Abstract
Aim and Objectives:
In the present study, we have evaluated the antihyperglycemic, hypolipidemic and antioxidant activities of aqueous extract of Phyllanthus amarus (PAAEt) in streptozotocin (STZ)-induced diabetic rats.
Materials and Methods:
PAAEt was administered at 200 mg/kg body weight/day to normal treated (NT-group) and STZ-induced diabetic treated rats (DT-group) by gavage for eight weeks. During the experimental period, blood was collected from fasted rats at 10 days intervals and plasma glucose level was estimated. The plasma lipid profile was estimated at the end of experimental period. After the treatment, period kidney lipid peroxidation (LPO), protein oxidation and reduced glutathione (GSH) were estimated and antioxidant enzymes viz., glutathione reductase (GR), glutathione peroxidase (GPx) and glutathione-S-transferase (GST), catalase (CAT) and superoxide dismutase (SOD) were also assayed.
Results:
The significant decrease in the body weight, hyperglycemia and hyperlipidemia observed in STZ-induced diabetic rats (D-group) were rectified with PAAEt treatment in diabetic treated group (DT-group). D-group rats showed increased renal oxidative stress with increased LPO and protein oxidation. DT-group showed a significant decrease in renal LPO, protein oxidation and a significant increase in GSH content and GR, GPx and GST activities when compared with D-group. The activities of SOD and CAT decreased significantly in D-group, but were normalized in DT-group. Normal rats treated with PAAEt (NT-rats) showed a significant decrease in lipid profile, renal LPO and protein oxidation, with significant increase in renal GSH and activities of antioxidant enzymes compared to normal rats (N-group).
Conclusion:
Our results demonstrated that PAAEt with its antidiabetic, hypolipidemic and antioxidant properties could be a potential herbal medicine in treating diabetes and renal problems.
Keywords: Antioxidant enzymes, oxidative stress, Phyllanthus amarus, STZ-induced diabetes
Introduction
Diabetes mellitus (DM) is characterized by chronic hyperglycemia, resulting from defects in insulin secretion, action, or both, leading to disturbance in carbohydrate, lipid and protein metabolism. There is increasing evidence that complications (macro and microvascular) related to diabetes are associated with oxidative stress, induced by the generation of free radicals.[1] Prolonged hyperglycemia in uncontrolled DM leads to glucose oxidation, which is believed to be the main source of free radicals.[2] Diabetic nephropathy is the chief cause of morbidity and premature mortality in patients with insulin-dependent DM.[3] Since nephropathy among several microvascular disorders could be improved by antioxidants, treatment[4] compounds with both antihyperglycemic and antioxidative properties would be useful antidiabetic agents. Renewed attention in recent decades to alternative medicines and natural therapies has stimulated a new way of research interest in traditional practices. The plant kingdom has become a target for the search for new drugs and biologically active compounds. Many plant extracts and plant products have been shown to possess significant antioxidant activity, which may be an important property of medicinal plants.
Phyllanthus amarus (Euphorbiaceae) can be found in all tropical regions of the world including the Southern India and China. In many countries around the world, plants of genus Phyllanthus are used in folk remedies; therefore, this genus is of great importance in traditional medicine. Phyllanthus amarus (P. amarus) has been claimed to be an excellent remedy for infective hepatitis.[5] Extract of P. amarus exhibited protective action against carbon tetrachloride (CCl4) induced mitochondrial dysfunction.[6] Methanolic extract of the leaf of Phyllanthus amarus showed hepato protection against ethanol induced oxidative stress,[7] alloxan[8] and cyclophosphamide-induced oxidative stress in rats.[9] P. amarus extract also possessed anti-tumor, anti-carcinogenic[10] and anti-inflammatory properties.[11] Methanol extract of P. amarus was reported to have hypoglycemic effect on alloxan induced diabetes mellitus.[8]
Though experimental studies indicated hepatoprotective role of P. amarus against oxidative stress in experimental animals, its nephroprotective effect under diabetic conditions has not yet been evaluated. The present study was, therefore undertaken for systematic evaluation of PAAEt for its antidiabetic, hypolipidemic and antioxidant potential in the kidney of STZ-induced diabetic rats.
Materials and Methods
Chemicals
Chemicals used in the current study were procured from Sigma Chemical Co. (St. Louis, MO, USA), Koch-Light Laboratories (Huntingdon, Cambridgeshire, England), and SISCO Research Laboratories (Maharashtra, India).
Plant Material
An aqueous extract of P. amarus (whole plant) (PAAEt; brown, in the form of with batch No. L201121) was purchased from Chemiloids (manufacturers and exporters of herbal extracts), Vijayawada, Andhra Pradesh (India). Prior to preparation of the plant extract, the plan was authenticated by Dr. K. Narasimha Reddy, Taxonomist, Laila impex R and D Center, Vijayawada. Herb-to-product ratio was 10:1. The extract was stored at 0-4°C and dissolved in water just before use.
Induction of Diabetes to Experimental Animals
Two to 2 1/2-months-old male albino Wistar rats of body weight 130 - 150 g were procured from Sri Venkateswara Enterprises (Bangalore, India), acclimatized for 7 days to our animal house, and maintained at standard conditions of temperature and relative humidity, with a 12 hour light/dark cycle. Water and commercial rat feed were provided ad libitum. All the procedures were performed in accordance with the Institutional Animal Ethics Committee. Diabetes was induced in overnight fasted rats by a single intraperitoneal (i.p) injection of freshly prepared STZ (55 mg/kg body weight, in ice-cold 0.1 M citrate buffer, pH 4.5, in a volume of 0.1 ml per rat). Seventy two hours after STZ administration, the plasma glucose level of each rat was determined for confirmation of diabetes. Rats with plasma glucose level above 300 mg/dl were considered as a diabetic and used subsequently for further study.
Experimental Design and Biochemical Analysis
In the present experiment, a total of 32 rats (16 diabetic surviving rats; 16 normal rats) were used. The rats were divided in to four groups of 8 each: normal (N-group); normal rats treated with PAAEt (NT-group); diabetic rats untreated (D-group) and diabetic rats treated with PAAEt (DT-group). Groups-DT, NT rats received PAAEt (200 mg/kg body weight) in 2 ml distilled water daily through oral intubation, whereas, 2 ml of distilled water was administered to N and D-rats. Based on preliminary experiments on dose-dependent antihyperglycemic effect of plant extract, a dose less than 200 mg/kg body weight was not found to be effective in rats. Body weight was monitored at 10 days intervals. During the experimental period blood, was collected from 12 hour fasted rats by means of capillary tube through orbital sinus at 10 day intervals. Plasma glucose was estimated by glucose oxidase-peroxidase (GOD-POD) method by using the Span diagnostic kit (Span diagnostic Ltd., Surat, India). Triglycerides, total cholesterol (TC) and HDL-cholesterol (HDL-C) were measured by using Span diagnostic reagent kit. Very-low-density lipoprotein (VLDL) was calculated using formula High triglyceride (TG)/5.[12] Low-density lipoprotein (LDL) concentration (mg/dl) was estimated indirectly from the measured levels of TG, HDL, and TC using equation LDL = TC - (VLDL+HDL).[12] Atherogenic index was calculated by the following formula: Atherogenic index = TC/HDL-C.[13]
At the end of the experimental period (8 weeks), the animals from each experimental group were starved for 16 hours and sacrificed by cervical dislocation. The supernatant obtained from centrifugation of 10% kidney homogenate in 0.15 M potassium chloride (KCl) was used for antioxidant enzyme assays and for estimation of GSH, LPO and protein oxidation. Reduced glutathione was estimated by Ellman method.[14] The extent of LPO was determined by the method of Utley et al.[15] Protein carbonyl content (protein oxidation) was evaluated by the method of Levine et al.[16] Renal GR (E.C 1.6.4.2), GST (E.C 2.5.1.18), GPx (E.C 1.11.1.9), CAT (E.C 1.11.1.6) and SOD (E.C 1.15.1.1) were assayed by following the methods of Pinto and Bartley,[17] Habig et al,[18] Rotruck et al,[19] Beers et al[20] and Soon and Tan[21] respectively. Protein was estimated by the method of Lowery et al,[22] using bovine serum albumin as standard.
Statistical Analysis
The results were expressed as means ± standard error (S.E.) Data were analyzed for significant differences using Duncan's multiple range (DMR) test. (P < 0.05).
Results
Characteristic symptoms of diabetes such as loss of body weight, polyphagia, polydipsia and polyuria were observed in the D-group. By the end of experimental period of 8 weeks, D-group showed a 14.2% reduction in body weight, whereas N, NT and DT-groups showed an increase in body weight by 60.1%, 90.1% and 38.7%, respectively [Figure 1a]. However, DT-group showed a significant increase (61.2%) in body weight compared to D-group during the corresponding period.
Figure 1.
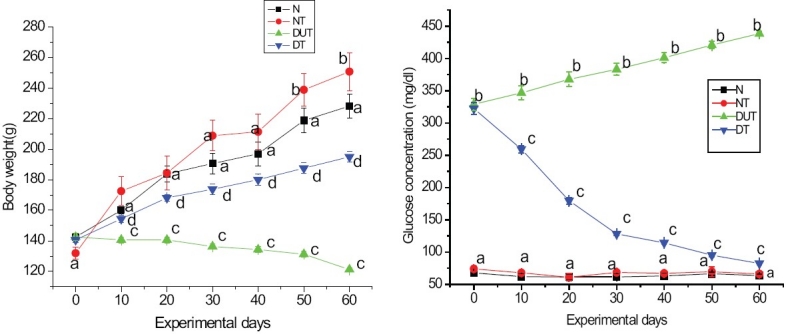
Mean body weight (a) and plasma glucose (b) of Normal (N), Normal treated (NT), Diabetic untreated (DUT) and Diabetic treated (DT) groups during the experimental period. Values are mean ± SE of 8 rats in each group. Values with different superscript letter are significantly different from each other at P <0.05
Data on plasma glucose content of four experimental groups are represented in Figure 1b. N and NT-rats remained persistently euglycemic throughout the experimental period. In the D-rats, plasma glucose level gradually increased during the experimental period from 328.86 ± 9.70 mg/dl to 438.45 ± 2.20 mg/dl. A significant antihyperglycemic effect was evident in DT-rats from 10 days onwards and the decrease in plasma glucose was 74.5% by 60 days of treatment. Thus, the per cent decrease in plasma glucose was 19.6, 24.8, 15.9, 4.5, 6 and 3.9 at 10, 20, 30, 40, 50 and 60 days, respectively.
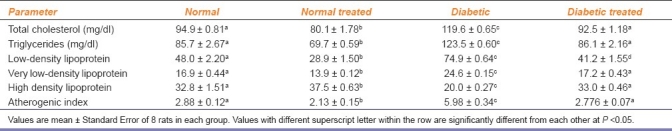
A significant increase in serum TC (26.1%), triglycerides (44.1%), LDL-C (56.0%) and VLDL-C (46.0%) and a significant decrease in HDL-C (39.1%) in D-rats compared to N-rats resulted in a significant increase in atherogenic index (100.7%). A significant decrease in serum TC (15.5%), triglycerides (18.6%), LDL-C (39.7%), VLDL-C (17.3%) and a significant increase in HDL-C concentration (14.1%) in NT-rats compared to N-rats resulted in a significant decrease in atherogenic index (26.04%). DT-rats showed a significant decrease in serum TC (23.50%), triglycerides (30.25%), LDL-C (45.0%), VLDL-C (30.14%) and atherogenic index (53.57%) and a significant increase in HDL-C concentration (65%) when compared with D-rats. Thus, PAAEt treatment resulted in restoration of lipid profile in DT-rats to normal values [Table 1].
Table 1.
Effect of Phyllanthus amarus treatment on serum lipid profile of normal and diabetic treated rats
Kidney Reduced Glutathione (GSH), Lipid peroxidation and Protein oxidation
The relative kidney weight (mg/g body weight) of D-rats was found to be significantly more (4.72 ± 0.02) and was almost the same (4.40 ± 0.06) in NT-rats when compared with N-rats (4.42 ± 0.04). DT-rats, however, showed a significant decrease in relative kidney weight (4.46 ± 0.05) compared to D-rats with no significant change from N-rats.
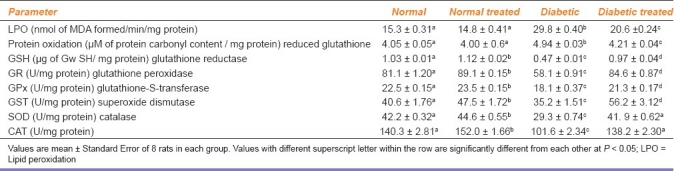
Normal rats treated with PAAEt (NT-group) showed a significant increase in renal concentration of GSH (8.2%), whereas, DUT rats and DT rats showed a significant decrease in the renal GSH (29.4% and 4.9% respectively) when compared with N-rats. However, DT-rats showed a significant increase in GSH level (32.1%) when compared with D-rats. No significant change in the extent of renal LPO and protein oxidation were observed in NT-rats compared to N-rats. D-rats and DT-rats showed a significant increase in extent of LPO (93.8% and 34.4%) and protein oxidation (21.8% and 3.9%), respectively when compared with N-rats. On the other hand, DT- rats showed a significant decrease in LPO (30.4%) and protein oxidation (14.7%) as compared to D-rats [Table 2].
Table 2.
Effect of Phyllanthus amarus treatment on renal LPO, protein oxidation, reduced glutathione (GSH) and antioxidant enzyme activities of normal and diabetic treated rats during the study
Antioxidant Enzymes
The activities of renal antioxidant enzymes in four experimental groups are presented in Table 2. The activities of renal GR (9.0%), GPx (4.2%), GST (17%), SOD (5.831%) and CAT (8.3%) were found to be increased significantly in NT-group than those in N-group, while the activities of these enzymes were significantly reduced (28.5%, 19.8%, 13.2%, 30.4%, and 27.6%, respectively) in D-group when compared with N-rats. DT-rats showed a pronounced activity of GPx as compared to D-rats, but this enzyme activity was still significantly lower than in N-rats. GST and GR activities of DT-rats were significantly greater than D-and N-rats. DT-rats showed a significant enhancement in SOD (43.2%) and CAT (34.1%) activities when compared to D-rats and restored later to normal level.
Discussion
As expected, STZ-induced D-rats showed the characteristic signs of diabetes such as polyphagia, polydipsia, polyuria, failure to gain body weight, hyperglycemia and hyperlipidemia. Inspite of the increased food consumption, the observed loss of body weight [Figure 1a] could be due to the defect in glucose metabolism and excessive breakdown of tissue protein which is a characteristic condition of diabetes.[23] In DT-group, ingestion of PAAEt effectively prevented these diabetic symptoms, indicating the antidiabetogenic action of this extract. No visible side effects of PAAEt treatment were observed in NT-group, representing the non-toxic nature of PAAEt.
The plasma glucose levels observed in NT and DT-groups during the experimental period clearly indicates that PAAEt does not promote hypoglycemic activity, but exerts antihyperglycemic effect. Chronic treatment of diabetic rats for a 2 month period with PAAEt lowered the plasma glucose level to the near normal levels. The most common lipid abnormalities of diabetes like hypertriglyceridemea, hypercholesterolemea, with enhanced serum LDL and VLDL, and decreased HDL concentration with increased atherogenic index are evident in D-group. High blood cholesterol, triglycerides with increased atherogenic index are important risk factors for cardiovascular diseases.[24] A significant alteration in the lipid profile of NT-rats compared to N-rats indicates the beneficial effect of PAAEt against cardiovascular diseases. Lipid abnormalities under diabetic conditions may be due to the unlimited action of lipolytic hormones on the fat deposits. Under normal circumstances insulin activates the enzyme lipoprotein lipase which hydrolyses triacylglycerols. However, in diabetes, lipoprotein lipase is not activated due to insulin deficiency, resulting in hypertriglyceridemea.[25] PAAEt treatment in DT-rats showed antihyperlipidemic effect by restoring the serum lipid profile to near normal values. Lowering of cholesterol and raise in HDL cholesterol are the desirable biochemical states for prevention of atherosclerosis and ischemic conditions.[24]
Kidneys are dynamic organs and represent the major control system in maintaining the body homeostasis. Kidneys exhibited morphological, biochemical and functional alterations during diabetes. However, treatment with PAAEt for 2 months in DT-group completely prevented the increase in relative kidney weight. Indices of oxidative stress like enhanced LPO, protein oxidation and decreased GSH content observed in the kidney of D-rats, confirms the existence of oxidative stress under diabetic conditions. Enhanced LPO may also play a potential role in progressive reduction in glomerular filtration rate. Similar trend of diminished levels of renal GSH was reported by earlier workers on experimental diabetic rats.[26] It appears that generation of oxygen radicals by hyperglycemia causes utilization of GSH and thus diminishes plasma GSH levels of DUT rats. The decreased renal GSH content of DUT rats increased the sensitivity of this organ to oxidative injury and may play a role in the development of diabetic nephropathy.[26] The significant decrease in the extent of LPO and protein oxidation with significant rise in renal GSH content in DT rats compared to DUT rats indicates the protective effect of PAAEt against STZ-induced diabetic oxidative stress. The significant enhancement in renal GSH content of DT rats may be a factor responsible for observed inhibition of LPO and protein oxidation through GSH redox cycle and also direct detoxification of reactive oxygen species (ROS) generated. The enhanced renal GSH content of NT compared to N-rats indicated increased reserves of free radical scavengers by PAAEt treatment.[27]
Peroxides produced in a cell can be detoxified by the action of GPx and CAT. GPx is specific for its hydrogen donor GSH, and non-specific for the hydroperoxides ranging from H2O2 to organic peroxides, thus offering a major defense role in cells against peroxidative damage of complex biochemical compounds such as lipids and nucleic acids. GST is a multifunctional protein with a broad specificity for organic hydroperoxides but not for H2O2. It also plays an important role in detoxification of xenobiotic compounds thereby protecting the cell from peroxidative damage.[28] The significant reduction in the activities of GR, GPx and GST of D-group compared to N-group reflects the enhanced oxidative stress under diabetic condition. However, in the available literature the response of antioxidant enzymes in diabetes have been unclear. Diabetes has been reported to be associated with either increased,[29] decreased[30] or unchanged[31] activities of antioxidant enzymes in various tissues. The decreased activity of GR in D-rats may lead to decreased generation of GSH from oxidized glutathione (GSSG). The decreased levels of renal GSH noticed in D-rats may be one of the factors for the decreased activities of GPx and GST. Thus, the decreased activities of renal GPx and GST of D-rats may be responsible for elevated renal LPO observed in this group of animals. Administration of PAAEt to DT-rats resulted in increased activities of renal GR, GPx and GST compared to D-rats, indicating the protective effect of PAAEt treatment against oxidative damage observed in STZ-induced diabetes.
Superoxide dismutase is an important defense enzyme that catalyses the dismutation of superoxide radicals.[32] The superoxide anion is known to inactivate CAT, which is a hemoprotein that catalyses the reduction of hydrogen peroxides and protects the tissues from highly reactive hydroxyl radicals.[33] The observed reduction in the activity of these enzymes in D-group may result in a number of deleterious effects due to the accumulation of superoxide radicals and H2O2. In DT-group chronic administration of PAAEt resulted in restoration of these enzyme activities to the normal level. Increased activities of renal GR, GRx, GST, SOD and CAT in NT-rats compared to N-rats indicate an improved antioxidant status of NT-rats by chronic ingestion of PAAEt.
Conclusion
The results suggest that the PAAEt not only possesses antihyperglycemic and hypolipidemic properties, but also reduces diabetic induced renal oxidative stress. This study indicates that the PAAEt is useful for diabetes and helps to prevent or delay the onset of diabetic complications such as diabetic nephropathy. Further studies are warranted to identify the active antihyperglycemic compounds and investigate the mechanism of antihyperglycemic and antioxidant actions of the PAAEt.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Jakus V. The role of free radicals, oxidative stress antioxidant systems in diabetic vascular disease. Bratisl Lek Listy. 2000;101:541–51. [PubMed] [Google Scholar]
- 2.King GL, Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol. 2004;122:333–8. doi: 10.1007/s00418-004-0678-9. [DOI] [PubMed] [Google Scholar]
- 3.Baynes JW. Perspectives in diabetes, role of oxidative stress on development of complications in diabetes. Diabetes. 1991;40:405–12. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 4.Kedziora-Komatowsk K, Szram S, Komtowski T, Szadujkis-Szadurski L, Kedziora J, Bartosz G. Effect of vitamin E and vitamin C supplementation on oxidative state and renal glomerular basement membrane thickness in diabetic kidney. Nephron Exp Nephrol. 2003;95:134–43. doi: 10.1159/000074840. [DOI] [PubMed] [Google Scholar]
- 5.Thyagarajan SP, Subramanian S, Thirunalasundari T, Venkateswaran PS, Blumberg BS. Effect of Phyllanthus amarus on chronic carriers of hepatitis B virus. Lancet. 1988;2:764–6. doi: 10.1016/s0140-6736(88)92416-6. [DOI] [PubMed] [Google Scholar]
- 6.Padma P, Setty OH. Protective effect of Phyllanthus against CCL4-induced mitochondrial dysfunction. Life Sci. 1999;64:2411–7. doi: 10.1016/s0024-3205(99)00195-2. [DOI] [PubMed] [Google Scholar]
- 7.Toyin YF, Stephen M, Michael AF, Udoka EO. Hepato protective potential of Phyllanthus amarus against ethanol-induced oxidative stress in rats. Food Chem Toxicol. 2008;46:2658–64. doi: 10.1016/j.fct.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 8.Raphael KR, Sabu MC, Kuttan R. Hypoglycemic effect of methanol extract of Phyllanthus amarus Schum and Thonn on alloxan induced diabetes mellitus in rats and its relation with antioxidant potential. Indian j Exp Biol. 2002;40:905–9. [PubMed] [Google Scholar]
- 9.Kumar KB, Kuttan R. Chemoprotective activity of an extract of Phyllanthus amarus against cyclophosphamide induced toxicity in mice. Phytomed. 2005;12:494–500. doi: 10.1016/j.phymed.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Rajeshkumar NV, Joy KL, Kuttan G, Ramsewak RS, Nair MG, Kuttan R. Antitumour and anticarcinogenic activity of Phyllanthus amarus extract. J Ethnoparmacol. 2002;81:17–22. doi: 10.1016/s0378-8741(01)00419-6. [DOI] [PubMed] [Google Scholar]
- 11.Kiemer AK, Hartung T, Huber C, Vollmar AM. Phyllanthus amarus has anti-inflammatory potential by inhibition of iNOS, COX-2, and cytokines via the NF-κB pathway. J Hepatol. 2003;38:289–97. doi: 10.1016/s0168-8278(02)00417-8. [DOI] [PubMed] [Google Scholar]
- 12.Friedwalds WT, Levy RT, Friedickson DS. Estimation of concentration of low density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 13.Boers M, Nurmohamed M, Doelman C, Lard L, Verhoeven A, Voskuyl A, et al. Influence of glucocorticoids and disease activity on total and high density lipoprotein cholesterol in patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:842–45. doi: 10.1136/ard.62.9.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellmans GL. Tissue sulphydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 15.Utley HG, Bernheim F, Hochstein P. Effect of sulfhydryl reagents on peroxidation in microsomes. Arch Biochem Biophys. 1967;118:29–32. [Google Scholar]
- 16.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, et al. Determation of carbonyl content in oxidatively modified proteins. Met Enzymol. 1990;186:464–78. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 17.Pinto RE, Bartley W. The effect of age and sex on glutathione reductase and glutathione peroxidasea activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem J. 1969;112:109–15. doi: 10.1042/bj1120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases: The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 19.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 20.Beers R, Jr, Sizer JW. Spectrophotometric method for measuring breakdown of H2O2 catalase. J Biol Chem. 1952;195:133–40. [PubMed] [Google Scholar]
- 21.Soon YY, Tan BK. Evaluation of the hypoglycemic and Antioxidant activities of Morinda officinalis in streptozotocin-induced diabetic rats. Singapore Med J. 2002;43:77–85. [PubMed] [Google Scholar]
- 22.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin-Phenol reagent. J Biol Chem. 1951;193:265–70. [PubMed] [Google Scholar]
- 23.Swnaston-Flat SK, Day C, Bailey CJ, Flatt PR. Traditional plant treatment for diabetes: Studies in normal and STZ diabetic mice. Diabetologia. 1990;33:462–4. doi: 10.1007/BF00405106. [DOI] [PubMed] [Google Scholar]
- 24.Massing MW, Sueta CA, Chowdary M, Biggs DP, Jr, Simpson RJ. Lipid management among coronary artery disease patients in diabetes mellitus or advanced age. Am J Cadiol. 2001;87:646–64. doi: 10.1016/s0002-9149(00)01447-8. [DOI] [PubMed] [Google Scholar]
- 25.AI-Shmaony L, AI-Khazrajoi SM, Twaij HA. Hypoglycemic effect of Artemisia herba alba.Effect of a valuable extracts on some blood parameters in diabetic animals. J Ethanopharmacol. 1994;43:167–71. doi: 10.1016/0378-8741(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 26.Obrosova IG, Fathallah L, Liu E, Nourooz ZJ. Early oxidative stress in diabetic kidney: Effect of DL-α-lipoic acid. Free Radic Biol Med. 2003;34:186–95. doi: 10.1016/s0891-5849(02)01195-4. [DOI] [PubMed] [Google Scholar]
- 27.Meister A. Glutathione deficiency produced by inhibition of its synthesis, and its reversal: Application in research and therapy. Pharmacol Ther. 1991;51:155–94. doi: 10.1016/0163-7258(91)90076-x. [DOI] [PubMed] [Google Scholar]
- 28.Uday B, Dipak D, Ranajit K, Benerjee T. Reactive oxygen species: Oxidative damage and pathogenesis. Curr Sci. 1999;77:658–65. [Google Scholar]
- 29.Ndahinana J, Dorchy H, Vertongen F. Erythrocyte and plasma antioxidant activity in diabetes mellitus type 1. Presse Med. 1996;25:188–92. [PubMed] [Google Scholar]
- 30.Li XM. Protective effect of Lycium barbarum polysaccharides on streptozotocin-induced oxidative stress in rats. Int J Biol Macromole. 2007;40:461–5. doi: 10.1016/j.ijbiomac.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Faure P, Benhamou PY, Perard A, Halimi S, Roussel AM. Lipid peroxidation in insulin-dependent diabetic patients with early retina degenerative lesions: Effects of an oral zinc supplementation. Eur J Clin Nutr. 1995;49:282–8. [PubMed] [Google Scholar]
- 32.McCorod JM, Keele BB, Jr, Fridovich I. An enzyme based theory of obligate anaerobiosis; the physiological functions of superoxide dismutase. Proc Natl Acad Sci USA. 1976;68:1024–27. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chance B, Greenstein DS, Roughton RJ. The mechanism of catalase action-study state analysis. Arch Biochem Biophys. 1952;37:301–21. doi: 10.1016/0003-9861(52)90194-x. [DOI] [PubMed] [Google Scholar]


