Abstract
Objective
Intracerebral hemorrhage (ICH) is associated with significant patient morbidity and mortality. Acute hematoma enlargement is an important predictor of neurological injury and poor clinical prognosis; however, neurosurgical clot evacuation may not be feasible in all patients and treatment options remain largely supportive. Thus, novel therapeutic approaches to promote hematoma resolution are needed. In the present study, we investigated whether the curry spice, curcumin, limited neurovascular injury following ICH in mice.
Methods
ICH was induced in adult male CD-1 mice by the intracerebral administration of collagenase or autologous blood. Clinically-relevant doses of curcumin (75–300 mg/kg) were administered up to 6 hours after ICH and hematoma volume, inflammatory gene expression, blood-brain barrier permeability, and brain edema were assessed over the first 72 hours. Neurological assessments were performed to correlate neurovascular protection with functional outcomes.
Results
Curcumin increased hematoma resolution at 72 hours post-ICH. This effect was associated with a significant reduction in the expression of the pro-inflammatory mediators, tumor necrosis factor-α, interleukin-6, and interleukin-1β. Curcumin also reduced disruption of the blood-brain barrier and attenuated the formation of vasogenic edema following ICH. Consistent with the reduction in neuroinflammation and neurovascular injury, curcumin significantly improved neurological outcome scores after ICH.
Conclusion
Curcumin promoted hematoma resolution and limited neurological injury following ICH. These data may indicate clinical utility for curcumin as an adjunct therapy to reduce brain injury and improve patient outcome.
Keywords: Hemorrhagic stroke, vasogenic edema, blood-brain barrier, hematoma
INTRODUCTION
Intracerebral hemorrhage (ICH) induces 50–60% mortality within the first year and is associated with long-term disability in many survivors4,33. Primary ICH may be caused by the rupture of small vessels damaged by chronic hypertension or amyloid angiopathy. This can lead to the accumulation of erythrocytes within the parenchyma and the formation of a space-occupying hematoma. Hematoma volume directly correlates with neurological outcome10, supporting clot evacuation as a strategy to attenuate brain injury and improve patient prognosis. Unfortunately, a limited number of suitable surgical candidates and/or unfavorable size/location of the mass lesion may restrict the utility of neurosurgical intervention. As such, treatment options remain largely supportive, reinforcing the notion that ICH is the least treatable form of stroke and stressing the need for novel therapeutic approaches.
Acute hematoma enlargement, an independent determinant of both mortality and functional outcome after ICH, is clinically associated with neurovascular damage and increased patient mortality8,10,18,23,33. Early phase predictive markers of acute hematoma growth remain poorly defined; however, an inflammatory reaction correlated with hematoma growth in pre-clinical injury models and with neurological deterioration in ICH patients26,31. Activation of the pro-inflammatory transcription factor, NFκB, increased the expression of inflammatory mediators associated with cell death, increased blood-brain barrier (BBB) permeability, and induced the development of vasogenic edema after experimental ICH20,38,42,48. Along these lines, interleukin-6 (IL-6) is an independent predictor of hematoma enlargement31, and both tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) increase BBB permeability and promote vasogenic edema after brain injury5,16,21,29,45. Thus, a reduction in inflammatory activation may limit neurovascular injury and improve clinical outcomes following ICH.
Recent work by our laboratory demonstrated curcumin, a low molecular weight curry spice derived from the Curcuma longa, reduced vascular inflammation and acute injury after subarachnoid hemorrhage (SAH) and traumatic brain injury (TBI) via a reduction in NFκB activity24,42. Curcumin has been safely consumed by humans for centuries, including use as an anti-inflammatory agent in Ayurveda, an ancient Indian system of medicine19,36. Recent clinical trials also demonstrated that oral administration of curcumin resulted in bioactivity with minimal adverse effects, even when administered at high doses6. Given the role for neuroinflammation in the development of secondary neurological injury, we hypothesized that curcumin may restrict neurological demise following ICH.
MATERIALS AND METHODS
ICH model
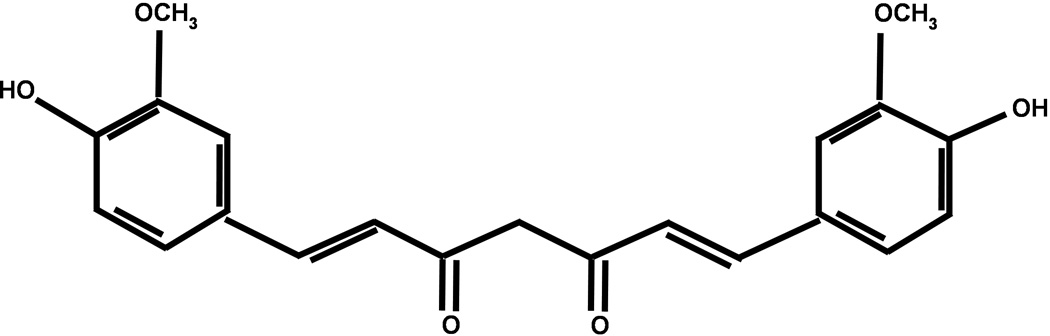
Animal studies were reviewed and approved by the Committee on Animal Use for Research and Education at the Medical College of Georgia, in compliance with NIH guidelines. Male CD-1 mice (8–10 weeks old; Charles River, Wilmington, MA, USA) were anesthetized with 8 mg/kg xylazine/60 mg/kg ketamine. Throughout all surgical procedures, body temperature was maintained at 37°C using a small-animal temperature controller (David Kopf Instruments, Tujunga, CA, USA). Mice were placed into a stereotactic frame and a 0.5 mm burr hole was formed 2.2 mm lateral to bregma using a high speed dental drill. A 26-gauge Hamilton syringe containing 0.04U of bacterial type IV collagenase in 0.5 µL saline was lowered 3 mm into the left striatum, as described previously3,9. A blood injection model of ICH was also tested, to confirm the effects observed in the collagenase model. Briefly, 30 µL of autologous tail blood was injected directly into the striatum via a 22 gauge Hamilton syringe, using the coordinates listed above, as detailed previously35. In both injury models, the syringe was depressed at a rate of 450 nL/min and remained in place for 10 minutes to prevent reflux. The burr hole was sealed with bone wax and the incision was surgically stapled. Curcumin (>98% purity; Acros Organics, Morris Plains, NJ; see Figure 1) was dissolved in corn oil (vehicle) and administered via intraperitoneal injection following randomization, prior to or up to 6 hours post-ICH. Sham animals underwent the same treatments (corn oil or curcumin) and surgical procedures, but only received a intracerebral saline (vehicle) injection. The surgeon was blinded to drug treatments throughout the experiment. Mice were maintained at 37°C until the recovery of the righting reflex.
Figure 1. Chemical structure of curcumin.
Hematoma assessment
Mice were intracardially perfused with saline and 2-mm coronal sections were immediately prepared. Anterior and posterior views of each brain section were digitized and a region of interest (ROI) was traced around the perimeter of the hematoma. Hematoma area was quantified by calculating the number of pixels within the ROI using Adobe Photoshop software. Hemoglobin content, a sensitive measure of hematoma volume, was quantified in the ipsilateral or contralateral cerebrum using a QuantiChrom Hemoglobin Assay kit (Bioassay Systems, Hayward, CA, USA)7,32.
Blood-brain barrier permeability
BBB permeability was quantified following administration of Evans blue (20 mg/mL in PBS, i.v.) 2h prior to sacrifice. Blood (100 µL) was obtained by cardiac puncture, centrifuged, and then the plasma was diluted in N, N-Dimethylformamide (1:1000). Following perfusion with saline, brains were weighed, solubilized in N,N-dimethylformamide, and then incubated at 78°C for 18h. Absorbance was then determined in brain and blood samples at 620 nm using a Synergy HT plate reader (Bio-Tek, Winooski, VT). The concentration of Evans blue (µg/µl) in each sample, a measure of BBB permeability, was calculated using a standard curve, and permeability was equal to [(Evans blue concentration of brain/weight of brain) / (Evans blue concentration of plasma/circulation time)], as reported previously47
Assessment of cerebral edema
Brain water content, an established measure of cerebral edema, was quantified in a 2 mm coronal tissue sections of the ipsilateral or corresponding contralateral striatum, as detailed by our laboratory and others11,37,42. Tissue was immediately weighed (wet weight), then dehydrated at 65°C. Samples were reweighed 48h later to obtain a dry weight. The percentage of water content in each sample was calculated as follows: % Brain water content = [((wet weight − dry weight)/wet weight)*100].
RNA isolation and qRT-PCR
Total RNA was isolated (SV RNA Isolation kit, Promega, Madison, WI, USA) and qRT-PCR performed on a Cepheid SmartCycler II (Cepheid, Sunnyvale, CA, USA) using a Superscript III Platinum SYBR Green One-Step qRT-PCR kit (Invitrogen, Carlsbad, CA, USA), as described by our laboratory25,42. Primers were as follows: IL-1β: (FP 5’-GCCCATCCTCTGTGACTCAT-3’; RP 5’-AGGCCACAGGTATTTTGTCG-3’), IL-6: (FP 5’-AGTTGCCTTCTTGGGACTGA-3’; RP 5’-TCCACGATTTCCCAGAGAAC-3’), TNF-α: (FP 5’-CGTCAGCCGATTTGCTATCT-3’; RP 5’-CGGACTCCGCAAAGTCTAAG-3’), and RPS3: (FP 5’-AATGAACCGAAGCACACCATA-3’; RP 5’-ATCAGAGAGTTGACCGCAGTT-3’). Product specificity was confirmed by melting curve analysis and visualization of a single, appropriately sized band on a 2% agarose gel. Gene expression levels were quantified using a cDNA standard curve12–14,25,34,42 and data were normalized to RPS3, a housekeeping gene that was unaffected by the experimental manipulations (data not shown). Data were expressed as fold change vs. sham.
Neurological outcomes
Neurological injury was determined using a modified 24-point scale, as detailed previously3,9. This scale was comprised of six behavioral tests, each of which were graded from 0 (performs with no impairment) to 4 (severe impairment). Prior to experimentation, mice were pre-tested and any mouse unable to climb without impairment was excluded from further study. A composite score was calculated as the sum of the grades on all six tests.
Statistical analysis
One-way analysis of variance (ANOVA) followed by Student Newman-Keuls or Two-way ANOVA followed by Bonferroni post-hoc test were used for multiple group comparisons and a t-test was used for two group comparisons, as indicated in the figure legends. Data are expressed as mean +/− SEM. A p value of <0.05 was considered to be significant.
RESULTS
Curcumin attenuates hematoma size and brain hemoglobin content after ICH
Administration of curcumin (150 mg/kg) significantly reduced gross hematoma size within the ipsilateral cortex following ICH. Notably, no beneficial effect was observed prior to 72h post-injury, indicating curcumin did not reduce collagenase activity or limit acute hematoma expansion (Figure 2a). Quantification of the hematoma at 72h post-injury indicated a significant reduction in the curcumin-treated group (10.6 ± 1.2 mm2), as compared to placebo-treated mice (16.1 ± 1.6 mm2, p<0.01, n=8/group) (Figure 2b) Conversely, significant differences in hematoma size were not observed at either the 24h or 48h timepoints. These changes mirrored the temporal pattern of hemoglobin content within the brain, a validated measure of hematoma volume. Specifically, acute administration of curcumin reduced hemoglobin content by 28%, as compared to placebo-treated mice (p<0.01 vs. ICH) using the collagenase ICH model (Figure 2c, n=8/group). Similarly, curcumin reduced hematoma volume by 42%, as compared to placebo-treated mice, using an autologous blood clot model of ICH (Figure 3).
Figure 2. Curcumin promotes hematoma resolution after collagenase-induced ICH.
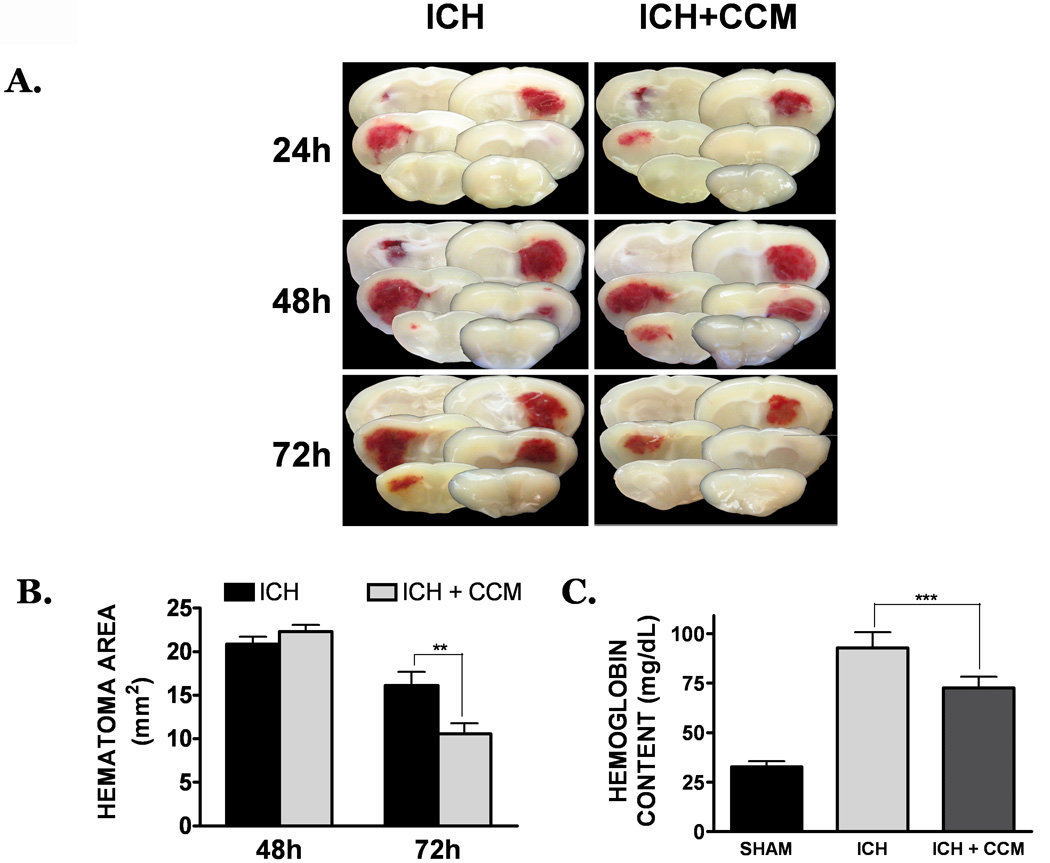
(A) Mice were treated with placebo (left panels) or curcumin (150 mg/kg; right panels) at the time of ICH. At 24h, 48h, or 72h post-ICH, coronal brain sections were prepared and digitally captured. Each panel depicts serial brain sections from a single, representative mouse per group. (B) Determination of hematoma area (mm2) at 48h or 72h post-ICH. Hematoma size was estimated by calculating the area inside a region of interest, as detailed in the Materials and Methods section (C) Hematoma volume was quantified by determining the hemoglobin content of each hemisphere at 72h post-ICH. Data are expressed as mean ± SEM and were analyzed by t-test or One-Way ANOVA (n=8 mice/group) followed by Student Newman-Keul’s post-hoc test (**p<0.01, ***p<0.001).
Figure 3. Curcumin promotes hematoma resolution after autologous blood clot-induced ICH.
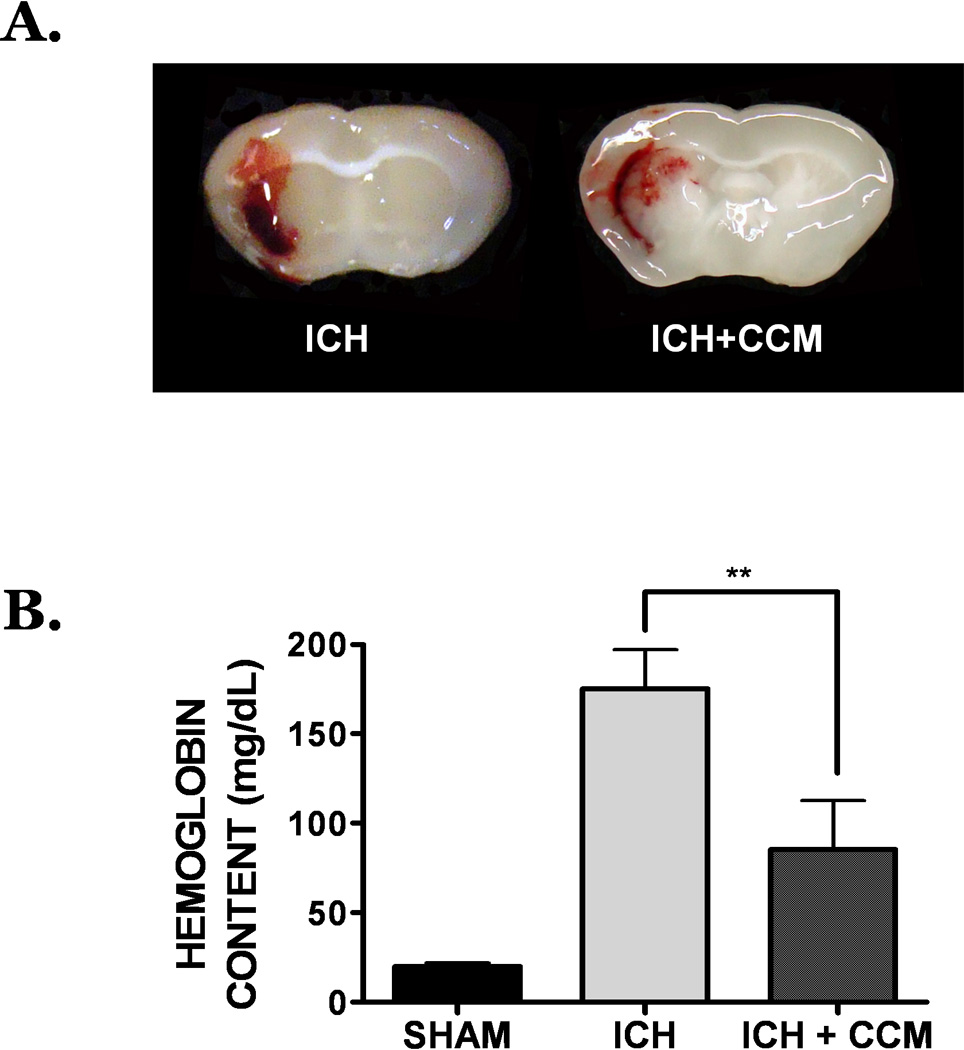
(A) Mice were treated with placebo (left panel) or curcumin (150 mg/kg; right panel) at the time of ICH. At 72h post-ICH, coronal brain sections were prepared and digitally captured. Each panel depicts a brain sections from a single, representative mouse per group (n=8 mice/group). (B) Hematoma volume was quantified by determining the hemoglobin content of each hemisphere at 72h post-ICH. Data are expressed as mean ± SEM (n=8 mice/group) and were analyzed by One-Way ANOVA followed by Student Newman-Keul’s post-hoc test (**p<0.01).
Hematoma volumes were significantly attenuated at 72h post-ICH by administration of curcumin at 15 minutes prior to injury; however, this pre-treatment regimen may not be clinically feasible. Thus, the therapeutic window for acute curcumin administration to limit hematoma size was established. As was observed using a pre-treatment paradigm, administration of curcumin up to 3h post-ICH significantly reduced hemoglobin content within the ipsilateral cortex at 72h. A 0.5h post-treatment with 150 mg/kg curcumin was associated with a 38.0% reduction in hematoma volume (p<0.001 vs. ICH, n=8) whereas a 3h post-treatment induced a 20.5% reduction in hematoma size (p<0.05 vs. ICH, n=8) (Figure 4). This protective effect was lost when post-treatment was delayed by 6h or more after ICH (data not shown), suggesting the molecular mechanisms responsible for hematoma resolution after curcumin treatment are restricted to the acute time period following injury.
Figure 4. Establishment of a therapeutic window for curcumin to reduce hematoma volume.

(A) Mice were treated with placebo or curcumin (150 mg/kg) at 15 minutes prior to injury or 0.5h, 1h, or 3h following collagenase-induced ICH. Hematoma volume was quantified 72h later using a hemoglobin content assay. Data are expressed as mean ± SEM (8 mice/group) and graphed as “% change from placebo-treated ICH mice”. Groups were analyzed using a One-Way ANOVA followed by Student Newman Keul’s post-hoc test (*p<0.05, **p<0.01, ***p<0.001 vs. ICH). Data are expressed as mean ± SEM (n=8 mice/group) and analyzed using One-Way ANOVA followed by Student Newman-Keul’s post-hoc test (*p<0.05, **p<0.01).
Curcumin attenuates inflammatory gene expression after ICH
We next investigated whether curcumin limits inflammatory gene expression following ICH. Pre-treatment with curcumin (150 mg/kg) significantly reduced the expression of IL-6, IL-1β, and TNF-α, inflammatory mediators strongly implicated in the pathophysiology of ICH, adjacent to the hematoma by 24h post-ICH. Expression of IL-6, a cytokine that this clinically correlated with hematoma expansion, was increased by 18.2 ± 6.0 fold (p<0.01 vs. sham) within the peri-hematoma region. This increase was significantly reduced by pre-treatment with curcumin (2.4 ± 1.3 fold vs. sham; p<0.01 vs. ICH; n=8) (Figure 5a). Similarly, curcumin reduced the expression of IL-1β (0.4 ± 0.2 fold vs. sham; p<0.001 vs. ICH, 29.5 ± 8.2 fold vs. sham; n=8) (Figure 5b) and TNF-α (1.5 ± 1.0 fold vs. sham; p<0.01 vs. ICH, 5.9 ± 1.5 fold vs. sham; n=8) (Figure 5c). Notably, inflammatory gene expression within the contralateral hemisphere following ICH did not significantly differ from sham-operated mice (data not shown).
Figure 5. Effect of curcumin on inflammatory gene expression.
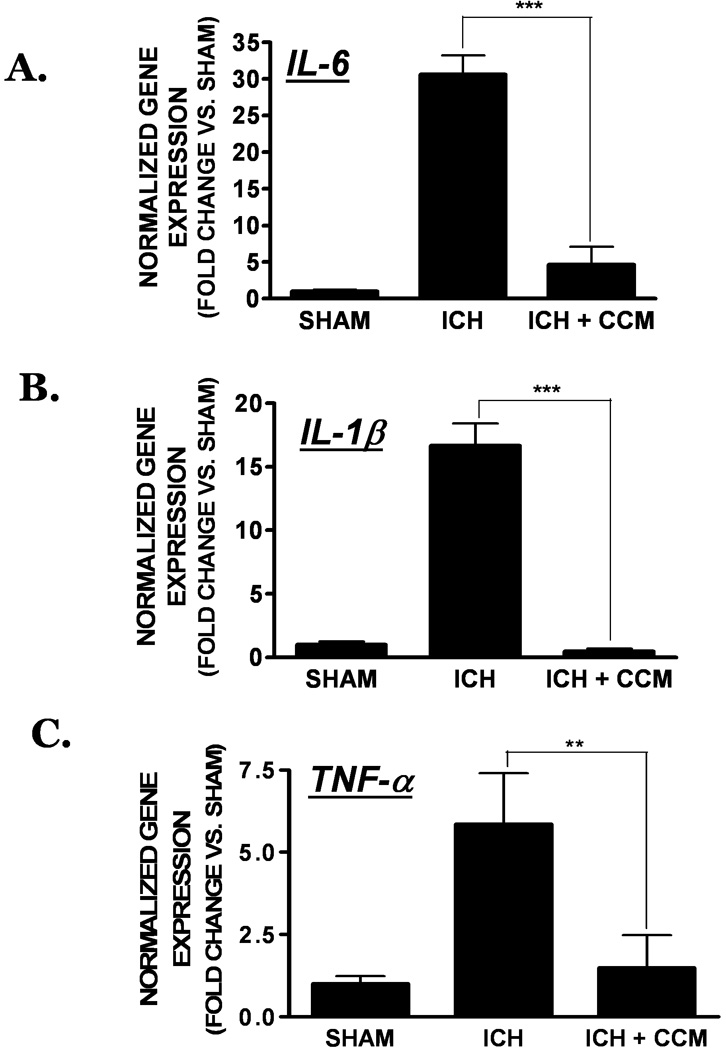
Mice were treated with curcumin (150 mg/kg) at the time of collagenase-induced ICH following by the quantification of inflammatory gene expression. (A) IL-6, (B) IL-1β, and (C) TNF-α expression was assessed in the peri-hematoma region at 24h post-ICH using qRT-PCR. Data were normalized to RPS3 and expressed as fold change vs. sham-operated mice (mean ± SEM; n=8 mice/group) Data were analyzed using One-Way ANOVA followed by Student Newman-Keul’s post-hoc test (*p<0.05, **p<0.01, ***p<0.001 vs. sham).
Curcumin reduces BBB permeability and cerebral edema after hemorrhagic injury
Production of pro-inflammatory mediators, such as those elevated following ICH, is associated with disruption of the BBB and the development of vasogenic edema. Consistent with this assertion, curcumin significantly reduced the extravasation of Evans blue dye, a sensitive estimate of BBB permeability, at 6h (p<0.001 vs. ICH, n=10/group), 12h (p<0.01 vs. ICH, n=10/group), and 24h (p<0.001 vs. ICH, n=10/group) post-ICH (Figure 6a–c). Similarly, brain water content was significantly decreased in curcumin-treated mice (81.3 ± 0.9%); p<0.05 vs. ICH, n=10/group), as compared to placebo-treated mice (83.7 ± 0.3%) at 24h (Figure 7a), with a maximal effect noted at 72h post-ICH (Figure 7b). Significant differences in edema were not observed in the contralateral hemispheres in any of the treatment groups across all time points (data not shown).
Figure 6. Curcumin reduces BBB permeability after ICH.

Mice were administered curcumin (150 mg/kg) at the time of collagenase-induced ICH. Evans blue extravasation, a sensitive measure of BBB disruption, was assessed at (A) 6h or (B) 12h or (C) 24h post-ICH. Comparisons between treatments groups were done using a one-way ANOVA followed by Student Newman Keul’s post-hoc test (**p<0.01, ***p<0.001). Data are expressed as mean ± SEM from 10 mice/group.
Figure 7. Curcumin attenuates brain edema following ICH.
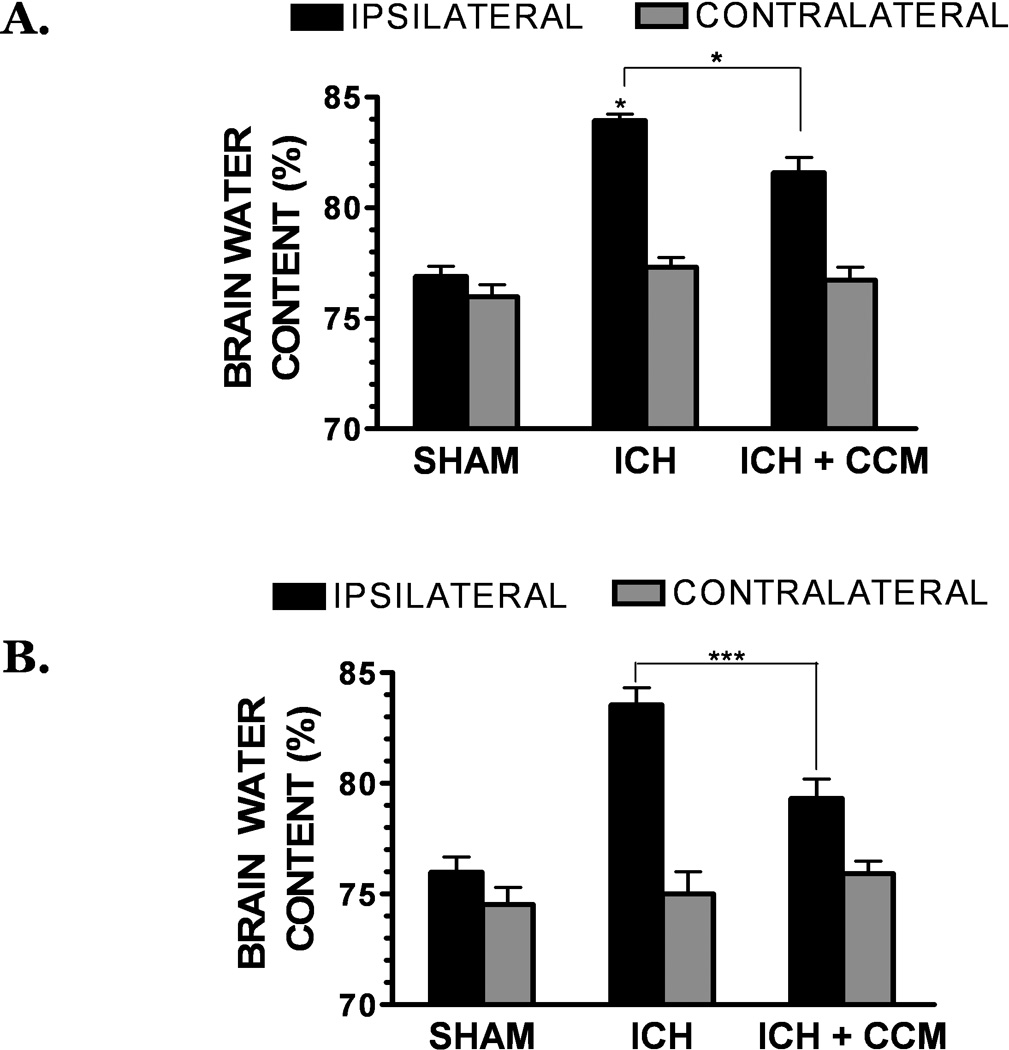
Mice were administered curcumin (150 mg/kg) at the time of collagenase-induced ICH. Brain water content, a measure of cerebral edema, was assessed in the ipsilateral and contralateral hemispheres at (A) 24h or (B) 72h following ICH. Comparisons within each hemisphere between different treatments groups were done using a one-way ANOVA followed by Student Newman Keul’s post-hoc test (*p<0.05, ***p<0.001). No significant differences were observed between groups in the contralateral hemispheres. Data are expressed as mean ± SEM from 10 mice/group.
Neurological outcome is improved in curcumin treated animals
Consistent with the effect on hematoma volume and cerebral edema, curcumin improved neurological outcomes following ICH, as determined using a 24-point scale. Specifically, curcumin significantly improved neurobehavioral scores at 48h, with a maximal improvement noted at 72h (n=10/group) (Figure 8). Significant differences in baseline neurological score were not observed between any of the experimental groups.
Figure 8. Curcumin improves neurological outcomes following ICH.
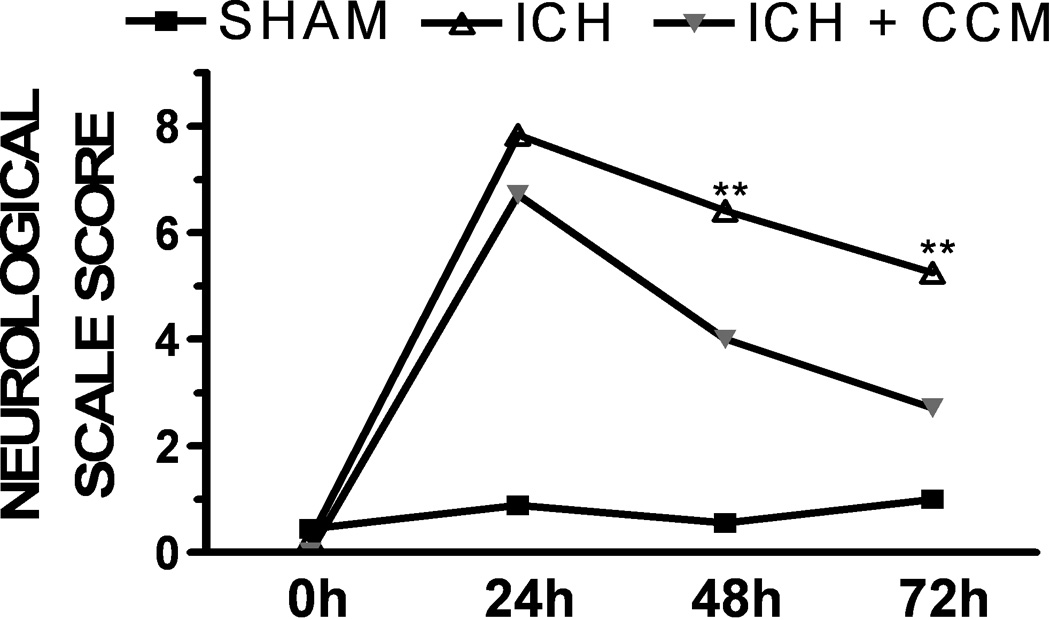
Mice were treated with placebo or curcumin (150 mg/kg) at the time of collagenase-induced ICH. Neurological outcomes were then assessed at 24h, 48h, or 72h following sham injury or ICH (n=10 mice/group. Data are the mean ± SEM and were analyzed using a repeated measures analysis of variance (ANOVA) followed by Bonferroni’s post-hoc test (**p<0.01 vs. sham).
DISCUSSION
Despite maximal surgical intervention and supportive care, ICH is associated with significant morbidity and mortality, in part, due to a lack of viable treatment options4,33. Acute or subacute hematoma expansion can occur in the days following the initial rupture. This persistent or recurrent bleeding exacerbates the space-occupying mass lesion, which subsequently increases neurovascular injury and promotes clinical deterioration. That surgical clot evacuation is associated with a more favorable outcome suggests hematoma size may dictate clinical prognosis30; however, many ICH patients are poor surgical candidates and/or present with a hemorrhage which is not amenable to neurosurgical intervention. Thus, new strategies to promote clot resolution are needed.
The present study demonstrates a novel beneficial effect of clinically-achievable doses of the curry spice, curcumin, in promoting hematoma resolution and neurological improvement in separate pre-clinical models of ICH. Hematoma formation was unaffected by curcumin treatment, implying neither the hemorrhagic activity of collagenase nor altered blood coagulation parameters were adversely influenced following drug administration. In contrast, curcumin reduced hematoma volume and neurological deficits by post-ICH days 3 and 7, respectively. Interestingly, administration of curcumin before injury or within the first hour following ICH was required for the maximal protective effect. While this narrow therapeutic window may diminish the ultimate translation of curcumin into the clinic, these data indicate the activation of cellular signaling pathways within the first hour after vascular rupture may contribute to subsequent neurovascular injury.
A dose of 150 mg/kg curcumin was optimal for promoting clot resolution and reducing neurological injury following ICH. This finding is consistent with reports from our laboratory and others showing a maximal beneficial effect of similar doses of curcumin in pre-clinical models of cerebral ischemia40,48, TBI24, and SAH42. Notably, pharmacokinetic studies in mice showed peak plasma concentrations (~1.6 µM) within 15 minutes and brain accumulation within 1 hour after intraperitoneal administration of 100 mg/kg curcumin25. These levels reflect the serum concentrations observed in patients following daily oral ingestion of 8 grams and more importantly, functionally inhibited pro-inflammatory signaling in peripheral blood mononuclear cells from pancreatic cancer patients6,15. Thus, the doses used to promote hematoma resolution in this study may simulate clinically-achievable steady-state serum concentrations. Unfortunately, curcumin exhibits relatively poor oral bioavailability and a short serum half-life (<45 minutes), which could also contribute to the limited therapeutic window observed in this study. Thus, the use of liposomes or nanoparticles may improve drug delivery, overcome bioavailability issues, and could extend the therapeutic window1. Alternatively, the development of curcumin analogues exhibiting an improved pharmacological profile (e.g. improved solubility, half life, brain distribution), may providing a longer therapeutic window and/or increased activity with respect to reducing the volume of the hematoma.
Inflammatory activation correlates with hematoma expansion, neurological deterioration, and a poor functional recovery20,26,31,48. Along these lines, plasma concentrations of IL-6 and TNF-α were significantly higher in patients exhibiting acute hematoma enlargement following a cerebral hemorrhage31. Similarly, expression of IL-1β and TNF-α were acutely increased within the perihematoma tissue using multiple species and models of experimental ICH2,27,28,41,43,46. Although the functional significance of the inflammatory responses remains largely unresolved, both IL-1β and TNF-α are associated with increased BBB permeability and the formation of vasogenic edema5,16,21,29,45. Further supporting this assertion, early elevations in plasma concentrations of TNF-α and IL-6 clinically correlated with the development of brain edema, mass effects, and patient outcome following ICH5,17,22,44.
The pro-inflammatory transcription factor, NFκB, is a positive regulator of IL-1β, IL-6, and TNF-α expression. Interestingly, NFκB activation was observed within minutes and was sustained for several days following ICH in rats48. Curcumin is a potent inhibitor of NFκB39 and our recent work suggests curcumin reduces neuroinflammatory expression, cerebral edema, and neurological injury after SAH and TBI42. Herein, we show for the first time, that curcumin reduces the expression of classical pro-inflammatory cytokines within the perihematomal tissue following collagenase- (Figure 4) or autologous blood-induced ICH (data not shown). As pro-inflammatory mediators, such as those studied in this report, often induce the expression of other inflammatory mediators, the ability of curcumin to limit acute expression could limit the progressive increase in neurovascular injury observed following ICH. These findings may also explain how the acute administration of curcumin produces a dramatic reduction in neurovascular injury observed days later.
CONCLUSION
Curcumin or structural analogues of curcumin may provide a novel, safe therapeutic approach to limit hematoma volume and neurovascular injury following ICH.
ACKNOWLEDGEMENTS
MDK and DJM performed all brain injury modeling and endpoint assessments. MFW and SEM performed the hematoma volume measurements. All authors contributed to data analysis and final manuscript editing. KMD and CHA conceptualized the study and wrote the manuscript.
Funding: This work was supported in part by grants from the National Institute of Health (NS065172) and American Heart Association (BGIA2300135) to KMD and by an Alpha Omega Alpha Carolyn L. Kuckein Research Fellowship to DJM.
Footnotes
Portions of this work were presented in abstract form at the Second Joint Symposium of the International and National Neurotrauma Societies, Santa Barbara, CA, September 7–11,2009
DISCLOSURES
The authors report no conflicts of interest.
REFERENCES
- 1.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 2.Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res. 2005;27:268–279. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- 3.Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, et al. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992;267:18148–18153. [PubMed] [Google Scholar]
- 4.Broderick JP, Adams HP, Jr, Barsan W, Feinberg W, Feldmann E, Grotta J GrottaJ, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1999;30:905–915. doi: 10.1161/01.str.30.4.905. [DOI] [PubMed] [Google Scholar]
- 5.Castillo J, Davalos A, Alvarez-Sabin J, Pumar JM, Leira R, Silva R, et al. Molecular signatures of brain injury after intracerebral hemorrhage. Neurology. 2002;58:624–629. doi: 10.1212/wnl.58.4.624. [DOI] [PubMed] [Google Scholar]
- 6.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 7.Choudhri TF, Hoh BL, Solomon RA, Connolly ES, Jr, Pinsky DJ. Use of a spectrophotometric hemoglobin assay to objectively quantify intracerebral hemorrhage in mice. Stroke. 1997;28:2296–2302. doi: 10.1161/01.str.28.11.2296. [DOI] [PubMed] [Google Scholar]
- 8.Christoforidis GA, Slivka A, Mohammad Y, Karakasis C, Avutu B, Yang M. Size matters: hemorrhage volume as an objective measure to define significant intracranial hemorrhage associated with thrombolysis. Stroke. 2007;38:1799–1804. doi: 10.1161/STROKEAHA.106.472282. [DOI] [PubMed] [Google Scholar]
- 9.Clark W, Gunion-Rinker L, Lessov N, Hazel K. Citicoline treatment for experimental intracerebral hemorrhage in mice. Stroke. 1998;29:2136–2140. doi: 10.1161/01.str.29.10.2136. [DOI] [PubMed] [Google Scholar]
- 10.Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–1181. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- 11.Dempsey RJ, Baskaya MK, Dogan A. Attenuation of brain edema, blood-brain barrier breakdown, and injury volume by ifenprodil, a polyamine-site N-methyl-D-aspartate receptor antagonist, after experimental traumatic brain injury in rats. Neurosurgery. 2000;47:399–404. doi: 10.1097/00006123-200008000-00024. discussion 404-396. [DOI] [PubMed] [Google Scholar]
- 12.Dhandapani KM, Khan MM, Wade FM, Wakade C, Mahesh VB, Brann DW. Induction of transforming growth factor-beta1 by basic fibroblast growth factor in rat C6 glioma cells and astrocytes is mediated by MEK/ERK signaling and AP-1 activation. J Neurosci Res. 2007;85:1033–1045. doi: 10.1002/jnr.21182. [DOI] [PubMed] [Google Scholar]
- 13.Dhandapani KM, Mahesh VB, Brann DW. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFkappaB transcription factors. J Neurochem. 2007;102:522–538. doi: 10.1111/j.1471-4159.2007.04633.x. [DOI] [PubMed] [Google Scholar]
- 14.Dhandapani KM, Wade FM, Mahesh VB, Brann DW. Astrocyte-derived transforming growth factor-{beta} mediates the neuroprotective effects of 17{beta}-estradiol: involvement of nonclassical genomic signaling pathways. Endocrinology. 2005;146:2749–2759. doi: 10.1210/en.2005-0014. [DOI] [PubMed] [Google Scholar]
- 15.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 16.Didier N, Romero IA, Creminon C, Wijkuisen A, Grassi J, Mabondzo A. Secretion of interleukin-1beta by astrocytes mediates endothelin-1 and tumour necrosis factor-alpha effects on human brain microvascular endothelial cell permeability. J Neurochem. 2003;86:246–254. doi: 10.1046/j.1471-4159.2003.01829.x. [DOI] [PubMed] [Google Scholar]
- 17.Dziedzic T, Bartus S, Klimkowicz A, Motyl M, Slowik A, Szczudlik A. Intracerebral hemorrhage triggers interleukin-6 and interleukin-10 release in blood. Stroke. 2002;33:2334–2335. doi: 10.1161/01.str.0000027211.73567.fa. [DOI] [PubMed] [Google Scholar]
- 18.Fewel ME, Thompson BG, Jr, Hoff JT. Spontaneous intracerebral hemorrhage: a review. Neurosurg Focus. 2003;15:E1. [PubMed] [Google Scholar]
- 19.Garodia P, Ichikawa H, Malani N, Sethi G, Aggarwal BB. From ancient medicine to modern medicine: ayurvedic concepts of health and their role in inflammation and cancer. J Soc Integr Oncol. 2007;5:25–37. doi: 10.2310/7200.2006.029. [DOI] [PubMed] [Google Scholar]
- 20.Hickenbottom SL, Grotta JC, Strong R, Denner LA, Aronowski J. Nuclear factor-kappaB and cell death after experimental intracerebral hemorrhage in rats. Stroke. 1999;30:2472–2477. doi: 10.1161/01.str.30.11.2472. discussion 2477-2478. [DOI] [PubMed] [Google Scholar]
- 21.Hua Y, Wu J, Keep RF, Nakamura T, Hoff JT, Xi G. Tumor necrosis factor-alpha increases in the brain after intracerebral hemorrhage and thrombin stimulation. Neurosurgery. 2006;58:542–550. doi: 10.1227/01.NEU.0000197333.55473.AD. discussion 542-550. [DOI] [PubMed] [Google Scholar]
- 22.Kim JS, Yoon SS, Kim YH, Ryu JS. Serial measurement of interleukin-6, transforming growth factor-beta, and S-100 protein in patients with acute stroke. Stroke. 1996;27:1553–1557. doi: 10.1161/01.str.27.9.1553. [DOI] [PubMed] [Google Scholar]
- 23.Labovitz DL, Sacco RL. Intracerebral hemorrhage: update. Curr Opin Neurol. 2001;14:103–108. doi: 10.1097/00019052-200102000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Laird MD, Sukumari-Ramesh S, Swift AE, Meiler SE, Vender JR, Dhandapani KM. Curcumin attenuates cerebral edema following traumatic brain injury in mice: a possible role for aquaporin-4? J Neurochem. 2010;113:637–648. doi: 10.1111/j.1471-4159.2010.06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laird MD, Wakade C, Alleyne CH, Jr, Dhandapani KM. Hemin-induced necroptosis involves glutathione depletion in mouse astrocytes. Free Radic Biol Med. 2008;45:1103–1114. doi: 10.1016/j.freeradbiomed.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Leira R, Davalos A, Silva Y, Gil-Peralta A, Tejada J, Garcia M, et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63:461–467. doi: 10.1212/01.wnl.0000133204.81153.ac. [DOI] [PubMed] [Google Scholar]
- 27.Lu A, Tang Y, Ran R, Ardizzone TL, Wagner KR, Sharp FR. Brain genomics of intracerebral hemorrhage. J Cereb Blood Flow Metab. 2006;26:230–252. doi: 10.1038/sj.jcbfm.9600183. [DOI] [PubMed] [Google Scholar]
- 28.Mayne M, Ni W, Yan HJ, Xue M, Johnston JB, Del Bigio MR, et al. Antisense oligodeoxynucleotide inhibition of tumor necrosis factor-alpha expression is neuroprotective after intracerebral hemorrhage. Stroke. 2001;32:240–248. doi: 10.1161/01.str.32.1.240. [DOI] [PubMed] [Google Scholar]
- 29.Megyeri P, Abraham CS, Temesvari P, Kovacs J, Vas T, Speer CP, et al. Recombinant human tumor necrosis factor alpha constricts pial arterioles and increases blood-brain barrier permeability in newborn piglets. Neurosci Lett. 1992;148:137–140. doi: 10.1016/0304-3940(92)90823-p. [DOI] [PubMed] [Google Scholar]
- 30.Mendelow AD. The International Surgical Trial in Intracerebral Haemorrhage (ISTICH) Acta Neurochir Suppl. 2003;86:441–443. doi: 10.1007/978-3-7091-0651-8_90. [DOI] [PubMed] [Google Scholar]
- 31.Platt N, da Silva RP, Gordon S. Recognizing death: the phagocytosis of apoptotic cells. Trends Cell Biol. 1998;8:365–372. doi: 10.1016/s0962-8924(98)01329-4. [DOI] [PubMed] [Google Scholar]
- 32.Qin Z, Karabiyikoglu M, Hua Y, Silbergleit R, He Y, Keep RF, et al. Hyperbaric oxygen-induced attenuation of hemorrhagic transformation after experimental focal transient cerebral ischemia. Stroke. 2007;38:1362–1367. doi: 10.1161/01.STR.0000259660.62865.eb. [DOI] [PubMed] [Google Scholar]
- 33.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 34.Rajeevan MS, Ranamukhaarachchi DG, Vernon SD, Unger ER. Use of real-time quantitative PCR to validate the results of cDNA array and differential display PCR technologies. Methods. 2001;25:443–451. doi: 10.1006/meth.2001.1266. [DOI] [PubMed] [Google Scholar]
- 35.Rynkowski MA, Kim GH, Komotar RJ, Otten ML, Ducruet AF, Zacharia BE, et al. A mouse model of intracerebral hemorrhage using autologous blood infusion. Nat Protoc. 2008;3:122–128. doi: 10.1038/nprot.2007.513. [DOI] [PubMed] [Google Scholar]
- 36.Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Ann N Y Acad Sci. 2005;1056:206–217. doi: 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- 37.Silakova JM, Hewett JA, Hewett SJ. Naproxen reduces excitotoxic neurodegeneration in vivo with an extended therapeutic window. J Pharmacol Exp Ther. 2004;309:1060–1066. doi: 10.1124/jpet.103.063867. [DOI] [PubMed] [Google Scholar]
- 38.Simard JM, Geng Z, Woo SK, Ivanova S, Tosun C, Melnichenko L, et al. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2009;29:317–330. doi: 10.1038/jcbfm.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 40.Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004;74:969–985. doi: 10.1016/j.lfs.2003.06.042. [DOI] [PubMed] [Google Scholar]
- 41.Wagner KR, Beiler S, Beiler C, Kirkman J, Casey K, Robinson T, et al. Delayed profound local brain hypothermia markedly reduces interleukin-1beta gene expression and vasogenic edema development in a porcine model of intracerebral hemorrhage. Acta Neurochir Suppl. 2006;96:177–182. doi: 10.1007/3-211-30714-1_39. [DOI] [PubMed] [Google Scholar]
- 42.Wakade C, King MD, Laird MD, Alleyne CH, Jr, Dhandapani KM. Curcumin attenuates vascular inflammation and cerebral vasospasm after subarachnoid hemorrhage in mice. Antioxid Redox Signal. 2009;11:35–45. doi: 10.1089/ars.2008.2056. [DOI] [PubMed] [Google Scholar]
- 43.Wasserman JK, Zhu X, Schlichter LC. Evolution of the inflammatory response in the brain following intracerebral hemorrhage and effects of delayed minocycline treatment. Brain Res. 2007 doi: 10.1016/j.brainres.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 44.Woiciechowsky C, Schoning B, Cobanov J, Lanksch WR, Volk HD, Docke WD. Early IL-6 plasma concentrations correlate with severity of brain injury and pneumonia in brain-injured patients. J Trauma. 2002;52:339–345. doi: 10.1097/00005373-200202000-00021. [DOI] [PubMed] [Google Scholar]
- 45.Wright JL, Merchant RE. Blood-brain barrier changes following intracerebral injection of human recombinant tumor necrosis factor-alpha in the rat. J Neurooncol. 1994;20:17–25. doi: 10.1007/BF01057957. [DOI] [PubMed] [Google Scholar]
- 46.Xi G, Hua Y, Keep RF, Younger JG, Hoff JT. Systemic complement depletion diminishes perihematomal brain edema in rats. Stroke. 2001;32:162–167. doi: 10.1161/01.str.32.1.162. [DOI] [PubMed] [Google Scholar]
- 47.Xu Q, Qaum T, Adamis AP. Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Invest Ophthalmol Vis Sci. 2001;42:789–794. [PubMed] [Google Scholar]
- 48.Zhao X, Zhang Y, Strong R, Zhang J, Brotta JC, Aronowski J. Distinct patterns of intracerebral hemorrhage-induced alterations in NF-kappaB subunit, iNOS, and COX-2 expression. J Neurochem. 2007;101:652–663. doi: 10.1111/j.1471-4159.2006.04414.x. [DOI] [PubMed] [Google Scholar]


