Abstract
Purpose
The method by which breast cancer is detected becomes a factor for long-term survival and should be considered in treatment plans. This report describes patient characteristics and time trends for various methods of breast cancer detection in the United States.
Methods
The 2003 National Health Interview Survey (NHIS), a nationally representative self-report health survey, included 361 women survivors diagnosed with breast cancer between 1980 and 2003. Responses to the question, How was your breast cancer found? were categorized as accident, self-examination, physician during routine breast examination, mammogram, and other. We examined responses by income, race, age, and year of diagnosis.
Results
Most women survivors (57%) reported a detection method other than mammographic examination. Women often detected breast cancers themselves, either by self-examination (25%) or by accident (18%).
Conclusions
Despite increased use of screening mammography, a large percentage of breast cancers are detected by the patients themselves. Patient-noted breast abnormalities should be carefully evaluated.
Introduction
Much of the increase in survival after breast cancer diagnosis has been attributed to screening mammography.1–3 Seventy percent of women in the United States now report having a screening mammogram in the previous 2 years.4 Given these high rates, we wondered why many women in our clinics report breast cancers that were detected by methods other than screening mammography. Is our clinical practice unique, or are many women detecting cancers by other methods? Insufficient data exist on methods of breast cancer detection.1,5–13 Most publications describe local or state populations and may not be representative of the United States as a whole. One publication on a national U.S. database describes only mammographic detection, without information on patient-detected and clinically detected breast cancers.8 This omission is surprising, given the relevance of detection method in estimating risk of distant breast cancer recurrence and determining treatment plans.14 Detection by screening mammography is usually associated with early stage cancers, which are easier to treat and cure.
The 2003 National Health Interview Survey (NHIS) provides a unique national data source to address this topic, as it included 361 women survivors diagnosed with breast cancer between 1980 and 2003. Our study describes detection methods in this population of survivors.
Materials and Methods
The 2003 NHIS was a nationally representative self-report health survey of the U.S. household population conducted through structurally diagnostic, in-person interviews. A detailed description of survey methods is provided elsewhere.15 The database is publicly available on the NHIS website.15 The Sample Adult component of the NHIS was conducted among 30,852 noninstitutionalized civilians aged ≥18 years. Exclusion criteria included individuals in long-term care institutions or correctional facilities and active duty Armed Forces, as well as U.S. nationals living in foreign countries.16 The response rate was 74.2%. Black and Hispanic populations were oversampled to allow for better estimation of health in these minority populations. Demographic variables (e.g., age, sex, race, income), type of cancer, and age at first diagnosis were assessed.
Method of detection was measured for all female participants who reported having breast cancer. They were asked, How was your breast cancer found? Eight response options were provided, which we collapsed into five categories for analyses:
• Found by accident (includes Found by myself by accident and Found by my spouse or partner)
• Found by myself during a breast self-examination
• Found by a physician during a routine breast examination
• Found by a mammogram
• Other (includes Other–specify, Refused, Don't know)
The resulting five categories of breast cancer detection were analyzed on the basis of age at first breast cancer diagnosis, race, income, and year of first diagnosis. Analysis of variance (ANOVA) was used to test for differences among the groups on demographic variables (age at first diagnosis, race, and income), and chi-square analyses were used to investigate trends in detection methods across time. All analyses were approved by the Human Subjects Division at the University of Washington.
Results
Of the 17,425 female participants, 412 reported a prior diagnosis of breast cancer. Thirteen respondents were excluded from our analyses because of incomplete data regarding age or year of diagnosis or both,17 and 38 were excluded because they reported diagnoses before 1980. Altogether, 361 breast cancer survivors were included in all analyses. Demographics for the overall sample, stratified by method of detection, are presented in Table 1. Method of breast cancer detection did not differ by year of diagnosis or race, but we found significant associations with age at diagnosis (χ2=22.03, p<0.05) and income (χ2=11.12, p<0.05). Older women and women who reported lower family income were more likely to have their cancer detected by mammography compared to younger women and women who reported higher family income.
Table 1.
Demographic Characteristics of United States Women Survivors of Breast Cancer, Diagnosed in 1980–2003, Stratified by Method of Breast Cancer Detection
| |
|
Method of breast cancer detectiona |
|||
|---|---|---|---|---|---|
| |
|
n (%) |
|||
| n (%) All women (n=361) | Mammogram (n=156) | Clinical breast examination (n=47) | Self-examination (n=90) | Accidentb (n=64) | |
| Age* | |||||
| 20–39 | 20 (6) | 4 (3) | 4 (9) | 4 (4) | 8 (13) |
| 40–49 | 71 (20) | 24 (15) | 6 (13) | 22 (24) | 19 (30) |
| 50–59 | 88 (24) | 40 (26) | 10 (21) | 22 (24) | 13 (20) |
| 60–69 | 113 (31) | 53 (34) | 15 (32) | 29 (32) | 16 (25) |
| 70+ | 69 (19) | 35 (22) | 12 (26) | 13 (14) | 8 (13) |
| Year cancer diagnosed | |||||
| 1980–1989 | 65 (18) | 20 (13) | 8 (18) | 21 (23) | 14 (22) |
| 1990–1994 | 73 (20) | 31 (20) | 13 (28) | 10 (22) | 9 (14) |
| 1995–1999 | 108 (30) | 50 (32) | 12 (26) | 23 (26) | 23 (36) |
| 2000–2003 | 115 (32) | 55 (35) | 14 (30) | 26 (29) | 18 (28) |
| Race | |||||
| White African | 331 (92) | 145 (93) | 45 (96) | 79 (88) | 59 (92) |
| American | 22 (6) | 10 (6) | 1 (2) | 9 (10) | 2 (3) |
| Other | 8 (2) | 1 (1) | 1 (2) | 2 (2) | 3 (5) |
| Family incomec,* | |||||
| ≤ $20,000 | 217 (60) | 108 (69) | 20 (43) | 50 (56) | 25 (39) |
| > $20,000 | 108 (30) | 35 (22) | 21 (45) | 26 (29) | 36 (56) |
Does not include 4 women who reported Other as their method of detection (Other–specify, n=2; Don't know, n=2).
Includes Found by myself by accident, n=62; Found by my spouse or partner, n=2.
n=36 with missing data for income.
p<0.05.
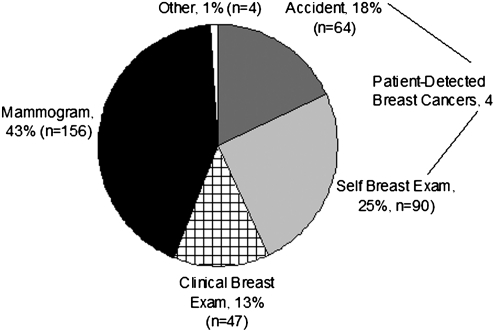
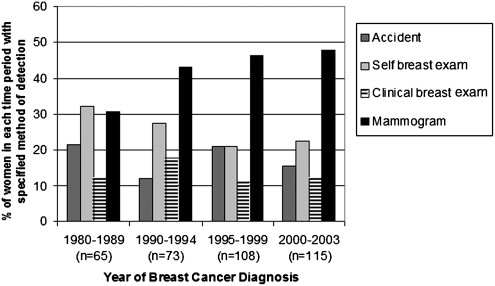
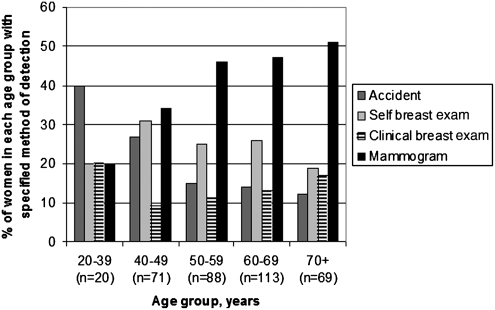
Among the 361 survivors, 43% (n=154) reported detecting their cancer themselves (18% by accident and 25% by breast self-examination), and 43% (n=156) reported mammographic detection (Fig. 1). Results were similar when analyses included the 13 women with incomplete data on age or year of diagnosis and the 38 women diagnosed before 1980 (total n=412). The percentage of women with mammographically detected breast cancer increased from 31% in the 1980s to 48% in 2000–2003 (Fig. 2). Detection by breast self-examination decreased over the same period from 32% to 23%, and self-detection methods in general (including breast self-examination and accident) decreased from 54% to 38%. Detection methods varied by age, with detection by mammography rising as the age of the woman increased (Fig. 3). Detection by mammography was the most frequent mode of diagnosis for four of the five age groups presented.
FIG. 1.
Self-reported method of breast cancer detection (n=361). Results are similar when analyses include the 13 women with incomplete data on age or year of diagnosis and the 38 women diagnosed before 1980 (total n=412).
FIG. 2.
Method of breast cancer detection by time period of diagnosis. Data for four categories of detection method are shown. Data for women reporting a detection method of Other are not shown; however, those results are as follows: 1980–1989=3% (n=2); 2000–2003=2% (n=2). Each time period sums to 100%, including Other category.
FIG. 3.
Method of breast cancer detection by age of the woman at diagnosis. Data for four categories of detection method are shown. Data for women reporting a detection method of Other are not shown; however, those results are as follows: 50–59=3% (n=3); 70+=1% (n=1). Each age group sums to 100%, including the Other Category.
In order to understand if detection methods differed for women of different ages, the data were analyzed in categories that included women aged 50–69 years (n=201) because this cohort has had consistent recommendations for screening over time (data not shown). Among these women, 46% reported mammographic detection, and 39% reported self-detection (14% by accident and 25% by breast self-examination). The percentage of mammographic detections increased when we limited the analyses to more recently diagnosed survivors (2000–2003, data not shown). Among women aged 50–69 years, 56% reported mammographic detection, and 37% reported self-detection. However, breast cancers diagnosed during the same time in women<age 50 (n=25) were mammographically detected for 36% and self-detected for 40%. Among women>age 70 (n=33), mammographic detection accounted for 42% of diagnoses and self-detection for 39%.
Discussion and Conclusions
Data from this national database suggest that since the 1980s, most (56%) breast cancers in the United States are discovered by screening clinical breast examinations or after patient-noted abnormalities are evaluated. Only 43% of all breast cancers were detected through mammography. An increase over time in breast cancers detected by mammography was noted that mirrors the increase in public acceptance and use of this screening method.18–21 Even in the last decade, however, among women in the screening ages of 50–69, we still found a significant proportion (39%) of self-detected breast cancers.
Our findings are in keeping with those of previous investigations. Data from a study of women receiving care through California's Breast and Cervical Cancer Treatment Program showed that 64% of women self-detected their breast cancer.22 Our findings are also similar to those reported from Finland, which has a 90% participation rate in mammography screening programs.23 Even in the Finnish population, however, the percentage of breast cancers found by screening mammography was only 21% for the entire population and only 41% among women aged 50–69 years at diagnosis.23
A key strength of our analysis lies in our data, in that the NHIS provides a nationwide sample of breast cancer survivors with a broad range of ages, socioeconomic characteristics, and diagnosis years. Our study also has limitations. Although the NHIS oversampled minority populations, we could not use the survey weights to adjust our results because of the small proportion (8%) of minority women with breast cancer. In addition, given the survey's reliance on self-report, it is possible that some participants made errors in classifying their detection methods. On the other hand, several studies suggest that self-report of breast cancer treatment is as accurate as medical record review24–26; therefore, despite the lack of data on quality of self-reported detection method, similar accuracy may be possible in this area.
We also excluded patients who died, possibly biasing the data toward breast cancers diagnosed at earlier stages.2 Nevertheless, we believe this bias should result in higher rather than lower percentages of cancers detected by screening mammography. Additionally, the NHIS did not specify type of breast cancer, so that women might have reported detection of ductal carcinoma in situ in addition to invasive cancer, yet ductal carcinoma in situ is more likely to be detected by screening mammography than by clinical examination or patient self-examination.14 Finally, even though the NHIS provided a nationally representative sample of >17,000 women, the number of women with breast cancer eligible for our analyses was only 361.
Screening mammography is emphasized in breast cancer education programs, whereas the roles of screening breast self-examination and clinical breast examination have been downplayed. This emphasis is understandable, as screening mammography is the best studied of the three screening methods and the only screening method associated with a reduction in breast cancer mortality.27 As highlighted in our findings, however, the majority of breast cancers have been detected by methods other than mammography. The self-reported method of breast cancer detection by the women in this U.S. sample included breast self-examination (25%), accidental finding of a breast abnormality (18%), and clinical breast examination by a healthcare provider (13%).
This study raises important issues for future public health initiatives. Although continued research efforts are needed to improve the quality of screening mammography and the uptake of screening mammography in the community, we cannot forget about the women whose breast cancer is detected by means other than mammography. Future research needs to be directed toward improving and expediting the evaluation process for the many women with breast abnormalities. In addition, women, especially those<age 40 who have not yet begun screening mammography, need to be educated about the importance of evaluating breast abnormalities.
These data demonstrate that individual U.S. women are almost as likely as healthcare professionals to notice the first signs of breast cancer. Even as women reach the recommended age for mammographic screening, they still frequently self-detect abnormalities that lead to a breast cancer diagnosis. Therefore, patients and physicians should not be falsely reassured by negative mammograms and should carefully evaluate all patient-noted breast abnormalities. More research is needed to determine why so many breast cancers continue to be found by methods other than screening mammography.
Acknowledgments
This research was supported by National Cancer Institute grant K05CA104699 (J. G. E.). M.Y.R. is supported, in part, by Eunice Kennedy Shriver National Institute of Child Health and Human Development grant 5K12HD053984.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.McPherson CP. Swenson KK. Jolitz G. Murray CL. Survival of women ages 40–49 years with breast carcinoma according to method of detection. Cancer. 1997;79:1923–1932. doi: 10.1002/(sici)1097-0142(19970515)79:10<1923::aid-cncr13>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 2.Shen Y. Yang Y. Inoue LY. Munsell MF. Miller AB. Berry DA. Role of detection method in predicting breast cancer survival: Analysis of randomized screening trials. J Natl Cancer Inst. 2005;97:1195–1203. doi: 10.1093/jnci/dji239. [DOI] [PubMed] [Google Scholar]
- 3.Berry DA. Cronin KA. Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute. Cancer trends progress report—2005 update. 2005.
- 5.Coates RJ. Uhler RJ. Brogan DJ, et al. Patterns and predictors of the breast cancer detection methods in women under 45 years of age (United States) Cancer Causes Control. 2001;12:431–442. doi: 10.1023/a:1011218005063. [DOI] [PubMed] [Google Scholar]
- 6.Newcomer LM. Newcomb PA. Trentham-Dietz A, et al. Detection method and breast carcinoma histology. Cancer. 2002;95:470–477. doi: 10.1002/cncr.10695. [DOI] [PubMed] [Google Scholar]
- 7.Reeves MJ. Newcomb PA. Remington PL. Marcus PM. Determinants of breast cancer detection among Wisconsin (United States) women, 1988–90. Cancer Causes Control. 1995;6:103–111. doi: 10.1007/BF00052770. [DOI] [PubMed] [Google Scholar]
- 8.Breen N. Yabroff KR. Meissner HI. What proportion of breast cancers are detected by mammography in the United States? Cancer Detect Prev. 2007;31:220–224. doi: 10.1016/j.cdp.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg A. Burke L. Vos P. Liles D. Self-exam is the most common method of breast cancer identification. J Clin Oncol; ASCO Annual Meeting Proceedings (post-meeting edition); 2007. p. 1543. [Google Scholar]
- 10.Baker L. Breast cancer detection demonstration project: Five-year summary report. Cancer J Clin. 1982;32:194–225. doi: 10.3322/canjclin.32.4.194. [DOI] [PubMed] [Google Scholar]
- 11.Beahrs O. Shapiro S. Smart C. McDivitt R. Report of the Working Group to Review the National Cancer Institute-American Cancer Society Breast Cancer Detection Demonstration Projects. J Natl Cancer Inst. 1979;62:639–709. [PubMed] [Google Scholar]
- 12.Shapiro S. Evidence on screening for breast cancer from a randomized trial. Cancer. 1977;39(Suppl 6):2772–2782. doi: 10.1002/1097-0142(197706)39:6<2772::aid-cncr2820390665>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 13.Nemoto T. Natarajan N. Smart CR. Mettlin C. Murphy GP. Patterns of breast cancer detection in the United States. J Surg Oncol. 1982;21:183–188. doi: 10.1002/jso.2930210311. [DOI] [PubMed] [Google Scholar]
- 14.Joensuu H. Lehtimaki T. Holli K, et al. Risk for distant recurrence of breast cancer detected by mammography screening or other methods. JAMA. 2004;292:1064–1073. doi: 10.1001/jama.292.9.1064. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control National Center for Health Statistics. National Health Interview Survey (NHIS): Questionnaires, datasets, and related documentation 1997–2007. www.cdc.gov/nchs/nhis.htm. [Jan;2010 ]. www.cdc.gov/nchs/nhis.htm
- 16.Schiller JS. Adams PF. Nelson ZC. Summary health statistics for the U.S. population: National Health Interview Survey, 2003. Vital Health Stat. 2005;10:1–104. [PubMed] [Google Scholar]
- 17.Hendrick RE. Chrvala CA. Plott CM. Cutter GR. Jessop NW. Wilcox-Buchalla P. Improvement in mammography quality control: 1987–1995. Radiology. 1998;207:663–668. doi: 10.1148/radiology.207.3.9609888. [DOI] [PubMed] [Google Scholar]
- 18.Conway BJ. Suleiman OH. Rueter FG. Antonsen RG. Slayton RJ. National survey of mammographic facilities in 1985, 1988, and 1992. Radiology. 1994;191:323–330. doi: 10.1148/radiology.191.2.8153301. [DOI] [PubMed] [Google Scholar]
- 19.Fintor L. Brown M. Fischer R, et al. The impact of mammography quality improvement legislation in Michigan: Implications for the National Mammography Quality Standards Act. Am J Public Health. 1998;88:667–671. doi: 10.2105/ajph.88.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cronin KA. Yu B. Krapcho M, et al. Modeling the dissemination of mammography in the United States. Cancer Causes Control. 2005;16:701–712. doi: 10.1007/s10552-005-0693-8. [DOI] [PubMed] [Google Scholar]
- 21.Hiatt RA. Klabunde C. Breen N. Swan J. Ballard-Barbash R. Cancer screening practices from National Health Interview Surveys: Past, present, and future. J Natl Cancer Inst. 2002;94:1837–1846. doi: 10.1093/jnci/94.24.1837. [DOI] [PubMed] [Google Scholar]
- 22.Thind A. Diamant A. Hoq L. Maly R. Method of detection of breast cancer in low-income women. J Womens Health. 2009;18:1807–1811. doi: 10.1089/jwh.2008.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hakama M. Pukkala E. Heikkila M. Kallio M. Effectiveness of the public health policy for breast cancer screening in Finland: Population based cohort study. BMJ. 1997;314:864–867. doi: 10.1136/bmj.314.7084.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maunsell E. Drolet M. Ouhoummane N. Robert J. Breast cancer survivors accurately reported key treatment and prognostic characteristics. J Clin Epidemiol. 2005;58:364–369. doi: 10.1016/j.jclinepi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Phillips KA. Milne RL. Buys S, et al. Agreement between self-reported breast cancer treatment and medical records in a population-based Breast Cancer Family Registry. J Clin Oncol. 2005;23:4679–4686. doi: 10.1200/JCO.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Schootman M. Jeffe DB. West MM. Aft R. Self-report by elderly breast cancer patients was an acceptable alternative to Surveillance, Epidemiology, and End Results (SEER) abstract data. J Clin Epidemiol. 2005;58:1316–1319. doi: 10.1016/j.jclinepi.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 27.The U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716–726. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]