Abstract
Aim:
The aim of the present study is to evaluate the gastroprotective effect of hydroalcoholic extract of Andrographis paniculata (HAEAP) in male albino wistar rats.
Materials and Methods:
Rats were pretreated with HAEAP (100,200,500mg/kg b. wt for 30 days) and then gastric ulcers were induced by ethanol, aspirin, pylorus ligation and cold restraint stress models. Ulcer score was determined in all the ulcer models. pH, gastric volume, titrable acidity, pepsin, mucin, myeloperoxidase, H+K+ATPase, thiobarbituric acid reacting substances (TBARS) and antioxidant enzyme activities were assayed in ethanol-administered rats.
Results:
The ulcer score was found to be low in HAEAP-pretreated rats. Among the doses studied, 200 mg/kg b.wt was found to be optimum for significant ulcer reduction. The test drug significantly reduced the acidity, pepsin concentration, myeloperoxidase and H+K+ATPase activities in ethanol-administered rats. The elevated TBARS and decreased glutathione (GSH) and mucin levels observed during ulcerogenesis were found to be altered in HAEAP-received animals.
Conclusions:
The ulcer preventing effect of HAEAP may partly be due to its regulating effect on H+K+ATPase activity and /or mucin preserving effects. The flavonoids present in the HAEAP might be responsible for the gastroprotective action probably by maintaining the antioxidants and thiol status in the gastrointestinal tract.
Keywords: Andrographis paniculata, antioxidants, ethanol-induced ulcer, mucin, ulcer score
Introduction
Gastric ulcer is a major disease of gastrointestinal system which affects 10% of the world population with different etiologies. Chronic alcohol consumption, smoking, stress, usage of non-steroidal anti-inflammatory drugs and H. pylori infection have been shown to be the causes of gastric ulcer characterized by inflammation and mucosal bleeding in long-term untreated patients. Excess acid secretion and reduced biosynthesis of prostaglandin E2 are important in gastric ulcer formation. Ulcer therapy is mainly focused on limiting the deleterious effects of offensive acid secretion, but the search for newer, safer alternative drugs has rekindled the interest in cytoprotective drugs, which protect the gastric mucosa from damaging agents without influencing acid secretion or neutralizing intragastric acidity.[1]
The conventional drugs used in the treatment of gastric ulcer include histamine (H2) receptor antagonists, proton pump inhibitors, antacids and anticholinergics. However, most of these drugs have undesirable side effects and drug interactions.[2] Although few drugs like sucralfate and prostaglandin analogs[3] are being used as antiulcer substances, they may also have the risk of drug interactions, adverse effects and increased incidence of relapse during ulcer therapy.
Hence, the search has been focused on natural products with antiulcer properties. From ancient times, plants have been a powerful therapeutic agent for the treatment of various human diseases including those of gastrointestinal system. Approximately 60% of the world population relies almost entirely on plants for medication and natural products have long been recognized as important sources of therapeutically effective medicines.[4] The ever increasing problem of gastroduodenal hyperacidity demands the identification of new drugs. Hence the objective of the present work is to evaluate the ulcer preventing activity of Andrographis paniculata in rats by administering hydroalcoholic extract prior to ulcer induction.
Andrographis paniculata (Burm.f.) wall. (Acanthaceae) is one of the most important medicinal plants widely used in Chinese and Ayurvedic systems for the treatment of gastric disorders, infectious diseases and common cold for many years. Pharmacological and clinical studies have demonstrated that Andrographis paniculata possesses anti-inflammatory, antiallergic and cardiovascular activities.[5] Diterpenoids and flavonoids are the main chemical constituents of Andrographis paniculata and these compounds are believed to be responsible for the biological activity of the plant. Several studies have been conducted to investigate the cytotoxic or antitumor activities of Andrographis paniculata and its chemical constituent andrographolide has been shown to inhibit in vitro proliferation of different tumor cell lines.[6] The present study was conducted to provide experimental evidence for antiulcer activity of HAEAP using different in vivo ulcer models in rats.
Materials and Methods
Plant Collection and Identification
The plant was purchased from the local market in Chennai and authenticated by Dr. Jayaraman, Taxonomist, Plant Anatomy Research Centre, Chennai (voucher no of the specimen: PARC/ 2008/ 185).
Hydroalcoholic Extract of Andrographis paniculata
The air-dried aerial parts of Andrographis paniculata were ground into homogenous powder and freeze-dried. The freeze-dried material was extracted by refluxing 4060 times with 70% ethanol for 6-8 h. The extract was evaporated to dryness to one third of the original volume and stored at 4°C. The filtrate was lyophilized and the dry material obtained was used for the study.
Animals
Male albino Wistar rats (120-140g) were obtained from Kings Institute, Chennai, India. They were acclimatized to animal house conditions, fed commercial pelleted rat chow (Hindustan Lever Ltd., Bangalore, India) and water ad libitum. Animals were maintained according to the rules and regulations laid down by the Institutional Animal Ethics Committee (290/04/V/CPCSEA/IAEC/PHA-24-27).
Dose Response Study and Dosage Fixation
An initial dose response study was conducted in rats treated orally with 100, 200 and 500 mg/kg b. wt of HAEAP for 30 days, to find out the optimal ulcer protective dose against ethanol, aspirin, pylorus ligation and cold stress induced gastric ulcer in rats. A dose of 200 mg/kg b. wt. of HAEAP was then selected on the basis of optimal ulcer protective effect for further studies. Similar dose response study was conducted with 15, 30, 50 mg/kg b.wt of ranitidine and 30 mg/kg b.wt was found to be the optimum dose. Ethanol-induced ulcer model was chosen for the evaluation of gastroprotective activity of HAEAP.
Treatment Protocol for Antiulcer Activity of Haeap by Ethanol Model
Rats were divided into five groups of six animals each.
Group I : Control rats.
Group II : Rats treated with ethanol (as mentioned in ulcer induction).
Group III : Rats pretreated with 200 mg/kg b. wt of HAEAP for 30 days and then administered ethanol.
Group IV : Rats pretreated with 30 mg/kg b. wt of standard drug ranitidine for 30 days and then administered ethanol.
Group V : Rats treated with 200 mg/kg b. wt of HAEAP for a period of 30 days.
Ulcer Induction
Ethanol- Induced Ulcer
Ulcers were produced by oral administration of ethanol 1.0 ml/200g/kg b.wt to rats after 12 h fasting. The animals were sacrificed after 1h of ethanol administration.[7]
Aspirin-Induced Ulcer
Aspirin was administered at the dose of 200 mg/kg b.wt and ulcer score was determined after 5 h. The stomach was cut open along the greater curvature and ulcer index was scored by a person unaware of the experiment protocol in the glandular portion of the stomach.[8]
Pylorus Ligation Induced Ulcer
Drugs were administered for a period of 30 days. On day 30, after the last dose, the rats were kept fasting for 18 h. Rats were anesthetized using diethyl ether, the abdomen was opened and pylorus ligation was done without causing any damage to its blood supply. After replacing the stomach carefully, the abdomen wall was closed in two layers with interrupted sutures. The animals were deprived of water during the postoperative period.[9] After 4 h, stomach was dissected out, the contents collected and ulcer score determined.
Cold Restraint Induced Ulcer (CRU)
On day 30, after 30 min of HAEAP or ranitidine treatment, rats were immobilized under light ether anesthesia and subjected to the cold stress at 4 ± 1°C for 3.5 h[10] and ulcer score determined.
Determination of Ulcer Score
The ulcer index of gastric mucosal lesions was evaluated by the score system reported by Nie et al.[11] Briefly, after collecting gastric juice, the stomach was opened along the greater curvature and rinsed with 0.1mol/L ice-cold PBS. The stomach was then examined under microscope (100X) to observe erosions and scored as 1-5: 1. small round hemorrhagic erosion, 2. hemorrhagic erosion < 1 mm, 3. hemorrhagic erosion = 1-2 mm, 4. hemorrhagic erosion = 2-3mm, 5. hemorrhagic erosion > 4 mm. The score was multiplied by 2 when the width of the erosion is larger than 1mm.
Determination of Acid Secretory Parameters
The ulcer induced in rats by ethanol model was considered for the evaluation of pH, gastric volume and titrable acidity. The stomach was dissected out and the gastric juice collected was centrifuged for 5 min at 2000 rpm and the volume of the supernatant was expressed as ml/ 100 g b.wt and pH was measured using pH meter. Total acid output was determined by titrating with 0.01N NaOH, using phenolphthalein as indicator and was expressed as mEq/L/100g.
Biochemical Investigations
Determination of pepsin activity
Pepsin was assayed according to the method of Shay et al.,[12] using hemoglobin as substrate. The absorbance of the solution was read at 650nm. The pepsin content was expressed as μM of tyrosine liberated/ml.
Determination of mucin content
Mucin content was determined by the method of Corne et al.[13] After the collection of gastric juice, the glandular portion was excised and opened down along the lesser curvature. The reverted stomach was soaked for 2 h in 0.1% alcian blue (0.16M sucrose buffered with 0.05M sodium acetate). The uncomplexed dye was removed by two successive washes of 15 and 45 min in 0.25M sucrose solution. The dye complexed with mucus was diluted by immersion in 10 ml of 0.5M magnesium chloride for 2 h. The resulting blue solution was shaken briefly with equal volume of diethyl ether and the optical density of aqueous phase was measured at 605 nm. The mucin content of the sample was determined from the standard curve obtained with different concentrations of mucin.
Determination of myeloperoxidase activity
Myeloperoxidase (MPO) activity in the gastric mucosa was measured according to the method of Bradley et al.[14] Pre- weighed tissue was homogenized (1: 10 w/v) in 0.5% hexadecyltrimethyl ammonium bromide in 50 mM potassium phosphate buffer (pH 6.0) before sonication in an ice bath for 20 s. Three freeze/thaw cycles were performed followed by sonication (20 s in ice bath). The samples were centrifuged at 17000g (5 min, 4°C) and myeloperoxidase in the supernatant was assayed by mixing 0.1 ml of supernatant and 2.9 ml of 50 mM potassium phosphate buffer (pH 6.0) containing 0.167 g/L o-dianisidine dihydrochloride and 0.0005% hydrogen peroxide (H2O2). The change in absorbance at 460 nm was measured for 4 min using an UV visible spectrophotometer.
Determination of H+K+-ATPase activity
Potassium proton ATPase was prepared from gastric mucosal scrapings[15] and homogenized in 200mM Tris-HCl, pH 7.4, centrifuged for 10 min at 5000 × g. The resulting supernatant was subsequently centrifuged at 5000 × g for 20 min. The protein concentration of the supernatant was determined by using bovine serum albumin as standard.[16]
The H+K+ ATPase activity in the gastric mucosa was assayed by the method of Reyes- Chilpa et al[17] The assay mixture consisted of an aliquot of enzyme in 20 mM Tris-HCl, pH 7.4, 2 mM MgCl2 and 2 mM KCl. The reaction was started with the addition of 2 mM ATP and incubated for 30 min at 30°C and terminated by the addition of 10% trichloroacetic acid followed by centrifugation at 2000 × g. The amount of inorganic phosphorous released from ATP was determined spectrophotometrically at 640 nm. The enzyme activity was expressed as nM of Pi liberated/min/mg protein.
Activity of H+K+ATPase in terms of ED50 value
Different doses of the test material (50, 100, 200, 500 mg/kg b.wt) were administered to rats for 30 days prior to ulcer induction by ethanol administration. H+K+ATPase activity was measured and the ED50 value was found to be 176 mg/kg b.wt (data not shown).
Estimation of lipid peroxides, reduced glutathione and antioxidant enzymes
The excised stomach tissue was mixed with 5 ml of 0.1M Tris-HCl buffer pH 7.4, homogenized on ice using Potter-Elvehjem glass homogenizer for 15 min. The homogenate was used for the estimation of thiobarbituric acid reacting substances (TBARS), glutathione (GSH) and antioxidants.
Lipid peroxides (LPO) in terms of TBARS were estimated using 1, 1’, 3, 3’- tetra methoxypropane as the standard and expressed as nM/mg protein.[18]
Glutathione (GSH) was determined by the method of Moron et al.[19] Aliquots of homogenate were mixed with equal volume of ice cold 5% TCA and the precipitated proteins were removed by centrifugation. The supernatant was added to equal volume of 0.2 M phosphate buffer, pH 8.0 and measured at 412 nm. GSH was used as a reference standard. Glutathione peroxidase (GPx) was assayed by the method of Flohe and Gunzler.[20] The activity of GPx was expressed as nM GSH oxidized/min/mg protein.
Superoxide dismutase (SOD) activity was measured according to the method of Kakkar et al.[21] The inhibition of reduction of nitroblue tetrazolium to blue colored formazan in the presence of phenazine methosulfate and NADH was measured at 560 nm using n- butanol as blank. The enzyme activity was expressed as units/mg protein.
Decomposition of H2O2 in the presence of catalase (CAT) was kinetically measured at 240 nm.[22] CAT activity was defined as the amount of enzyme required to decompose 1 μM of H2O2/min. The enzyme activity was expressed as μM of H2O2 consumed/min/mg protein.
Statistical Analysis
Data were analyzed by using a commercially available statistics software package (SPSS for window V.7.5). Student's t test was performed and results were presented as mean ± S.E.M. Spearman's rank one tailed test was conducted to find out the significance of correlation between the pairs of related parameters.
Results
Ulcer index, a measure of lesion in gastric mucosa observed in all the ulcer models, is presented in Table 1. The lesions were characterized by multiple hemorrhagic red bands of different sizes along the long axis of the glandular stomach. Ulcer score was found to be significantly low in HAEAP-pretreated animals. Rats received 200 mg of HAEAP/kg b.wt showed an optimum reduction in ulcer index comparable to that of the standard drug ranitidine. Hence this concentration was chosen to evaluate the antiulcer activity of the test material by using ethanol ulcer model. The % protection was found to be dose dependent and optimum protection was observed at the concentration of 200 mg/kg b.wt in all the ulcer models studied.
Table 1.
Ulcer score in ulcerogen-treated animals
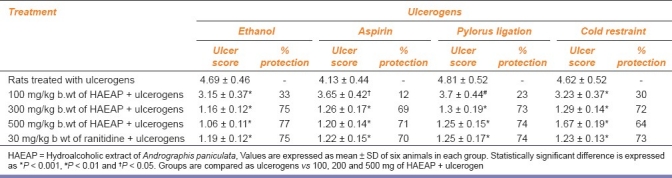
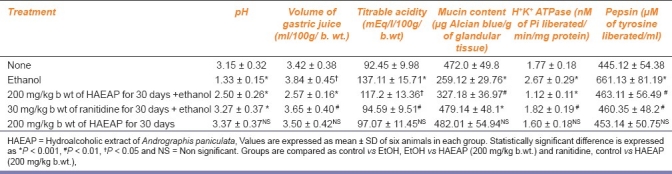
Table 2 shows the effect of HAEAP on pH, volume of gastric fluid, titrable acidity, mucin content, H+K+ATPase activity and pepsin concentration. Ulcer-induced rats pretreated with HAEAP showed significant increase in pH when compared to that of ulcerogen-treated control animals. The animals pretreated with the test drug showed a significant reduction in gastric volume and titrable acidity when compared to those which did not receive HAEAP. The mucin content was found to be depleted significantly in ulcer-induced control animals. The activity of H+K+ATPase and pepsin in ulcerated rats was found to be elevated and was significantly maintained in HAEAP-treated rats.
Table 2.
Effect of hydroalcoholic extract of Andrographis paniculata on several parameters in rats with ethanol-induced gastric ulcer
The levels of myeloperoxidase, lipid peroxidation products and GSH in gastric tissue of experimental animals are presented in Table 3. The levels of myeloperoxidase and lipid peroxidation products measured as TBARS were significantly increased in ulcer-induced rats with a significant decrease in GSH when compared with those of control rats.
Table 3.
Effect of hydroalcoholic extract of Andrographis paniculata on myeloperoxidase, thiobarbituric acid reacting substances and GSH in gastric tissue
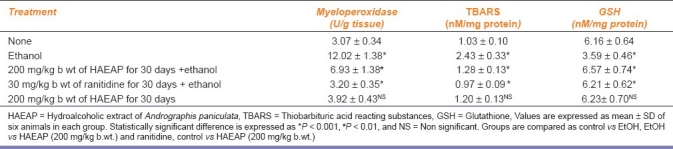
Table 4 shows the level of enzymatic antioxidants in gastric tissue. The activities of enzymatic antioxidants SOD, CAT, GPx were maintained in HAEAP pretreated rats. No significant change was registered in drug control rats.
Table 4.
Effect of hydroalcoholic extract of Andrographis paniculata on enzymatic antioxidants in gastric tissue
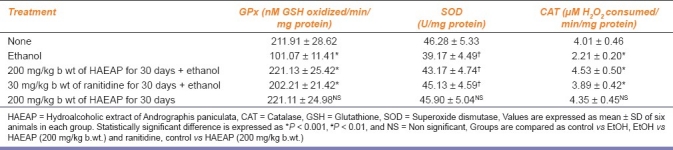
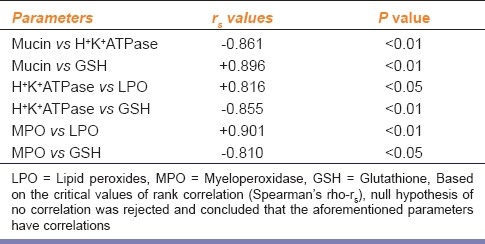
Table 5 shows the Spearman's rank correlation coefficient between the pairs of related parameters such as mucin-H+K+ATPase, mucin-GSH, H+K+ATPase- GSH, H+K+ATPase-LPO, MPO-LPO and MPO-GSH. A significant negative correlation was observed between mucin-H+ K+-ATPase, H+K+ATPase-GSH and MPO-GSH. The level of mucin was found to have positive correlation with GSH.
Table 5.
Spearman rank test conducted for related parameters
Discussion
The HAEAP reduced the ulcer score and inflammation significantly in all the models of gastric ulcer tested. Ethanol- and aspirin-induced ulcer have been used for the evaluation of new antiulcer drugs.[23] Pylorus ligation and cold stress models have also been the standard models of experimental ulcer induction as they cause human ulcer like conditions by enhancing pepsin secretion and reducing prostaglandins production respectively. By reducing the ulcer score, the test drug has proved itself a potent gastroprotective agent against ulcer of different etiology.
The pepsin reducing effect of HAEAP was found to be significant in ethanol-induced ulcer model. Ethanol induces pepsin secretion that causes digestion of endogenous protein in the mucous layer of gastric wall that precedes the inflammation. Ethanol also influences the secretion of hydrochloric acid by parietal cells and increases the severity of inflammation.[24] From the results of this investigation, it is evident that the HAEAP reduced the activity of H+K+ATPase, the enzyme involved in gastric acid secretion. Human ulcer has been shown to be associated with excess acid secretion due to upregulation of this enzyme.
The elevation in pH and decrease in acidity and volume of gastric fluid collected from test drug received rats indicate that HAEAP contains some active components which reduce the gastric acidity by down regulating the activity of acid secreting H+K+ATPase and pepsin. We have already reported that the HAEAP contains flavonoids such as quercitin, formononetin and biochin A and inhibits H+K+ATPase activity in vitro.[25]
Recent evidence has shown that pepsin may have a role in the etiology of gastric ulceration and cancer.[26] This suggests that inhibitors of acid secretion may prevent ulceration not only by the removal of acid but also by inactivation of pepsin following the subsequent rise in gastric pH. Therefore acid secretion may not have to be suppressed to prevent the development of gastric ulcers since the inhibition of pepsin activity alone may be sufficient to heal the ulcers and the side effects of suppressing acid secretion can be avoided. Proteolytic activity of pepsin as the primary aggressor in gastric mucosal ulceration has been reported. The pepsin activity is significantly reduced in HAEAP-pretreated rats. The reduced inflammation and ulcer score observed in the gastric mucosa of HAEAP-treated rats might be due to its suppressive action on pepsin activity.
Gastric H+K+ATPase, located in the apical membrane of parietal cells, pump proton into the gastric lumen using energy derived from the hydrolysis of ATP, and is thus involved in the gastric acid secretion. The HAEAP was found to inhibit the acid secreting enzyme H+K+ATPase. The H+K+ATPase is selectively inhibited by acid blockers and so used to treat gastric ulcers. Natural plant products derived from indegenous medicinal plants have been reported to alter the H+K+ATPase activity.[27] The inhibitory action on the enzyme is not abnormal and is confirmed by the effect of the drug in normal rats treated with the test drug alone. The role of enzymic gastric proton pump with H+K+ATPase activity is very crucial in ulcers of different etiology, irrespective of the root cause.[28] Therefore, blockade of H+K+ATPase has been considered and explored to design antiulcer drugs such as omeprazole, lansoprazole, etc.,
The mucus membrane of gastrointestinal tract contains rich amount of mucin, the protein involved in mucosal protection and the role of mucin in the pathology of gastrointestinal diseases has been reported.[29] The luminal surface with the covered mucin acts as a protective barrier against the brush luminal environment. The high molecular weight mucins are responsible for the viscoelastic properties of the mucous barrier. The major mucins present in gastrointestinal tract are mucin 2 and mucin 6 which form enormous network like covalent polymers and found to be bactericidal in action.[30] The level of mucin in gastric mucosa was found to be maintained significantly in test drug received rats against ethanol-induced depletion.
Myeloperoxidase (MPO) activity, a measure of leukocyte infiltration, was markedly elevated in ethanol-induced ulcer. MPO produces hypochlorus acid (HOCl) from H2O2 and chloride ions in neutrophils during respiratory burst. It also oxidizes tyrosine to tyrosyl radical using hydrogen peroxide as an oxidizing agent. HOCl and tyrosyl radicals are cytotoxic and so used by the neutrophil to kill bacteria and other pathogens. The enzyme has been shown to involve in the nitration of tyrosine residues observed in a wide variety of inflammatory disorders that involve neutrophils and macrophage activation. Role of nitric oxide related free radicals and the nitration of proteins has been implicated in the pathogenesis of human ulcer.[31] The rats pretreated with HAEAP showed reduced activity of MPO when compared to those without test drug treatment.
Free radicals contribute for the pathogenesis of human ulcer. Lipid peroxidation is an important reason for cell membrane damage. Malondialdehyde is the end product of lipid peroxidation and is a measure of lipid peroxidation level.[32] Increased MPO activity might have also contributed for the elevated formation of free radicals from neutrophils which are the major sources. Treatment with HAEAP significantly decreased the lipid peroxidation products in gastric tissue against ulcer control rats showing the antioxidant role of the test drug.
Natural food based antioxidants have been shown to delay or inhibit the onset of gastric ulcers and carcinoma. In the present study, ethanol-induced ulcer is shown to deplete the enzymatic antioxidants and glutathione in gastric tissues. The antioxidant levels are significantly maintained in test drug treated rats. Experimental alcoholism has been reported to cause both sympathetic and parasympathetic stimulation of stomach leading to local hypoxia and ischemia like condition to increase the level of free radicals which oxidize important cellular constituents such as structural and functional proteins.[33] Any form of chemical or physical stress results in decrease of prostaglandin synthase activity followed by decrease in prostaglandin biosynthesis, the major substance that offers gastroprotection against various insults to mucosa. The assay of prostaglandin E2 level may give more evidence for the gastroprotection action of the drug.
Superoxide dismutase (SOD) plays an important role in providing gastroprotection partially by preventing oxidative damage. SOD destroys the highly reactive radical superoxide (O2-) by converting it into the less reactive peroxide (H2O2) which can be destroyed by CAT reaction. CAT is a highly reactive enzyme that reacts with H2O2 to form water and molecular oxygen. The results of this study showed that HAEAP restored the activities of antioxidant enzymes of SOD and CAT.
Glutathione, a ubiquitous intracellular thiol present in the stomach tissue, offers gastroprotection against oxidative insult by various means. Gastric wall has high concentration of GSH that provides protection against oxidative damage induced by necrotizing agents, such as ethanol, acetic acid, carcinogens and NSAIDs as well as by ischemia reperfusion. The depletion of GSH in gastric tissue is associated with the increased risk of gastric injury.[34] GSH and related enzymes in tissues particularly glutathione peroxidase have been proposed as potential chemopreventive agents due to their antioxidant and detoxification properties.[35] In the present study, we have observed that treatment with the test drug maintains the level of GSH and GPx in the rat gastric tissue when compared to that of ulcer control rats.
The HAEAP pretreatment was found to maintain the levels of glutathione, SOD, GPx and catalase in gastric tissue for defense action. The test extract is found to contain flavonoids such as biochin A, quercetin and formononetin which are proved as potent antioxidants. Therefore, the biocomponents of the plant may be partly responsible for the antiulcer activity by preserving the mucin content and antioxidants in stomach.
Acknowledgments
The author Panneerselvam Saranya thanks Indian Council of Medical Research, New Delhi, India, for the financial support provided in the form of Senior Research Fellowship.
Footnotes
Source of Support: Indian Council of Medical Research, New Delhi, India, for the financial support provided in the form of Senior Research Fellowship.
Conflict of Interest: None declared.
References
- 1.Robert A. Cytoprotection by prostaglandins. Gastroenterology. 1979;77:761–7. [PubMed] [Google Scholar]
- 2.Vergin H, Kori-Lindner C. Putative mechanisms of cytoprotective effect of certain antacids and sucralfate. Dig Dis Sci. 1990;35:1320–7. doi: 10.1007/BF01536735. [DOI] [PubMed] [Google Scholar]
- 3.Prakash A, Faulds D. Rabeprazole. Drugs. 1998;55:261–7. doi: 10.2165/00003495-199855020-00009. [DOI] [PubMed] [Google Scholar]
- 4.Meena K, Sobhana S, Anatanandga MA. Pareek LK, Swarnakar PL, editors. Bioassay for antitumor compounds in callus extracts of Phyllanthus spp. And Euphorbia spp. Trends in plant tissue. 1988:33–35. [Google Scholar]
- 5.Tang W, Eisenbrand G. Chinese drugs of plant origin: Chemistry, pharmacology and use in traditional and modern medicine. Berlin: Springer Verlag; 1992. pp. 97–103. [Google Scholar]
- 6.Rajagopal S, Kumar RA, Deevi DS, Satyanarayana C, Rajagopalan R. Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. J Exp Ther Oncol. 2003;3:147–58. doi: 10.1046/j.1359-4117.2003.01090.x. [DOI] [PubMed] [Google Scholar]
- 7.Brzozowski T, Konturek PC, Konturek SJ, Kwiecien S, Pajdo R, Brzozowska I, et al. Involvement of endogenous cholecystokinin and somatostatin in gastroprotection induced by intraduodenal fat. J Clin Gastroenterol. 1998;27(Suppl 1):S125–37. doi: 10.1097/00004836-199800001-00020. [DOI] [PubMed] [Google Scholar]
- 8.Goel RK, Chakrabarti A, Sanyal AK. The effect of biological variables on the anti-ulcerogenic effect of vegetable plantain banana. Planta Med. 1985;2:85–8. doi: 10.1055/s-2007-969412. [DOI] [PubMed] [Google Scholar]
- 9.Sanyal AK, Pandey BL, Goel RK. The effect of cyproheptadine on gastric activity, an experimental study. In: Pfeiffer CJ, editor. Peptic ulcer. Copenhagen: Munksguard; 1971. pp. 312–8. [Google Scholar]
- 10.Senay EC, Levine RJ. Synergism between cold and restraint for rapid production of stress ulcer in rats. Proc Soc Exp Biol Med. 1967;124:1221–3. doi: 10.3181/00379727-124-31970. [DOI] [PubMed] [Google Scholar]
- 11.Nie SN, Qian XM, Wu XH, Yang SY, Tang WJ, Xu BH, et al. Role of TFF in healing of stress induced gastric lesions. World J Gastroenterol. 2003;9:1772–6. doi: 10.3748/wjg.v9.i8.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shay H, Komarov SA, Fels SS, Mreanze D, Gruenstein M, Siplet H. A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology. 1945;5:43–61. [Google Scholar]
- 13.Corne SJ, Morrissey SM, Woods RJ. Proceedings: A method for the quantitative estimation of gastric barrier mucus. J Physiol. 1974;242:116P–7. [PubMed] [Google Scholar]
- 14.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–9. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 15.Cheon HG, Lim H, Lee DH. Biochemical properties of a newly synthesized H+/K+ATPase inhibitor, 1-(2-methyl-4-methoxyphenyl)-4-[(3-hydroxypropyl) amino]-6-methyl-2, 3-dihydropyrrolo [3, 2-c} quinoline. Eur J Pharmacol. 2001;411:181–6. doi: 10.1016/s0014-2999(00)00919-5. [DOI] [PubMed] [Google Scholar]
- 16.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 17.Reyes-Chilpa R, Baggio CH, Alavez-Solano D, Estrada-Muniz E, Kauffman FC, Sanchezc RI, et al. Inhibition of gastric H+, K+-ATPase activity by flavonoids, coumarins and xanthones isolated from Mexican medicinal plants. J Ethnopharmacol. 2006;105:167–72. doi: 10.1016/j.jep.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–31. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 19.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 20.Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–21. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 21.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 22.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 23.Vasanthakumar M, Parameswari RP, Vijayakumar V, Sangeetha MK, Gayathri V, Balaji Raghavendran H, et al. Anti-ulcer role of herbomineral Siddha drug-Thamira parpam on experimentally induced gastric mucosal damage in rats. Hum Exp Toxicol. 2010;29:161–73. doi: 10.1177/0960327109357217. [DOI] [PubMed] [Google Scholar]
- 24.Dias PC, Foglio MA, Possenti A, de Carvalho JE. Antiulcerogenic activity of crude hydroalcoholic extract of Rosmarinus officinalis L. J Ethnopharmacol. 2000;69:57–62. doi: 10.1016/s0378-8741(99)00133-6. [DOI] [PubMed] [Google Scholar]
- 25.Saranya P, Geetha A, Narmadha Selvamathy SMK. The antioxidant and H+K+ATPase inhibitory effect of Andrographis paniculata and andrographolide-in vitro and in vivo studies. Pharmacologyonline. 2010;1:356–76. [Google Scholar]
- 26.Plebani M. Pepsinogens in health and disease. Crit Rev Clin Lab Sci. 1993;30:273–328. doi: 10.3109/10408369309084670. [DOI] [PubMed] [Google Scholar]
- 27.Sachs G, Chang HH, Rabon E, Schackman R, Lewin M, Saccomani GA. Non-electrogenic H+ pump in plasma membranes of hog stomach. J Biol Chem. 1976;251:7690–8. [PubMed] [Google Scholar]
- 28.Sachs G, Spenney JG, Lewin M. H+ transport: Regulation and mechanism in gastric mucosa and membrane vesicles. Physiol Rev. 1978;58:106–73. doi: 10.1152/physrev.1978.58.1.106. [DOI] [PubMed] [Google Scholar]
- 29.Longman RJ, Douthwaite J, Sylvester PA, Poulsom R, Corfield AP, Thomas MG, et al. Coordinated localization of mucins and trefoil peptides in the ulcer associated cell lineage and the gastrointestinal mucosa. Gut. 2000;47:792–800. doi: 10.1136/gut.47.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6:e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elliott SN, Wallace JL. Nitric oxide: A regulator of mucosal defense and injury. J Gastroenterol. 1998;33:792–803. doi: 10.1007/s005350050178. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen F, Mikkelsen BB, Nielson JB, Anderson HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin Chem. 1997;43:1209–14. [PubMed] [Google Scholar]
- 33.Chaturvedi A, Kumar MM, Bhawani G, Chaturvedi H, Kumar M, Goel RK. Effect of ethanolic extract of Eugenia jambolana seeds on gastric ulceration and secretion in rats. Indian J Physiol Pharmacol. 2007;51:131–40. [PubMed] [Google Scholar]
- 34.Robert A, Eberle D, Kaplowitz N. Role of glutathione in gastric mucosal cytoprotection. Am J Physiol Gastrointest Liver Physiol. 1984;247:G296–304. doi: 10.1152/ajpgi.1984.247.3.G296. [DOI] [PubMed] [Google Scholar]
- 35.Pohle T, Brzozowski T, Becker JC, Vander Voort IR, Markmann A, Konturek SJ, et al. Oxygen metabolities in aspirin-induced gastric damage in humans: Gastro protection by vitamin C. Aliment Pharmacol Ther. 2001;15:677–87. doi: 10.1046/j.1365-2036.2001.00975.x. [DOI] [PubMed] [Google Scholar]


