Abstract
Serotonin [5-hydroxytryptamine (5-HT)]-absorbing neurons use serotonin reuptake transporter (SERT) to uptake 5-HT from extracellular space but do not synthesize it. While 5-HT-absorbing neurons have been identified in diverse organisms from Caenorhabditis elegans to humans, their function has not been elucidated. Here, we show that SERT in 5-HT-absorbing neurons controls behavioral response to food deprivation in C. elegans. The AIM and RIH interneurons uptake 5-HT released from chemosensory neurons and secretory neurons. Genetic analyses suggest that 5-HT secreted by both synaptic vesicles and dense core vesicles diffuse readily to the extrasynaptic space adjacent to the AIM and RIH neurons. Loss of mod-5/SERT function blocks the 5-HT absorption. mod-5/SERT mutants have been shown to exhibit exaggerated locomotor response to food deprivation. We found that transgenic expression of MOD-5/SERT in the 5-HT-absorbing neurons fully corrected the exaggerated behavior. Experiments of cell-specific inhibition of synaptic transmission suggest that the synaptic release of 5-HT from the 5-HT-absorbing neurons is not required for this behavioral modulation. Our data point to the role of 5-HT-absorbing neurons as temporal–spatial regulators of extrasynaptic 5-HT. Regulation of extrasynaptic 5-HT levels by 5-HT-absorbing neurons may represent a fundamental mechanism of 5-HT homeostasis, integrating the activity of 5-HT-producing neurons with distant targets in the neural circuits, and could be relevant to some actions of selective serotonin reuptake inhibitors in humans.
Introduction
Dynamic changes in serotonin [5-hydroxytryptamine (5-HT)] signaling have been implicated as an instrumental mechanism underlying the plasticity of neuronal circuits involved in stress response, learning, adaptation, and memory in both vertebrates and invertebrates (Kandel, 2001; Zhang et al., 2005). The levels of 5-HT signals are controlled by the function of two types of transporters. The vesicular monoamine transporter (VMAT) pumps 5-HT from the cytoplasm into the small synaptic vesicles (SVs) and dense core vesicles (DCVs) (Liu and Edwards, 1997), thereby controlling the releasable pool of 5-HT. The serotonin reuptake transporter (SERT) in the plasma membrane absorbs extracellular 5-HT into the cytoplasm (Blakely et al., 1991; Torres et al., 2003), this being the major mechanism of terminating 5-HT neurotransmission. Consequently, the density and activity of SERT are important determinants of extracellular 5-HT concentration, the magnitude of 5-HT signals, and the number and duration of 5-HT receptors activated. SERT is considered as the major target for the therapeutic action of selective serotonin reuptake inhibitors (SSRIs) and tricyclic agents. Despite clinical significance, much remains to be learned about the mechanisms by which SERT function regulates 5-HT signaling in neural circuits and behavior.
SERT is present not only in the presynaptic plasma membrane of 5-HT-producing neurons to reuptake 5-HT from the synaptic cleft, but also in a range of neurons that are capable of absorbing 5-HT from extrasynaptic space but do not synthesize it. In particular, a transient dense serotonergic innervation was observed in the dorsal thalamus and in all primary sensory areas (optical, auditory, and somatosensory areas) of the developing cerebral cortex in mice between postnatal day 1 (P1) and P10 (Lebrand et al., 1996), and in rats between P3 and P13 (D'Amato et al., 1987). These neurons use glutamate as the major neurotransmitter; they do not express tryptophan hydroxylase, the rate-limiting enzyme for 5-HT biosynthesis, but transiently express SERT and VMAT (Lebrand et al., 1996, 1998; Cases et al., 1998; Hansson et al., 1999). Transient coexpression of SERT and VMAT also occurs in the developing human cerebral cortex (Verney et al., 2002). Hereafter in this article, we term these neurons as “5-HT-absorbing neurons” to distinguish them from “5-HT-producing neurons” that are capable of biosynthesizing 5-HT de novo. It has been proposed that 5-HT-absorbing neurons create “a morphogenetic gradient of 5-HT” to influence targets at a distance or they reuse 5-HT at their synapses (Gaspar et al., 2003). To date, the 5-HT source for 5-HT-absorbing neurons in the brain remains uncertain, and their function is unknown.
In Caenorhabditis elegans, 5-HT signaling is required for coupling feeding, the memory of prior food availability, and the rate of locomotion. Following food deprivation, wild-type (WT) animals markedly reduce locomotion on their return to a lawn of bacterial food; while 5-HT-deficient mutants exhibit less slowing response, mutants of the sole SERT gene mod-5 exaggerate the slowing response (Sawin et al., 2000; Ranganathan et al., 2001; Rivard et al., 2010). This suggests that loss of MOD-5/SERT function increases 5-HT inputs to the neuronal circuit, resulting in behavioral sensitization. Our laboratory has identified that, of five classes of a total of nine neurons that accumulate 5-HT in C. elegans, a pair of AIM interneurons and a single RIH interneuron are 5-HT-absorbing neurons (Kullyev et al., 2010). Here, we report that the MOD-5/SERT function in AIM and RIH neurons controls the response to the experience of food deprivation. We show that the AIM and RIH neurons absorb extrasynaptic 5-HT originated from multiple classes of 5-HT-producing neurons. However, synaptic release of 5-HT by the 5-HT-absorbing neurons is not required for this behavioral modulation. Based on our results, we propose that behavioral response to aversive experience is gauged by MOD-5/SERT regulation of extrasynaptic 5-HT levels.
Materials and Methods
Strains.
WT animals were the Bristol Strain N2. The mutants and transgenic animals used were as follows: decarboxylase mutants bas-1(ad446); VMAT mutants cat-1(e1111) and cat-1(nu90); SERT mutants mod-5(n822) and mod-5(n3314); synaptobrevin mutants snb-1(js17) and snb-1(md247); synaptotagmin mutants snt-1(ad596) and snt-1(md290); tryptophan hydroxylase mutants tph-1(mg280), tph-1(n4622), and tph-1(mg280); mod-5(n3314); Munc-13 mutants unc-13(e450), unc-13(e1091), and unc-13(s69); Munc-18 mutant unc-18(e81); CAPS mutant unc-31(e928); syntaxin mutant unc-64(e246); synaptic vesicle transporter mutant unc-104(e1265); transgenic lines CX6741 tph-1(mg280);Ex[ADF::tph-1], CX7749 tph-1(mg280);Ex[NSM::tph-1], and tph-1(mg280);mod-5(n3314);Ex[ADF::tph-1]; and tph-1(mg280);mod- 5(n3314);Ex[NSM::tph-1].
Constructs and transgenes.
All the constructs were generated by PCR, and purified PCR fragments were used to generate transgenic animals. Pmod-5::gfp(A) was generated by fusion of 4258 bp upstream of the translation start of mod-5 with the sequence of GFP and the 3′-untranslated regulatory region (UTR) of unc-54. Pmod-5::gfp(B) was generated by fusion of a genomic fragment encompassing 4258 bp promoter, exon 1, the first intron and exon 2 of mod-5 with the GFP sequence and unc-54 3′-UTR. rol-6(su1006) was used as a transformation marker.
To express mod-5 in specific neurons, full-length mod-5 cDNA was amplified from WT RNA by RT-PCR, and fused to the sequence of unc-54 3′-UTR, and the promoter sequence of mbr-1 (Kage et al., 2005), gcy-27 (Ortiz et al., 2006), or gcy-36 (Macosko et al., 2009). To express the tetanus toxin light chain (TeTx) in 5-HT-producing neurons ADF and NSM (PN::TeTx), the TeTx sequence (Davis et al., 2008) was fused to the BCD region of the tph-1 promoter (Sze et al., 2002) and the pes-10 minimal promoter element provided by the vector pPD122.53 (from A. Fire, Stanford University, Stanford, CA). To express TeTx in 5-HT-absorbing neurons (AN::TeTx), the TeTx sequence was fused to the mbr-1 promoter, which is expressed in AIM and several nonserotonergic neurons (Kage et al., 2005). For those transgenes, elt-2::gfp (Fukushige et al., 1998) was used as a transformation marker.
Indirect immunofluorescence histochemistry and microscopy.
To generate antibodies against MOD-5/SERT, a peptide corresponding to the C-terminal 22 amino acids (AADPTQIIDSSLLDPIHTLTPV) of the mod-5 protein was chemically synthesized and used to immunize rabbits by Proteintech Group. Antiserum obtained after the fourth boost was partially purified using MicroLink Peptide Coupling Kit (Pierce). Anti-MOD-5 antibody staining was performed using the Finny–Ruvkun protocol (Finney et al., 1988).
Staining of antibody raised against 5-HT (purchased from Dr. H.W.M. Steinbusch, Maastricht University, Maastricht, The Netherlands) was performed as described previously (Sze et al., 2002). To analyze the effect of drugs on 5-HT immunoreactivity, drugs were dissolved in water and the solution was poured onto plates containing nematode growth medium (NGM). The plates were dried under a hood for 2–3 h and used immediately. Well-fed mixed-stage worms were washed off their culture plates, transferred onto the drug plates, and incubated at 20°C for a period as indicated, before being fixed for staining with anti-5-HT antibody. The control animals were grown and stained in parallel but without drug treatment. We have tried a range of 5-HT and 5-hydroxytryptophan (5-HTP) concentrations incubated for various periods. WT animals incubated on plates supplemented with 2 mg/ml 5-HT or 0.1 mg/ml 5-HTP for 5.5 h gave the most reproducible results. Therefore, results from those conditions are shown. The staining patterns were visualized via Alexa Fluor 594- or 488-conjugated secondary antibodies (Invitrogen) under an AxioImager Z1 microscope equipped with proper filters, and images were captured using Axiocam MR digital camera (Zeiss).
To quantify the intensity of 5-HT immunoreactivity, the images of the AIM neurons in individual worms were captured at a fixed exposure time of 3 ms with 100% UV exposure level. For each image, the fluorescence intensity of a circular 10 pixel area within the AIM cell body was quantified using the ImageJ software (National Institutes of Health). To exclude the background, the fluorescence intensity over a circular 10 pixel area posterior to the AIM cell body in the same image was quantified, and the value of the background was subtracted from the value of the AIM area.
Behavioral assay.
Locomotion assays were performed as described previously (Sawin et al., 2000). Well-fed L4 animals were transferred onto fresh NGM agar plates seeded with Escherichia coli OP50 food and allowed to develop at 20°C, and 1-day-old adults were analyzed. The assays were performed on NGM agar plates seeded with a ring of E. coli HB101 food. For food deprivation assays, animals were washed twice with S-basal buffer or transferred to an empty NGM agar plate to get rid of the bacteria food, then were transferred onto NGM agar plates without food and incubated for 30 min at room temperature. Afterward, the animals were transferred to the center inside of the bacterial ring of the assay plates. Five minutes after the transfer, the number of the bends of the anterior portion of the animal on the bacterial lawn in an interval of 20 s was scored. WT, mod-5 mutants, and transgenic animals raised and assayed in parallel were compared. Throughout this article, data are expressed as the mean ± SEM, and Student's t tests were performed for comparisons between two test groups.
Results
5-HT immunoreactivity in the AIM and RIH interneurons requires MOD-5/SERT function
In C. elegans, 5-HT in neurons can be visualized by whole-mount staining of the entire animal with antibody against 5-HT. In WT hermaphrodites, 5-HT immunoreactivity was observed in a total of seven neurons in four classes in the head region shortly after hatching and throughout their lives: a pair of NSM secretory neurons, a pair of ADF chemosensory neurons, a single RIH interneuron, and a pair of AIM interneurons (Fig. 1a). In addition, 5-HT was present in a pair of HSN egg-laying motor neurons in adults (Fig. 1b) (Desai et al., 1988; Sze et al., 2000). 5-HT biosynthesis in C. elegans requires the tryptophan hydroxylase gene tph-1, and tph-1 knock-out worms did not show any discernible 5-HT immunoreactivity (Sze et al., 2000), validating the specificity of the staining.
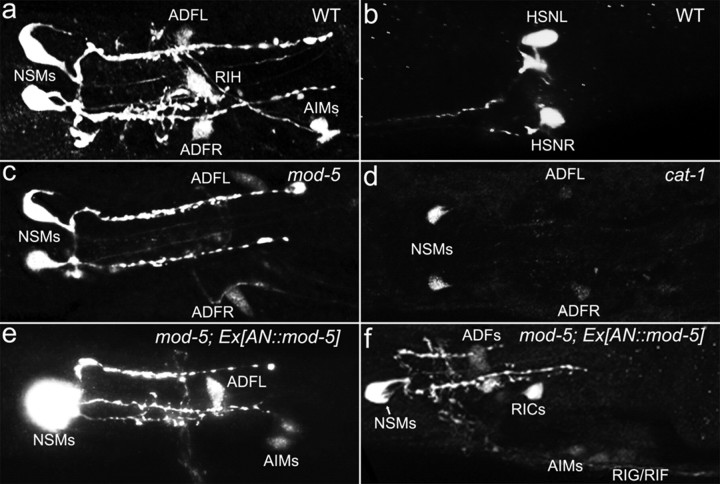
Figure 1.
5-HT accumulation in AIM and RIH neurons requires mod-5/SERT and cat-1/VMAT. Except in b, which shows a representative photomicrograph of 5-HT immunostaining of the HSN neurons, all the other panels show photomicrographs of 5-HT immunostaining of the head region. All the animals shown were adults with anterior to the left. a, In WT animals, seven neurons in four classes (NSM, ADF, RIH, and AIM) showed 5-HT immunoreactivity. c, In mod-5/SERT mutants, only NSM and ADF neurons showed 5-HT immunoreactivity. d, In cat-1/VMAT mutants, 5-HT immunoreactivity in NSM and ADF neurons was significantly reduced and undetectable in AIM and RIH. e, mod-5/SERT mutants bearing the AN::mod-5 transgene restored AIM 5-HT immunoreactivity. f, 5-HT immunoreactivity in ectopic neurons (RIC, RIG, and RIF). The percentages of each neuronal type stained in these strains are summarized in Table 1.
Previously, we showed that AIM and RIH uptake 5-HT from extracellular space but do not synthesize it (Kullyev et al., 2010). We first noticed that 5-HT immunoreactivity in AIM and RIH was abolished in WT animals treated with the SSRI fluoxetine (Kullyev et al., 2010). The C. elegans genome encodes a single SERT gene, mod-5 (Ranganathan et al., 2001). In both mod-5(n3314) deletion and mod-5(n822) opal mutants, 5-HT immunoreactivity was observed in NSM, ADF, and adult HSN neurons, but not in AIM and RIH neurons (Fig. 1c) (Kullyev et al., 2010). To determine MOD-5/SERT function in these neurons, we generated a transgene expressing WT mod-5 cDNA in putative 5-HT-absorbing neurons (AN::mod-5) from the mbr-1 promoter, which is expressed in AIM and several nonserotonergic neurons (including RIC, RIF, and RIG), but not in NSM, ADF, and HSN (Kage et al., 2005). The AN::mod-5 transgene restored 5-HT in AIM neurons (Fig. 1e) as well as in the ectopic neurons (Fig. 1f). This result demonstrates that MOD-5/SERT is not only responsible for 5-HT accumulation in the native 5-HT-absorbing neurons but is also sufficient to cause 5-HT accumulation in nonserotonergic neurons. This result supports the idea that in C. elegans, as in mammals, there are two distinctive populations of serotonergic neurons: NSM, ADF, and HSN produce 5-HT; and AIM and RIH absorb extracellular 5-HT via MOD-5. This idea is consistent with the observation that the sole tryptophan hydroxylase gene tph-1 is predominantly expressed in NSM, ADF, and adult HSN (Sze et al., 2000). In addition, the decarboxylase gene bas-1, which catalyzes the second step of 5-HT biosynthesis, was not detected in RIH (Hare and Loer, 2004).
To further investigate the mechanism of 5-HT accumulation in 5-HT-absorbing neurons, we analyzed 5-HT immunoreactivity in mutants of cat-1, which encodes the sole VMAT (Duerr et al., 1999; Sze et al., 2002). Consistent with the importance of CAT-1/VMAT for 5-HT intracellular storage and 5-HT release (Duerr et al., 1999), 5-HT was dramatically reduced or absent in NSM, ADF, and HSN as well as in AIM and RIH in two cat-1 alleles (Fig. 1d). Thus, 5-HT accumulation in AIM and RIH is also influenced by CAT-1/VMAT activity.
The AIM and RIH neurons absorb extrasynaptic 5-HT released from chemosensory neurons and secretory neurons
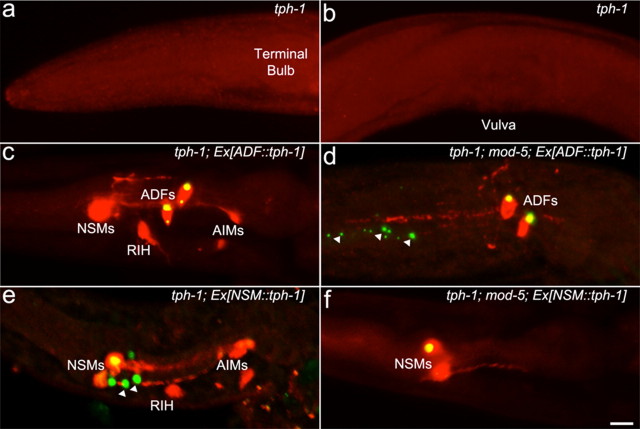
To characterize the mechanisms of 5-HT-absorbing neurons, we analyzed their relation to 5-HT-producing neurons. ADF neurons are chemosensory neurons with ciliated dendritic endings that are exposed to the external environment and sense food signals (Bargmann and Horvitz, 1991; Schackwitz et al., 1996). The pharyngeal NSM neurons have been implicated in feeding behavior (Avery and Horvitz, 1990). NSM neurons have the characteristics of excitable endocrine cells: their main processes extent along the pseudocoelom, with numerous branches filled with large and small vesicles projected toward the pseudocoelomic cavity (Albertson and Thomson, 1976; Hall and Altun, 2008). Consequently, NSM neurons are likely to secrete 5-HT hormonally. We asked whether particular 5-HT-absorbing neurons uptake 5-HT released from specific 5-HT-producing neurons. To address this question, we made use of established transgenes that express GFP-tagged tph-1 under heterologous promoters to direct 5-HT production specifically in either ADF (ADF::tph-1) or NSM (NSM::tph-1) (Zhang et al., 2005). The tph-1(mg280) deletion mutant was devoid of 5-HT. When the tph-1 mutant expressed the ADF::tph-1 transgene, 5-HT immunoreactivity was restored in ADF as well as in NSM, RIH, and AIM, but not in HSN (Fig. 2c; Table 1). This result is unlikely due to overexpression of tph-1 by the transgene producing excessive amounts of 5-HT, because mutations in the POU transcription factor UNC-86 eliminate tph-1 expression in NSM and produced the same pattern of 5-HT immunoreactivity as seen in tph-1 mutants expressing ADF::tph-1 (Sze et al., 2002), showing that the chromosomal tph-1 in ADF produces sufficient amounts of 5-HT reaching NSM, AIM, and RIH. To distinguish between neurons that produce 5-HT and those that absorb it, we crossed the ADF::tph-1 transgene into the tph-1(mg280);mod-5(n3314) double mutant. In tph-1;mod-5 background, 5-HT was present only in ADF (Fig. 2d; Table 1). These results indicate that 5-HT originated from ADF can be taken up by both classes of 5-HT-absorbing neurons, as well as by the NSM 5-HT-producing neurons.
Figure 2.
AIM and RIH neurons take up 5-HT originated from ADF and NSM. tph-1 mutants, and transgenic tph-1 and tph-1;mod-5 mutants expressing GFP-tagged TPH-1 in ADF, Ex[ADF::tph-1]), or in NSM, Ex[NSM::tph-1], were analyzed by anti-5-HT antibody staining. Photomicrographs show merging of the green fluorescence of the transgenes and the red fluorescence of 5-HT immunoreactivity to discern the neurons that express TPH-1 and the neurons that accumulate 5-HT. a, b, tph-1 mutants showed no discernable 5-HT immunoreactivity in the head neurons (a) or in HSN neurons (b). c, tph-1 mutants bearing the Ex[ADF::tph-1] transgene showed 5-HT immunoreactivity in ADF, as well as in NSM, RIH, and AIM neurons. d, tph-1;mod-5 double mutants bearing Ex[ADF::tph-1] showed 5-HT immunoreactivity in ADF neurons only. e, tph-1 mutants expressing the Ex[NSM::tph-1] transgene showed 5-HT immunoreactivity in NSM, as well as in RIH and AIM neurons. f, tph-1;mod-5 double mutants bearing Ex[NSM::tph-1] showed 5-HT immunoreactivity in NSM neurons only. All the animals shown were adults with anterior to the left. The arrows indicate GFP-tagged TPH-1 in ADF dendrites (d) and NSM processes (e). Quantification of the percentage of each neuronal type stained in the strains is presented in Table 1. Scale bar, 10 μm.
Table 1.
5-HT immunoreactivity in tph-1 and mod-5 mutants
| Strains | N | Neurons |
||||
|---|---|---|---|---|---|---|
| NSM | ADF | HSN | RIH | AIM | ||
| WT | 53 | 100 | 100 | 100 | 87 | 87 |
| WT + fluoxetine | 40 | 100 | 93 | 100 | 40 | 13 |
| tph-1(mg280) | 16 | 0 | 0 | 0 | 0 | 0 |
| mod-5(n3314) | 40 | 100 | 100 | 100 | 0 | 0 |
| mod-5(n822) | 40 | 100 | 93 | 100 | 0 | 3 |
| mod-5(n3314);Ex[AN::mod-5]a | 28 | 100 | 96 | NA | 0 | 75 |
| cat-1(e1111) | 105 | 98 | 50 | 21 | 4 | 3 |
| cat-1(nu90) | 106 | 99 | 56 | 50 | 5 | 8 |
| tph-1;Ex[ADF::tph-1] | 72 | 100 | 100 | 0 | 68 | 76 |
| tph-1;mod-5;Ex[ADF::tph-1] | 23 | 0 | 100 | 0 | 0 | 0 |
| tph-1;Ex[NSM::tph-1] | 29 | 100 | 7 | 0 | 72 | 55 |
| tph-1;mod-5; Ex[NSM::tph-1] | 19 | 63 | 0 | 0 | 0 | 0 |
WT animals and the mutants were stained in parallel with anti-5-HT antibody. The results of two staining trials are shown. Data represent the percentages of indicated neuronal types stained in the strains, regardless of the intensity. Mixed-stage animals were analyzed. HSN neuron immunoreactivity was scored only in adults, and other neurons were scored in larvae and adults. N, Number of animals per strain analyzed; NA, not analyzed.
aIn mod-5(n3314);Ex[AN::mod-5] animals, 5-HT immunoreactivity was observed in additional neurons (Fig 1f), but the percentage for these neurons stained was not scored.
In tph-1 mutant animals expressing the NSM::tph-1 transgene, 5-HT immunoreactivity was observed in NSM, as well as in AIM and RIH, rarely and very weakly in ADF, but not in HSN (Fig. 2e; Table 1). In tph-1;mod-5 animals expressing NSM::tph-1, only NSM showed 5-HT immunoreactivity (Fig. 2f; Table 1). Thus, AIM and RIH absorb 5-HT originated from both NSM and ADF.
Although ADF, AIM, and RIH are all located in the head region of the somatic nervous system, there is no direct connection among them (White et al., 1986). NSM neurons are located in the pharynx, which is separated from the somatic system (Albertson and Thomson, 1976). Consequently, our results suggest that AIM and RIH, as well as NSM neurons absorb 5-HT from extrasynaptic space, and 5-HT can traverse between the somatic extracellular space and the pharynx.
Some 5-HT-producing neurons cannot efficiently uptake extracellular 5-HT
While NSM efficiently took up 5-HT originated from ADF neurons, ADF neurons showed minimal ability to uptake 5-HT originated by NSM neurons, and HSN neurons did not uptake 5-HT from either ADF or NSM neurons (Table 1). We thought that the differences could reflect their accessibility to 5-HT released from the other neurons. To test this idea, we analyzed their ability to uptake exogenous 5-HT. In tph-1 mutants incubated for 5.5 h in culture media supplemented with 2 mg/ml 5-HT, 5-HT immunoreactivity was observed in NSM, and less frequently in ADF, AIM, and RIH neurons (Fig. 3a; Table 2). Many attempts to change 5-HT dosages did not improve the frequency of the neurons to be stained. Noticeably, 5-HT treatments reduced 5-HT immunoreactivity in WT animals (Table 2). Nevertheless, the same 5-HT treatment did not produce any discernible 5-HT immunoreactivity in the tph-1;mod-5 double mutant (Fig. 3b), indicating that the uptake of exogenous 5-HT requires MOD-5 function. These results are in accord with a previous report (Ranganathan et al., 2001).
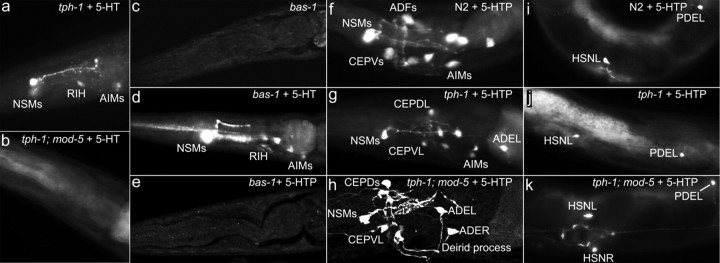
Figure 3.
Differential ability of 5-HT-producing neurons to take up 5-HT and 5-HTP. Representative photomicrographs of 5-HT immunostaining of WT and indicated mutants untreated and treated with 2 mg/ml 5-HT or 0.1 mg/ml 5-HTP for 5.5 h. All the animals shown have anterior to the left. a, b, tph-1 and tph-1;mod-5 mutants treated with exogenous 5-HT. Applying 5-HT restored 5-HT immunoreactivity in tph-1 mutants but not in tph-1;mod-5 double mutants. c–e, bas-1/decarboxylase mutants untreated and treated with 5-HT or 5-HTP. Untreated bas-1 mutants showed no discernable 5-HT immunoreactivity. bas-1 mutants treated with 5-HT restored 5-HT immunoreactivity. 5-HTP treatment did not improve 5-HT immunoreactivity in bas-1 mutants. f–k, 5-HTP treatment of WT, tph-1 mutants, and tph-1;mod-5 double mutants. The head region (f–h) and the middle-to-posterior portion of the body (i–k). 5-HT immunoreactivity was observed in all of the 5-HT-producing neurons in these genetic backgrounds. In addition, 5-HT immunoreactivity was detected in the dopaminergic neurons (CEP, ADE, and PDE), consistent with the expression pattern of bas-1/decarboxylase (Hare and Loer, 2004). Quantification of the percentage of each neuronal types stained in the strains is presented in Table 2.
Table 2.
5-HT immunoreactivity following drug treatment
| Strains + treatment | N | Neurons |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NSM | ADF | RIH | AIM | HSN | Dp | VC | PHx | I5/M5 | ||
| WT | 48 | 100 | 98 | 96 | 85 | 100 | 0 | 0 | 0 | 0 |
| WT + 5-HTP | 35 | 100 | 86 | 89 | 86 | 100 | 100 | 31 | 46 | 54 |
| WT + 5-HT | 97 | 70 | 18 | 18 | 18 | 15 | 0 | 0 | 0 | NA |
| bas-1 | 50 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| bas-1 + 5-HTP | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| bas-1 + 5-HT | 68 | 94 | 3 | 4 | 15 | 0 | 0 | 0 | 0 | NA |
| tph-1 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| tph-1 + 5-HTP | 49 | 100 | 36 | 65 | 76 | 95 | 96 | 22 | 33 | NA |
| tph-1 + 5-HT | 137 | 86 | 1 | 2 | 5 | 0 | 0 | 0 | 1 | NA |
| mod-5 + 5-HTP | 15 | 100 | 100 | 0 | 0 | 100 | 100 | 27 | 0 | 33 |
| tph-1; mod-5 + 5-HTP | 38 | 95 | 21 | 0 | 3 | 100 | 89 | 18 | 0 | 16 |
The animals were stained with anti-5-HT antibody. WT, mutants, and drug-treated animals were stained and analyzed in parallel. The 5-HT concentration used in these experiments was 2 mg/ml, and the 5-HTP concentration was 0.1 mg/ml in media. Summary of the two staining trials are shown. Data represent the percentages of indicated neuronal types stained in the strains, regardless of the intensity. Mixed-stage animals were stained. HSN neuron immunoreactivity was scored only in adults; other neurons were scored in larvae and adults. Dp, Dopaminergic neurons; N, number of animal analyzed per strain; NA, not analyzed.
As a control for the uptake of 5-HT, we analyzed the uptake of the 5-HT precursor 5-HTP by the 5-HT-producing neurons. The uptake of 5-HTP does not require a specific transporter system (Coates, 2005), and 5-HTP is converted to 5-HT by a decarboxylase. The bas-1/decarboxylase mutant showed dramatically reduced/undetectable 5-HT immunoreactivity (Fig. 3c) (Sawin et al., 2000; Hare and Loer, 2004). bas-1/decarboxylase mutant worms treated with 5-HT showed an increase in 5-HT immunoreactivity very similar to the tph-1 mutant treated with 5-HT (Fig. 3d; Table 2). Applying 5-HTP did not restore 5-HT immunoreactivity in bas-1/decarboxylase mutants (Fig. 3e), showing the specificity of the antibody to 5-HT. WT and tph-1 mutant animals treated with 5-HTP showed strong 5-HT immunoreactivity in all the 5-HT-producing neuron, the 5-HT-absorbing neurons, as well as in dopaminergic neurons (Fig. 3f,g,i,j; Table 2), consistent with prior reports (Hare and Loer, 2004; Rivard et al., 2010). In addition, we observed 5-HT immunoreactivity in several other neurons, including the pharyngeal neurons I5 and M5, the ventral cord motor neurons VC4 and VC5, and the phasmid sensory neurons PHx in both WT and tph-1 mutant animals treated with 5-HTP (Table 2).
Importantly, strong 5-HT immunoreactivity was observed in the tph-1;mod-5 mutant treated with 5-HTP (Fig. 3h,k), showing that MOD-5/SERT is not required for the uptake of 5-HTP. In tph-1;mod-5 mutant animals treated with 5-HTP, 5-HT immunoreactivity was not observed in RIH, and the frequency of AIM staining was substantially reduced compared with tph-1 mutants treated with 5-HTP (Table 2), suggesting that 5-HTP was mostly converted to 5-HT in other cells before being transported into AIM and RIH neurons.
Overall, NSM showed a greater capability than ADF to uptake both exogenous 5-HT and 5-HTP (Table 2). On the other hand, HSN neurons, which were unable to absorb 5-HT, showed strong 5-HT immunoreactivity in both tph-1 and tph-1;mod-5 mutants treated with 5-HTP (Table 2), suggesting that HSN neurons can access to exogenous chemicals but are unable to efficiently take up 5-HT. These results suggest that the ability of 5-HT-producing neurons to take up extracellular 5-HT is a cell-specific property.
Expression and localization of MOD-5/SERT are regulated by a cell-specific manner
We next analyzed the expression and distribution of mod-5. To determine which cells express the mod-5 gene, we generated GFP reporters under the control of mod-5 promoter elements (Fig. 4a). Pmod-5::gfp(A) is driven by 4.5 kb upstream of the translational start of the mod-5 gene. GFP fluorescence was observed in ADF, AIM, and RIH, as well as in several other neuronal and non-neuronal cells (Fig. 4b) (data not shown). However, we did not observe GFP in NSM and HSN neurons in multiple Pmod-5::gfp(A) transgenic lines.
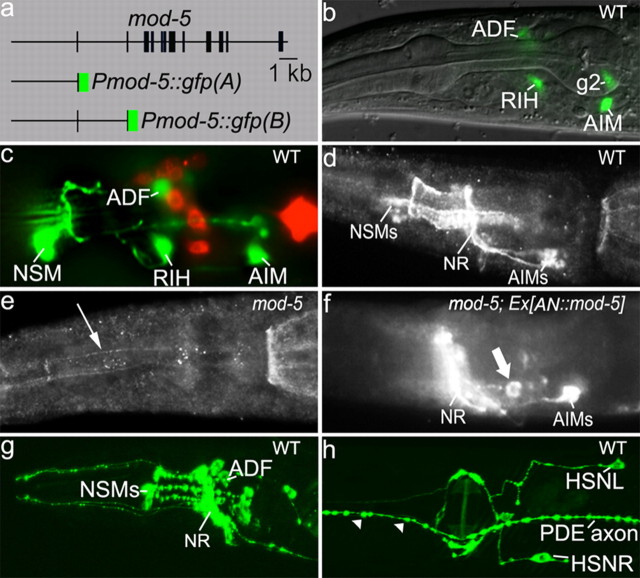
Figure 4.
Expression and distribution of mod-5/SERT. a, The structure of mod-5 GFP reporters. Intron (line)/exon (bar) structure of the mod-5 gene is adapted from Ranganathan et al. (2001). The structure of mod-5 GFP reporters is schematically depicted. b, Pmod-5::gfp(A) expression in WT animals. Photomicrograph shows GFP expression in ADF, RIH, and AIM neurons, as well as in nonserotonergic cell g2, in a merged image of Nomarski microscopy and fluorescence microscopy. c, Pmod-5::gfp(B) expression in WT animals. Photomicrograph shows merging of green fluorescence of the transgene and red fluorescence of DiI staining of the amphid chemosensory neurons, indicating GFP expression in NSM, as well as in ADF, RIH, and AIM neurons. d–f, MOD-5 immunostaining of WT and mod-5 mutants. d, In WT animals, MOD-5/SERT immunoreactivity was evenly distributed in AIM soma and axon, but it appeared to be enriched in axons in NSM neurons. e, No staining was detected in any neuron in mod-5(n822) opal mutant (data not shown), although rare and very faint staining of NSM axons (indicated by a thin arrow) was observed in the mod-5(n3114) mutant. f, The AN::mod-5 transgene restored MOD-5 immunoreactivity in AIM neurons, as well as in several ectopic cells (indicated by a thick arrow), but not in NSM in mod-5 mutants. NR, Nerve ring. g, h, Distribution of GFP-tagged CAT-1/VMAT in WT animals. CAT-1::GFP showed punctate pattern in the soma and processes in NSM, ADF, and HSN, as well as in dopaminergic neurons including PDE. Notice that the pattern of CAT-1::GFP overlaps with MOD-5 immunoreactivity in NSM, but not in ADF neurons. All the animals shown were adults with anterior to the left.
Pmod-5::gfp(B) is driven by a mod-5 genomic fragment containing the same 4.5 kb upstream noncoding sequence, plus exon 1, the first intron, and exon 2. Pmod-5::gfp(B) was expressed in all of the cells that express Pmod-5::gfp(A). In addition, it was strongly expressed in NSM in two analyzed transgenic lines (Fig. 4c). Thus, mod-5 expression in ADF and NSM is regulated by distinctive cis-elements, but none of these elements is expressed in HSN neurons.
To examine the cellular distribution of the MOD-5/SERT protein, polyclonal antibodies were raised against a peptide corresponding to the C-terminal region of MOD-5/SERT for whole-mount staining of C. elegans animals. In WT animals, the strongest staining was observed in NSM axons and their branches, and in the soma and axons of AIM (Fig. 4d). MOD-5/SERT immunoreactivity was not present in any neurons in the mod-5(n822) opal mutant, although rare, very weak staining of NSM axons could be detected in mod-5(n3314) background (Fig. 4e). In mod-5(n3314) mutants expressing the AN::mod-5 transgene, MOD-5/SERT immunoreactivity was observed in AIM as well as in the ectopic neurons (Fig. 4f), consistent with 5-HT accumulation in those cells (Fig. 1f).
However, we were not able to detect MOD-5/SERT immunoreactivity in ADF, RIH, and HSN in WT animals. This could be due to weak levels of the signals at particular cellular sites that are not distinguishable from the background. The low MOD-5/SERT abundance in ADF neurons could be an explanation for their modest ability to uptake extracellular 5-HT (Tables 1, 2). HSN neurons did not express the mod-5 GFP reporters, did not show MOD-5/SERT immunoreactivity, and did not uptake 5-HT under conditions tested thus far.
To compare the 5-HT reuptake sites with 5-HT release sites, we analyzed the distribution of GFP-tagged CAT-1/VMAT. CAT-1::GFP showed a punctate pattern in the soma, dendrites and axonal processes of NSM, ADF, and HSN neurons (Fig. 4g,h), suggesting that 5-HT can be released from these cellular sites. In NSM, CAT-1::GFP mostly overlapped with MOD-5/SERT immunoreactivity. By contrast, CAT-1::GFP puncta in ADF and HSN neurons were present at the sites where showed very little, if any, MOD-5/SERT immunoreactivity.
AIM and RIH 5-HT accumulation is reduced in mutants of SV and DCV secretion
In the mammalian CNS, 5-HT can be released from small, clear SVs that are clustered at the synaptic sites, as well as from DCVs throughout the soma, dendrites, and axonal processes (Nirenberg et al., 1995; De-Miguel and Trueta, 2005). Since AIM and RIH neurons absorb 5-HT from extrasynaptic space, we asked whether 5-HT in RIH and AIM neurons is attributed to DCV secretion. The UNC-31/calcium-dependent activator protein for secretion (CAPS) is required for regulated DCV release, but not for evoked SV release (Charlie et al., 2006; Speese et al., 2007). In the unc-31(e928) deletion mutant, 5-HT immunoreactivity in AIM and RIH neurons was substantially reduced but not eliminated (Fig. 5b). This result suggests that DCV secretion cannot fully account for the 5-HT in the extrasynaptic space.
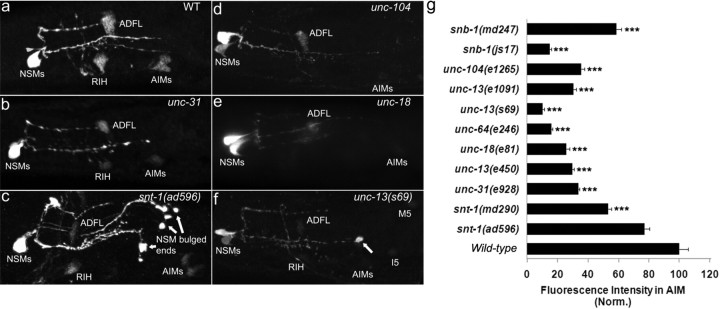
Figure 5.
Effects of SV and DCV secretion on 5-HT accumulation in 5-HT-absorbing neurons. a–f, Photomicrographs of 5-HT immunostaining of mutants affecting SV secretion (snt-1 and snb-1), DCV secretion (unc-31), and both forms of secretion (unc-13, unc-18, unc-64, and unc-104). All the animals shown were adults with anterior to the left. Arrows point to bulged 5-HT immunoreactivity at the end of NSM axons, which could reflect the accumulation of unreleased 5-HT vesicles in the mutant neurons. g, Quantification of 5-HT immunoreactivity in AIM. Data represent the average of 15 animals per strain ± SEM, and the value of the mutants is normalized to that of WT stained in parallel. The experiments have been performed multiple times, and the results from the other trials were similar. ***p < 0.001, Student's t test.
Next, we analyzed 5-HT immunoreactivity in mutants of snt-1/synaptotagmin (Nonet et al., 1993) and snb-1/synaptobrevin (Nonet et al., 1998). AIM and RIH 5-HT immunoreactivity was reduced in two analyzed snb-1 alleles and in the snt-1(md290) deletion mutant, although the reduction in the snt-1(ad596) hypomorph allele was not significant, compared with WT animals (Fig. 5g).
The unc-104/synaptic vesicle transporter (Hall and Hedgecock, 1991), unc-18/Munc18 (Weimer et al., 2003; McEwen and Kaplan, 2008), unc-64/syntaxin (Iwasaki et al., 1997; Saifee et al., 1998; Hammarlund et al., 2008), and unc-13 (Richmond et al., 1999; Sieburth et al., 2007) regulate both SV and DCV secretion. 5-HT immunoreactivity in RIH and AIM was reduced further even in non-null mutants of unc-18, unc-64, and unc-104, compared with that in the snt-1(md290) and unc-31(e928) mutants (Fig. 5g). We noticed strong 5-HT immunoreactivity often bulged at the end of NSM axons in snt-1, unc-13, unc-18, and unc-64 mutants (Fig. 5c,f). Together, these results suggest a model in which the synaptic mutants have reduced 5-HT secretion, and deficits in either SV secretion or DCV secretion would reduce 5-HT availability to AIM and RIH neurons. However, the possibility of reduced MOD-5 activity in the mutants cannot be excluded.
Expression of MOD-5/SERT in 5-HT-absorbing neurons corrects exaggerated behavior of mod-5 mutants
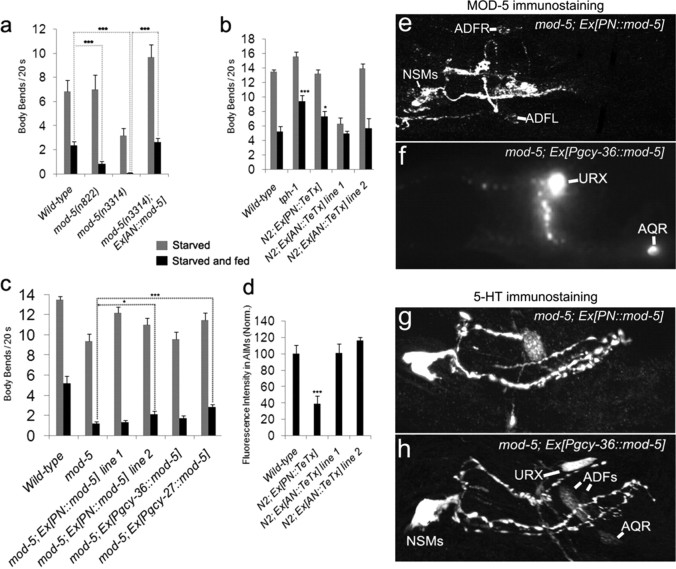
The level of 5-HT has been shown to control the C. elegans behavioral response to food deprivation (Sawin, 1996; Sawin et al., 2000). C. elegans adjusts the rate of locomotion according to the presence or absence of food, as well as the memory of prior food availability. When worms returned to a lawn of bacterial food following food deprivation, animals markedly reduced the rate of locomotion (Fig. 6a) (Sawin, 1996). 5-HT-deficient mutant tph-1 showed less slowing response; by contrast, mod-5 mutants essentially stopped locomotion as soon as they reached bacterial food (Fig. 6a,b) (Sawin et al., 2000; Ranganathan et al., 2001; Rivard et al., 2010). To evaluate the role of mod-5/SERT in 5-HT-producing and 5-HT-absorbing neurons in this behavioral modulation, we generated transgenic mod-5 mutants expressing mod-5 cDNA either in 5-HT-absorbing neurons (AN::mod-5) or in the 5-HT-producing neurons ADF and NSM (PN::mod-5). We found that the AN::mod-5 transgene fully rescued the exaggerated locomotor response of mod-5 mutants (Fig. 6a). By contrast, the PN::mod-5 gene restored MOD-5/SERT immunoreactivity in NSM and ADF neurons in the mod-5 mutants (Fig. 6e), but only partially rescued the exaggerated behavior (Fig. 6c). These results demonstrate that the MOD-5/SERT function in those 5-HT-absorbing neurons is critical for preventing the exaggerated response.
Figure 6.
Modulation of locomotion response to food deprivation by MOD-5/SERT in 5-HT-absorbing neurons. a–c, Locomotion rates of WT, mod-5 mutants, and transgenic mod-5 worms following brief food deprivation. PN denotes transgenes expressed in the 5-HT-producing neurons NSM and ADF. AN denotes transgenes under the control of the mbr-1 promoter, which is expressed in the 5-HT-absorbing neurons AIM and several nonserotonergic neurons (Kage et al., 2005). Each bar represents the average of at least three trials ± SEM, with five animals per strain per trial. a, mod-5 mutants bearing the Ex[AN::mod-5] transgene fully corrected exaggerated slowing response. b, Expressing the tetanus toxin light chain in WT animals by the Ex[PN::TeTx] transgene reduced slowing response, as seen in 5-HT-deficient mutant tph-1. However, the Ex[AN::TeTx] transgene did not enhance slowing response compared with WT animals. c, Differential rescue of exaggerated slowing response of mod-5 mutants by transgenic expression of MOD-5 in 5-HT-producing neurons (PN::mod-5) and in nonserotonergic neurons (Pgcy-27::mod-5 and Pgcy-36::mod-5). The difference between mod-5 mutants and the transgenic animals is indicated (*p < 0.05, ***p < 0.001). mod-5; Ex[PN::mod-5] and mod-5; Ex[Pgcy-27::mod-5] differed from WT animals (p < 0.001 and p < 0.01, respectively). d, Quantification of 5-HT immunoreactivity in AIM. Data represent the average of 15 animals per strain ± SEM, and the values of the transgenic animals were normalized to those of nontransgenic WT animals stained in parallel. ***p < 0.001. e, f, MOD-5 immunostaining of transgenic mod-5 mutants. e, The Ex[PN::mod-5] transgene restored MOD-5 immunoreactivity in NSM and ADF neurons, showing the specificity of the promoter for 5-HT-absorbing neurons. f, The Ex[Pgcy-36::mod-5] transgene produced ectopic MOD-5 expression in the URX and AQR neurons, consistent with the expression pattern of gcy-36 (Ortiz et al., 2006). g, h, 5-HT immunostaining of transgenic mod-5 mutants. Notice the 5-HT immunoreactivity in the ectopic neurons URX and AQR.
The 5-HT-absorbing neurons could influence the behavior by rereleasing 5-HT at their synapses. Alternatively, these neurons subserve to control 5-HT levels in the extracellular space. To distinguish between these possibilities, we used TeTx to inhibit synaptic transmission of 5-HT-producing neurons (PN::TeTx) or 5-HT-absorbing neurons (AN::TeTx) in WT background. TeTx is a specific protease of synaptobrevin (Sweeney et al., 1995) and has been used successfully to block synaptic transmission in C. elegans (Davis et al., 2008; Macosko et al., 2009). In WT animals expressing the PN::TeTx transgene, we observed reduced AIM and RIH 5-HT immunoreactivity (Fig. 6d). Furthermore, the transgenic animals showed less slowing response to food deprivation (Fig. 6b), as one would expect for animals with reduced 5-HT signals. These results suggest that the PN::TeTx transgene inhibits 5-HT release from the 5-HT-producing neurons.
We next investigated whether synaptic release of 5-HT from 5-HT-absorbing neurons is required for preventing exaggerated slowing response. If this were the case, blockage of the synaptic transmission would produce exaggerated slowing, as seen in mod-5 mutants. By contrast, no enhanced slowing response was observed in WT animals expressing the AN::TeTx transgene (Fig. 6b).
We then asked whether expressing MOD-5/SERT in any neurons in the head region could rescue exaggerated slowing of mod-5 mutants. The gcy-27 gene is expressed in the ASI, ASJ, and ASK chemosensory neurons (Ortiz et al., 2006), which are located adjacent to the ADF neuron. Ectopic expression of MOD-5/SERT by the gcy-27 promoter (Pgcy-27::mod-5) partially rescued exaggerated behavior of mod-5 mutants (Fig. 6c). However, ectopic expression of MOD-5/SERT in the AQR and URX neurons under the gcy-36 promoter (Pgcy-36::mod-5) did not (Fig. 6c), even though the transgene caused ectopic MOD-5/SERT expression and 5-HT accumulation in those neurons (Fig. 6f,h). Collectively, these results support the idea that the synaptic release of 5-HT by 5-HT-absorbing neurons is not required for the regulation of the behavioral response to food deprivation. The partial rescue by MOD-5/SERT function in some ectopic neurons but not others could reflect the importance of the position of these neurons. However, we cannot exclude the importance of other properties of AIM and RIH neurons in this behavioral circuitry.
Discussion
While SSRIs are effective in the treatment of depression, reduced SERT function has been implicated in elevated anxiety in mammals (Ansorge et al., 2007). SERT knock-out mice are impaired in coping with stress (Wellman et al., 2007). A low-expressing polymorphism in the human SERT gene SLC6A4 is linked to heightened fear and anxiety in individuals with stressful life history (Lesch et al., 1996; Caspi et al., 2003). In this article, we provide genetic evidence that MOD-5/SERT function in 5-HT-absorbing neurons governs the behavioral response to the experience of food deprivation in C. elegans. 5-HT-absorbing neurons are a common feature conserved from C. elegans to mammals (Verney et al., 2002; Gaspar et al., 2003). It may be reasonable to envision that SERT subserves two distinctive functions in the nervous system: SERT at the synapse of 5-HT-producing neurons regulates “hard-wired” neuronal activity; and SERT in 5-HT-absorbing neurons may integrate the activity of multiple 5-HT-producing neurons and neural circuitry to coordinate behavior (Fig. 7).
Figure 7.
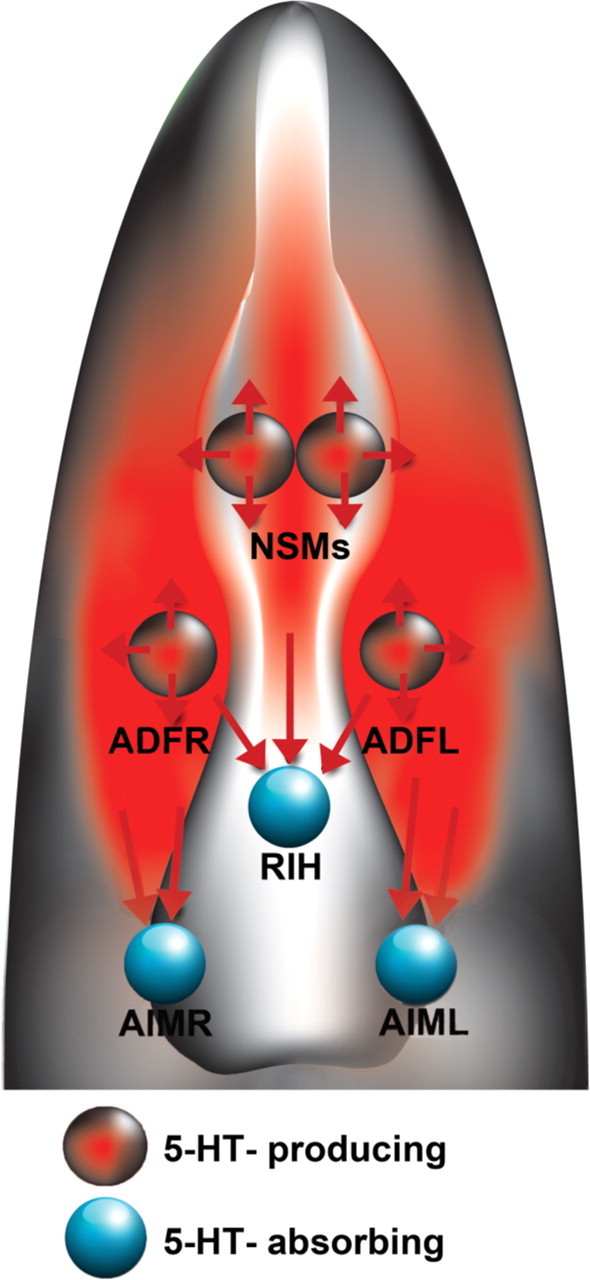
A model for 5-HT regulation of slowing response. The ADF chemosensory neurons sense external cues; they may couple environmental food signals and 5-HT neurotransmission. The NSM secretory neurons detect food passing through the pharynx; they may couple food ingestion and 5-HT neurotransmission. Following food deprivation, ADF and NSM neurons release 5-HT at the synapses, as well as into the extrasynaptic space, inducing slowing response when food becomes available. MOD-5/SERT in RIH and AIM neurons absorb 5-HT from the extrasynaptic space, preventing exaggerated response. The drawing depicts a ventral view of the head region with anterior up.
What could be the 5-HT source for 5-HT-absorbing neurons? Several lines of evidence suggest that AIM and RIH absorb 5-HT from extrasynaptic space. First, AIM and RIH neurons are not connected to ADF and NSM neurons (White et al., 1986; Hall and Altun, 2008). In addition, NSM in the pharynx can efficiently take up 5-HT originated from ADF neurons located in the somatic system, indicating that 5-HT travels long distances. Second, CAT-1/VMAT is localized to puncta in the soma, axons, and dendrites of the 5-HT-producing neurons. The distribution of CAT-1/VMAT mostly overlapped with MOD-5/SERT immunoreactivity in NSM neurons. But, we observed very little, if any, MOD-5/SERT immunoreactivity in ADF and HSN neurons, suggesting that many sites in ADF and HSN release 5-HT but do not efficiently reuptake it. Third, ectopic expression of MOD-5 resulted in ectopic 5-HT accumulation in nonserotonergic neurons, indicating that many neurons could have access to 5-HT released from ADF and NSM. Analyses of the synaptic mutants suggest that 5-HT released either by SV or DCV diffuses readily to the extrasynaptic space adjacent to AIM and RIH. This idea is consistent with the ultrastructural analyses of rodent and monkey brains, in which 5-HT was found in two classes of varicosities: one class shows 5-HT vesicles in a well defined synaptic specialization with established synaptic contacts, and the other class of 5-HT varicosities does not exhibit synaptic specialization (Calas et al., 1974; Descarries et al., 1990; Maley et al., 1990).
What could be the role of 5-HT-absorbing neurons in 5-HT signaling? Two alternative models have been proposed: (1) 5-HT-absorbing neurons create “a morphogenetic gradient of 5-HT” to influence the targets at a distance; and (2) they rerelease 5-HT at their synapses (Gaspar et al., 2003). Our data thus far favor the first model. The AN::mod-5 transgene expressing MOD-5/SERT in AIM and several nonserotonergic neurons fully corrected the exaggerated behavior of the mod-5 mutant, indicating that MOD-5/SERT function in these 5-HT-absorbing neurons is sufficient to govern the behavior. The results of neuron-specific inhibition of synaptic transmission by tetanus toxin suggest that lacking synaptic transmission of 5-HT-absorbing neurons cannot account for exaggerated slowing response. However, expressing MOD-5/SERT in 5-HT-producing neurons also partially rescued the exaggerated behavior of mod-5 mutants. Extra chromosomal arrays of the transgenes frequently result in overexpression of the genes. Indeed, ADF MOD-5/SERT immunoreactivity was undetectable in WT animals, but can be clearly seen in the transgenic animals expressing the PN::mod-5 transgene. Elevated MOD-5/SERT abundance in the 5-HT-producing neurons could reduce 5-HT signals. These observations could imply that regulated 5-HT release from 5-HT-producing neurons underscores the sensation of food deprivation and the memory of the aversive experience that initiated the slowing response upon return to food, whereas MOD-5/SERT function in 5-HT-absorbing neurons subserves to measure the response, preventing overreaction.
We showed that ectopic MOD-5/SERT function in some neurons partially rescued mod-5 behavior, but in some neurons did not, although they all can efficiently absorb 5-HT. These results indicate that the effects of these ectopic 5-HT-absorbing neurons on this behavioral circuitry are not identical to those of AIM and RIH neurons, and differ from each other. One possible model for this is that MOD-5/SERT in AIM and RIH controls spatial–temporal 5-HT levels in extracellular space to modulate the activity of the behavioral circuits. However, CAT-1/VMAT is expressed in AIM and RIH (Duerr et al., 1999; Sze et al., 2002), indicating that these neurons are capable of packing absorbed 5-HT into synaptic vesicles. Thus, it remains plausible that AIM and RIH can release 5-HT. For instance, they may release 5-HT via DCV at nonsynaptic sites, which could not be blocked by the tetanus toxin. It is also plausible that AIM and RIH indeed release 5-HT at the synapse to coordinate other behavior and physiological function, but not the slowing response. Alternatively, CAT-1/VMAT may direct absorbed 5-HT to intracellular degradation pathways. Elizabeth Sawin (1996) reported in her PhD thesis that laser ablation of AIM and RIH neurons in WT animals caused exaggerated slowing behavior. The question has been why and how eliminating these “serotonergic neurons” could result in an effect of increased 5-HT. Our genetic analyses shed some light on MOD-5/SERT function in these neurons, and together these studies provide a foundation for further investigating the mechanisms of 5-HT-absorbing neurons in 5-HT signaling and its modulation of behavior. 5-HT-absorbing neurons are present in the developing mammalian nervous systems as well as in the adult brain (Vanhatalo and Soinila, 1998; Hansson et al., 1999; Gaspar et al., 2003). It is plausible that some fundamental aspects of 5-HT-absorbing neurons are conserved evolutionarily across phyla.
Footnotes
This work was supported by a grant from National Institute on Mental Health to J.Y.S. We thank C. Bargmann for providing ADF::tph-1 and NSM::tph-1 constructs, E. Jorgensen for tetanus toxin-expressing vectors, R. Horvitz and the Caenorhabditis Genetics Center for worm strains, and R. Partel for construction of the mod-5 cDNA clone. We specially thank Shohei Mitani for deletion alleles of amino acid transporters and Zeynep Altun for her help in identification of 5-HT immunoreactivity in the I5 and M5 neurons.
References
- Albertson DG, Thomson JN. The pharynx of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976;275:299–325. doi: 10.1098/rstb.1976.0085. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Hen R, Gingrich JA. Neurodevelopmental origins of depressive disorders. Curr Opin Pharmacol. 2007;7:8–17. doi: 10.1016/j.coph.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Avery L, Horvitz HR. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J Exp Zool. 1990;253:263–270. doi: 10.1002/jez.1402530305. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Horvitz HR. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991;7:729–742. doi: 10.1016/0896-6273(91)90276-6. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Berson HE, Fremeau RT, Jr, Caron MG, Peek MM, Prince HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Calas A, Alonso G, Arnauld E, Vincent JD. Demonstration of indolaminergic fibres in the media eminence of the duck, rat and monkey. Nature. 1974;250:241–243. doi: 10.1038/250241a0. [DOI] [PubMed] [Google Scholar]
- Cases O, Lebrand C, Giros B, Vitalis T, De Maeyer E, Caron MG, Price DJ, Gaspar P, Seif I. Plasma membrane transporters of serotonin, dopamine, and norepinephrine mediate serotonin accumulation in atypical locations in the developing brain of monoamine oxidase A knock-outs. J Neurosci. 1998;18:6914–6927. doi: 10.1523/JNEUROSCI.18-17-06914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Charlie NK, Schade MA, Thomure AM, Miller KG. Presynaptic UNC-31 (CAPS) is required to activate the G alpha(s) pathway of the Caenorhabditis elegans synaptic signaling network. Genetics. 2006;172:943–961. doi: 10.1534/genetics.105.049577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates PM. Encyclopedia of dietary supplements. New York: Marcel Dekker; 2005. [Google Scholar]
- D'Amato RJ, Blue ME, Largent BL, Lynch DR, Ledbetter DJ, Molliver ME, Snyder SH. Ontogeny of the serotonergic projection to rat neocortex: transient expression of a dense innervation to primary sensory areas. Proc Natl Acad Sci U S A. 1987;84:4322–4326. doi: 10.1073/pnas.84.12.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MW, Morton JJ, Carroll D, Jorgensen EM. Gene activation using FLP recombinase in C. elegans. PLoS Genet. 2008;4:e1000028. doi: 10.1371/journal.pgen.1000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Miguel FF, Trueta C. Synaptic and extrasynaptic secretion of serotonin. Cell Mol Neurobiol. 2005;25:297–312. doi: 10.1007/s10571-005-3061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai C, Garriga G, McIntire SL, Horvitz HR. A genetic pathway for the development of the Caenorhabditis elegans HSN motor neurons. Nature. 1988;336:638–646. doi: 10.1038/336638a0. [DOI] [PubMed] [Google Scholar]
- Descarries L, Audet MA, Doucet G, Garcia S, Oleskevich S, Séguéla P, Soghomonian JJ, Watkins KC. Morphology of central serotonin neurons—brief review of quantified aspects of their distribution and ultrastructural relationships. Ann N Y Acad Sci. 1990;600:81–92. doi: 10.1111/j.1749-6632.1990.tb16874.x. [DOI] [PubMed] [Google Scholar]
- Duerr JS, Frisby DL, Gaskin J, Duke A, Asermely K, Huddleston D, Eiden LE, Rand JB. The cat-1 gene of Caenorhabditis elegans encodes a vesicular monoamine transporter required for specific monoamine-dependent behaviors. J Neurosci. 1999;19:72–84. doi: 10.1523/JNEUROSCI.19-01-00072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney M, Ruvkun G, Horvitz HR. The C. elegans cell lineage and differentiation gene unc-86 encodes a protein with a homeodomain and extended similarity to transcription factors. Cell. 1988;55:757–769. doi: 10.1016/0092-8674(88)90132-8. [DOI] [PubMed] [Google Scholar]
- Fukushige T, Hawkins MG, McGhee JD. The GATA-factor elt-2 is essential for formation of the Caenorhabditis elegans intestine. Dev Biol. 1998;198:286–302. [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Hall DH, Hedgecock EM. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell. 1991;65:837–847. doi: 10.1016/0092-8674(91)90391-b. [DOI] [PubMed] [Google Scholar]
- Hall DH, Altun ZF. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 2008. C. elegans atlas. [Google Scholar]
- Hammarlund M, Watanabe S, Schuske K, Jorgensen EM. CAPS and syntaxin dock dense core vesicles to the plasma membrane in neurons. J Cell Biol. 2008;180:483–491. doi: 10.1083/jcb.200708018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson SR, Mezey E, Hoffman BJ. Serotonin transporter messenger RNA expression in neural crest-derived structures and sensory pathways of the developing rat embryo. Neuroscience. 1999;89:243–265. doi: 10.1016/s0306-4522(98)00281-4. [DOI] [PubMed] [Google Scholar]
- Hare EE, Loer CM. Function and evolution of the serotonin-synthetic bas-1 gene and other aromatic amino acid decarboxylase genes in Caenorhabditis. BMC Evol Biol. 2004;4:24. doi: 10.1186/1471-2148-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K, Staunton J, Saifee O, Nonet M, Thomas JH. aex-3 encodes a novel regulator of presynaptic activity in C-elegans. Neuron. 1997;18:613–622. doi: 10.1016/s0896-6273(00)80302-5. [DOI] [PubMed] [Google Scholar]
- Kage E, Hayashi Y, Takeuchi H, Hirotsu T, Kunitomo H, Inoue T, Arai H, Iino Y, Kubo T. MBR-1, a novel helix-turn-helix transcription factor, is required for pruning excessive neurites in Caenorhabditis elegans. Curr Biol. 2005;15:1554–1559. doi: 10.1016/j.cub.2005.07.057. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kullyev A, Dempsey CM, Miller S, Kuan CJ, Hapiak VM, Komuniecki RW, Griffin CT, Sze JY. A genetic survey of fluoxetine action on synaptic transmission in Caenorhabditis elegans. Genetics. 2010;186:929–941. doi: 10.1534/genetics.110.118877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Seif I, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Wehrlé R, Blakely RD, Edwards RH, Gaspar P. Transient developmental expression of monoamine transporters in the rodent forebrain. J Comp Neurol. 1998;401:506–524. [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Liu Y, Edwards RH. The role of vesicular transport proteins in synaptic transmission and neural degeneration. Annu Rev Neurosci. 1997;20:125–156. doi: 10.1146/annurev.neuro.20.1.125. [DOI] [PubMed] [Google Scholar]
- Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, Clardy J, Bargmann CI. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature. 2009;458:1171–1175. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley BE, Engle MG, Humphreys S, Vascik DA, Howes KA, Newton BW, Elde RP. Monoamine synaptic structure and localization in the central-nervous-system. J Electron Microsc Tech. 1990;15:20–33. doi: 10.1002/jemt.1060150104. [DOI] [PubMed] [Google Scholar]
- McEwen JM, Kaplan JM. UNC-18 promotes both the anterograde trafficking and synaptic function of Syntaxin. Mol Biol Cell. 2008;19:3836–3846. doi: 10.1091/mbc.E08-02-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ, Liu Y, Peter D, Edwards RH, Pickel VM. The vesicular monoamine transporter 2 is present in small synaptic vesicles and preferentially localizes to large dense core vesicles in rat solitary tract nuclei. Proc Natl Acad Sci U S A. 1995;92:8773–8777. doi: 10.1073/pnas.92.19.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet ML, Grundahl K, Meyer BJ, Rand JB. Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell. 1993;73:1291–1305. doi: 10.1016/0092-8674(93)90357-v. [DOI] [PubMed] [Google Scholar]
- Nonet ML, Saifee O, Zhao H, Rand JB, Wei L. Synaptic transmission deficits in Caenorhabditis elegans synaptobrevin mutants. J Neurosci. 1998;18:70–80. doi: 10.1523/JNEUROSCI.18-01-00070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz CO, Etchberger JF, Posy SL, Frøkjaer-Jensen C, Lockery S, Honig B, Hobert O. Searching for neuronal left/right asymmetry: genomewide analysis of nematode receptor-type guanylyl cyclases. Genetics. 2006;173:131–149. doi: 10.1534/genetics.106.055749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan R, Sawin ER, Trent C, Horvitz HR. Mutations in the Caenorhabditis elegans serotonin reuptake transporter MOD-5 reveal serotonin-dependent and -independent activities of fluoxetine. J Neurosci. 2001;21:5871–5884. doi: 10.1523/JNEUROSCI.21-16-05871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JE, Davis WS, Jorgensen EM. UNC-13 is required for synaptic vesicle fusion in C-elegans. Nat Neurosci. 1999;2:959–964. doi: 10.1038/14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivard L, Srinivasan J, Stone A, Ochoa S, Sternberg PW, Loer CM. A comparison of experience-dependent locomotory behaviors and biogenic amine neurons in nematode relatives of Caenorhabditis elegans. BMC Neurosci. 2010;11:22. doi: 10.1186/1471-2202-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saifee O, Wei L, Nonet ML. The Caenorhabditis elegans unc-64 locus encodes a syntaxin that interacts genetically with synaptobrevin. Mol Biol Cell. 1998;9:1235–1252. doi: 10.1091/mbc.9.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin ER. Massachusetts Institute of Technology; 1996. Genetic and cellular analysis of modulated behaviors in Caenorhabditis elegans. PhD Thesis. [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Schackwitz WS, Inoue T, Thomas JH. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron. 1996;17:719–728. doi: 10.1016/s0896-6273(00)80203-2. [DOI] [PubMed] [Google Scholar]
- Sieburth D, Madison JM, Kaplan JM. PKC-1 regulates secretion of neuropeptides. Nat Neurosci. 2007;10:49–57. doi: 10.1038/nn1810. [DOI] [PubMed] [Google Scholar]
- Speese S, Petrie M, Schuske K, Ailion M, Ann K, Iwasaki K, Jorgensen EM, Martin TF. UNC-31 (CAPS) is required for dense-core vesicle but not synaptic vesicle exocytosis in Caenorhabditis elegans. J Neurosci. 2007;27:6150–6162. doi: 10.1523/JNEUROSCI.1466-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Sze JY, Victor M, Loer C, Shi Y, Ruvkun G. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000;403:560–564. doi: 10.1038/35000609. [DOI] [PubMed] [Google Scholar]
- Sze JY, Zhang S, Li J, Ruvkun G. The C. elegans POU-domain transcription factor UNC-86 regulates the tph-1 tryptophan hydroxylase gene and neurite outgrowth in specific serotonergic neurons. Development. 2002;129:3901–3911. doi: 10.1242/dev.129.16.3901. [DOI] [PubMed] [Google Scholar]
- Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Soinila S. Serotonin is not synthesized, but specifically transported, in the neurons of the hypothalamic dorsomedial nucleus. Eur J Neurosci. 1998;10:1930–1935. doi: 10.1046/j.1460-9568.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- Verney C, Lebrand C, Gaspar P. Changing distribution of monoaminergic markers in the developing human cerebral cortex with special emphasis on the serotonin transporter. Anat Rec. 2002;267:87–93. doi: 10.1002/ar.10089. [DOI] [PubMed] [Google Scholar]
- Weimer RM, Richmond JE, Davis WS, Hadwiger G, Nonet ML, Jorgensen EM. Defects in synaptic vesicle docking in unc-18 mutants. Nat Neurosci. 2003;6:1023–1030. doi: 10.1038/nn1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–184. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]