Abstract
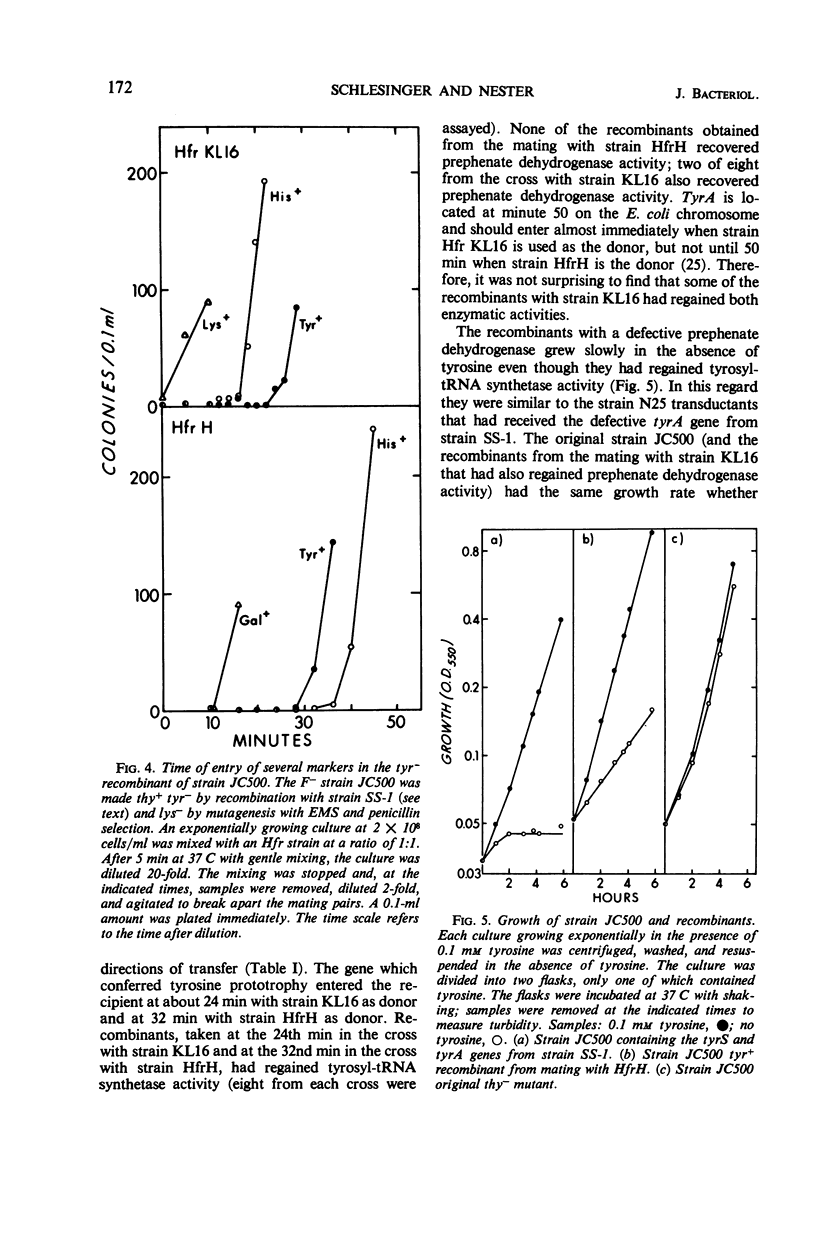
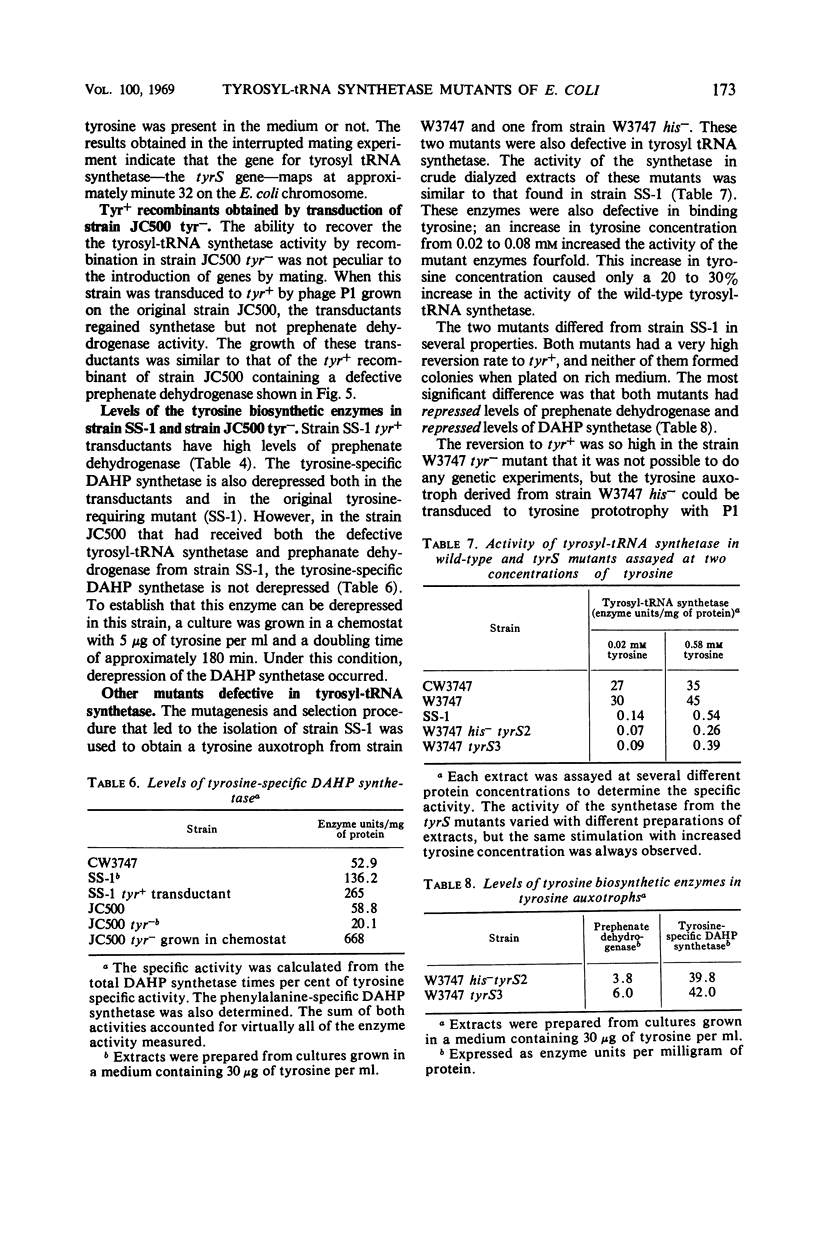
We have isolated several mutants defective in the gene for tyrosyl-transfer ribonucleic acid (tRNA) synthetase (tyrS). One of these mutants is described in detail. It was isolated as a tyrosine auxotroph with defects both in the tyrosyl-tRNA synthetase and in the tyrosine biosynthetic enzyme, prephenate dehydrogenase. It also had derepressed levels of the tyrosine-specific 3-deoxy-d-arabinoheptulosonic acid-7-phosphate (DAHP) synthetase. The latter finding suggested that a wild-type tyrS gene was required for repression of the tyrosine biosynthetic enzymes. The following results demonstrated that this hypothesis was not correct. (i) When the defective tyrS gene was transferred to another strain, the tyrosine-specific DAHP synthetase in that strain was not derepressed, and (ii) two other mutants with defective tyrosyl-tRNA synthetases had repressed levels of the tyrosine biosynthetic enzymes. The tyrS gene was located near minute 32 on the Escherichia coli chromosome by interrupted mating experiments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apirion D. Altered ribosomes in a suppressor strain of Escherichia coli. J Mol Biol. 1966 Apr;16(2):285–301. doi: 10.1016/s0022-2836(66)80173-0. [DOI] [PubMed] [Google Scholar]
- Apirion D. Three genes that affect Escherichia coli ribosomes. J Mol Biol. 1967 Dec 14;30(2):255–275. [PubMed] [Google Scholar]
- Calendar R., Berg P. Purification and physical characterization of tyrosyl ribonucleic acid synthetases from Escherichia coli and Bacillus subtilis. Biochemistry. 1966 May;5(5):1681–1690. doi: 10.1021/bi00869a033. [DOI] [PubMed] [Google Scholar]
- Calendar R., Berg P. The catalytic properties of tyrosyl ribonucleic acid synthetases from Escherichia coli and Bacillus subtilis. Biochemistry. 1966 May;5(5):1690–1695. doi: 10.1021/bi00869a034. [DOI] [PubMed] [Google Scholar]
- Doolittle W. F., Yanofsky C. Mutants of Escherichia coli with an altered tryptophanyl-transfer ribonucleic acid synthetase. J Bacteriol. 1968 Apr;95(4):1283–1294. doi: 10.1128/jb.95.4.1283-1294.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECHOLS H., GAREN A., GAREN S., TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. doi: 10.1016/s0022-2836(61)80055-7. [DOI] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. ROLE OF VALYL-SRNA SYNTHETASE IN ENZYME REPRESSION. Proc Natl Acad Sci U S A. 1965 Mar;53:539–543. doi: 10.1073/pnas.53.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundlich M. Valyl-Transfer RNA: Role in Repression of the Isoleucine-Valine Enzymes in Escherichia coli. Science. 1967 Aug 18;157(3790):823–825. doi: 10.1126/science.157.3790.823-a. [DOI] [PubMed] [Google Scholar]
- GORINI L., KAUFMAN H. Selecting bacterial mutants by the penicillin method. Science. 1960 Feb 26;131(3400):604–605. doi: 10.1126/science.131.3400.604. [DOI] [PubMed] [Google Scholar]
- Groves W. E., Davis F. C., Jr, Sells B. H. Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Anal Biochem. 1968 Feb;22(2):195–210. doi: 10.1016/0003-2697(68)90307-2. [DOI] [PubMed] [Google Scholar]
- Hirshfield I. N., Horn P. C., Hopwood D. A., Maas W. K., DeDeken R. Studies on the mechanism of repression of arginine biosynthesis in Escherichia coli. 3. Repression of enzymes of arginine biosynthesis in arginyl-tRNA synthetase mutants. J Mol Biol. 1968 Jul 14;35(1):83–93. doi: 10.1016/s0022-2836(68)80038-5. [DOI] [PubMed] [Google Scholar]
- JENSEN R. A., NESTER E. W. THE REGULATORY SIGNIFICANCE OF INTERMEDIARY METABOLITES: CONTROL OF AROMATIC ACID BIOSYNTHESIS BY FEEDBACK INHIBITION IN BACILLUS SUBTILIS. J Mol Biol. 1965 Jun;12:468–481. doi: 10.1016/s0022-2836(65)80270-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUBIN M. Enrichment of auxotrophic mutant populations by recycling. J Bacteriol. 1962 Mar;83:696–697. doi: 10.1128/jb.83.3.696-697.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass G. Regulation of histidine biosynthetic enzymes in a mutant of Escherichia coli with an altered histidyl-tRNA synthetase. Mol Gen Genet. 1967;100(2):216–224. doi: 10.1007/BF00333608. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C. Roles of amino acid activating enzymes in cellular physiology. Bacteriol Rev. 1966 Dec;30(4):701–719. doi: 10.1128/br.30.4.701-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel J. M., White M. N., Shive W. Activation of tyrosine analogs in relation to enzyme repression. Biochem Biophys Res Commun. 1965 Jul 26;20(3):352–359. doi: 10.1016/0006-291x(65)90372-4. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium II. Histidine regulatory mutants having altered histidyl-tRNA synthetase. J Mol Biol. 1966 Dec 28;22(2):325–333. doi: 10.1016/0022-2836(66)90135-5. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER S., MAGASANIK B. EFFECT OF ALPHA-METHYLHISTIDINE ON THE CONTROL OF HISTIDINE SYNTHESIS. J Mol Biol. 1964 Sep;9:670–682. doi: 10.1016/s0022-2836(64)80174-1. [DOI] [PubMed] [Google Scholar]
- SCHWINCK I., ADAMS E. Aromatic biosynthesis. XVI. Aromatization of prephenic acid to p-hydroxyphenylpyruvic acid, a step in tyrosine biosynthesis in Escherichia coli. Biochim Biophys Acta. 1959 Nov;36:102–117. doi: 10.1016/0006-3002(59)90074-5. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Fink G. R., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium 3. A class of regulatory mutants deficient in tRNA for histidine. J Mol Biol. 1966 Dec 28;22(2):335–347. doi: 10.1016/0022-2836(66)90136-7. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai A., Szentirmai M., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XV. Biochemical properties of mutants resistant to thiaisoleucine. J Bacteriol. 1968 May;95(5):1672–1679. doi: 10.1128/jb.95.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Regulator gene controlling enzymes concerned in tyrosine biosynthesis in Escherichia coli. J Bacteriol. 1969 Mar;97(3):1234–1241. doi: 10.1128/jb.97.3.1234-1241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


