Abstract
In a wild tobacco plant, Nicotiana attenuata, two mitogen-activated protein kinases (MAPKs), salicylic acid-induced protein kinase (SIPK) and wound-induced protein kinase (WIPK), play central roles in modulating herbivory-induced phytohormone and anti-herbivore secondary metabolites. However, the identities of their upstream MAPK kinases (MAPKKs) were elusive. Ectopic overexpression studies in N. benthamiana and N. tabacum suggested that two MAPKKs, MKK1 and MEK2, may activate SIPK and WIPK. The homologues of MKK1 and MEK2 were cloned in N. attenuata (NaMKK1 and NaMEK2) and a virus-induced gene silencing approach was used to knock-down the transcript levels of these MAPKK genes. Plants silenced in NaMKK1 and NaMEK2 were treated with wounding or simulated herbivory by applying the oral secretions of the specialist herbivore Manduca sexta to wounds. MAPK activity assay indicated that after wounding or simulated herbivory NaMKK1 is not required for the phosphorylation of NaSIPK and NaWIPK; in contrast, NaMEK2 and other unknown MAPKKs are important for simulated herbivory-elicited activation of NaSIPK and NaWIPK, and after wounding NaMEK2 probably does not activate NaWIPK but plays a minor role in activating NaSIPK. Consistently, NaMEK2 and certain other MAPKKs, but not NaMKK1, are needed for wounding- and simulated herbivory-elicited accumulation of jasmonic acid (JA), JA–isoleucine, and ethylene. Furthermore, both NaMEK2 and NaMKK1 regulate the levels of trypsin proteinase inhibitors. The findings underscore the complexity of MAPK signalling pathways and highlight the importance of MAPKKs in regulating wounding- and herbivory-induced responses.
Keywords: Defence, ethylene, herbivory, jasmonic acid, mitogen-activated protein kinase kinase, trypsin proteinase inhibitors
Introduction
Mitogen-activated protein kinase (MAPK) cascades play critical roles in regulating various cellular processes in eukaryotes (Herskowitz, 1995; Chang and Karin, 2001; MAPK Group, 2002). They are located downstream of receptors and sensors and control cell physiology in response to various intra- and extracellular stimuli. The highly conserved MAPK cascades are composed of three kinases: MAPKs are phosphorylated by MAPK kinases (MAPKKs) at the threonine and tyrosine residues located in the activation loop (T-loop) between subdomains VII and VIII of the kinase catalytic domain; these MAPKKs are activated by the triple kinases, MAP kinase kinase kinases (MAPKKKs). Activated (phosphorylated) MAPKs can directly phosphorylate certain downstream targets, which include mainly transcription factors which, in turn, initiate stimulus-induced transcriptional changes, enzymes, and even proteins that function in cytokinesis (Chang and Karin, 2001; Liu et al., 2004; Sasabe and Machida, 2006; Beck et al., 2010).
Although the functions of MAPK cascades have been intensively studied in animals and yeast, how MAPK signalling is involved in plant development and stress responses is still not well understood. In plants, MAPK signalling pathways are important for development (Bergmann et al., 2004; Lukowitz et al., 2004; HC Wang et al., 2007; Rodriguez et al., 2010), responses to abiotic stresses, such as drought and salt (Kiegerl et al., 2000; Kovtun et al., 2000; Cardinale et al., 2002; Xiong and Yang, 2003), and resistance to viral, bacterial, and fungal pathogens (reviewed in Pedley and Martin, 2005; Rodriguez et al., 2010).
Emerging evidence has also indicated the involvement of MAPK signalling in plant defence against herbivores (Kandoth et al., 2007; Wu et al., 2007). In Nicotiana attenuata, attack from its natural herbivore, Manduca sexta, induces a myriad of responses on transcriptomic, proteomic, and metabolomic levels (Wu and Baldwin, 2010). Nicotiana attenuata recognizes the fatty acid–amino acid conjugates (FACs) in M. sexta oral secretions (OS) that are introduced into wounds during feeding and rapidly activates two MAPKs, salicylic acid-induced kinase (NaSIPK) and wound-induced protein kinase (NaWIPK); importantly, these kinases are required for the herbivory-induced biosynthesis of jasmonic acid (JA) and ethylene (Wu et al., 2007). The central role of JA in plant defence against herbivores has been well documented (reviewed in Wasternack, 2007; Howe and Jander, 2008; Wu and Baldwin, 2010). JAR (JASMONATE RESISTANT) proteins conjugate JA with isoleucine to form JA–Ile (Staswick and Tiryaki, 2004), which binds to the COI1 receptor and thus activates most of the JA-induced responses (Chini et al., 2007; Thines et al., 2007), including biosynthesis of various defensive compounds, such as direct defensive compounds, trypsin proteinase inhibitors (TPIs) (Zavala et al., 2004), caffeoylputrescine (CP) (Kaur et al., 2010), nicotine (Steppuhn et al., 2004), and diterpene glycosides (DTGs) (Jassbi et al., 2008; Heiling et al., 2010), and indirect defensive compounds, such as trans-α-bergamotene (Kessler and Baldwin, 2001). In N. attenuata, M. sexta attack, but not mechanical wounding, induces a burst of ethylene; genetic analysis indicated that ethylene is important for herbivory-induced nicotine production (von Dahl et al., 2007). Yet little is known about the signalling pathway that transduces FAC recognition into MAPK activation and eventually JA production in plants.
The Arabidopsis genome harbours ∼60 MAPKKKs, 10 MAPKKS, and 20 MAPKs (MAPK Group, 2002). The small number of MAPKKs suggests that MAPKKs may have multiple MAPK targets and that interactions among different signalling pathways are concentrated at the level of MAPKKs (MAPK Group, 2002; Hamel et al., 2006; Andreasson and Ellis, 2010). A growing body of evidence has revealed the important functions of MAPKKs in plant development and stress-induced responses. In tobacco, NQK1/NtMEK1 is required for cell cytokinesis (Soyano et al., 2003). The Arabidopsis double mutant mkk4 mkk5 develops densely clustered stomata and is seedling lethal, demonstrating the important role of MAPKKs in development (HC Wang et al., 2007). Detached leaves of an mkk9 mutant have delayed senescence (Zhou et al., 2009). Several MAPKKs are involved in abiotic stress responses (Kiegerl et al., 2000; Teige et al., 2004; Gomi et al., 2005; Xu et al., 2008), and resistance to pathogens (Asai et al., 2002; Jin et al., 2003; Liu et al., 2004; Meszaros et al., 2006; Doczi et al., 2007; Takahashi et al., 2007). In Arabidopsis, after perception of pathogen elicitor flg22, AtMKK4 and AtMKK5 activate AtMPK3 and AtMPK6, the homologues of Nicotiana WIPK and SIPK, respectively (Asai et al., 2002). Furthermore, overexpressing the constitutively active form of NtMEK2 (the homologue of AtMKK4/AtMKK5) in tobacco leads to activation of NtSIPK and NtWIPK (Yang et al., 2001; Jin et al., 2003). Overexpression of another Arabidopsis MAPKK, AtMKK9, activates AtMPK3 and AtMPK6 in protoplasts, and this MAPK cascade mediates the stability of EIN3 (ETHYLENE INSENSITIVE3), an important component in ethylene signalling (Yoo et al., 2008). Furthermore, Arabidopsis plants overexpressing AtMKK9 have enhanced ethylene and camalexin levels (Xu et al., 2008). In N. benthamiana, a close homologue of Arabidopsis AtMKK9, NbMKK1, interacts with NbSIPK in yeast, and ectopically overexpressing NbMKK1 activates NbSIPK (Takahashi et al., 2007).
In N. attenuata, NaSIPK and NaWIPK are pivotal MAPKs that regulate plant responses to herbivory. However, their upstream MAPKKs involved in herbivore defence responses were unknown. Using a reverse genetic approach, the transcript levels of two MAPKK genes, NaMEK2 and NaMKK1, were knocked down and it was found that NaMEK2 is important in mediating M. sexta herbivory-induced defence responses, while NaMKK1 plays only a minor role. After simulated herbivory, NaMEK2 and certain other MAPKKs, but not NaMKK1, are required for the activation of NaSIPK and NaWIPK, and thus JA and ethylene biosynthesis. The data highlight the important roles of MAPKKs in plant–herbivore interaction and the complexity of the regulation of JA and ethylene biosynthesis.
Materials and methods
Molecular cloning and virus-induced gene silencing (VIGS)
Nicotiana attenuata NaMKK1 and NaMEK2 (GenBank accession numbers: HQ023234 and HQ023235) were amplified using Phusion DNA polymerase (Finnzymes Oy, Espoo, Finland) (primer sequences are listed in Supplementary Table S1 available at JXB online) and the purified PCR products were cloned into pJET1.2 vector (Fermentas GmbH, St. Leon-Rot, Germany) and sequenced. Partial NaMEK2 and NaMKK1 sequence were amplified using plasmids as templates and gene-specific primers (listed in Supplementary Table S2). The PCR products were digested with appropriate restriction endonucleases and were further ligated into pTV00 to obtain the constructs pTV-NaMEK2 and pTV-NaMKK1.
Agrobacterium tumefaciens carrying these constructs was inoculated into plants to obtain VIGS (virus-induced gene silencing) plants following a procedure optimized for N. attenuata (Saedler and Baldwin, 2004). Plants inoculated with A. tumefaciens carrying pTV00 (empty vector) were used for comparisons (EV plants). Plants silenced in NaPDS (phytoene desaturase) were used to monitor the degree of VIGS visually, since these plants showed a photo-bleaching phenotype (Saedler and Baldwin, 2004). About 14 d after inoculation, when the leaves of NaPDS-silenced plants were completely white, experiments were performed.
Phylogenetic analysis of MAPKKs
MAPKK protein sequences were deduced from their respective nucleotide sequences (accession numbers are listed in Supplementary Table S3 at JXB online). Protein sequences were aligned using the Clustal W algorithm (DNAStar Inc., Madison, WI, USA). An unrooted Neighbor–Joining tree and bootstrap analysis (1000 replications) were conducted using MEGA 4 software (Tamura et al., 2007).
Plant growth and treatments
Plants of the 31st generation of an N. attenuata inbred line were used in all experiments. Plants were grown at 22 °C under 16 h of light in a growth chamber. In all the experiments, leaves of rosette-stage (∼4–5 weeks old) plants were used. Wounding was performed by rolling a fabric pattern wheel three times on each side of the midvein. The wounded leaves were immediately supplied with either 15 μl of water (W+W) or 15 μl of 1:5 diluted OS from M. sexta. For the collection of M. sexta OS, larvae were reared on N. attenuata wild-type plants until the third to fifth instar. OS were collected on ice as described in Roda et al. (2004).
Manduca sexta growth bioassays
Manduca sexta eggs from in-house reared populations were kept in a growth chamber (Snijders Scientific, Tilburg, The Netherlands) at 26 °C under 16 h of light, and at 24 °C in 8 h of darkness, until larvae hatched. Freshly hatched M. sexta neonates were placed on fully developed leaves of 30 replicated rosette-stage NaMEK2-VIGS, NaMKK1-VIGS, and EV plants (one larva per plant). The larval masses were measured on day 5, 8, and 12.
Transcriptional analysis
Total RNA was extracted from leaves using the TRIzol reagent (Invitrogen, Paisley, UK). A 0.5 μg aliquot of total RNA of each sample was reverse-transcribed using oligo(dT)12–18 and Superscript II reverse transcriptase (Invitrogen) following the manufacturer's instructions. Quantitative real-time PCR (qPCR) was carried out on an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA), using qPCR Core kits (Eurogentec, Liege, Belgium). Elongation factor 1A (NaEF1A) transcript levels were used to normalize total cDNA concentration variations. The sequences of primers used for qPCR are provided in Supplementary Table S4 at JXB online.
Phytohormone analysis
About 100 mg of frozen plant tissue were homogenized in 2 ml microcentrifuge tubes containing two metal balls and 1 ml of ethyl acetate spiked with 200 ng of D2-JA, and 40 ng of D4-salicylic acid (SA) and 13C6-JA–Ile. Homogenization was done twice with 200 strokes min−1 for 1 min using a Geno/Grinder 2000 (SPEX CertiPrep, Metuchen, NJ, USA). Samples were centrifuged at 13 000 g for 20 min at 4 °C. The supernatants were dried on a vacuum concentrator (Eppendorf AG, Hamburg, Germany). The residues were resuspended in 500 μl of 70% methanol by vortexing for 5 min, and centrifuged for 10 min at 4 °C (13 000 g). Supernatants were transferred to crimp vials, and sample measurements were carried out as described in Wu et al. (2007). Ethylene emissions were measured on a photoacoustic spectrometer (INVIVO GmbH, Sankt Augustin, Germany) as described in von Dahl et al. (2007). Leaves of N. attenuata plants were treated with W+OS or left untreated for control. Immediately after treatments three leaves were weighed and enclosed in a three-neck 250 ml round-bottom glass flask for 5 h, and then the concentration of collected ethylene was measured.
Analysis of trypsin proteinase inhibitor activity
Trypsin proteinase inhibitor (NaTPI) activity was quantified using a radial diffusion assay protocol described by van Dam et al. (2001).
Protein extraction and in-gel kinase activity assay
The tissue of five replicates was pooled and ground in liquid nitrogen. About 100 mg of tissue were resuspended in 300 μl of extraction buffer [100 mM HEPES pH 7.5, 5 mM EDTA, 5 mM EGTA, 10 mM Na3VO4, 10 mM NaF, 50 mM β-glycerolphosphate, 1 mM phenylmethylsulphonyl floride, 10% glycerol, and one proteinase inhibitor cocktail tablet per 10 ml of extraction buffer (Roche, Mannheim, Germany)]. Samples were then centrifuged at 4 °C, 13 000 g for 20 min and the supernatants were transferred to fresh tubes. Protein concentrations were measured using the Bio-Rad Protein Assay Dye Reagent (Bio-Rad, Hercules, CA, USA) with bovine serum albumin (Sigma-Aldrich, Hamburg, Germany) as a standard. A 10 μg aliquot of total protein from each sample was used for in-gel kinase activity assay according to a procedure described by Zhang and Klessig (1997). The images of in-gel kinase activity assays were obtained on a phosphorimager (FLA-3000 phosphor imager system, Fuji Photo Film, Stamford, CT, USA), and the band intensities were quantified using the AIDA software (Raytest Isotopenmessgeräte GmbH, Straubenhardt, Germany).
Statistical analysis
Data were analysed by unpaired t-tests using SPSS Statistics Version 17.0 (www.spss.com).
Results
Phylogenetic analysis of N. attenuata NaMEK2 and NaMKK1
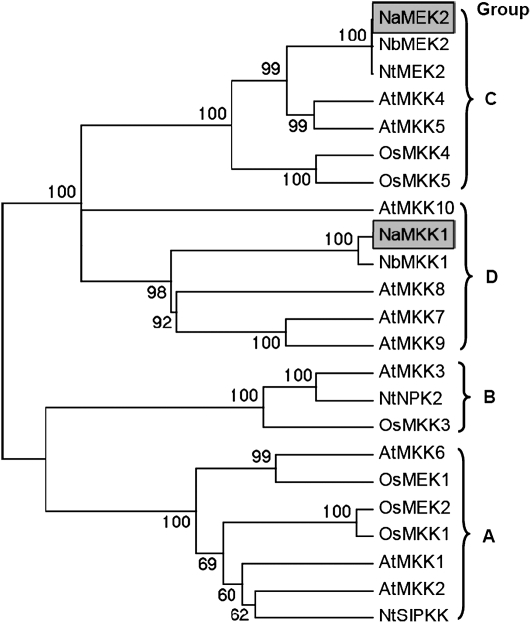
Using sequences of NtMEK2 in N. tabacum (Yang et al., 2001; Zhang and Liu, 2001) and NbMKK1 in N. benthamiana (Takahashi et al., 2007) as references, the open reading frames of NaMEK2 and NaMKK1 in N. attenuata were cloned. Sequence alignments indicated that the protein sequences of NaMEK2 and NaMKK1 shared 99% and 95% similarity to that of NtMEK2 and NbMKK1, respectively, and the conserved motif sequence [S/TxxxxxS/T] of MAPKKs (MAPK Group, 2002) was also found in both kinases (Supplementary Fig. S1 at JXB online). Moreover, phylogenetic analysis of NaMEK2, NaMKK1, and MAPKKs in A. thaliana, N. tabacum, N. benthamiana, and Oryza sativa indicated that NaMEK2 is a close homologue of AtMKK4 and AtMKK5 (group C of MAPKKs) and NaMKK1 is closely related to AtMKK7, AtMKK8, and AtMKK9 (group D of MAPKKs) (MAPK Group, 2002) (Fig. 1). It is likely that both NaMKK1 and NaMEK2 are single genes in N. attenuata, since searching the tobacco expressed sequence tag (EST) database and an N. attenuata transcriptome database obtained by 454 sequencing revealed no other close homologues. However, the possibility that NaMKK1 and NaMEK2 have paralogues, which might have low expression levels or are expressed in specific organs or tissues, cannot be completely ruled out.
Fig. 1.
Phylogenetic analysis of plant mitogen-activated protein kinase kinases (MAPKKs). Protein sequences of MAPKKs in Arabidopsis, Nicotiana spp., and rice were aligned using the Clustal W algorithm. An unrooted Neighbor–Joining tree and bootstrap analysis were performed with the MEGA 4 program. The species of origin of the MAPKKs are indicated by the abbreviation in front of the protein names: At, Arabidopsis thaliana; Na, Nicotiana attenuata; Nb, Nicotiana benthamiana; Nt, Nicotiana tabacum; Os, Oryza sativa. NaMKK1 and NaMEK2 are highlighted with grey backgrounds. Letters A to D represent different MAPKK groups (see MAPK Group, 2002).
Transcriptional regulation of NaMKK1 and NaMEK2 in N. attenuata
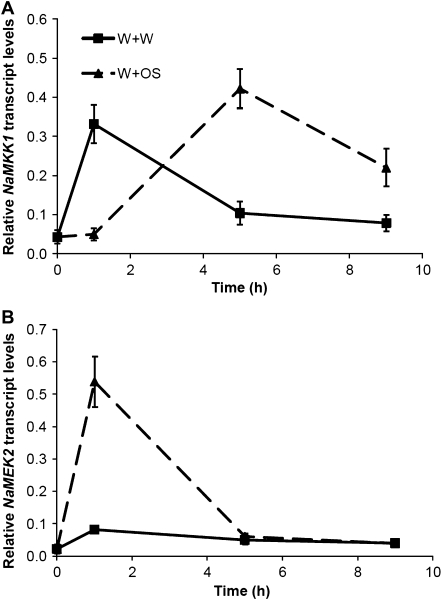
To determine whether these two MAPKK genes are involved in herbivory-induced transcriptional responses, their transcriptional changes were examined in N. attenuata after wounding and simulated herbivory treatment. A fabric pattern wheel was rolled over N. attenuata leaves to generate puncture wounds; thereafter, 15 μl of water were immediately applied to wounds (W+W). To mimic herbivory, 15 μl of M. sexta OS were applied (W+OS) (Halitschke et al., 2003). qPCR analyses indicated that the transcript levels of NaMKK1 were elevated ∼7-fold 1 h after W+W; while after W+OS treatment NaMKK1 reached its highest level of transcription (10-fold increase) by 5 h (Fig. 2A). W+W treatment marginally enhanced the levels of NaMEK2 transcripts, while NaMEK2 transcript levels were elevated >25 times 1 h after W+OS (Fig. 2B). These transcript data suggest possible involvement of NaMKK1 and NaMEK2 in wounding and herbivore defence responses. Whether these differential transcriptional regulations of NaMKK1 and NaMEK2 are further translated into different levels of NaMKK1 and NaMEK2 protein abundance/activity needs to be studied further.
Fig. 2.
Transcript levels of NaMKK1 and NaMEK2 after wounding and simulated herbivory. Nicotiana attenuata plants were wounded with a fabric pattern wheel, and 15 μl of water or M. sexta oral secretions (OS) were applied immediately to wounds (W+W and W+OS, respectively); untreated plants served as controls. Samples were harvested after1, 5, and 9 h and the transcript levels of (A) NaMKK1 and (B) NaMEK2 were analysed by qPCR.
NaMEK2, but not NaMKK1, is required for the phosphorylation of NaSIPK and NaWIPK after simulated herbivory
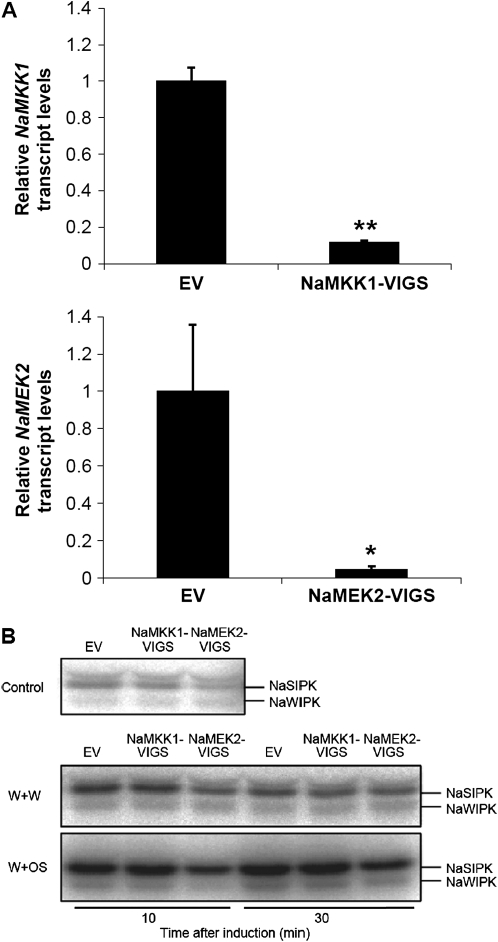
To study the function of NaMKK1 and NaMEK2 in wounding- and M. sexta herbivory-induced responses in N. attenuata, RNA interference (RNAi) constructs harbouring partial NaMKK1 and NaMEK2 sequences in an inverted repeat orientation were prepared and N. attenuata was transformed by A. tumefaciens carrying these constructs. Although stable RNAi lines of NaMKK1-silenced plants were obtained, after screening 20 independent lines transformed with the NaMEK2-RNAi construct, all 20 lines were found to be tetraploids. Therefore, a transient silencing approach, VIGS, was employed to knock-down the transcript levels of these two MAPKK genes. Plants inoculated with A. tumefaciens carrying pTV00, pTV-NaMKK1, and pTV-NaMEK2 formed EV, NaMKK1-VIGS, and NaMEK2-VIGS plants, respectively. qPCR analyses indicated that compared with those in EV plants, NaMKK1 and NaMEK2 transcript levels were reduced 89% and 95% in NaMKK1-VIGS and NaMEK2-VIGS plants (Fig. 3A). Silencing either gene using VIGS did not result in obvious developmental abnormalities in N. attenuata (data not shown).
Fig. 3.
MAPK activity in wounding- or simulated herbivory-induced responses in EV, NaMKK1-VIGS, and NaMEK2-VIGS plants. (A) NaMKK1 and NaMEK2 transcript levels are highly suppressed in NaMKK1-VIGS and NaMEK2-VIGS plants. Rosette leaves of EV, NaMKK1-VIGS, and NaMEK2-VIGS plants were harvested and the transcript levels of NaMKK1 and NaMEK2 were analysed by qPCR. The transcript levels of NaMKK1 and NaMEK2 in EV plants were designated as 1. Asterisks indicate significant differences between EV and NaMKK1-VIGS or NaMEK2-VIGS plants (t-test; *P <0.05; **P <0.01; n=5). (B) MAPK activity in EV, NaMKK1-VIGS, and NaMEK2-VIGS plants after wounding and simulated herbivory treatment. Plants were wounded with a fabric pattern wheel, and 15 μl of water or M. sexta oral secretions (OS) were applied immediately to wounds [W+W (middle panel) and W+OS (bottom panel), respectively]; untreated plants served as controls (top panel). Samples were harvested after 0, 10, and 30 min and immediately frozen in liquid nitrogen. MAPK activity was detected with an in-gel kinase assay.
An in-gel kinase activity assay was performed to determine whether NaMKK1 and NaMEK2 are the upstream MAPKKs for NaSIPK and NaWIPK when plants are challenged with wounding and herbivory. Plants were treated with W+W and W+OS and samples were collected after 10 min and 30 min. When untreated, no obvious different levels of MAPK activity were detected among EV, NaMKK1-VIGS, and NaMEK2-VIGS plants (Fig. 3B, top panel). In all plants, W+W and W+OS treatment rapidly enhanced the activity of NaSIPK and NaWIPK (as early as 10 min), indicating that N. attenuata recognized M. sexta OS and responded with higher MAPK activity levels than those induced by just wounding (Wu et al., 2007). Importantly, compared with EV, 10 min after W+W and 10 min and 30 min after W+OS, NaMEK2-VIGS plants showed ∼50% reduced NaSIPK activity levels (Fig. 3B, middle panel; for quantification of band intensities see Supplementary Fig. S2A at JXB online). In-gel kinase assays revealed only weak NaWIPK activity even after inductions. NaMEK2-VIGS plants seemed to have decreased levels of NaWIPK activity after W+OS, but not after W+W (Fig. 3B, bottom panel); in contrast, silencing NaMKK1 had no detectable effect on the activity levels of NaSIPK (and probably also NaWIPK) after either treatment.
Therefore, after mechanical wounding and simulated herbivory, NaMEK2 is important for the activation of NaSIPK, while NaWIPK seems to require NaMEK2 for phosphorylation only after simulated herbivory. Consistent results were obtained from an independently repeated experiment (Supplementary Fig. S2B at JXB online). These data suggest that very probably some other MAPKKs are also involved in the regulation of NaSIPK and NaWIPK in N. attenuata’s responses to wounding and herbivory, since silencing neither NaMEK2 nor NaMKK1 greatly compromises MAPK activity.
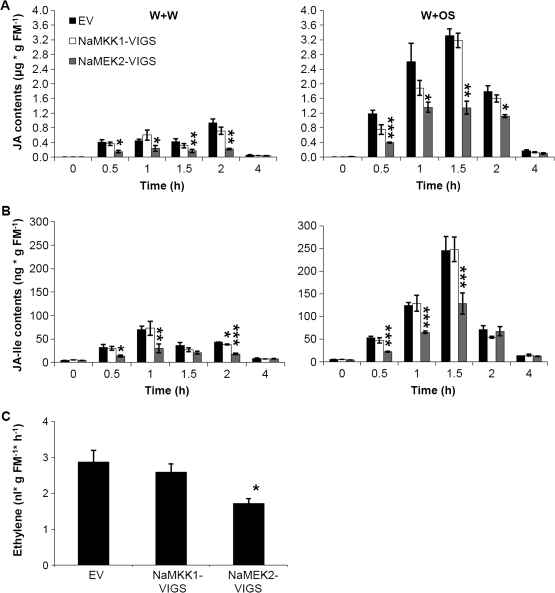
NaMEK2 but not NaMKK1 regulates wounding- and herbivory-induced accumulation of phytohormones in N. attenuata
Given that NaSIPK and NaWIPK are important regulators of wounding- and M. sexta herbivory-induced JA and JA–Ile accumulation and these phytohormones play a central role in mediating resistance to herbivores, whether silencing NaMKK1 and NaMEK2 alters the levels of JA and JA–Ile after these treatments was examined next. In EV plants, 1.5 h after W+W treatment JA reached 800 ng g−1 fresh mass (FM); consistent with the more highly augmented NaSIPK and NaWIPK activity levels after W+OS, EV plants accumulated up to 3600 ng g−1 FM of JA (Fig. 4A). The JA–Ile contents in EV plants followed similar patterns: 1 h after treatments, W+OS induced 4-fold higher JA–Ile levels than did W+W treatment (Fig. 4B). Congruent with their MAPK activity levels, NaMKK1-VIGS plants had no different JA and JA–Ile levels compared with EV plants after W+W and W+OS treatment, whereas NaMEK2-VIGS exhibited ∼50% lower levels of JA and JA–Ile at most of the time points examined after either treatment (Fig. 4).
Fig. 4.
Silencing NaMKK1 and NaMEK2 decreases wounding- or simulated herbivory-induced levels of phytohormones. (A) and (B) EV, NaMKK1-VIGS, and NaMEK2-VIGS plants were wounded with a fabric pattern wheel, and 15 μl of water or M. sexta oral secretions (OS) were applied immediately to wounds (W+W and W+OS, respectively). Samples were harvested after the indicated times. Contents (mean ±SE) of JA (A) and JA–Ile (B) were measured with HPLC-MS/MS. (C) EV, NaMKK1-VIGS and NaMEK2-VIGS plants were treated with W+OS, and ethylene accumulated in 5 h was collected and analysed. Asterisks indicate significant differences between EV and NaMKK1-VIGS or NaMEK2-VIGS plants (t-test; *P <0.05; **P <0.01; ***P <0.001; n=5).
Since wounding barely elicits ethylene biosynthesis, ethylene production was only measured in W+OS-elicited plants (von Dahl et al., 2007). After W+OS treatment, the same amount of ethylene was detected in NaMKK1-VIGS and EV plants, and NaMEK2-VIGS plants had 40% reduced ethylene emissions (Fig. 4C).
Many studies have indicated that SA suppresses JA accumulation (Spoel et al., 2003; Cipollini et al., 2004; Leon-Reyes et al., 2010). To rule out the possibility that the decreased JA levels in NaMEK2-VIGS plants resulted from augmented SA levels in these plants, SA contents were quantified. EV, NaMKK1-VIGS, and NaMEK2-VIGS plants showed no difference in basal and W+W-induced SA levels (Supplementary Fig. S3 at JXB online). After W+OS treatment, SA levels in NaMKK1- and NaMEK2-silenced plants were not higher than those of EV plants and tended to be lower by 50% in NaMEK2-silenced plants 1 h after W+OS (Supplementary Fig. S3).
Thus, NaMEK2 but not NaMKK1 is important for the regulation of wounding- and M. sexta herbivory-induced defence-related phytohormones.
Silencing NaMKK1 and NaMEK2 in N. attenuata compromises NaTPI activity
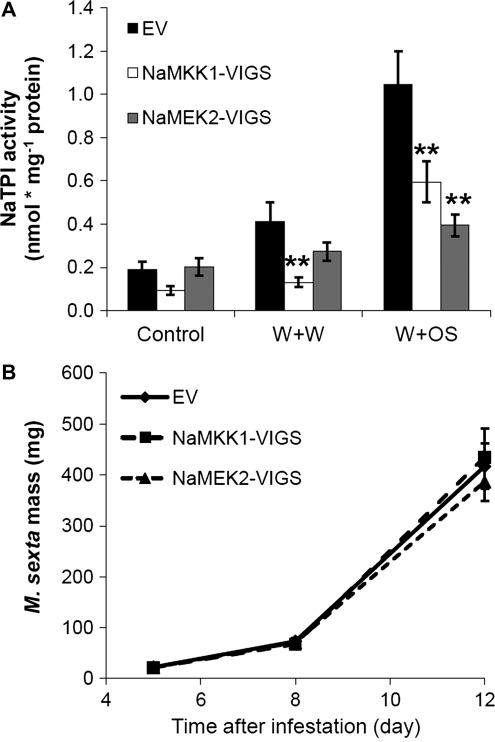
TPIs are important anti-herbivore compounds in Solanaceae, including N. attenuata (Ryan, 1989; Haq et al., 2004; Zavala et al., 2004). To analyse the function of NaMKK1 and NaMEK2 in regulating NaTPI, the activity of NaTPI was determined in EV, NaMKK1-VIGS, and NaMEK2-VIGS plants 3 d after they were treated with W+W or W+OS (non-treated plants served as controls). No significant differences were found among the non-treated samples (Fig. 5A). However, after W+W treatment, compared with EV, NaMKK1-VIGS plants showed ∼60% decreased NaTPI activity levels, while NaMEK2-VIGS plants had similar levels. Furthermore, W+OS-treated NaMKK1-VIGS and NaMEK2-VIGS exhibited ∼40% and 50% lower levels of NaTPI activity, respectively (Fig. 5A).
Fig. 5.
NaMKK1-VIGS and NaMEK2-VIGS plants have decreased NaTPI activity, but do not exhibit compromised resistance to M. sexta. (A) NaTPI activity in EV, NaMKK1-VIGS, and NaMEK2-VIGS plants. Plants were wounded with a fabric pattern wheel, and 15 μl of water or M. sexta oral secretions (OS) were applied immediately to wounds (W+W and W+OS, respectively); untreated plants served as controls. Three days after treatments, samples were collected and NaTPI activity was analysed. Asterisks indicate significant differences between EV and NaMKK1-VIGS or NaMEK2-VIGS plants (t-test; *P <0.05; **P <0.01; n=5). (B) Manduca sexta mass gain on EV, NaMKK1-VIGS, and NaMEK2-VIGS plants. Each type of plant was infested with 30 M. sexta neonates (one larva per plant) and larval masses (mean ±SE) were measured after 5, 8, and 12 d.
In N. attenuata, nicotine, CP, and DTGs are also important defensive compounds against M. sexta larvae. Neither W+W nor W+OS treatment elevated the contents of these compounds even in EV plants (data not shown). Very probably this was caused by the low temperature required for efficient VIGS (Kaplan et al., 2004; Shohael et al., 2006).
Bioassays were performed to examine whether knocking down NaMKK1 and NaMEK2 alters the performance of the specialist herbivore M. sexta. Freshly hatched neonates were placed on EV, NaMKK1-VIGS, and NaMEK2-VIGS plants, and their masses were recorded over 12 d. Despite the decreased NaTPI activity in NaMKK1-VIGS and NaMEK2-VIGS, M. sexta larvae gained similar mass on all plants (Fig. 5B).
Discussion
MAPKs play central roles in the activation of plant defence responses against abiotic and biotic stresses (Bergmann et al., 2004; Lukowitz et al., 2004; Pedley and Martin, 2005; Andreasson and Ellis, 2010; Rodriguez et al., 2010; HC Wang et al., 2007). At least two MAPKs, SIPK and WIPK, are important for plant resistance to herbivores (Wu et al., 2007). However, the identities of their upstream MAPKKs in plant–herbivore interactions were largely unknown. Using a reverse genetic approach, it is shown here that two MAPKKs, NaMKK1 and NaMEK2, are involved in wounding- and M. sexta feeding-induced responses in N. attenuata.
NaMEK2, but not NaMKK1, is upstream of NaSIPK and NaWIPK
Thus far, only two MAPKs, NaSIPK and NaWIPK and their homologues in tomato, are known to play a role in resistance to herbivore attack (Kandoth et al., 2007; Wu et al., 2007). Whether AtMPK6 and AtMPK3 (homologues of NaSIPK and NaWIPK in Arabidopsis) are also important for herbivory-induced responses is still unclear.
Using ectopic overexpression systems, a few studies have demonstrated that in Arabidopsis AtMKK4 and AtMKK5 phosphorylate AtMPK6 and AtMPK3 (Asai et al., 2002), and their close homologue in tobacco, NtMEK2, activates NtSIPK and NtWIPK (Yang et al., 2001; Zhang and Liu, 2001). Consistent with an AtMKK4/AtMKK5–AtMPK6/AtMPK3 and NtMEK2–NtSIPK/NtWIPK cascade, NaMEK2 was identified to be located upstream of NaSIPK and NaWIPK in the herbivory-induced signalling pathway using a knock-down approach. Importantly, silencing NaMEK2 only reduced NaSIPK activity levels after W+OS treatment by ∼50%, although qPCR analysis indicated that only 5% of NaMEK2 transcript levels were detected in NaMEK2-VIGS plants. It is speculated that one or more other unknown MAPKKs also phosphorylate NaSIPK when N. attenuata is challenged by M. sexta feeding. The possibility that NaMEK2-VIGS plants had substantially greater levels of NaMEK2 protein than the levels of NaMEK2 transcripts cannot be ruled out. In the wounding-activated signalling pathway, NaWIPK seems not to be located downstream of NaMEK2, given that NaMEK2-silenced plants did not have noticeably altered NaWIPK activity after wounding. Given the low activity of NaWIPK in in-gel kinase assays, this should be confirmed with immunocomplex kinase activity assays using an NaWIPK-specific antibody. Furthermore, in contrast to its reduced activity after simulated herbivory (at least by 30 min), the activity of NaSIPK in NaMEK2-silenced plants decreased only shortly after wounding (10 min) but regained the levels found in EV plants by 30 min. These data suggest that wounding and herbivory may activate overlapping but distinct MAPK pathways involving different MAPKKs, given that NaMEK2 is important for herbivory-induced activation of NaSIPK (and probably NaWIPK), but in response to wounding it only plays a minor role. Identification of the other MAPKK (or MAPKKs) that activate NaSIPK and NaWIPK will provide valuable insight into the mechanism by which plants distinguish mechanical wounding and herbivory and thus deploy appropriate defences.
Overexpression of NbMKK1 results in phosphorylation of NbSIPK in N. benthamiana (Takahashi et al., 2007). Transiently expressing AtMKK9 (a close homologue of NaMKK1) in tobacco and Arabidopsis leads to SIPK/AtMPK6 and WIPK/AtMPK3 activation (Xu et al., 2008), and transforming Arabidopsis protoplasts with a constitutively active form of AtMKK9 results in phosphorylation of AtMPK6 and AtMPK3 (Yoo et al., 2008). However, silencing experiments indicated that NaMKK1 is not required for the phosphorylation of NaSIPK and NaWIPK after wounding and simulated herbivory. It is very unlikely that the silencing of NaMKK1 was not sufficient to knock-down the protein levels of NaMKK1 since, after wounding and simulated herbivory treatment, NaMKK1-silenced plants exhibited compromised NaTPI activity. It is speculated that this was because ectopic overexpression may have led to very high levels of protein and thus produced non-physiological interactions. It is also possible that NaMKK1 activates NaSIPK and NaWIPK in other stimulus-activated signalling cascades, such as those elicited by pathogens (Takahashi et al., 2007).
Function of NaMEK2 in wounding- and herbivory-induced biosynthesis of phytohormones
SIPK and WIPK are important regulators of wounding- and herbivory-induced JA (and JA–Ile) accumulation (Kandoth et al., 2007; Wu et al., 2007). Consistent with this, NaMEK2-silenced plants had nearly 50% decreased JA and JA–Ile levels after both wounding and simulated herbivory. NaMKK1 is not required for the phosphorylation of NaSIPK and NaWIPK after wounding and herbivory; congruently, no changes of JA and JA–Ile levels were detected in NaMKK1-silenced plants. Moreover, compared with those in EV, no large differences in SA levels were found in NaMKK1- and NaMEK2-silenced plants. Kobayashi et al. (2010) examined the function of SIPK and WIPK in modulating the levels of JA and SA after N gene-carrying tobacco plants were challenged with tobacco mosaic virus (TMV): simultaneously silencing SIPK and WIPK highly compromises TMV viral accumulation, and this is associated with increased SA and decreased JA contents. It is speculated that silencing MKK1 in tobacco may not affect viral amplification and the accumulation of JA and SA, but MEK2-silenced plants could somewhat resemble plants silenced in both SIPK and WIPK (TMV amplification and the accumulation of JA and SA).
An elegant study revealed that Arabidopsis AtMPK6 phosphorylates 1-aminocyclopropane-1-carboxylic acid synthases (AtACS2 and AtACS6) and thus stabilizes these enzymes and greatly enhances ethylene biosynthesis (Liu and Zhang, 2004). However, after application of a pathogen elicitor, an mpk6 null mutant still produces 50% of the amount of ethylene synthesized by wild-type plants (Liu and Zhang, 2004). Similarly, silencing NaSIPK in N. attenuata results in 40% reduced ethylene production after simulated herbivory treatment (Wu et al., 2007). These facts suggest that certain AtMPK6/NaSIPK-independent pathways regulate the other 50% of ethylene production after pathogen/herbivory elicitation. Notably, even though the activity of NaSIPK in NaMEK2-VIGS plants is not decreased to the same extent as that in NaSIPK-VIGS plants or in mpk6 null mutants (Liu and Zhang, 2004; Wu et al., 2007), ethylene production is still 40% reduced. Either full activation of NaSIPK is critical for herbivory-induced ethylene biosynthesis or, in addition to NaSIPK, NaMEK2 also phosphorylates another MAPK, which also regulates ethylene production. Identification of this NaSIPK-independent ethylene regulation pathway will provide valuable insight into the mechanism by which plants control the biosynthesis of this important hormone.
NaMKK1 and NaMEK2 regulate the defence metabolite NaTPI
TPIs play an important role as direct defences against herbivores in solanaceous plants (Ryan, 1989; Haq et al., 2004). Many studies have indicated that JA signalling plays a major role in regulating the levels of TPIs (Koiwa et al., 1997; Paschold et al., 2007; L Wang et al., 2007). Despite the unaltered JA and JA–Ile levels in NaMKK1-silenced plants after wounding and simulated herbivory, NaTPI activity was reduced by 50%. Similar inconsistency between JA–Ile and NaTPI activity levels were also seen in NaMEK2-VIGS plants: after wounding, NaMEK2-VIGS plants did not have lower levels of NaTPI activity than did EV plants, although wounding-induced JA–Ile levels were reduced. These results reveal a complex regulatory network, including MAPK cascades (more specifically, certain transcription factors that are probably controlled directly by MAPK cascades) and JA signalling, in modulating TPI defence in responses to wounding and herbivory. Probably due to the low temperatures required for VIGS, other defence-related secondary metabolites (nicotine, CP, and DTGs) were not elevated after either W+W or W+OS, even in EV plants. Given the critical role of JA signalling in regulating CP and DTGs (Paschold et al., 2007), it is expected that the contents of these compounds in NaMEK2-silenced plants are also decreased, and NaMKK1 may also control the levels of these compounds in a largely JA signalling-independent manner. This hypothesis should be tested in plants whose NaMKK1 and NaMEK2 are stably silenced with an RNAi approach.
Although NaTPI activity levels were decreased in both NaMKK1-VIGS and NaMEK2-VIGS plants, M. sexta gained similar masses on these plants compared with those on EV plants. One possibility is that the decrease of NaTPI activity in these plants was not sufficient to weaken plant defence. Moreover, green leave volatiles (GLVs) are released from wounded leaves during insect feeding and these C6 compounds are thought to function as indirect defence, but also feeding stimulants or herbivore attractants (Meldau et al., 2009; Allmann and Baldwin., 2010; Dicke and Baldwin, 2010). In N. attenuata, GLVs stimulate M. sexta feeding, and silencing NaSIPK and NaWIPK impairs GLV emission and results in similar larval growth to those fed on wild-type plants, despite their decreased contents of direct defensive compounds (Meldau et al., 2009). This might also account for the normal growth of M. sexta on NaMEK2-VIGS plants. Whether NaMKK1 also controls GLV emission requires further investigation.
In Arabidopsis and rice, two close homologues of MEK2 exist (AtMKK4 and AtMKK5 in Arabidopsis and OsMKK4 and OsMKK5 in rice). However, only one MEK2 was found in the EST database of N. tabacum and N. benthamiana and in an N. attenuata transcriptome database prepared by 454 sequencing. It is possible that one of the two paralogues of MEK2 was deleted from the genomes of Nicotiana spp. or it is not expressed. More sequence information and phylogeny analyses will provide valuable information about the evolution of MEK2—whether MEK2 is an ancient MAPKK that appeared before the divergence of monocots and dicots or the gene duplication of MEK2 in Arabidopsis and rice is completely independent. Assuming that another copy of MEK2 does exist in Nicotiana spp., perhaps it is only expressed in specific organs or tissues. Alternatively, its transcript levels might be very low, and, if so, the possibility of co-silencing by the pTV-NaMEK2 construct cannot be ruled out, given the high sequence similarity between AtMKK4 and AtMKK5. A similar scenario may also apply to NaMKK1, which could also have one or more paralogues in N. attenuata, although current data point to the likelihood that NaMEK2 and NaMKK1 are single-copy genes.
Taken together, the present analyses indicate the involvement of two MAPKKs, NaMKK1 and NaMEK2, in plant responses to herbivores. Gene silencing revealed that these MAPKKs are upstream of different MAPKs and play overlapping but distinct roles in wounding- and herbivory-induced defence. Identification of other important components in wounding- and herbivory-specific signalling pathways, such as MAPKKKs, MAPKKs, MAPKs, and transcription factors, will greatly facilitate our understanding of how plants have evolved to cope with these stresses. Field studies will further reveal the ecological significance of these regulators in plant interactions with various herbivore feeding guilds.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Alignment of the protein sequences of N. attenuata NaMKK1 and NaMEK2 with N. benthamiana NbMKK1 and N. tabacum NtMEK2.
Figure S2 Wounding- and simulated herbivory-induced MAPK activity in EV, NaMKK1-VIGS, and NaMEK2-VIGS plants.
Figure S3 Salicylic acid accumulation in EV, NaMKK1-VIGS, and NaMEK2-VIGS plants after W+W and W+OS treatment.
Table S1. Primers used to clone the open reading frames of NaMKK1 and NaMEK2 in N. attenuata.
Table S2. Primers used to clone partial NaMKK1 and NaMEK2 into pTV00 to obtain VIGS constructs.
Table S3. GenBank accession numbers or Swiss-Prot accession numbers of the MAPKKs for phylogenetic analysis.
Table S4. Primers used for qPCR.
Supplementary Material
Acknowledgments
This work was funded by the Max Planck Society. We thank Yvonn Stampnik, Hendrik Wünsche, and Christina Bartnitzek for technical assistance. We thank Christian Hettenhausen for assistance with the in-gel kinase assays and discussions.
Glossary
Abbreviations
- CP
caffeoylputrescine
- DTG
17-hydroxygeranyllinalool diterpene glycoside
- HPLC-MS/MS
high-performance liquid chromatography–tandem mass spectrometry
- MAPK
mitogen-activated protein kinase
- SIPK
salicylic acid-induced protein kinase
- TPI
trypsin proteinase inhibitor
- VIGS
virus-induced gene silencing
- WIPK
wound-induced protein kinase
References
- Allmann S, Baldwin IT. Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science. 2010;329:1075–1078. doi: 10.1126/science.1191634. [DOI] [PubMed] [Google Scholar]
- Andreasson E, Ellis B. Convergence and specificity in the Arabidopsis MAPK nexus. Trends in Plant Science. 2010;15:106–113. doi: 10.1016/j.tplants.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- Beck M, Komis G, Muller J, Menzel D, Samaj J. Arabidopsis homologs of nucleus- and phragmoplast-localized kinase 2 and 3 and mitogen-activated protein kinase 4 are essential for microtubule organization. The Plant Cell. 2010;22:755–771. doi: 10.1105/tpc.109.071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann DC, Lukowitz W, Somerville CR. Stomatal development and pattern controlled by a MAPKK kinase. Science. 2004;304:1494–1497. doi: 10.1126/science.1096014. [DOI] [PubMed] [Google Scholar]
- Cardinale F, Meskiene I, Ouaked F, Hirt H. Convergence and divergence of stress-induced mitogen-activated protein kinase signaling pathways at the level of two distinct mitogen-activated protein kinase kinases. The Plant Cell. 2002;14:703–711. [PMC free article] [PubMed] [Google Scholar]
- Chang LF, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chini A, Fonseca S, Fernández G, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448:666–671. doi: 10.1038/nature06006. [DOI] [PubMed] [Google Scholar]
- Cipollini D, Enright S, Traw MB, Bergelson J. Salicylic acid inhibits jasmonic acid-induced resistance of Arabidopsis thaliana to. Spodoptera exigua. Molecular Ecology. 2004;13:1643–1653. doi: 10.1111/j.1365-294X.2004.02161.x. [DOI] [PubMed] [Google Scholar]
- Dicke M, Baldwin IT. The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends in Plant Science. 2010;15:167–175. doi: 10.1016/j.tplants.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Doczi R, Brader G, Pettko-Szandtner A, Rajh I, Djamei A, Pitzschke A, Teige M, Hirt H. The Arabidopsis mitogen-activated protein kinase kinase MKK3 is upstream of group C mitogen-activated protein kinases and participates in pathogen signaling. The Plant Cell. 2007;19:3266–3279. doi: 10.1105/tpc.106.050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomi K, Ogawa D, Katou S, et al. A mitogen-activated protein kinase NtMPK4 activated by SIPKK is required for jasmonic acid signaling and involved in ozone tolerance via stomatal movement in tobacco. Plant and Cell Physiology. 2005;46:1902–1914. doi: 10.1093/pcp/pci211. [DOI] [PubMed] [Google Scholar]
- Halitschke R, Gase K, Hui D, Schmidt DD, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (lepidoptera, sphingidae) and its natural host Nicotiana attenuata. VI. Microarray analysis reveals that most herbivore-specific transcriptional changes are mediated by fatty acid–amino acid conjugates. Plant Physiology. 2003;131:1894–1902. doi: 10.1104/pp.102.018184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel LP, Nicole MC, Sritubtim S, et al. Ancient signals: comparative genomics of plant MAPK and MAPKK gene families. Trends in Plant Science. 2006;11:192–198. doi: 10.1016/j.tplants.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Haq SK, Atif SM, Khan RH. Protein proteinase inhibitor genes in combat against insects, pests, and pathogens: natural and engineered phytoprotection. Archives of Biochemistry and Biophysics. 2004;431:145–159. doi: 10.1016/j.abb.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Heiling S, Schuman MC, Schoettner M, Mukerjee P, Berger B, Schneider B, Jassbi AR, Baldwin IT. Jasmonate and ppHsystemin regulate key malonylation steps in the biosynthesis of 17-hydroxygeranyllinalool diterpene glycosides, an abundant and effective direct defense against herbivores in. Nicotiana attenuata. The Plant Cell. 2010;22:273–292. doi: 10.1105/tpc.109.071449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- Howe GA, Jander G. Plant immunity to insect herbivores. Annual Review of Plant Biology. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- Jassbi AR, Gase K, Hettenhausen C, Schmidt A, Baldwin IT. Silencing geranylgeranyl diphosphate synthase in Nicotiana attenuata dramatically impairs resistance to tobacco hornworm. Plant Physiology. 2008;146:974–986. doi: 10.1104/pp.107.108811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin HL, Liu YD, Yang KY, Kim CY, Baker B, Zhang SQ. Function of a mitogen-activated protein kinase pathway in N gene-mediated resistance in tobacco. The Plant Journal. 2003;33:719–731. doi: 10.1046/j.1365-313x.2003.01664.x. [DOI] [PubMed] [Google Scholar]
- Kandoth PK, Ranf S, Pancholi SS, Jayanty S, Walla MD, Miller W, Howe GA, Lincoln DE, Stratmann JW. Tomato MAPKs LeMPK1, LeMPK2, and LeMPK3 function in the systemin-mediated defense response against herbivorous insects. Proceedings of the National Acadademy of Sciences, USA. 2007;104:12205–12210. doi: 10.1073/pnas.0700344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N, Sung DY, Guy CL. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiology. 2004;136:4159–4168. doi: 10.1104/pp.104.052142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Heinzel N, Schottner M, Baldwin IT, Galis I. R2R3-NaMYB8 regulates the accumulation of phenylpropanoid–polyamine conjugates, which are essential for local and systemic defense against insect herbivores in Nicotiana attenuata. Plant Physiology. 2010;152:1731–1747. doi: 10.1104/pp.109.151738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- Kiegerl S, Cardinale F, Siligan C, et al. SIMKK, a mitogen-activated protein kinase (MAPK) kinase, is a specific activator of the salt stress-induced MAPK, SIMK. The Plant Cell. 2000;12:2247–2258. doi: 10.1105/tpc.12.11.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Seo S, Hirai K, Yamamoto-Katou A, Katou S, Seto H, Meshi T, Mitsuhara I, Ohashi Y. Silencing of WIPK and SIPK mitogen-activated protein kinases reduces tobacco mosaic virus accumulation but permits systemic viral movement in tobacco possessing the N resistance gene. Molecular Plant-Microbe Interaction. 2010;23:1032–1041. doi: 10.1094/MPMI-23-8-1032. [DOI] [PubMed] [Google Scholar]
- Koiwa H, Bressan RA, Hasegawa PM. Regulation of protease inhibitors and plant defense. Trends in Plant Science. 1997;2:379–384. [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proceedings of the National Academy of Sciences, USA. 2000;97:2940–2945. doi: 10.1073/pnas.97.6.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Reyes A, Van der Does D, De Lange ES, Delker C, Wasternack C, Van Wees SCM, Ritsema T, Pieterse CMJ. Salicylate-mediated suppression of jasmonate-responsive gene expression in Arabidopsis is targeted downstream of the jasmonate biosynthesis pathway. Planta. 2010;232:1423–1432. doi: 10.1007/s00425-010-1265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang S. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. The Plant Cell. 2004;16:3386–3399. doi: 10.1105/tpc.104.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YL, Schiff M, Dinesh-Kumar SP. Involvement of MEK1 MAPKK, NTF6 MAPK, WRKY/MYB transcription factors, COI1 and CTR1 in N-mediated resistance to tobacco mosaic virus. The Plant Journal. 2004;38:800–809. doi: 10.1111/j.1365-313X.2004.02085.x. [DOI] [PubMed] [Google Scholar]
- Lukowitz W, Roeder A, Parmenter D, Somerville C. A MAPKK kinase gene regulates extra-embryonic cell fate in Arabidopsis. Cell. 2004;116:109–119. doi: 10.1016/s0092-8674(03)01067-5. [DOI] [PubMed] [Google Scholar]
- MAPK Group Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends in Plant Science. 2002;7:301–308. doi: 10.1016/s1360-1385(02)02302-6. [DOI] [PubMed] [Google Scholar]
- Meldau S, Wu J, Baldwin IT. Silencing two herbivory-activated MAP kinases, SIPK and WIPK, does not increase Nicotiana attenuata’s susceptibility to herbivores in the glasshouse and in nature. New Phytologist. 2009;181:161–173. doi: 10.1111/j.1469-8137.2008.02645.x. [DOI] [PubMed] [Google Scholar]
- Meszaros T, Helfer A, Hatzimasoura E, et al. The Arabidopsis MAP kinase kinase MKK1 participates in defence responses to the bacterial elicitor flagellin. The Plant Journal. 2006;48:485–498. doi: 10.1111/j.1365-313X.2006.02888.x. [DOI] [PubMed] [Google Scholar]
- Paschold A, Halitschke R, Baldwin IT. Co(i)-ordinating defenses: NaCOI1 mediates herbivore-induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. The Plant Journal. 2007;51:79–91. doi: 10.1111/j.1365-313X.2007.03119.x. [DOI] [PubMed] [Google Scholar]
- Pedley KF, Martin GB. Role of mitogen-activated protein kinases in plant immunity. Current Opinion in Plant Biology. 2005;8:541–547. doi: 10.1016/j.pbi.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Roda A, Halitschke R, Steppuhn A, Baldwin IT. Individual variability in herbivore-specific elicitors from the plant's perspective. Molecular Ecology. 2004;13:2421–2433. doi: 10.1111/j.1365-294X.2004.02260.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez MC, Petersen M, Mundy J. Mitogen-activated protein kinase signaling in plants. Annual Review Plant Biology. 2010;61:621–649. doi: 10.1146/annurev-arplant-042809-112252. [DOI] [PubMed] [Google Scholar]
- Ryan CA. Proteinase inhibitor gene families: strategies for transformation to improve plant defenses against herbivores. Bioessays. 1989;10:20–24. doi: 10.1002/bies.950100106. [DOI] [PubMed] [Google Scholar]
- Saedler R, Baldwin IT. Virus-induced gene silencing of jasmonate-induced direct defences, nicotine and trypsin proteinase-inhibitors in Nicotiana attenuata. Journal of Experimental Botany. 2004;55:151–157. doi: 10.1093/jxb/erh004. [DOI] [PubMed] [Google Scholar]
- Sasabe M, Machida Y. MAP65: a bridge linking a MAP kinase to microtubule turnover. Current Opinion in Plant Biology. 2006;9:563–570. doi: 10.1016/j.pbi.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Shohael AM, Ali MB, Yu KW, Hahn EJ, Paek KY. Effect of temperature on secondary metabolites production and antioxidant enzyme activities in Eleutherococcus senticosus somatic embryos. Plant Cell, Tissue and Organ Culture. 2006;85:219–228. [Google Scholar]
- Soyano T, Nishihama R, Morikiyo K, Ishikawa M, Machida Y. NQK1/NtMEK1 is a MAPKK that acts in the NPK1 MAPKKK-mediated MAPK cascade and is required for plant cytokinesis. Genes and Development. 2003;17:1055–1067. doi: 10.1101/gad.1071103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel SH, Koornneef A, Claessens SM, et al. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. The Plant Cell. 2003;15:760–770. [Google Scholar]
- Staswick PE, Tiryaki I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. The Plant Cell. 2004;16:2117–2127. doi: 10.1105/tpc.104.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT. Nicotine's defensive function in nature. PLoS Biology. 2004;2:E217. doi: 10.1371/journal.pbio.0020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Bin Nasir KH, Ito A, Kanzaki H, Matsumura H, Saitoh H, Fujisawa S, Kamoun S, Terauchi R. A high-throughput screen of cell-death-inducing factors in Nicotiana benthamiana identifies a novel MAPKK that mediates INF1-induced cell death signaling and non-host resistance to Pseudomonas cichorii. The Plant Journal. 2007;49:1030–1040. doi: 10.1111/j.1365-313X.2006.03022.x. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Teige M, Scheikl E, Eulgem T, Doczi R, Ichimura K, Shinozaki K, Dangl JL, Hirt H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Molecular Cell. 2004;15:141–152. doi: 10.1016/j.molcel.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature. 2007;448:661–665. doi: 10.1038/nature05960. [DOI] [PubMed] [Google Scholar]
- van Dam NM, Horn M, Mares M, Baldwin IT. Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. Journal of Chemical Ecology. 2001;27:547–568. doi: 10.1023/a:1010341022761. [DOI] [PubMed] [Google Scholar]
- von Dahl CC, Winz RA, Halitschke R, Kuhnemann F, Gase K, Baldwin IT. Tuning the herbivore-induced ethylene burst: the role of transcript accumulation and ethylene perception in Nicotiana attenuata. The Plant Journal. 2007;51:293–307. doi: 10.1111/j.1365-313X.2007.03142.x. [DOI] [PubMed] [Google Scholar]
- Wang HC, Ngwenyama N, Liu YD, Walker JC, Zhang SQ. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. The Plant Cell. 2007;19:63–73. doi: 10.1105/tpc.106.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Halitschke R, Kang JH, Berg A, Harnisch F, Baldwin IT. Independently silencing two JAR family members impairs levels of trypsin proteinase inhibitors but not nicotine. Planta. 2007;226:159–167. doi: 10.1007/s00425-007-0477-3. [DOI] [PubMed] [Google Scholar]
- Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Annals of Botany. 2007;100:681–697. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Baldwin IT. New insights into plant responses to the attack from insect herbivores. Annual Review of Genetics. 2010;44:1–24. doi: 10.1146/annurev-genet-102209-163500. [DOI] [PubMed] [Google Scholar]
- Wu J, Hettenhausen C, Meldau S, Baldwin IT. Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of. Nicotiana attenuata. The Plant Cell. 2007;19:1096–1122. doi: 10.1105/tpc.106.049353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Yang Y. Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid-inducible mitogen-activated protein kinase. The Plant Cell. 2003;15:745–759. [Google Scholar]
- Xu J, Li Y, Wang Y, Liu HX, Lei L, Yang HL, Liu GQ, Ren DT. Activation of MAPK kinase 9 induces ethylene and camalexin biosynthesis and enhances sensitivity to salt stress in Arabidopsis. Journal of Biological Chemistry. 2008;283:26996–27006. doi: 10.1074/jbc.M801392200. [DOI] [PubMed] [Google Scholar]
- Yang KY, Liu Y, Zhang S. Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proceedings of the National Academy of Sciences, USA. 2001;98:741–746. doi: 10.1073/pnas.98.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Tena G, Xiong Y, Sheen J. Dual control of nuclear EIN3 by bifurcate MAPK cascades in C2H4 signalling. Nature. 2008;451:789–795. doi: 10.1038/nature06543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Patankar AG, Gase K, Hui D, Baldwin IT. Manipulation of endogenous trypsin proteinase inhibitor production in Nicotiana attenuata demonstrates their function as antiherbivore defenses. Plant Physiology. 2004;134:1181–1190. doi: 10.1104/pp.103.035634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Klessig DF. Salicylic acid activates a 48-kD MAP kinase in tobacco. The Plant Cell. 1997;9:809–824. doi: 10.1105/tpc.9.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Liu Y. Activation of salicylic acid-induced protein kinase, a mitogen-activated protein kinase, induces multiple defense responses in tobacco. The Plant Cell. 2001;13:1877–1889. doi: 10.1105/TPC.010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Cai Z, Guo Y, Gan S. An arabidopsis mitogen-activated protein kinase cascade, MKK9–MPK6, plays a role in leaf senescence. Plant Physiology. 2009;150:167–177. doi: 10.1104/pp.108.133439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.