Abstract
Functional CD8 T cell effector and memory responses are generated and maintained during murine γ-herpesvirus 68 (γHV68) persistent infection despite continuous presentation of viral lytic Ags. However, the identity of the CD8 T cell subpopulations that mediate effective recall responses and that can participate in the generation of protective memory to a γ-herpesvirus infection remains unknown. During γHV68 persistence, ~75% of γHV68-specific CD8 T cells coexpress the NK receptors killer cell lectin-like receptor G1 (KLRG1) and NKG2A. In this study, we take advantage of this unique phenotype to analyze the capacity of CD8 T cells expressing or not expressing KLRG1 and NKG2A to mediate effector and memory responses. Our results show that γHV68-specific KLRG1+NKG2A+ CD8 T cells have an effector memory phenotype as well as characteristics of polyfunctional effector cells such us IFN-γ and TNF-α production, killing capacity, and are more efficient at protecting against a γHV68 challenge than their NKG2A−KLRG1− counterparts. Nevertheless, γHV68-specific NKG2A+KLRG1+ CD8 T cells express IL-7 and IL-15 receptors, can survive long-term without cognate Ag, and subsequently mount a protective response during antigenic recall. These results highlight the plasticity of the immune system to generate protective effector and proliferative memory responses during virus persistence from a pool of KLRG1+NKG2A+ effector memory CD8 T cells.
After the resolution of an infection, the expanded pathogen-specific T cell population contracts via programmed cell death leaving only a small population of long-lived memory T cells (1). These memory T cells persist independently of Ag and self-renew by homeostatic proliferation in response to the cytokines IL-7 and IL-15 (2–4). Memory T cells are maintained at increased frequencies compared with those of their Ag-specific naive repertoire and have reduced costimulatory requirements compared with those of their naive counterparts (5–7). However, when the Ag or pathogen persists, the generation and maintenance of memory is affected. During persistent viral infections, CD8 T cell function and memory responses become progressively dysfunctional with increasing amounts of Ags and time (8, 9). CD8 T cells upregulate a variety of receptors that have the capacity to modify their function during a variety of persistent infections (10–12). Inhibitory NK (iNK) receptors are also thought to contribute to the modulation of T cell function (13). The expression of two of these NK receptors, killer cell lectin-like receptor G1 (KLRG1) and NKG2A, on the surfaces of CD8 T cells has been associated with an effector phenotype and function (14–16). KLRG1 is typically expressed on terminally differentiated senescent CD8 T cells (17–19). NKG2A forms a heterodimer with CD94 and has been reported to prevent cytolysis by CD8 T cells after TCR engagement and ligand binding (16, 20).
Murine γ-herpesvirus 68 (γHV68) infection of mice results in a low-level persistent viral infection in which there is continuous presentation of lytic viral Ags (21). Despite this, CD8 T cells retain their functional and protective capacities during the long-term latency phase of infection (21, 22). In addition, protective Ag-independent CD8 T cell memory is generated and maintained during γHV68 persistence (23). However, the identity and phenotype of the specific CD8 T cell subpopulations that can generate protective memory to γHV68 during a recall response remains unknown. In the current study, we show that ~75% of γHV68-specific CD8 T cells during the long-term latency phase of infection coexpress KLRG1 and NKG2A. These cells have characteristics of polyfunctional effector cells such us IFN-γ and TNF-α production, killing capacity, and are more efficient at protecting against an immediate γHV68 challenge than their NKG2A−KLRG1− counterparts. Even so, γHV68-specific NKG2A+KLRG1+ CD8 T cells also express IL-7 and IL-15 receptors, can survive long-term without cognate Ag, and subsequently proliferate and mount a protective response during antigenic recall.
Materials and Methods
Mice and viral infection
C57BL/6J and B6.PL-Thy1a/CyJ mice were obtained from The Jackson Laboratory, Harlan Farms, or were bred at The Research Institute at Nationwide Children’s Hospital. Mice were housed in BL2 containment under pathogen-free conditions. γHV68, clone WUMS, was propagated and titered on a monolayer of NIH3T3 fibroblasts. Mice were anesthetized with 2,2,2-tribromoethanol and intranasally inoculated with 1000 PFU γHV68. The institutional animal care and use committee approved all of the animal studies described in this work.
Flow cytometry analysis
Single-cell suspensions were obtained from bronchoalveolar lavage, lung, spleen, and bone marrow. RBCs were lysed, and the number of cells per organ was determined. Cells were stained with Fc-block (CD16/32) and washed and stained with a combination of γHV68-specific MHC tetramers ORF6487–495/Db and ORF61524–531/Kb and Abs against CD8 (53-6.7), CD90.2 (53.2-1), NKG2A (20d5 and 16b11), KLRG1 (2F1), CD62L (MEL-14), CD43 (1B-11), CD44 (IM7), CD122 (TM-b1), and CD127 (A7R34). Flow cytometry data were acquired on a BD LSR II (BD Biosciences) and analyzed using FlowJo software. Gates were set using negative controls and isotype controls. For any given cell surface marker and tetramer combination, all the analysis gates are identical in size and position.
Intracellular cytokine staining
A total of 2 × 106 cells/sample was incubated in medium in the presence of IL-2 (10 U/ml), brefeldin A (10 µg/ml), and purified ORF6487–495 or ORF61524–531 peptides for 5 h at 37°C. As positive control, cells were stimulated with PMA/ionomycin. After Fc blocking, the cells were stained with Abs against cell surface markers, fixed, permeabilized, and stained with anti–IFN-γ (XMG1.2) and anti–TNF-α (MP6-XT22) or isotype control Abs. In separate wells, the intracellular cytokine assay was performed as described above with the addition of affinity-purified blocking Abs against NKG2A, KLRG1, Syrian hamster IgG, or rat IgG2A during the 5-h incubation.
Degranulation assay
A total of 2 × 106 cells/sample was incubated in 1 ml medium with 1 µg/ml of each of Abs against CD28 (37.51), CD49d (9C10), CD107a (1D4B), and CD107b (ABL-93), purified ORF6487–495 or ORF61524–531 peptides, IL-2 (10 U/ml), and brefeldin A (10 µg/ml). As positive control, cells were stimulated with PMA/ionomycin. A negative control (anti-CD28, anti-CD49d, anti-CD107a, and anti-CD107b) was included to control for spontaneous production of cytokine and/or expression of CD107a/b. The cultures were incubated for 1 h at 37°C, followed by an additional 5 h in the presence of monensin. After Fc blocking, the cells were stained with cell surface and intracellular Abs as described above. In separate wells, the degranulation assay was performed as described above with the addition of affinity-purified blocking Abs against NKG2A (20d5) or KLRG1 (2F1), Syrian hamster IgG, or rat IgG2A during the 6-h incubation.
In vivo cytotoxicity assay
Single-cell suspensions obtained from naive spleens were pulsed with ORF6487–495 peptide or irrelevant influenza NP366–374 peptide (1 µg/106 cells) for 2 h at 37°C. The NP-pulsed control cells were stained with 50 nM CFSE and the relevant targets with 500 nM CFSE for 15 min at 37°C. The cells were thoroughly washed, combined in a 1:1 ratio, and a total of 1 × 107 cells were injected intravenously into mice. Recipient mice were injected with purified Abs against NKG2A (20d5) or KLRG1 (2F1), Syrian hamster IgG, or rat IgG2A Abs on day −3 and day −1 before transfer of target cells. The presence of CFSE-positive cells was analyzed in spleen cell suspensions from the recipient mice 18 h later. The results are shown as the percentage-specific killing, which was calculated according to the following formula: Percentage-specific killing = [1 − (Ratio of infected recipients/Ratio of naive recipients) × 100], where Ratio = (Number of CFSEhigh/Number of CFSElow).
Adoptive cell transfers
Splenocytes from C57BL/6 mice that were at least 3 mo post-γHV68 infection were plated in flasks coated with anti-mouse IgG and IgM Abs for 1 h to enrich for T cells. The nonadherent cells were incubated with Fc-block, washed, and stained with Abs against CD44, CD8, NKG2A, and KLRG1. CD44high CD8 T cells were purified into NKG2A+KLRG1+ and NKG2A−KLRG1− populations using a FACSVantage with Diva option (purity 98%). Purified cells (1 × 106) from each group were intravenously injected into naive recipient B6.PL mice. The presence of CD90.2+ CD8 T cells was analyzed in spleen cell suspensions from recipient mice at different time points after transfer. The presence of contaminating γHV68 was routinely analyzed in the spleens of host mice after adoptive transfer by limiting dilution nested PCR and infective center assays, with negative results.
Virus titers
The number of latently infected cells was determined on splenic cell suspensions on day 16 postinfection (p.i.) using a standard infectious center assay as described (24).
Results
γHV68-specific CD8 T cells coexpress KLRG1 and NKG2A during long-term latency
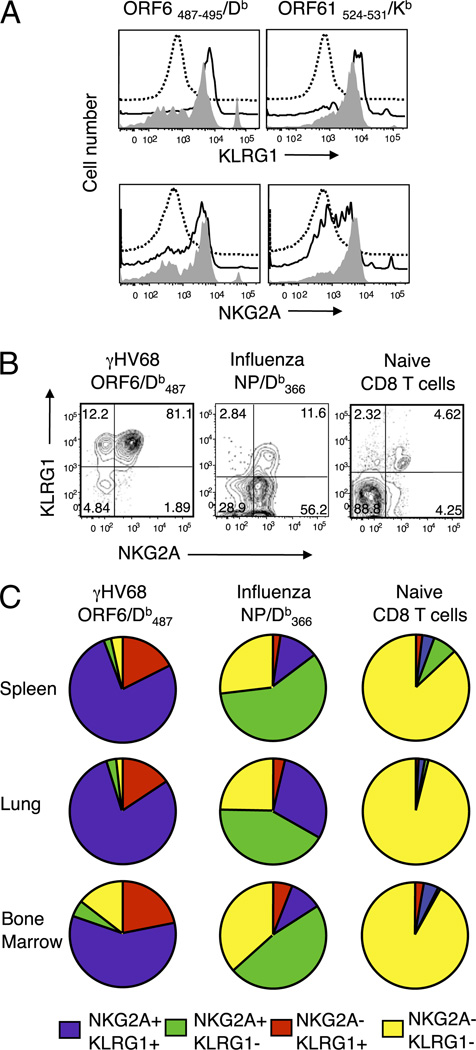
Our previous studies have shown that during γHV68 long-term latency, CD8 T cells maintain their effector, proliferative, and memory capacities (21, 23). In this study, we sought to gain a better understanding of the heterogeneity of the CD8 T cell populations present in the lymphoid organs and the respiratory tract during γHV68 latency. We analyzed the expression of a variety of inhibitory coreceptors on the CD8 T cells during γHV68 latency. We found that γHV68-specific CD8 T cells expressed NKG2A and KLRG1 on their cell surfaces during the initial phase of infection (3 wk p.i.), and their expression was sustained during long-term viral latency (Fig. 1A). We next analyzed the coexpression of NKG2A and KLRG1 on γHV68-specific CD8 T cells and compared it with their expression on naive CD8 T cells and with that on virus-specific CD8 T cells during the memory phase to a well-studied virus infection, influenza virus. The data show that the majority of γHV68-specific CD8 T cells in the spleen coexpressed both of the receptors (75–80%), whereas their expression on naive or influenza virus-specific CD8 T cells was ~4 and ~12%, respectively (Fig. 1B). A detailed analysis of the expression pattern of NKG2A and KLRG1 on a per cell basis during γHV68 infection, influenza virus infection, or in naive mice in CD8 T cells isolated from spleen, lung, and bone marrow is shown in Fig. 1C. The majority of γHV68-specific CD8 T cells (58–80%) coexpressed NKG2A and KLRG1 in all organs analyzed. The percentage of influenza virus-specific CD8 T cells that expressed both receptors ranged between 9 and 30%. The frequency of naive CD8 T cells that coexpressed NKG2A and KLRG1 was 1–4%. Table I displays the mean percentages of the receptor expression profile and compares the percentages of each NKG2A–KLRG1 subset for each organ analyzed between the experimental groups. The majority of the NKG2A–KLRG1 double-negative cells were found in the bone marrow in mice experimentally infected with γHV68 or influenza virus. Altogether, these results indicate that during long-term latency, γHV68-specific CD8 T cells have an unusual proportion of NKG2A+KLRG1+ cells that is distinct from that of influenza virus-specific memory CD8 T cells or their naive counterparts.
FIGURE 1.
Expression of KLRG1 and NKG2A on γHV68-specific CD8 T cells during long-term infection. Mice were infected with γHV68, influenza virus, or kept as naive age-matched controls, and tissues were analyzed at the indicated times p.i. A, Representative histograms showing KLRG1 and NKG2A expression on CD8 T cells isolated from naive mice (dotted line) and on γHV68-specific CD8 T cell splenocytes (ORF6487–495/Db [left panels] and ORF61524–531/Kb [right panels]) at 3 wk p.i. (continuous line) and at 3 mo p.i. (gray fill). B, Representative dot plots showing KLRG1 and NKG2A coexpression in naive mice and in γHV68- or influenza virus-infected mice at 3 mo p.i. Splenocytes were gated on γHV68 ORF6487–495/Db-specific CD8 T cells, influenza virus NP366–377/Db-specific CD8 T cells, or total CD8 T cells (naive mice), and numbers indicate percentage of cells in the quadrant. C, Pie charts show the distribution of NKG2A and KLRG1 expression in the spleen, lung, and bone marrow of mice infected 3 mo earlier with γHV68 (left column), influenza virus (center column), or kept as naive age-matched controls (right column). Results were gated on virus-specific or naive CD8 T cells as indicated above.
Table I.
Frequency of KLRG1–NKG2A subsets in CD8 T cells in the spleen, lung, and bone marrow of C57BL/6 mice
| Tissue | Virus | NKG2A−KLRG1+ | NKG2A+KLRG1+ | NKG2A+KLRG1− | NKG2A−KLRG1− |
|---|---|---|---|---|---|
| Spleen | γHV68a | 17.4 (8.3) | 76.5 (7.5) | 2.2 (1.0) | 3.7 (0.4) |
| Influenzab | 2.8 (0.4) | 11.7 (2.6) | 58.0 (2.0) | 27.2 (2.6) | |
| Naivec | 2.2 (0.9) | 3.7 (2.2) | 7.1 (4.9) | 87.0 (8.0) | |
| Lung | γHV68 | 15.6 (7.5) | 79.5 (5.2) | 2.9 (2.0) | 2.0 (0.7) |
| Influenza | 3.9 (3.4) | 29.2 (2.3) | 41.7 (16.2) | 24.3 (15.6) | |
| Naive | 1.2 (0.3) | 1.8 (0.2) | 1.2 (0.3) | 95.9 (0.4) | |
| Bone marrow | γHV68 | 21.6 (4.1) | 58.8 (2.8) | 5.0 (1.8) | 14.6 (0.9) |
| Influenza | 6.1 (2.60) | 9.8 (3.4) | 47.6 (7.1) | 36.7 (3.6) | |
| Naive | 3.0 (1.5) | 4.5 (3.8) | 0.9 (0.5) | 91.7 (5.2) |
Mice were infected intranasally with γHV68 (1000 PFU), influenza x-31 (300 EID50), or kept as naive mice. Mice were analyzed 3 mo later. Data show the percentage of NKG2A–KLRG1 cells among virus-specific or naive CD8 T cells. Data are the mean value from three individual mice. Data in parentheses represent SD.
Cells from γHV68-infected mice were analyzed as ORF6487–495/Db CD8 T cells.
Cells from influenza virus-infected mice were analyzed as NP366–377/Db CD8 T cells.
Cells from naive mice were analyzed as CD8 T cells.
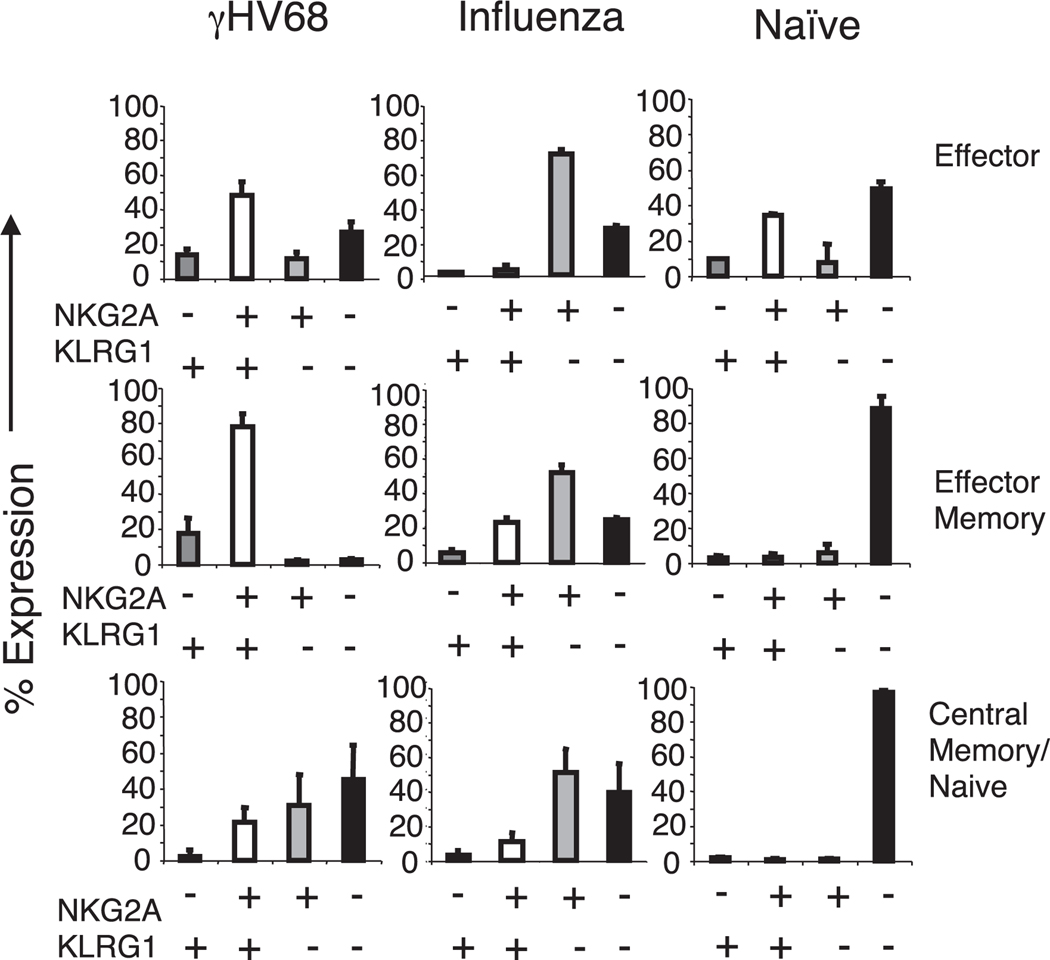
The expression of KLRG1 and NKG2A on the surfaces of CD8 T cells has been associated with effector phenotype and function (14–16). KLRG1 expression is also used as a surrogate marker of replicative senescence and terminal differentiation of CD8 T cells (17–19). We grouped the virus-specific CD8 T cells into subsets based on the expression of L-selectin (CD62L) and CD43-associated glycoform (21, 25, 26) to determine how the expression pattern of NKG2A and KLRG1 on γHV68-specific CD8 T cells correlated with different subsets of effector and memory CD8 T cell populations. The results show the frequency of NKG2A and KLRG1 receptor expression in the virus-specific CD8 T cell population in the spleen or in total CD8 T cells in the case of naive mice (Fig. 2). The data show that during γHV68 long-term latency, the majority of effector (CD62LlowCD43high) and effector memory (CD62LlowCD43low) γHV68-specific CD8 T cells coexpress NKG2A and KLRG1 (~50–80%), whereas the majority of central memory (CD62LhighCD43low) γHV68-specific CD8 T cells do not express either receptor (~40%). This pattern of expression is quite different from that of influenza virus-specific CD8 T cells at 3 mo p.i. or from that of CD8 T cells isolated from naive mice. KLRG1−NKG2A+ cells are the dominant subpopulation in every subset of influenza virus effector or memory cells among the NP366–374/Db CD8 T cell population. Most of the CD8 T cells isolated from naive mice lacked expression of NKG2A and KLRG1 regardless of their CD62L–CD43 expression pattern. The results obtained in the spleen were similar to those from lung and bone marrow (data not shown). These findings corroborate that the NKG2A–KLRG1 expression profile of CD8 T cells is different depending on the history of previous pathogen exposure. The majority of γHV68-specific KLRG1+NKG2A+ CD8 T cells have an effector or effector memory phenotype, which is in accord with studies suggesting that KLRG1 expression correlates with the replicative history of the T cell and its terminally differentiated status (17, 19).
FIGURE 2.
KLRG1–NKG2A double-positive cells constitute the majority of effector/effector-memory CD8 T cells during γHV68 persistent infection. Bar diagrams show the frequency of different NKG2A–KLRG1 subsets in the effector (CD62LlowCD43high), effector memory (CD62LlowCD43low), and central memory (CD62LhighCD43low) CD8 T cells. Mice were infected 3 mo earlier with γHV68 (left panels), influenza virus (center panels), or kept as naive age-matched controls (right panels). Cells were gated on virus-specific (γHV68 ORF6487–495/Db-specific or influenza virus NP366–374/Db-specific) CD8 T cells as indicated. Bars represent the mean value of three experimental mice, and error bars indicate SD.
Long-term KLRG1+NKG2A+ CD8 T cells have sustained effector function
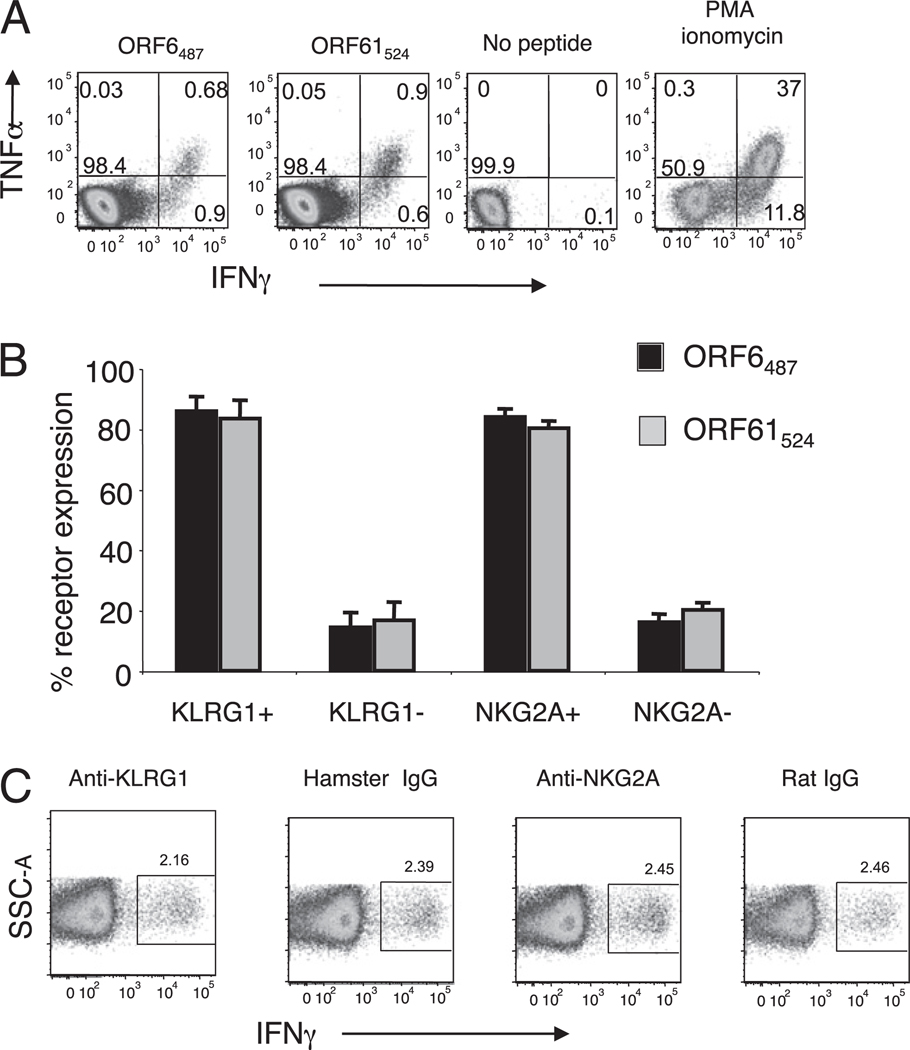
NK cells express a variety of receptors that enhance or mitigate their ability to exert effector activity against target cells expressing low levels of MHC class I molecules while maintaining tolerance toward normal cells (reviewed in Ref. 13). However, the function of these iNK receptors on CD8 T cells is less well defined. NKG2A–CD94 ligation on T cells may result in dampened effector activity (16, 27, 28), although this effect has not been consistently observed in other T cell studies (29–31). KLRG1 triggering inhibits suboptimal TCR signaling (32) and dampens cytokine production and killing (15, 18, 33, 34), although the described effects are modest. The γHV68 model of infection provides an excellent system to analyze the impact of KLRG1 and NKG2A expression on CD8 T cell function because the majority of the γHV68-specific CD8 T cells express KLRG1, NKG2A, or both receptors on their cell surfaces by 3 mo p.i. This pattern of expression also allows us to explore the effect of their coexpression on cellular function. Therefore, we analyzed the effector function of γHV68-specific CD8 T cells by measuring IFN-γ and TNF-α production by intracellular cytokine staining. The data show the existence of γHV68-specific CD8 T cells capable of producing IFN-γ or TNF-α and IFN-γ simultaneously in response to peptide stimulation (Fig. 3A). We next determined the expression of NKG2A or KLRG1 on the polyfunctional IFN-γ+TNF-α+ CD8 T cells. The majority of γHV68-specific CD8 T cells that were making TNF-α and IFN-γ expressed NKG2A and KLRG1 (80%) (Fig. 3B). Next, we tested whether Ab blocking of these NK receptors (28, 34) had any impact on the ability of the γHV68-specific CD8 T cells to produce IFN-γ. The cells were incubated with saturating levels of anti-KLRG1, anti-NKG2A, or the corresponding isotype controls. The results show that the IFN-γ production was unchanged between the five groups of Ab treatment, suggesting that the engagement of these iNK receptors on the cell surface of γHV68 ORF61524–531/Kb-specific CD8 T cell was not sufficient to alter IFN-γ production (Fig. 3C).
FIGURE 3.
KLRG1+ and NKG2A+ expressing cells constitute the majority of the IFN-γ/TNF-α polyfunctional CD8 T cells during γHV68 persistent infection. Splenocytes were isolated from mice infected with γHV68 for 3 mo. A, Representative plots showing TNF-α and IFN-γ production by CD8 T cells in response to γHV68-specific peptides ORF6487–495 and ORF61524–531. Numbers indicate percentage of cells in each quadrant. B, Bar diagram displays the frequency of KLRG1 and NKG2A receptor expression among IFN-γ/TNF-α polyfunctional CD8 T cells. Bars represent the mean value of three experimental samples, and error bars indicate SD. C, Representative plots showing IFN-γ production by CD8 T cells stimulated in the presence of ORF61524–531/Kb and Abs against KLRG1, NKG2A, or their respective isotype controls. Numbers indicate percentage of cells in the gate. Similar results were obtained in three independent experiments.
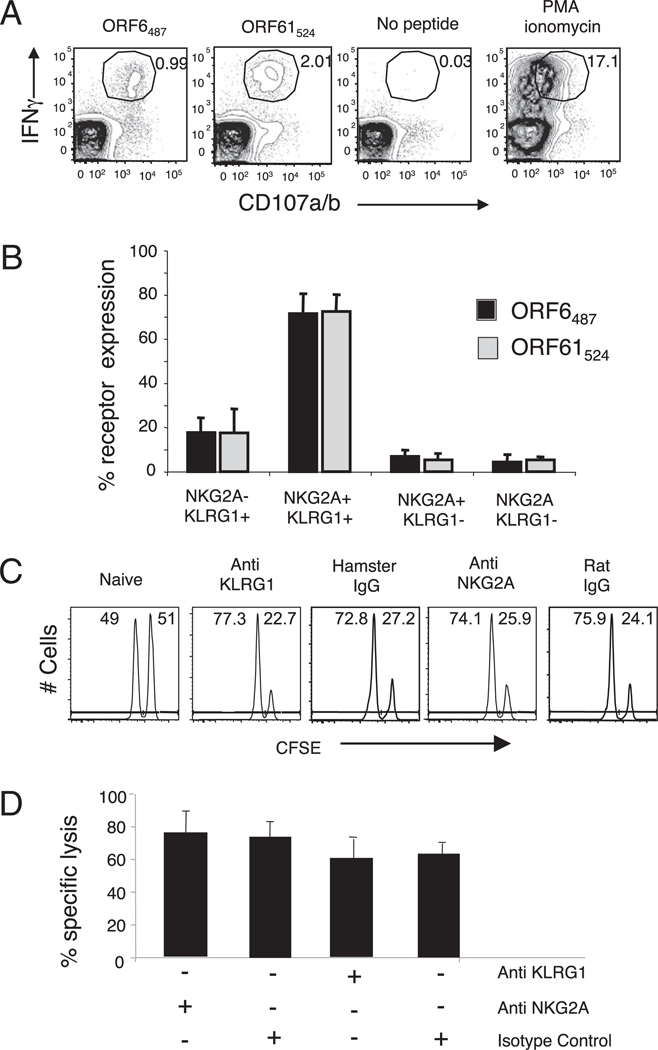
To analyze the impact of KLRG1 and NKG2A expression on the killing capacity of γHV68-specific CD8 T cells, we initially measured CD107a/b degranulation, a surrogate marker of cytolytic T cells (35). The results show that γHV68-specific CD8 T cells are capable of producing IFN-γ and mobilizing CD107 to the cell surface, and 80% of these polyfunctional CD8 T cells expressed KLRG1 and NKG2A (Fig. 4A, 4B). We next wanted to assess the in vivo cytotoxic capacity of γHV68-specific CD8 T cells and the role that NKG2A and KLRG1 could play in this process. We performed in vivo CTL assays on γHV68-infected mice at 3 mo p.i. (23) and administered Abs specific to NKG2A, KLRG1, or the corresponding isotype control into mice at day −3 and −1 before the assay. Ab administration did not deplete NKG2A+ or KLRG1+ CD8 T cells or affect the number of γHV68-specific CD8 T cells (data not shown). As shown in Fig. 4C and 4D, there were no significant differences observed in specific lysis between the mice that received anti-KLRG1 or anti-NKG2A Abs and their respective isotype controls. Altogether, these results indicate that the expression of NKG2A and KLRG1 receptors or their blockade with Abs in the presence of virus-specific peptides does not impact the overall capacity of γHV68-specific CD8 T cells to mount a functional effector response during the long-term latency phase of infection.
FIGURE 4.
KLRG1+NKG2A+ cells constitute the majority of the IFN-γ/CD107a/b polyfunctional CD8 T cells during γHV68 persistent infection. A, Representative plots show intracellular IFN-γ production and cell surface CD107a/b mobilization on previously gated CD8 T cells. Splenocytes isolated from mice infected with γHV68 3 mo earlier were stimulated with γHV68-specific ORF6487–495 and ORF61524–531 peptides. B, Bar diagram displays the frequency of KLRG1 and NKG2A receptor expression among IFN-γ/CD107a/b polyfunctional CD8 T cells. Bars represent the mean value of three experimental samples, and error bars indicate SD. C, Representative histograms of CFSE intensity of an in vivo CTL assay in the presence or absence of Abs against KLRG1 and NKG2A. D, Bar diagram shows the percentage specific lysis of ORF6487–495-loaded target cells injected into γHV68-infected mice at 3 mo p.i. that were previously treated or not treated with Abs against KLRG1 and NKG2A. Data are the mean of three individual mice per experiment, and error bars represent SD. Similar results were obtained in three independent experiments.
KLRG1+NKG2A+ phenotype correlates with protective efficacy during recall responses
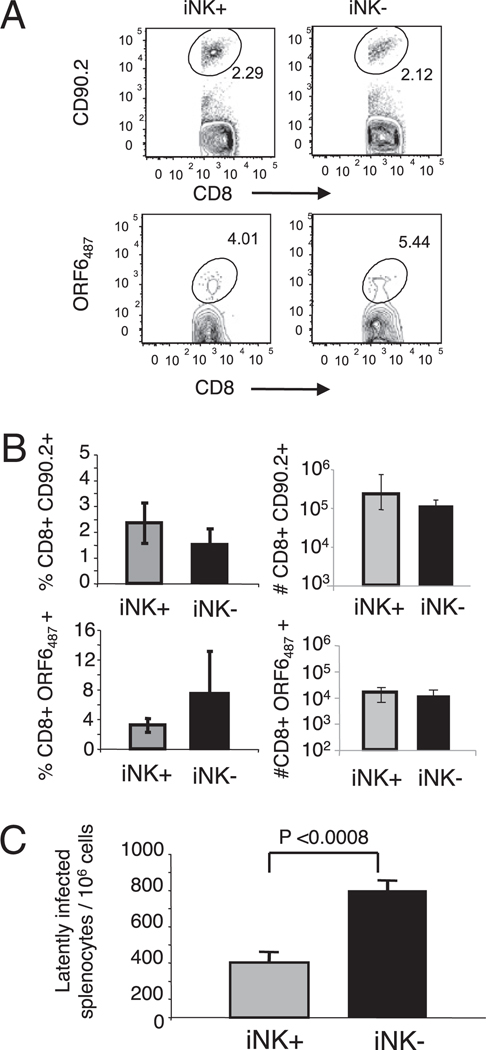
We have previously shown that CD8 T cells generated during long-term viral persistence can mediate protection to γHV68 during a recall response (21, 23). However, the identity and phenotype of the specific CD8 T cell subpopulation that has this potential remains obscure. KLRG1 is used as a marker of nonreplicative, senescent or terminally differentiated CD8 T cells (19, 36, 37). Thus, we wanted to investigate if KLRG1+NKG2A+ or KLRG1−NKG2A− CD8 T cells isolated from γHV68 latently infected mice were responsible for mediating the protective recall response to γHV68 infection. We FACS purified Ag-experienced CD8 T cells from mice infected with γHV68 3 mo earlier into KLRG1+NKG2A+ and KLRG1−NKG2A− subsets and then adoptively transferred 106 cells into naive CD90.1 congenic recipients. The average frequency of virus-specific cells transferred per mouse was 5000 ORF6487–495/Db-positive and 50,000 ORF61524–531/Kb-positive. The mice were challenged with γHV68 24 h after adoptive transfer. We assessed the frequency and number of virus-specific donor cells in the spleens of the recipient mice 16 d p.i. Our results show that the frequency and cell numbers of donor CD90.2 CD8 T cells and of γHV68-specific CD90.2 CD8 T cells were not significantly different when comparing the mice that received KLRG1+NKG2A+ or KLRG1−NKG2A− CD8 T cells (Fig. 5A, 5B). This finding suggests that long-term γHV68-specific KLRG1+NKG2A+ CD8 T cells have a similar potential to participate in a recall response as that of their KLRG1−NKG2A− CD8 T cell counterparts. Next, we analyzed the number of γHV68 latently infected cells in the spleens of mice that had received adoptively transferred cells 17 d before. The data show that the mice that received KLRG1+NKG2A+ CD8 T cells had significantly less latently infected splenocytes than that of the mice that received KLRG1−NKG2A− CD8 T cells (p < 0.0008) (Fig. 5C), suggesting that KLRG1+NKG2A+ expression correlates with better protective function. Altogether, these results indicate that although the capacity to participate in a recall response is similar between KLRG1+NKG2A+ or KLRG1−NKG2A− CD8 T cells, the KLRG1+NKG2A+ CD8 T cell subset has a functional protective advantage over their KLRG1−NKG2A− CD8 T cell counterparts.
FIGURE 5.
KLRG1+NKG2A+ expression on CD8 T cells correlates with protective efficacy during a recall response to γHV68. KLRG1+NKG2A+ and KLRG1−NKG2A− CD8 T cells were FACS purified from the spleens of mice 3 mo after γHV68 infection, adoptively transferred into naive congenic animals, and the mice were infected with γHV68 24 h later. The analysis was performed using the spleens of recipient mice on day 16 p.i. A, Representative plots show the frequency of CD90.2+ cells among the CD8+ T cell population (top panels) and the frequency of γHV68 ORF6487–495/Db-specific cells among the CD90.2+ CD8+ T cell population (bottom panels). The left panels show the KLRG1+NKG2A+ adoptive transfer group (iNK+), and the right panels shows the KLRG1−NKG2A− adoptive transfer group (iNK−). Numbers represent the percentage of cells in each gate. B, Bar diagrams show the frequency (left panels) and cell numbers (right panels) of donor CD90.2+ CD8+ T cells (top panels) and those of γHV68 ORF6487–495/Db-specific cells among the donor CD90.2+ CD8+ population (bottom panels). C, Bar diagram displays the number of latently infected cells per 106 splenocytes. Data are the mean of three individual mice per experiment, and error bars represent SD. Similar results were obtained in three independent experiments.
KLRG1+NKG2A+ CD8 T cells mount protective memory responses
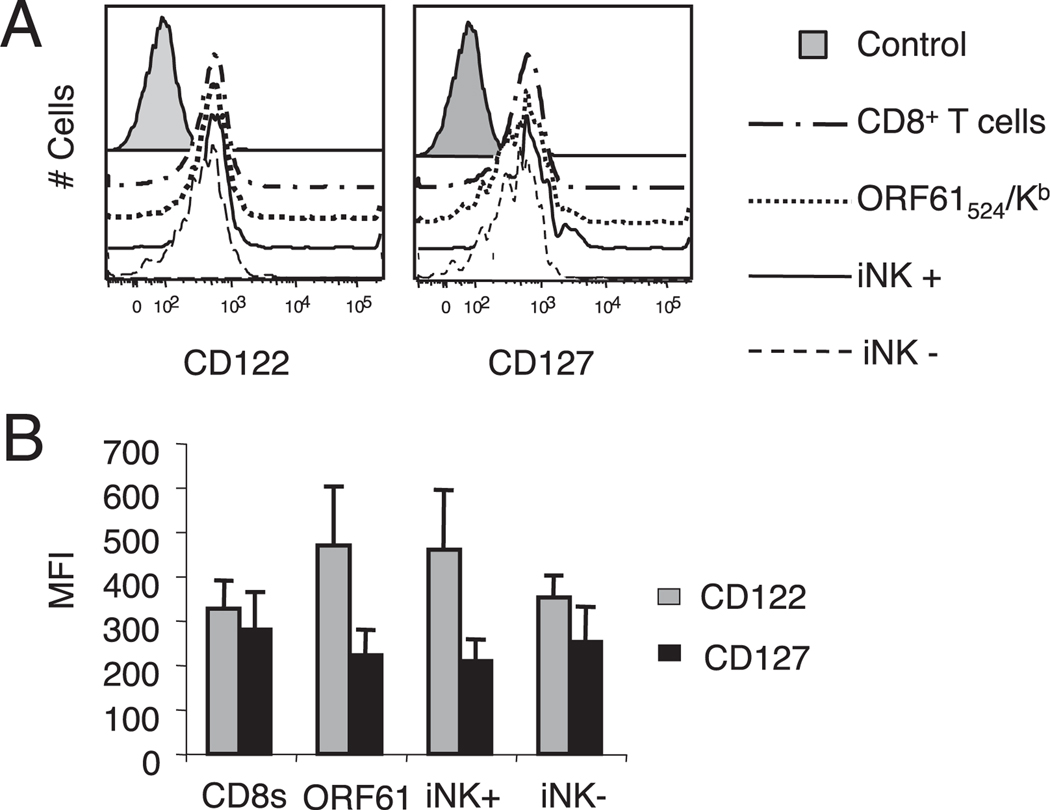
Having established that γHV68-specific KLRG1+NKG2A+ CD8 T cells have functional characteristics of effectors and can participate in protective recall responses, we wanted to examine their capacity to survive in the absence of cognate Ag and to participate in memory responses. Therefore, we FACS sorted Ag-experienced CD8 T cells into KLRG1+NKG2A+ and KLRG1−NKG2A− subsets and adoptively transferred 106 cells into naive CD90.1 congenic recipients. We allowed the adoptively transferred cells to rest in a cognate Ag-free environment for at least 30 d before analyzing their frequency in the recipient animals and determining their NKG2A–KLRG1 phenotype. As shown in Fig. 6, both the KLRG1+NKG2A+ and KLRG1−NKG2A− CD8 T cell subsets persisted in their respective naive hosts for up to 30 d. Importantly, each group of transferred cells retained their original NKG2A–KLRG1 phenotype during this time frame. These results indicate that KLRG1+NKG2A+ CD8 T cells isolated from long-term γHV68-infected mice have the capacity to endure in the absence of cognate Ag without reverting to a KLRG1−NKG2A− phenotype. These data suggest the possibility that at least some of the γHV68-specific KLRG1+NKG2A+ CD8 T cells have characteristics of memory precursors that allow them to survive without the need for constant Ag stimulation. To investigate this possibility, we analyzed the expression of the receptors for the homeostatic cytokines IL-7 and IL-15 (38, 39) on γHV68-specific CD8 T cells expressing or not expressing NKG2A and KLRG1 during long-term infection. The data show that the majority of the γHV68-specific ORF61524–531/Kb CD8 T cells expressed CD122 and CD127 (Fig. 7). In addition, the level of IL-7 and IL-15 receptor expression was similar between the KLRG1+NKG2A+ and KLRG1−NKG2A− subsets of γHV68-specific CD8 T cells. These results suggest that γHV68-specific CD8 T cells isolated long-term p.i. could respond to homeostatic cytokine signaling regardless of their NKG2A–KLRG1 expression profile and explain why these cells can survive in the absence of cognate Ag.
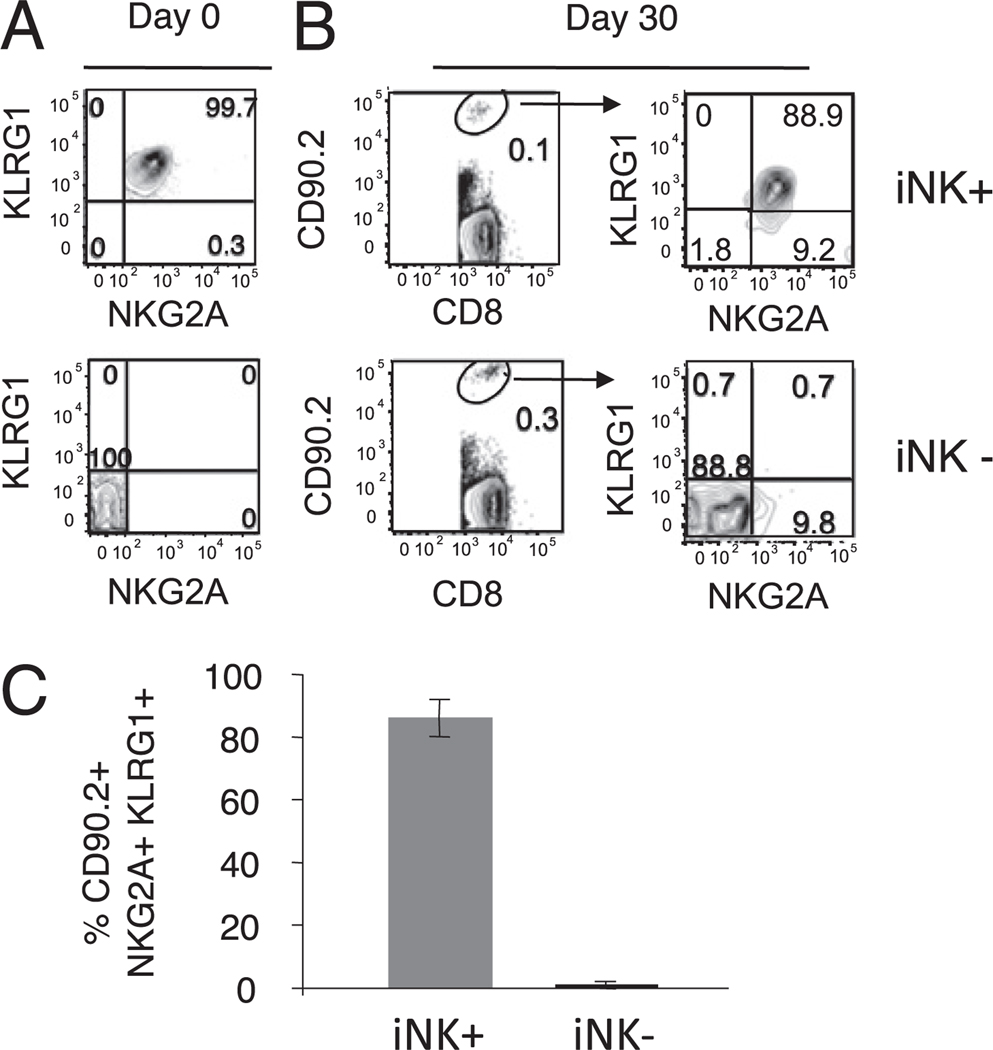
FIGURE 6.
KLRG1+NKG2A+ CD8 T cells survive in an environment free of cognate γHV68 Ags. KLRG1+NKG2A+ and KLRG1−NKG2A− CD8 T cells were FACS purified from the spleens of mice 3 mo after γHV68 infection and adoptively transferred into naive congenic animals. The spleens of recipient mice were analyzed 30 d after adoptive cell transfer. A, Representative plots showing the NKG2A–KLRG1 expression profile of sorted CD44+ CD8 T cells before being adoptively transferred (day 0). B, Plots show the frequency (left panels) and phenotype (right panels) of donor CD90.2+ CD8 T cells 30 d after transfer. The top panels correspond with the KLRG1+NKG2A+ adoptive transfer group (iNK+), and the bottom panels correspond with the KLRG1−NKG2A− adoptive transfer group (iNK−). Numbers represent the percentage of cells in each respective gate or quadrant. C, Bar diagram shows the frequency of donor KLRG1+NKG2A+ CD8 T cells recovered in the spleens of mice of each transfer group (iNK+ or iNK−) on day 30 after adoptive transfer. Data are the mean of three individual mice per experiment, and error bars represent SD.
FIGURE 7.
γHV68-specific KLRG1+NKG2A+ CD8 T cells express homeostatic cytokine receptors. Splenocytes were isolated from mice infected with γHV68 for 3 mo. A, Representative histograms display the expression profile of CD122 (left panel) and CD127 (right panel) on CD8 T cells, γHV68 ORF61524–531/Kb-specific CD8 T cells, γHV68 ORF61524–531/Kb-specific KLRG1+NKG2A+ CD8 T cells (iNK+), and γHV68 ORF61524–531/Kb-specific KLRG1−NKG2A− CD8 T cells (iNK−). B, Bar diagram shows the mean fluorescence intensity (MFI) of CD122 and CD127 within the groups described above. Data are the mean of three individual mice per experiment, and error bars represent SD.
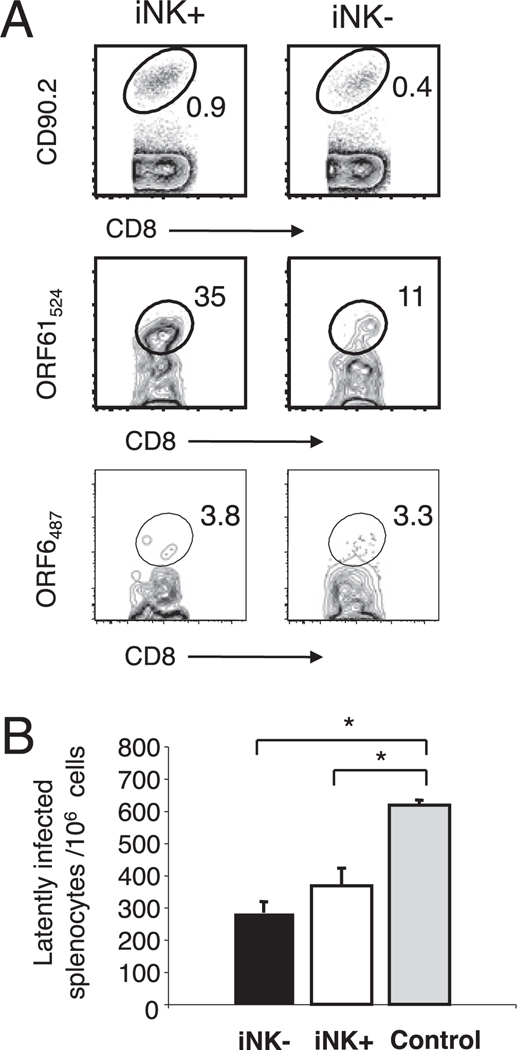
To test whether rested γHV68-specific KLRG1+NKG2A+ CD8 T cells could mount a protective recall response against γHV68 via proliferation and protection against infection, we challenged the naive congenic recipient mice with γHV68 30 d after the adoptive transfer of KLRG1+NKG2A+ or KLRG1−NKG2A− Ag-experienced CD8 T cells. The data in Fig. 8 show that the mice that received Ag-experienced CD8 T cells purified from persistently infected mice had significantly lower numbers of γHV68-infected cells than those of the control mice. In addition, there were no significant differences in the number of γHV68 latently infected cells between the mice that received KLRG1+NKG2A+ or KLRG1−NKG2A− Ag-experienced CD8 T cells. These data demonstrate that γHV68-specific CD8 T cells preserve their protective capacity after prolonged cognate Ag withdrawal regardless of their NKG2A–KLRG1 phenotype.
FIGURE 8.
Rested γHV68-specific KLRG1+NKG2A+ CD8 T cells mediate a protective recall response. BL6.PL mice received 1 × 106 KLRG1+NKG2A + (iNK+) or KLRG1−NKG2A− (iNK−) CD44+ CD8 T cells isolated from the spleens of mice 3 mo after γHV68 infection. The recipient mice were rested for 30 d after adoptive cell transfer and then infected with γHV68. Data correspond with spleen cells from day 16 p.i. (day 47 after adoptive cell transfer). A, Representative FACS plots show the frequency of donor CD90.2+ CD8 T cells (top panels), of γHV68 ORF61524–531/Kb-specific CD8 T cells among the CD90.2 donor population (middle panels), and of γHV68 ORF6487–495/Db-specific CD8 T cells among the CD90.2 donor population (bottom panels) in the iNK+ and iNK− adoptive transfer groups. B, Bar diagram shows the number of latently infected cells per 106 splenocytes. Data are the mean of three individual mice per experiment, and error bars represent SD. Similar results were obtained in three independent experiments.
Discussion
Despite recent advances, our understanding of the generation and maintenance of protective memory responses during persistent infections is still incomplete. In this study, we show that during long-term γHV68 persistent infection, the majority of virus-specific CD8 T cells coexpress KLRG1 and NKG2A. These cells have characteristics of polyfunctional effector cells such us IFN-γ and TNF-α production, killing capacity, and the ability to participate in protective recall responses. Nevertheless, they also express IL-7 and IL-15 receptors, can survive long-term without cognate Ag, and subsequently mount a protective response during antigenic recall. Altogether, these findings support the concept that during long-term γHV68 persistence, γHV68-specific KLRG1+NKG2A+ CD8 T cells have concurrent functional characteristics of effector and of memory cells.
We found that ~75% of the γHV68 virus-specific CD8 T cells express both KLRG1 and NKG2A during γHV68 latency. The expression of NKG2A or of KLRG1 on CD8 T cells has been reported during a variety of viral infections, in particular during persistent viral infections (16, 29, 33, 40–45). The expression of these NK receptors on CD8 T cells appears to be a common consequence of CD8 T cell antigenic exposure and activation, and therefore, their expression is sustained during infections characterized by diverse degrees of Ag persistence. Our results show that the KLRG1+NKG2A+ CD8 T cells have an effector/effector-memory phenotype, whereas their KLRG1−NKG2A− counterparts display a central memory phenotype during γHV68 persistence. Our attempts to use the expression of NKG2A or KLRG1 as a surrogate marker to define different functional subsets of γHV68-specific CD8 T cells did not show qualitative differences. However, the KLRG1+NKG2A+ CD8 T cell population had a more activated phenotype (as measured by CD62L and CD43 expression) and a higher proportion of polyfunctional (i.e., can make IFN-γ, TNF-α, and kill) cells than their KLRG1−NKG2A− counterparts. These two characteristics contribute to explain why the KLRG1+NKG2A+ CD8 T cells were more efficient than their KLRG1−NKG2A− counterparts in controlling the establishment of γHV68 latency when the recipient mice were infected immediately after their adoptive cell transfer. These findings support the idea that γHV68-specific KLRG1+NKG2A+ CD8 T cells do not exhibit dampened effector function and, on the contrary, that KLRG1+NKG2A+ expression on γHV68-specific CD8 T cells predicts protective efficacy.
A critical aspect of our findings is that γHV68-specific KLRG1+NKG2A+ CD8 T cells coexpress IL-7 and IL-15 receptors during the persistent phase of infection and survive up to a month in the absence of cognate Ag without reverting back to a KLRG1−NKG2A− phenotype. KLRG1 expression on CD8 T cells is thought to correlate with replicative senescence and impaired proliferative potential (18, 19, 33, 36, 37). Our data support the idea that KLRG1 expression is not sufficient to lock CD8 T cells in a terminally differentiated state and that an activated phenotype can coexist with attributes of memory cells, such as expression of homeostatic cytokine receptors and proliferative capacity. KLRG1+NKG2A+ CD8 T cells were protective when the recipient mice were challenged with γHV68 directly after adoptive transfer, and also when the mice were challenged 1 mo after adoptive transfer. Resting in an environment free of cognate Ags will likely select for adoptively transferred cells that can either survive or proliferate in the absence of persistent viral Ags. This process may enrich for cells within both the iNK+ (KLRG1+NKG2A+) and the iNK− (KLRG1−NKG2A−) populations that are capable of protecting against γHV68 challenge. It seems that under this experimental condition, both iNK− and iNK+ T cells are equally protective. In addition, the expression of NKG2A on iNK+ γHV68-specific CD8 T cells may contribute to explain their proliferative capacity because it has been demonstrated that NKG2A expression is associated with proliferative potential of CD8 T cells during persistent polyoma virus infection (41). Thus, although the specific circumstances that are necessary for the expression of these NK receptors during γHV68 persistence are unknown, it appears that KLRG1 and NKG2A expression may reflect the dual personality of γHV68-specific CD8 T cells as activated effectors and proliferative memory cells, respectively. IL-7 receptor expression on effector T cells contributes to their long-term maintenance during chronic Leishmania major infection (46). Altogether, these results highlight the plasticity of the immune system to generate protective effector memory responses despite the presence of persistent Ags.
Our findings with the γHV68 model of infection support the concept that a large population of activated effector memory CD8 T cells induced by low-level Ag persistence is beneficial for the control of secondary infections and for the generation of protective memory. Two different results sustain this idea. First, there is more robust protection after adoptive transfer of KLRG1+NKG2A+ than of KLRG1−NKG2A− CD8 T cells when the mice are challenged immediately (Fig. 5). Second, KLRG1+NKG2A+ CD8 T cells are as efficient as KLRG1−NKG2A− CD8 T cells at surviving after Ag withdrawal and equally protective during a subsequent γHV68 challenge (Figs. 6, 7). Other lines of evidence in the literature also support the concept that a certain degree of Ag persistence may contribute to protection by stimulating T cell responses. γHV68 persistence enhances viral protection by shaping CD8 T cell memory responses (22). Persistent infection contributes to T cell-mediated protective immunity against ehrlichiosis (47). Vaccine vectors based on persistent CMV elicit cellular protection to SIV mediated by effector memory T cell responses (48). In addition, activation phenotype can predict the recall efficacy of memory CD8 T cells (49). Thus, optimal vaccination strategies aimed to elicit cell-mediated protection will benefit from the generation of a large cohort of activated effector memory CD8 T cells, and a certain level of Ag persistence may be beneficial to accomplish this objective.
Acknowledgments
This work was supported in part by National Institutes of Health Grant AI-59603 and by The Research Institute.
We thank the Flow Cytometry Core Laboratory at The Research Institute for assistance with flow cytometry and sorting. We thank Dr. David Raulet for providing the hybridomas 2F1 and 20d5.
Abbreviations used in this article
- γHV68
murine γ-herpesvirus 68
- iNK
inhibitory NK
- KLRG1
killer cell lectin-like receptor G1
- p.i.
postinfection
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Sprent J, Tough DF. T cell death and memory. Science. 2001;293:245–248. doi: 10.1126/science.1062416. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 3.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 5.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu. Rev. Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 6.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr. Opin. Immunol. 2005;17:326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Lefrançois L. Development, trafficking, and function of memory T-cell subsets. Immunol. Rev. 2006;211:93–103. doi: 10.1111/j.0105-2896.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 8.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 2007;204:941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 12.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Up-regulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 13.Vivier E, Anfossi N. Inhibitory NK-cell receptors on T cells: witness of the past, actors of the future. Nat. Rev. Immunol. 2004;4:190–198. doi: 10.1038/nri1306. [DOI] [PubMed] [Google Scholar]
- 14.Gründemann C, Bauer M, Schweier O, von Oppen N, Lässing U, Saudan P, Becker KF, Karp K, Hanke T, Bachmann MF, Pircher H. Cutting edge: identification of E-cadherin as a ligand for the murine killer cell lectin-like receptor G1. J. Immunol. 2006;176:1311–1315. doi: 10.4049/jimmunol.176.3.1311. [DOI] [PubMed] [Google Scholar]
- 15.Henson SM, Franzese O, Macaulay R, Libri V, Azevedo RI, Kiani-Alikhan S, Plunkett FJ, Masters JE, Jackson S, Griffiths SJ, et al. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood. 2009;113:6619–6628. doi: 10.1182/blood-2009-01-199588. [DOI] [PubMed] [Google Scholar]
- 16.Suvas S, Azkur AK, Rouse BT. Qa-1b and CD94-NKG2a interaction regulate cytolytic activity of herpes simplex virus-specific memory CD8+ T cells in the latently infected trigeminal ganglia. J. Immunol. 2006;176:1703–1711. doi: 10.4049/jimmunol.176.3.1703. [DOI] [PubMed] [Google Scholar]
- 17.Marcolino I, Przybylski GK, Koschella M, Schmidt CA, Voehringer D, Schlesier M, Pircher H. Frequent expression of the natural killer cell receptor KLRG1 in human cord blood T cells: correlation with replicative history. Eur. J. Immunol. 2004;34:2672–2680. doi: 10.1002/eji.200425282. [DOI] [PubMed] [Google Scholar]
- 18.Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1) Blood. 2002;100:3698–3702. doi: 10.1182/blood-2002-02-0657. [DOI] [PubMed] [Google Scholar]
- 19.Heffner M, Fearon DT. Loss of T cell receptor-induced Bmi-1 in the KLRG1(+) senescent CD8(+) T lymphocyte. Proc. Natl. Acad. Sci. USA. 2007;104:13414–13419. doi: 10.1073/pnas.0706040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masilamani M, Nguyen C, Kabat J, Borrego F, Coligan JE. CD94/NKG2A inhibits NK cell activation by disrupting the actin network at the immunological synapse. J. Immunol. 2006;177:3590–3596. doi: 10.4049/jimmunol.177.6.3590. [DOI] [PubMed] [Google Scholar]
- 21.Cush SS, Anderson KM, Ravneberg DH, Weslow-Schmidt JL, Flaño E. Memory generation and maintenance of CD8+ T cell function during viral persistence. J. Immunol. 2007;179:141–153. doi: 10.4049/jimmunol.179.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obar JJ, Fuse S, Leung EK, Bellfy SC, Usherwood EJ. Gammaherpesvirus persistence alters key CD8 T-cell memory characteristics and enhances antiviral protection. J. Virol. 2006;80:8303–8315. doi: 10.1128/JVI.00237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cush SS, Flaño E. Protective antigen-independent CD8 T cellmemory is maintained during gamma-herpesvirus persistence. J. Immunol. 2009;182:3995–4004. doi: 10.4049/jimmunol.0803625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, Woodland DL. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J. Immunol. 2001;166:1813–1822. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- 26.Onami TM, Harrington LE, Williams MA, Galvan M, Larsen CP, Pearson TC, Manjunath N, Baum LG, Pearce BD, Ahmed R. Dynamic regulation of T cell immunity by CD43. J. Immunol. 2002;168:6022–6031. doi: 10.4049/jimmunol.168.12.6022. [DOI] [PubMed] [Google Scholar]
- 27.Moser JM, Gibbs J, Jensen PE, Lukacher AE. CD94-NKG2A receptors regulate antiviral CD8(+) T cell responses. Nat. Immunol. 2002;3:189–195. doi: 10.1038/ni757. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J, Matsuoka M, Cantor H, Homer R, Enelow RI. Cutting edge: engagement of NKG2A on CD8+ effector T cells limits immunopathology in influenza pneumonia. J. Immunol. 2008;180:25–29. doi: 10.4049/jimmunol.180.1.25. [DOI] [PubMed] [Google Scholar]
- 29.McMahon CW, Zajac AJ, Jamieson AM, Corral L, Hammer GE, Ahmed R, Raulet DH. Viral and bacterial infections induce expression of multiple NK cell receptors in responding CD8(+) T cells. J. Immunol. 2002;169:1444–1452. doi: 10.4049/jimmunol.169.3.1444. [DOI] [PubMed] [Google Scholar]
- 30.Cavanaugh VJ, Raulet DH, Campbell AE. Upregulation of CD94/NKG2A receptors and Qa-1b ligand during murine cytomegalovirus infection of salivary glands. J. Gen. Virol. 2007;88:1440–1445. doi: 10.1099/vir.0.82444-0. [DOI] [PubMed] [Google Scholar]
- 31.Miller JD, Peters M, Oran AE, Beresford GW, Harrington L, Boss JM, Altman JD. CD94/NKG2 expression does not inhibit cytotoxic function of lymphocytic choriomeningitis virus-specific CD8+ T cells. J. Immunol. 2002;169:693–701. doi: 10.4049/jimmunol.169.2.693. [DOI] [PubMed] [Google Scholar]
- 32.Tessmer MS, Fugere C, Stevenaert F, Naidenko OV, Chong HJ, Leclercq G, Brossay L. KLRG1 binds cadherins and preferentially associates with SHIP-1. Int. Immunol. 2007;19:391–400. doi: 10.1093/intimm/dxm004. [DOI] [PubMed] [Google Scholar]
- 33.Voehringer D, Blaser C, Brawand P, Raulet DH, Hanke T, Pircher H. Viral infections induce abundant numbers of senescent CD8 T cells. J. Immunol. 2001;167:4838–4843. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- 34.Rosshart S, Hofmann M, Schweier O, Pfaff AK, Yoshimoto K, Takeuchi T, Molnar E, Schamel WW, Pircher H. Interaction of KLRG1 with Ecadherin: new functional and structural insights. Eur. J. Immunol. 2008;38:3354–3364. doi: 10.1002/eji.200838690. [DOI] [PubMed] [Google Scholar]
- 35.Betts MR, Koup RA. Detection of T-cell degranulation: CD107a and b. Methods Cell Biol. 2004;75:497–512. doi: 10.1016/s0091-679x(04)75020-7. [DOI] [PubMed] [Google Scholar]
- 36.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. USA. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 40.Moser JM, Byers AM, Lukacher AE. NK cell receptors in antiviral immunity. Curr. Opin. Immunol. 2002;14:509–516. doi: 10.1016/s0952-7915(02)00357-6. [DOI] [PubMed] [Google Scholar]
- 41.Byers AM, Andrews NP, Lukacher AE. CD94/NKG2A expression is associated with proliferative potential of CD8 T cells during persistent polyoma virus infection. J. Immunol. 2006;176:6121–6129. doi: 10.4049/jimmunol.176.10.6121. [DOI] [PubMed] [Google Scholar]
- 42.van Stijn A, Rowshani AT, Yong SL, Baas F, Roosnek E, ten Berge IJ, van Lier RA. Human cytomegalovirus infection induces a rapid and sustained change in the expression of NK cell receptors on CD8+ T cells. J. Immunol. 2008;180:4550–4560. doi: 10.4049/jimmunol.180.7.4550. [DOI] [PubMed] [Google Scholar]
- 43.Snyder CM, Cho KS, Bonnett EL, van Dommelen S, Shellam GR, Hill AB. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29:650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bengsch B, Spangenberg HC, Kersting N, Neumann-Haefelin C, Panther E, von Weizsäcker F, Blum HE, Pircher H, Thimme R. Analysis of CD127 and KLRG1 expression on hepatitis C virus-specific CD8+ T cells reveals the existence of different memory T-cell subsets in the peripheral blood and liver. J. Virol. 2007;81:945–953. doi: 10.1128/JVI.01354-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thimme R, Appay V, Koschella M, Panther E, Roth E, Hislop AD, Rickinson AB, Rowland-Jones SL, Blum HE, Pircher H. Increased expression of the NK cell receptor KLRG1 by virus-specific CD8 T cells during persistent antigen stimulation. J. Virol. 2005;79:12112–12116. doi: 10.1128/JVI.79.18.12112-12116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colpitts SL, Dalton NM, Scott P. IL-7 receptor expression provides the potential for long-term survival of both CD62Lhigh central memory T cells and Th1 effector cells during Leishmania major infection. J. Immunol. 2009;182:5702–5711. doi: 10.4049/jimmunol.0803450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thirumalapura NR, Crossley EC, Walker DH, Ismail N. Persistent infection contributes to heterologous protective immunity against fatal ehrlichiosis. Infect. Immun. 2009;77:5682–5689. doi: 10.1128/IAI.00720-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J. Exp. Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]