Abstract
Heparosan is a polysaccharide, which serves as the critical precursor in heparin biosynthesis and chemoenzymatic synthesis of bioengineered heparin. Because the molecular weight of microbial heparosan is considerably larger than heparin, the controlled depolymerization of microbial heparosan is necessary prior to its conversion to bioengineered heparin. We have previously reported that other acidic polysaccharides could be partially depolymerized with maintenance of their internal structure using a titanium dioxide-catalyzed photochemical reaction. This photolytic process is characterized by the generation of reactive oxygen species that oxidize individual saccharide residues within the polysaccharide chain. Using a similar approach, a microbial heparosan from Escherichia coli K5 of molecular weight >15,000 was depolymerized to a heparosan of molecular weight 8,000. The 1H-NMR spectra obtained showed that the photolyzed heparosan maintained the same structure as the starting heparosan. The polysaccharide chains of the photochemically depolymerized heparosan were also characterized by electrospray ionization-Fourier-transform mass spectrometry. While the chain of K5 heparosan starting material contained primarily an even number of saccharide residues, as a result of coliphage K5 lyase processing, both odd and even chain numbers were detected in the photochemically-depolymerized heparosan. These results suggest that the photochemical depolymerization of heparosan was a random process that can take place at either the glucuronic acid or the N-acetylglucosamine residue within the heparosan polysaccharide.
Keywords: heparosan, heparin, photochemical, depolymerization, titanium dioxide
INTRODUCTION
Heparin is a heterogeneous polydisperse mixture of sulfated polysaccharides ranging in molecular weight from 5,000 to 30,000 with an average molecular weight of 15,000, and is widely used as an anticoagulant drug.1–5 In 2008, several lots of porcine intestinal heparin were contaminated with oversulfated chondroitin sulfate, which caused anaphylactoid reactions and over 100 deaths in patients receiving the contaminated drug.6–8 While effective assays are being developed for the detection of known and unknown contaminants and adulterants in heparin,9–11 new highly regulated processes must be implemented in heparin production. Recently, our laboratory has chemoenzymatically synthesized heparin in small quantities by following the heparin and heparan sulfate biosynthetic pathways using cloned and expressed enzymes.12 Starting from E. coli K5 heparosan, two chemical steps and four enzymatic steps led our group to prepare a bioengineered heparin with anticoagulant properties comparable to porcine intestinal heparin. The structure of the capsular polysaccharide from E. coli O10:K5:H4 heparosan is similar to that of desulfated heparin.13 Since heparin chain size plays a critical role in mediating protein binding for blood anticoagulant activity, control of the polysaccharide chain length of the bacterial precursor from E. coli K5 is necessary in the development of bioengineered heparin, and other heparin-related drugs.14
Previously, it has been determined that the molecular weight of microbial heparosan is greater than that of heparin, and that controlled depolymerization of heparosan is necessary prior to its conversion into bioengineered heparin.15 Although controlled depolymerization of heparosan by heparin lyase III treatment can be easily performed, the resulting product contains modified saccharide units within the polysaccharide backbone.16 In previous studies we reported the depolymerization of simple, nonsulfated polysaccharides, sodium alginate and pectin, using a photochemical reaction relying on titanium dioxide catalyst.17,18 Furthermore, we found that titanium dioxide can catalyze depolymerization of unfractionated heparin to prepare photodegraded low molecular weight heparin (pLMWH) without structural modifications.19 This photochemical reaction has been investigated using liquid chromatography/mass spectrometry (LC/MS). The polysaccharide chains of reduced length have both an odd and even number of saccharide residues.
In this paper, crude and pure heparosan are depolymerized by titanium dioxide with visible light of >370 nm. Structures of photodegraded heparosan were same as a starting material, suggesting that photochemical depolymerization of heparosan is useful for preparation of a bioengineered heparin. Finally, the odd and even chain lengths of photodegraded heparosan were characterized by electrospray ionization Fourier-transform mass spectrometry (ESI-FT-MS).
MATERIALS AND METHODS
Chemicals and Equipment
Titanium dioxide (anatase type, particle size average, 50 μm) was purchased from Wako Pure Chemical Co. (Osaka, Japan). Electrophoresis-grade acrylamide, N,N′-methylene-bis-acrylamide, sucrose, glycine, ammonium persulfate (APS), N,N,N′,N′-tetramethylenediamine (TEMED), and 4–15% Ready Gel Tris-HCl precast gels were from Bio-Rad, (Hercules, CA, USA). Boric acid, disodium salt of ethylenediaminetetraacetic acid (EDTA), phenol red, and Alcian blue were from Fisher (Pittsburgh, PA, USA). D2O (99.96 atom %) was from Sigma-Aldrich (St. Louis, MO USA).
The photochemical reaction device (Sen Lights Corporation, Osaka, Japan) consisted of a VG1500 reaction tank with 5 inlets, a UV light source (high pressure mercury lamp HL100 CH-4, 100W), a power source (HB100P-1), and lamp jacket-Pyrex glass JW-1G. The apparatus was connected with a circulating water system to cool the lamp.
Growth and harvest of E. coli K5
The E. coli strain Bi 8337/41 (O10:K5:H4) was from ATCC (Manassas, VA, USA). The E.coli K5 was grown in a 7 L fermentor.14 Briefly, the fermentation consists of a batch growth stage and a fed batch growth stage. The composition of the medium for the batch growth in the fermentation was: 20 g/L glucose, 10 or 300 mg/L thiamine, 13.5 g KH2PO4; 4.0 g (NH4)2HPO4, 1.4 g MgSO4·7H2O, 1.7 g citric acid, and 10.0 mL trace metal solution. Trace metal solution consisted of (per L of 5 M HCl) 10.0 g FeSO4·7H2O, 2.0 g CaCl2, 2.2 g ZnSO4·7H2O, 0.5 g MnSO4·4H2O, 1.0 g CuSO4·5H2O, 0.1 g (NH4)6Mo7O24·4H2O, and 0.02 g Na2B4O7·10H2O. The feeding solution used in the fed batch stage consisted of (per L): 250–1000 g glucose, 20 g MgSO4·7H2O and 0.15 or 0.25 g thiamine.1 The pH was kept at a value of ~ 7.0 throughout the fermentation.
The batch growth stage began with the inoculation of seed culture (300 mL of 5.6 g/L DCW) obtained from a shake flask in late exponential growth. The temperature was maintained at ~ 37°C, and the pH was maintained at approximately 7 (by adding 29% ammonia solution). Air was sparged into the fermentor to supply oxygen, and the stirrer speed was set to 520 rpm.
The second stage of the fermentation began after glucose in the batch growth medium had been depleted and the dissolved oxygen showed a sharp increase. The feeding solution was then fed exponentially as described previously.14 Fermentation supernatant was harvested after no further increase in cell density was observed at 32 h.
Preparation of extracellular polysaccharide of E. coli K5
Crude heparosan was prepared from fermentation supernatant (100 mL) by adding absolute ethanol (Fisher Scientific, PA) in a 4:1 ratio. A precipitate of heparosan was allowed to form at 4° C for 24 h in an explosion-proof refrigerator. Crude heparosan was recovered in a pellet form using Sorvall Evolution RC superspeed centrifuge at 2,400 × g for 1 hour. The recovered pellet was resolubilized in water and dialyzed against water using 3,500 Da molecular weight cut-off (MWCO) Spectra/por®Dialysis membranes (Spectrum Laboratories Inc.) for 3 days. The retentate was concentrated using Buchi rotavapor R-200 and then lyophilized. Pure heparosan was prepared by an alternative route.2 Fermentation supernatant (100 mL) was diluted two folds using buffer A (50 mM NaCl and 20 mM NaOAc, pH 4.0) and the pH was readjusted to 4.0. This diluted fermentation broth was then purified using DEAE Sepharose fast flow resin packed weak anion exchange column: heparosan (20 mg) was bound to 1 mL of DEAE resin (GE Healthcare Bio-Sciences Corp., NJ), which was equilibrated at pH 4.0 using 3 column volumes of buffer A. Diluted fermentation broth (200 mL containing 1 g of heparosan) was then loaded onto a 150 mL column under gravity flow conditions. The bound heparosan was eluted using 2 column volumes of buffer B (1 M NaCl and 20 mM NaOAc, pH 4.0). Pure heparosan was recovered from the eluent by ethanol precipitation, dialysis, rotary evaporation and lyophilization as previously described for the recovery of crude heparosan.
Depolymerization of K5 heparosan by photochemical reaction
K5 heparosans (10 mg) were each dissolved in 1 mL water with 1 mg of titanium dioxide (TiO2) particles and closed by a screw cap. The sample tube (borosilicate glass) was then placed in the photochemical reaction tank and exposed to light at room temperature. A mechanical stirrer was used in addition to a magnetic stirrer to ensure the dissolution of air into the solution. After the reaction, the sample was centrifuged at 1,500 × g for 5 min at 20° C and the supernatant was filtered through 0.45 μm membrane filter (Dismic-13HP; Advantec, Tokyo, Japan) to eliminate all of the TiO2, and the product solution was dialyzed and lyophilized. The change in molecular weights of degraded heparosan samples were monitored by gel permeation chromatography (GPC) high performance liquid chromatography (HPLC).
Estimation of the average molecular weight
GPC-HPLC was performed using a TSK-GEL G4000PWxl size exclusion column with a sample injection volume of 20 μL (100 μg) and a flow rate of 0.6 mL/min on an apparatus composed of a Shimadzu LC-10Ai pump, a Shimadzu CBM-20A controller and a Shimadzu RID-10A refractive index detector. The mobile phase consisted of 0.1 M NaNO3. The column was maintained at 40°C with an Eppendorf column heater during the chromatography. The GPC chromatograms were recorded with the LCsolution Version 1.25 software and analyzed with its “GPC Postrun” function. Hyaluronan of molecular weights 30.6 kDa, 54 kDa, 128 kDa and 262 kDa were purchased from Hyalose L.L.C. (Oklahoma City, Oklahoma) was used as calibrants for the standard curve.
NMR analysis
All 1H-NMR were conducted on a Brüker 600 MHz NMR spectrometer. Heparosan samples (2 mg) were dissolved in 0.5 mL of D2O (99.96%, Sigma-Aldrich, Co.) and exchanged 3-times by lyophilization. The samples were prepared in 5 mm standard NMR tubes. Acquisition of the spectra was carried out using TOPSPIN 2.0 software. All the spectra were acquired at the temperature of 298 K. A recycle delay time of 8 s was used. The acquired 1H-NMR spectra were processed with MestReC NMR software for phase and baseline correction.
Continuous-elution preparative polyacrylamide gel electrophoresis PAGE
Photolytically depolymerized heparosan (pure) was fractionated by continuous elution preparative polyacrylamide gel electrophoresis (PAGE) to decrease chain size heterogeneity as described elsewhere.15,20 Lower molecular weight fractions of heparosan were separated by a 10% total acrylamide (10% T) resolving gel containing 9.66% (w/v) acrylamide, 0.33% (w/v)N,N′-methylene-bis-acrylamide, and 5% sucrose. Monomer T solution (2 mL) was prepared in resolving buffer (0.1 M boric acid, 0.1 M Tris, 0.01 M disodium EDTA, pH 8.3) and allowed to polymerize as a 5 cm × 7 mm diameter resolving gel column using 2 μL TEMED and 12 μL 10% APS. A stacking gel of 0.5 mL was used with 0.5 μL TEMED and 30 μL 10% APS.
The heparosan (1.5 mg) was loaded as 25% (w/v) sucrose above the stacking gel. Electrode running buffer was 1 M glycine and 0.2 M Tris at pH 9, and the lower chamber and elution buffer chamber were filled with resolving buffer. A peristaltic pump was set to 80 μL/min and subjected to electrophoresis at a constant power of 1W for 4 h. Collected fractions (numbering 80) were analyzed by analytical PAGE on 0.75-mm × 6.8-cm × 8.6-cm minigels cast in-house using 12% T resolving gel monomer solution and 5% T stacking gel monomer solution. Upper chamber and lower chamber were filled with buffer A (0.2 M Tris (pH 9 without adjustment) and 1 M glycine) and buffer B (0.1 M Tris-HCl (pH 8.3), 0.1 M boric acid 10 mM EDTA), respectively. The mini-gels were subjected to electrophoresis at constant 200 V, stained in 0.5% (w/v) alcian blue containing 2% (v/v) aqueous acetic acid solution for 30 min, and destained in water. Heparosan of various molecular weights (fraction 10, 13, 16, 20, 23, 25) were chosen and mixed on a vortex mixer with 240 μL of 4 M salt and desalted using 10,000 MWCO Amicon Ultra-0.5 centrifugal filter devices (Millipore, Billerica, MA, USA) prior to electrospray ionization (ESI) Fourier transform (FT) mass spectrometric (MS) analysis15 for intact chain molecular weights of heparosan.
Electrospray ionization Fourier-transform mass spectrometry
ESI-FT mass spectra were acquired at a resolution of 60,000 on an LTQ-Orbitrap-XL mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA) in the negative ionization mode as previously.15 Parameters used include spray voltage 3.5 kV, capillary temperature 270°C, capillary voltage −15°C, tube lens −100 V; the sheath and auxiliary gas flow rates were set to 10 and 0, respectively. Mobile phase consisting of 1:1 H2O: methanol with 0.2% formic acid was delivered by an Agilent 1200 nano-LC pump at a flow rate of 50 μL/min, and 1 μL of each samples was infused through an Agilent 1200 autosampler.
RESULTS AND DISCUSSION
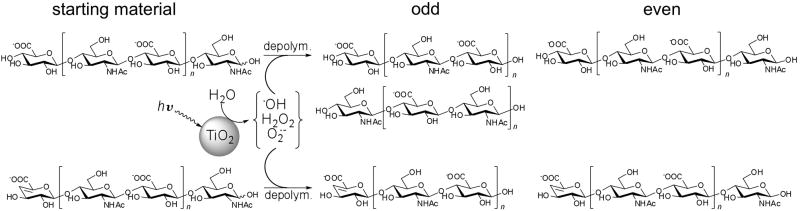
Heparosan was prepared by fermentation of E. coli K5. Crude heparosan was recovered from the fermentation supernatant by ethanol precipitation and dialysis. Pure heparosan was recovered and purified from the fermentation supernatant by weak anion exchange chromatography, ethanol precipitation and dialysis. Heparosan isolated from E. coli K5 was photolyzed using TiO2-catalyzed photochemical depolymerization conditions developed for other acidic polysaccharides.17,18,19 The structure of heparosan and the putative reaction products are shown in Figure 1.
Figure 1. Controlled photolytic depolymerization of heparosan.
When titanium dioxide particles absorb irradiated light (>370 nm), a photogenerated electron (e−) and hole (h+) react with the oxygen or water on the surface of the activated titanium dioxide particles, generating reactive oxygen species (H2O2, O2−, ·OH).22,23,24
The molecular weight of starting material of crude and pure heparosan were determined using GPC-HPLC (Fig. 2). Number-averaged molecular weight (MN) of starting materials, crude heparosan and pure heparosan were 15.2 kDa and 14.6 kDa, respectively (Table 1). Table 1 also shows weight-average molecular weight (MW), and the polydispersity (P) of the crude and pure heparosan before and after photolysis. The molecular weight of starting heparosan determined by GPC-HPLC was consistent with previously reported molecular weight of heparosan.15 Heparosan is used in our laboratory as a precursor in the chemoenzymatic preparation of bioengineered heparin.12 Thus, the heparosan precursor must have a molecular weight of ~8–9 kDa to afford a bioengineered heparin of molecular weight ~12–20 kDa, corresponding to the molecular weight of porcine intestinal heparin used pharmaceutically as a clinical anticoagulant. The goal of the current study was to rely on photolysis to reduce the molecular weight of either crude or pure heparosan obtained from E. coli K5 to the requisite size. Depolymerization of heparosan by time-controlled photochemical reaction was performed (Figure 1) and resulting MW heparosan were determined by GPC-HPLC (Figure 2). Heparosan obtained by 9 h photolysis of crude heparosan had a molecular weight of 8.2 kDa (Table 1). In contrast, photolysis of pure heparosan occurred at a much faster rate than crude heparosan and a product of 8.5 kDa was obtained after 1 h of photolysis (Figure 2, Table 1).
Figure 2. GPC-HPLC analysis of heparosan and photolyzed heparosan.
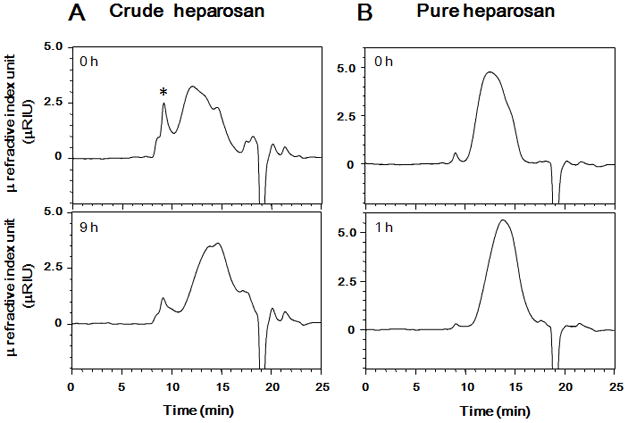
A. Crude heparosan (upper chromatogram) and crude heparosan photolyzed for 9 h (lower chromatogram). B. Pure heparosan (upper chromatogram) and pure heparosan photolyzed for 1 h (lower chromatogram). HPLC conditions: column, a TSK-GEL G4000PWXL size exclusion column; mobile phase, 0.1 M NaNO3 at a flow rate of 0.6 mL/min; detection, refractive index.
Table 1.
Molecular weight (MN, MW and P) of starting heparosan and photochemically-depolymerized heparosan.
| Photolysis time (h) | MN | MW | P | |
|---|---|---|---|---|
| Crude heparosan | 0 | 15246 | 77829 | 5.10 |
| 9 | 8158 | 28671 | 3.44 | |
| Pure heparosan | 0 | 14559 | 29024 | 1.99 |
| 1 | 8501 | 16400 | 1.93 |
Next, the 1H NMR spectra (600 MHz) of the heparosan samples were examined (Figure 3). Crude heparosan (~85% pure by NMR) showed additional minor signals associated with protein impurities. Pure heparosan (~95 % pure) gave a much cleaner spectrum in which all major signals could be assigned.13,15 The signals for the photolyzed heparosan sample showed the same chemical shift values as observed for the starting crude or pure heparosan. This suggests that no major modification of the polysaccharide backbone had taken place, consistent with our observations when examining the photolysis of other, structurally related polysaccharides.17,18,19
Figure 3. 1H-NMR spectra of heparosan and photolyzed heparosan.
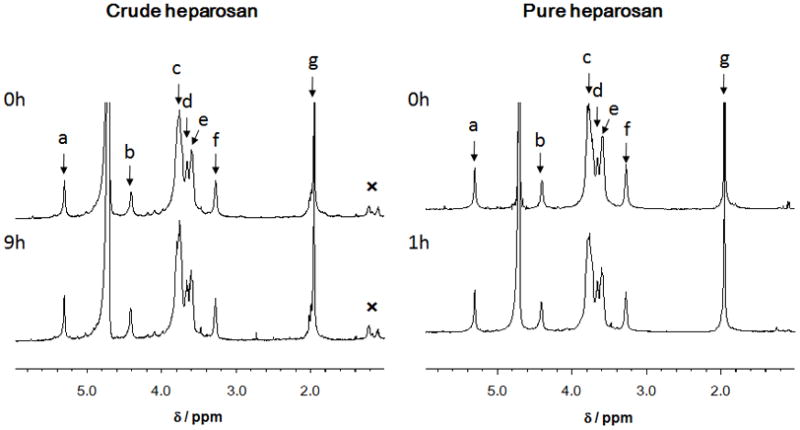
A. Crude heparosan (upper spectrum) and crude heparosan photolyzed for 9 h. B. Pure heparosan (upper spectrum) and crude heparosan photolyzed for 1 h (lower spectrum). Peak assignments are: a. GlcNAc H1; b. GlcA H1; c. GlcNAc H2, H3, H5 and H6; d, GlcA H5; e, GlcA H3, H4 and GlcNAc H4; f, GlcA H2; g, GlcNAc CH3. An impurity is marked with an “✕” near 1 ppm.
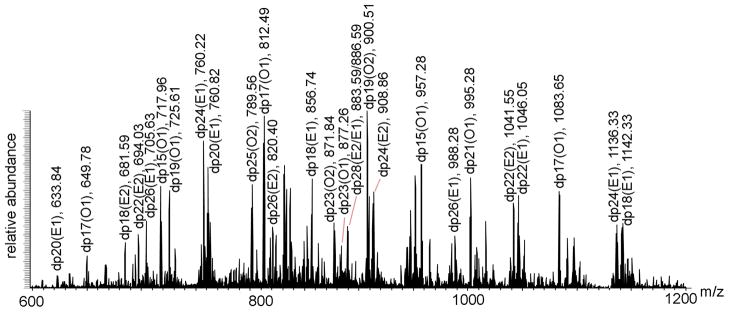
Next, we examined the molecular weight of the photochemically depolymerized pure heparosan by ESI-FT-MS.15 Continuous elution preparative PAGE had been previously described by our laboratory as a method to recover chains of a single size (or degree of polymerization, dp) from a complex polydisperse mixture of polysaccharide chains. Using this method, oligosaccharides were prepared and analyzed by ESI-FT-MS. The resulting spectra are shown in Figure 4. Interpretation of these spectral data allows assignment of exact mass and, hence, chain structure (Supplemental Table 1). As a result of this analysis it is clear that while the starting heparosan (pure) has chains containing primarily an even number of saccharide residues and that these chains are terminated with an unsaturated uronic acid residue (ΔUA) at their non-reducing end. These chains have the general structure ΔUA- (GlcNAc-GlcA) n-GlcNAc where n = 5 to 434. In contrast, depolymerized heparosan (pure) had chains with both an odd and even number of saccharide residues some terminated with ΔUA and some terminated with GlcNAc or with GlcA. These chains, which could be detected by ESI-FT-MS, have multiple structures including GlcNAc-(GlcA-GlcNAc)n (Type O1), GlcA-(GlcNAc-GlcA)n (Type O2), ΔUA-(GlcNAc-GlcA)n (Type O3), and GlcA-(GlcNAc-GlcA)n-GlcNAc and GlcNAc- (GlcA-GlcNAc)n-GlcA (type E1), ΔUA- (GlcNAc-GlcA)n-GlcNAc (Type E2) where n = 8 to 50. Since chains having both an even and odd number of saccharide residues are detected in commercial bovine lung heparin (30% depolymerized by heparinase), using liquid chromatography/mass spectrometry (LC/MS),21 photochemically-depolymerized heparosan may be directly used as a starting material for bioengineered heparin. Although GlcNAc-(GlcA-GlcNAc)n (Type O1), GlcA-(GlcNAc-GlcA)n (Type O2), ΔUA-(GlcNAc-GlcA)n (Type O3), and GlcA-(GlcNAc-GlcA)n-GlcNAc and GlcNAc-(GlcA-GlcNAc)n-GlcA (type E1), ΔUA (GlcNAc-GlcA)n-GlcNAc (Type E2) were detected in molecular weight <5,000, GlcA-(GlcNAc-GlcA)n (Type O2), ΔUA-(GlcNAc-GlcA)n (Type O3) were not observed in molecular weight >5,000. The photo-degradation mechanism leading to these results is unclear, however, it is possible that reactive oxygen species more easily attack the [→4)-β-D-GlcA(1→ 4)-α-D-GlcNAc(1→] than the [→4)-α-D-GlcNAc(1→4)-β-D-GlcA(1→] linkage.
Figure 4. ESI-FT-MS analysis of heparosan and photolyzed heparosan.
A. FTMS spectra of photolysis reaction of E.coli K5 heparosan. Heparosan chains identified from dp17 to dp28 labeled according to different types of fragments formed as explained below. B–F. Zooms of FTMS spectra to show different types of odd and even chains present in photolysis reaction product. B. Identification of dp17 (z=3) as odd oligomer ending in GlcNAc (type O1). C. Identification of dp17 (z=3) as odd oligomer ending in GlcA (type O2). D. Identification of dp17 (z=3) as oligomer with unsaturated uronic acid (type O3). E. Identification of dp16 (z=4) as even oligomer (type E1). F. Identification of dp16 (z=3) with unsaturated uronic acid (type E2).
Conclusions
In summary, this study shows that titanium dioxide with visible light catalyzes the depolymerization of heparosan. Photochemically-depolymerized low molecular weight of heparosan maintained the same structure as the starting heparosan, as confirmed by 1H-NMR. The polysaccharide chains of the photochemically depolymerized heparosan were also characterized by electrospray ionization-Fourier-transform mass spectrometry. The results suggest that the photochemical depolymerization of heparosan is a random process that can take place at either the glucuronic acid or the N-acetylglucosamine residue within the heparosan polysaccharide. These results indicate that photochemical depolymerization of heparosan was available for a bioengineered heparin. Future studies will be required to prepare the heparin for clinical use using photochemically-depolymerized heparosan as a starting material.
Supplementary Material
Acknowledgments
The authors are grateful for support by a Grant-in-Aid for Scientific Research (20590032), and Special Funds for Education and Research (Development of SPECT Probes for Pharmaceutical Innovation) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (TT), and by the US National Institutes of Health in the form of grants GM38060, HL096972 and HL101721 (RJL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnson E, Mulloy B. The molecular-weight range of commercial heparin preparations. Carbohydr Res. 1976;51:119–127. doi: 10.1016/s0008-6215(00)84041-0. [DOI] [PubMed] [Google Scholar]
- 2.Capila I, Linhardt RJ. Heparin-protein interactions. Angew Chem Int Ed Engl. 2002;41:391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Munoz EM, Linhardt RJ. Heparin-binding domains in vascular biology. Arterioscler Thromb Vasc Biol. 2004;24:1549–1557. doi: 10.1161/01.ATV.0000137189.22999.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raman R, Sasisekharan R. Structural insights into biological roles of protein-glycosaminoglycan interactions. Chem Biol. 2005;12:267–277. doi: 10.1016/j.chembiol.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Linhardt RJ, Claude S. Hudson Award address in carbohydrate chemistry. Heparin: structure and activity. J Med Chem. 2003;46:2551–2564. doi: 10.1021/jm030176m. [DOI] [PubMed] [Google Scholar]
- 6.Kishimoto TK, Viswanathan K, Ganguly T, Elankumaran S, Smith S, Pelzer K, Lansing JC, Sriranganathan N, Zhao GL, Galcheva-Gargova Z, Al-Hakim A, Bailey GS, Fraser B, Roy S, Rogers-Cotrone T, Buhse L, Whary M, Fox J, Nasr M, Dal, Pan GJ, Shriver Z, Langer R, Venkataraman G, Austen KF, Woodcock J, Sasisekharan R. Contaminated heparin associated with adverse clinical events and activation of the contact system. N Engl J Med. 2008;358:2457–2467. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerrini M, Beccati D, Shriver Z, Naggi A, Viswanathan K, Bisio A, Capila I, Lansing JC, Guglieri S, Fraser B, Al-Hakim A, Gunay NS, Zhang A, Robinson L, Buhse L, Nasr M, Woodcook J, Langer R, Venkataraman G, Linhardt RJ, Casu B, Torri G, Sasisekharan R. Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events. Nat Biotechnol. 2008;22:669–675. doi: 10.1038/nbt1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerrini M, Zhang Z, Shriver Z, Masuko S, Langer R, Casu B, Linhardt RJ, Torri G, Sasisekharan R. Orthogonal analytical approaches to detect potential contaminants in heparin. Proc Natl Acad Sci USA. 2009;106:16956–16961. doi: 10.1073/pnas.0906861106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li B, Suwan J, Zhang F, Wang Z, Liu H, Mulloy B, Linhardt RJ. Analysis of pharmaceutical heparins and potential contaminants using (1)H-NMR and PAGE. J Pharm Sci. 2009;98:4017–4026. doi: 10.1002/jps.21729. [DOI] [PubMed] [Google Scholar]
- 10.Rudd TR, Skidmore MA, Guimond SE, Holman J, Turnbull JE, Lauder RM, Fernig DG, Yates EA. The potential for circular dichroism as an additional facile and sensitive method of monitoring low-molecular-weight heparins and heparinoids. Thromb Haemost. 2009;102:874–878. doi: 10.1160/TH08-12-0797. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Zhang Z, Linhardt RJ. Lessons learned from the contamination of heparin. Nat Prod Rep. 2009;26:313–321. doi: 10.1039/b819896a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z, Mccallum SA, Xie J, Nieto L, Corzana F, Jimenez-Barbero J, Chen M, Liu J, Linhardt RJ. Solution structures of chemoenzymatically synthesized heparin and its precursors. J Am Chem Soc. 2008;130:12998–3007. doi: 10.1021/ja8026345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vann WF, Schmidt MA, Jann B, Jann K. The structure of the capsular polysaccharide (K5 antigen) of urinary-tract-infective Escherichia coli O10:K5:H4. A polymer similar to desulfo-heparin. Eur J Biochem. 1981;116:359–364. doi: 10.1111/j.1432-1033.1981.tb05343.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Ly M, Zhang F, Zhong W, Suen A, Hickey AM, Dordick JS, Linhardt RJ. E. coli K5 fermentation and the preparation of heparosan, a bioengineered heparin precursor. Biotechnol Bioeng. 2010;107:968–977. doi: 10.1002/bit.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ly M, Wang Z, Laremore TN, Zhang F, Zhong W, Pu D, Zagorevski DV, Dordick JS, Linhardt RJ. Analysis of E. coli K5 capsular polysaccharide heparosan. Anal Bioanal Chem. 2011;399:737–745. doi: 10.1007/s00216-010-3679-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linhardt RJ. Cur Meth in Mole Biol. UNIT. 2001. Analysis of glycosaminoglycans with polysaccharide lyases; pp. 17.13B.1–17.13B.16. [DOI] [PubMed] [Google Scholar]
- 17.Burana-osot J, Hosoyama S, Nagamoto Y, Suzuki S, Linhardt RJ, Toida T. Photolytic depolymerization of alginate. Carbohydr Res. 2009;344:2023–2027. doi: 10.1016/j.carres.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 18.Burana-osot J, Soonthornchareonnon N, Hosoyama S, Linhardt RJ, Toida T. Partial depolymerization of pectin by a photochemical reaction. Carbohydr Res. 2010;345:1205–1210. doi: 10.1016/j.carres.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Higashi K, Hosoyama S, Ohno A, Masuko A, Yang B, Sterner E, Nakatsukasa Y, Wang Z, Linhardt RJ, Toida T. Photochemical preparation of a novel low molecular weight heparin. Eur J Med Chem. 2011 doi: 10.1016/j.carbpol.2011.09.087. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laremore TN, Ly M, Solakyildirim K, Zagorevski DV, Linhardt RJ. High-resolution preparative separation of glycosaminoglycan oligosaccharides by polyacrylamide gel electrophoresis. Anal Biochem. 2010;401:236–241. doi: 10.1016/j.ab.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thanawiroon C, Rice KG, Toida T, Linhardt RJ. Liquid chromatography/mass spectrometry sequencing approach for highly sulfated heparin-derived oligosaccharides. J Biol Chem. 2004;279:2608–2615. doi: 10.1074/jbc.M304772200. [DOI] [PubMed] [Google Scholar]
- 22.Harbour JR, Tromp J, Hair ML. Photogeneration of hydrogen peroxide in aqueous TiO2 dispersions. Can J Chem. 1985;63:204–208. [Google Scholar]
- 23.Jaeger CD, Bard AJ. Spin trapping and electron spin resonance detection of radical intermediates in the photodecomposition of water at titanium dioxide particulate systems. J Phys Chem. 1979;83:3146–3152. [Google Scholar]
- 24.Noda H, Oikawa K, Kamada H. ESR Study of Active Oxygen Radicals from Photoexcited Semiconductors Using the Spin-Trapping Technique. Bull Chem Soc Jpn. 1992;65:2505–2509. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.