GlpF. Structure. 2005;13:1107–1118. doi: 10.1016/j.str.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Wu B, Beitz E. Aquaporins with selectivity for unconventional permeants. Cellular and Molecular Life Sciences. 2007;64:2413–2421. doi: 10.1007/s00018-007-7163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao FJ, Ago Y, Mitani N, Li RY, Su YH, Yamaji N, McGrath SP, Ma JF. The role of the rice aquaporin Lsi1 in arsenite efflux from roots. New Phytologist. 2010a;186:392–399. doi: 10.1111/j.1469-8137.2010.03192.x. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Ma JF, Meharg AA, McGrath SP. Arsenic uptake and metabolism in plants. New Phytologist. 2009;181:777–794. doi: 10.1111/j.1469-8137.2008.02716.x. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, McGrath SP, Meharg AA. Arsenic as a food-chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annual Review of Plant Biology. 2010b;61:535–559. doi: 10.1146/annurev-arplant-042809-112152. [DOI] [PubMed] [Google Scholar]
- Zhao XQ, Mitani N, Yamaji N, Shen RF, Ma JF. Involvement of silicon influx transporter OsNIP2;1 in selenite uptake in rice. Plant Physiology. 2010c;153:1871–1877. doi: 10.1104/pp.110.157867. [DOI] [PMC free article] [PubMed] [Google Scholar]