Abstract
Acute administration of loop diuretics like furosemide leads to a stimulation of renin secretion, an effect thought to result from inhibition of NKCC2-mediated salt transport at the luminal surface of the macula densa (MD). However, loop diuretics also inhibit NKCC1, the second isoform of the Na/K/2Cl cotransporter, with similar potency. In the present study we examined the influence of furosemide on renin secretion in NKCC1-deficient mice in order to distinguish between effects of the loop diuretic involving NKCC2 and by implication the macula densa pathway, and effects that might occur via inhibition of NKCC1. Baseline PRC was 1212 ± 211 in NKCC1+/+ (n=13) and 3851 ± 579 ng Ang I/ml h in NKCC1−/− mice (n=14; p=.00024). Acute administration of furosemide (50 mg/kg i.p.) increased PRC significantly to 9324 ± 1018 ng Ang I/ml h in NKCC1+/+ (n=13; p<.0001 compared to basal), and to 14188 ± 2274 ng Ang I/ml h in NKCC1−/− mice (n=14; p=.0002 compared to basal; p=.034 compared to wild type plus furosemide). Renin mRNA expression was about 3 fold higher in NKCC1−/− compared to WT mice. There was considerable recruitment of granular cells to upstream regions of afferent arterioles in NKCC1−/− mice. Patch clamp studies on single JG cells from wild type mice showed an about 10% increase in membrane capacitance during incubation with furosemide (10−4 M) indicating a direct effect of the loop diuretic on renin secretion. No effect of furosemide on membrane capacitance was observed in JG cells from NKCC1-deficient mice. Furosemide (10−3 M) significantly stimulated renin release from primary cultures of JG cells from wild type mice whereas no response was observed in NKCC1−/− mice. Our data suggest that a functional NKCC1 suppresses basal renin release, at least in part through a direct effect on juxtaglomerular granular cells.
Keywords: NKCC1 knockout mice, plasma renin, renin mRNA, patch clamp, JG cells
Introduction
NaCl concentration in the tubular fluid in the tubulo-vascular contact region of the distal nephron has been shown to exert major regulatory effects on both preglomerular resistance and renin secretion (30). The interaction between tubules and vessels is thought to be initiated by the specialized epithelial cell plaque called the macula densa (MD) located at the distal end of the loop of Henle. This group of cells acts as a chemosensor for luminal NaCl concentration: a low NaCl concentration in the tubular lumen generates a signal that leads to the release of renin from juxtaglomerular cells and to a dilatation of the smooth muscle cells of the afferent arteriole, while a high NaCl concentration has the opposite effects. Tubular NaCl concentration is believed to be detected as some direct function of the transport activity of the Na/K/2Cl cotransporter NKCC2 in the apical membrane of MD cells (21, 29). Much of the evidence supporting a role of NKCC2-mediated transport activity in MD signaling is derived from studies of the effects of inhibitors of NKCC2 such as furosemide or bumetanide. In fact, administration of loop diuretics has become a whole animal approach to determine the functional characteristics of MD control of both preglomerular resistance and renin secretion.
Nevertheless, the mechanisms responsible for the effects of systemic administration of loop diuretics are not entirely clear. These agents can inhibit both isoforms of the cotransporter, NKCC1 and NKCC2, with similar potency (13). The NKCC1 subtype of the Na/K/2Cl cotransporter is widely expressed in both epithelial and non-epithelial cells (11). In secretory epithelia, NKCC1 is typically found in a basolateral position. NKCC1 expression in the kidney is primarily localized in the inner medullary collecting duct, but has also been found in cells of the afferent arteriole, and extraglomerular mesangium (20). NKCC1 mRNA was detected by RT-PCR in various nephron segments, most abundantly in outer and inner medullary collecting ducts (16). Furthermore, in a recently developed cell line with MD characteristics NKCC1 was found to be the predominant isoform of the cotransporter (14, 35). In contrast to the extended presence of NKCC1, expression of NKCC2 is restricted to the apical membrane of thick ascending limbs and MD cells (19, 34). Because of its conspicuous immunopositivity in the region of the juxtaglomerular apparatus a role of NKCC1 in renin secretion seemed conceivable. Therefore, systemic administration of furosemide may exert effects that reflect inhibition of NKCC1 in addition or as an alternative to its action as inhibitor of NKCC2 activity in the loop of Henle and MD cells.
The present study was undertaken to explore the possibility of NKCC1 playing a role in basal renin release and in the stimulation of renin secretion known to be exerted by loop diuretics. Because of the absence of selective inhibitors of the NKCC isoforms we utilized experimental models in which only one of the cotransporters is expressed so that the target of the loop diuretic is better defined. Our specific in vivo approach was to examine the renin-regulatory effect of furosemide in NKCC1 knockout mice in which the diuretic can only act through NKCC2 (5, 6). In addition, we used isolated juxtaglomerular granular cells to determine if furosemide can affect renin secretion in the absence of the MD, an effect presumably mediated by inhibition of NKCC1.
Our studies in NKCC1−/− mice show that basal levels of renal renin mRNA were elevated compared to wild type mice, and this was accompanied by an increase in renin expression that included recruitment of renin- forming cells outside the juxtaglomerular region. Furthermore, in isolated granular cells from mouse kidneys furosemide caused an increase in membrane capacitance, an index of renin exocytosis, in cells from wild type, but not from NKCC1−/− mice. These results suggest that renin synthesis and secretion is tonically suppressed at the level of the renin-generating cells by NKCC1-mediated NaCl flux.
Methods
Animals
NKCC1 deficient mice (NKCC1−/−) and their wild type controls were from a colony originally generated by Flagella et al. (6), and maintained at the University of Cincinnati. Animal care and experimentation was approved and carried out in accordance with institutional and NIH principles and guidelines for the Care and Use of Laboratory Animals. An additional group of 10 NKCC1−/− and 10 wild type mice from a subcolony of the original knockout strain maintained at Emory University was used for the blood pressure and in vitro studies.
Blood collection and renin determination
Tail blood was taken from conscious mice by nicking the tail with a razor blade, and collecting the emerging blood into a 75 µl hematocrit tube that contained 1 µl 125 mM EDTA in its tip. Red cells and plasma were separated by centrifugation; the plasma was ejected into an Eppendorf tube, and frozen until used for renin determinations. Plasma renin concentration (PRC) was measured with a commercial radioimmunoassay kit (Gammacoat, DiaSorin, Stillwater MN). In our approach, 1 µl of a 1:5 plasma dilution (equivalent to 0.2 µl of undiluted plasma) was mixed on ice with 14 µl of dialyzed rat substrate and 10 µl of maleate buffer containing EDTA, neomycin sulfate, and PMSF provided by the manufacturer. For determination of background angiotensin I levels 10 µl of this mix was removed and kept frozen until assaying. The remaining aliquot was incubated for 1 hour at 37 °C. Generated angiotensin I was measured by radioimmunoassay using standards and reagents provided by the manufacturer. In each assay, substrate without plasma was incubated for the same time, and any background angiotensin I formation was subtracted from the plasma containing samples. Angiotensin I levels determined in the non-incubated plasma aliquot were also subtracted. The high renin levels in the mouse especially after furosemide treatment pose a problem for measurements of PRC since saturating amounts of substrate are difficult to maintain for the entire incubation period. The amount of substrate used in these studies is based on pilot experiments aimed at establishing saturation conditions for plasma samples with very high renin levels.
For basal renin determinations, all animals were kept in individual cages at least 3 days before blood collections. Separation of mice, especially of male animals, was found to reduce data variation and to minimize baseline renin values (2). Following baseline blood collections animals were allowed to recover for 3 days before they received a single i.p. injection of furosemide (50 mg/kg; Lasix, Hoechst). Blood was collected 45 min later, while urine was collected over this period in metabolic cages to verify the efficacy of the injection.
Visualization of JG cell granules
Kidneys from NKCC1−/− and +/+ mice were treated with 5M HCl for 1 hour at 37°C. After acid removal, kidneys were kept in distilled water for 48 hours at 4°C. Vascular trees were microdissected under a stereomicroscope and the JG cells were directly visualized as described by Casellas et al (1). In four vessel trees from each genotype we counted the number of afferent arterioles that showed typical juxtaglomerular granularity, additional upstream recruitment, or no granularity.
Real time PCR
Total RNA was extracted from whole kidney using Trizol reagent and denatured at 70 °C for 5 minutes. cDNA synthesis was done at 42 °C for 45 min using superscript reverse transcriptase. For real time PCR, renin primers were chosen by using PRIMER EXPRESS 2.0 (PE Applied Biosystems) with an MGB probe being positioned at an exon-intron boundary. The primer/MGB probe mixture for 18S rRNA was purchased from Applied Biosystems as was the β-actin primer/probe set. Real time PCR amplification was performed in 50 ng cDNA using TagMan Universal PCR Master Mix. Cycling conditions were 50 °C for 2 min, 95 °C for 10 min, followed by 40 repeats at 95 °C for 0.15 min and 60 °C for 1 min. The relative amount of renin mRNA, normalized by 18S rRNA or β-actin, was expressed as 2−??CT calculated from threshold cycle numbers (CT) according to the manufacturer’s suggestion.
Isolation of JG cells and renin secretion studies
Kidneys from C57Bl/6 mice (wild type) and NKCC1−/− mice were removed, decapsulated, minced, and transferred to 30 mL of isolation buffer supplemented with 0.1% (wt/vol) of collagenase A (0.57 U/mg; Roche Diagnostics) and 0.25% (wt/vol) of trypsin (1300 BAEE U/mg; Sigma). The tissue was incubated and stirred gently for 70 minutes at 37°C, then filtered through a 22 µm nylon mesh. The filtrate was washed, centrifuged, and resuspended in 4 mL of isolation buffer. For patch clamp studies, JG cells were identified by their appearance and transferred individually to cover slips. For renin secretion studies, cells were further separated on a Percoll density gradient (25% Percoll) by centrifugation for 30 minutes at 27 000g (4°C). Four cell layers with different specific renin activities were obtained. The cellular layer (equivalent to a density of 1.049 g/mL) with the highest renin concentration (100-fold increase in specific renin activity) was used for the experiments. These cells were washed twice and resuspended in RPMI-1640 medium to a concentration of 150,000 cells/ml. Media contained FCS (2%), insulin, penicillin and streptomycin. Aliquots (100 µL) of this suspension were seeded in 96-multiwell plates. After incubating the cells for 20 hours, cells were washed and experimental agents (furosemide 10−5 M - 10−3 M, forskolin 10−5 M) were added. Cell-conditioned medium was removed after another 20 hours, centrifuged at 10 000g at room temperature to remove cellular debris, and renin concentration was determined by radioimmunoassay of angiotensin I (18). The cells were lysed by the addition of 100 µL of PBS with 0.1% of Triton X-100 and 0.1% human serum albumin to each well as described by Friis et al (7). Plates were shaken for 45 minutes at room temperature, and lysates were centrifuged at 10 000 g for 10 minutes. The supernatants were stored at −20°C until further processing. The renin concentration in the supernatants was measured after incubation for 3 hours with excess rat renin substrate followed by RIA against angiotensin I. Renin secretion rates were calculated as fractional release of total renin content [i.e., renin released/(renin released + renin remaining in the cells)].
Patch-clamp experiments
A glass cover slip with individual JG cells was superfused with the control external solution, then transferred to the recording chamber and supplemented with buffer to a volume of approximately 400 µl. Experiments were performed at room temperature in the tight-seal whole-cell configuration of the patch-clamp technique using heat-polished, Sylgard-coated patch pipettes with resistances of 3 to 7 MΩ. Series resistances were in the range of 6 to 25 MΩ and seal resistances were in the range of 1 to 15 GΩ. High-resolution membrane currents were recorded with an EPC-9 patch-clamp amplifier (HEKA Elektronik, Lambrecht, Germany) controlled by PULSE v8.11 software on a Power Macintosh G3 computer. High-resolution currents were low-pass filtered at 2.9 kHz and acquired at a sampling rate of 20 kHz. The current-voltage relationship was monitored by the response to nine voltage steps of 30 mV (range −150 to +90 mV) for 200 ms from a holding potential of −30 mV. Low time resolution acquisition of membrane capacitance, Cm, was performed with the "sine+dc" method using the LockIn extension of the PULSE v8.11 software. The Cm measurements were started no later than 30 sec after the I-V recording. Data from an entire sweep are averaged to result in one Cm point per sweep, resulting in an acquisition rate of about 5 Hz using the Xchart extensio n of the PULSE software. The reference electrode was an Ag/AgCl pellet connected to the bath solution through a 150 mmol/L NaCl/agar bridge. All potentials were corrected for the liquid junction potential that develops at the tip of the pipette when it is immersed into the bath solution. The following solutions were used: 1. “Internal” control solution for patch clamp (in mmol/L): K-glutamate 135; NaCl 10; KCl 10; MgCl2 1; HEPES 10; Mg-ATP 0.5; Na2GTP 0.3; osmolality was 306 mOsm/kg; pH 7.05 (22°C). 2. “External” (bath) solution for patch clamp (in mmol/L): HEPES 10; NaCl 140; KCl 2.8; MgCl2 1; CaCl2 2; glucose 11; sucrose 10; osmolality was 304 mOsm/kg; pH 7.21 (25°C).
RT-PCR in single JG cells
Single mouse JG cells were used to determine the presence of NKCC1 mRNA by RT-PCR. 20 cells were selected one by one using a patch pipette, and RNA was isolated according to a microadaptation of the protocol of Chomczynski and Sacchi (3). PCR amplification was performed for 36 cycles using 3.3 µL of cDNA equivalent to 2.5 JG cells. Negative controls included water instead of cDNA in the PCR reaction and -RT. As positive control kidney medulla was used as template. The published sequence for mouse NKCC1 (NM_009194) was used to design primers for PCR amplification, the primer sequence was as follows: NKCC1: sense 5’ GAA CCT TTT GAG GAT GGC’3, antisense 5’ CAC GAT CCA TGA CAA TCT’3 covering base pair numbers 865–1044 resulting in a product length of 179 +15 (restriction site), i.e. 194 bp.
Blood pressure and heart rate
Systolic blood pressures and heart rates of 6 wild type and 5 NKCC1-deficient mice were determined by tail cuff manometry (Visitech Systems). Animals were conditioned by placing them into the holding device on three consecutive days before the first measurement. Blood pressure was determined on three days in a row and values were calculated as averages of these 3 measurements for each individual mouse.
Statistics
All values are means ± SE. Analysis of variance (ANOVA) was performed to test for statistical significance of differences among the values observed in each treatment group followed by a Bonferroni post test. A p value <0.05 was considered significant.
Results
Plasma renin concentration in NKCC1+/+ and NKCC1−/− mice
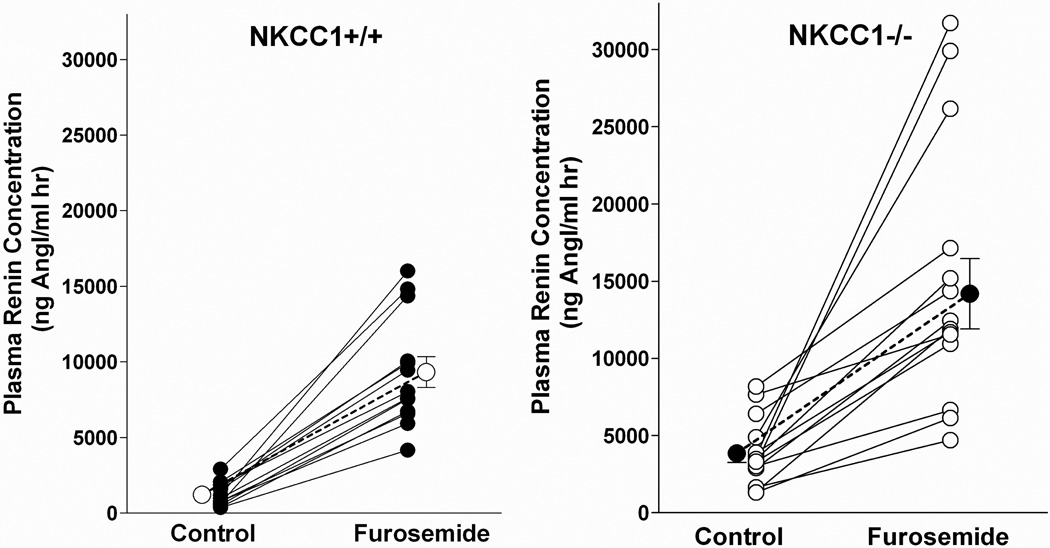
Baseline plasma renin concentration (PRC) averaged 1212 ± 211 in NKCC1+/+ (n=13) and 3851 ± 579 ng Ang I/ml h in NKCC1−/− mice (n=14; p=.00024 compared to wild type). Measurements in individual mice are shown in Fig. 1. After a single intraperitoneal injection of furosemide (50 mg/kg) PRC increased to 9324 ± 1018 ng Ang I/ml h in wild type animals (n=13; p<.0001), and to 14188 ± 2274 ng Ang I/ml h in NKCC1−/− mice (n=14; p=.0002 compared to basal and p=.034 versus wild type). Thus, the furosemide effect consisted of a PRC increase of 8112 ± 930 ng AngI/ml h in wild type and 11118 ± 2208 ng AngI/ml h in NKCC1−/− mice (p=.23) representing a 9.9 ± 1.5 fold rise in wild type and a 4.4 ± 0.7 fold rise in NKCC1−/− mice (p=.003).
Fig. 1.
PRC in NKCC1++ (left) and NKCC1−/− mice (right) mice under basal conditions and after acute administration of furosemide (50 mg/kg). Lines connect data from individual animals. Bold symbols and connecting lines represent means.
Visualization of granular cells
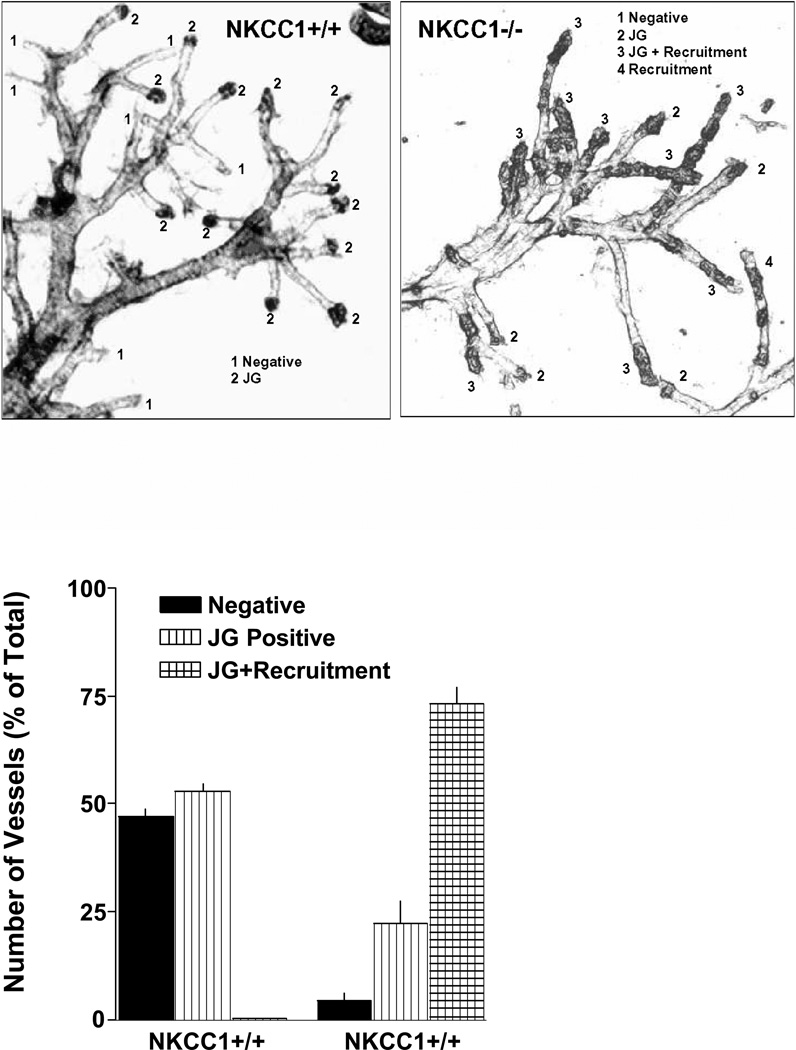
The method of Casellas et al. was used to visualize and quantify the extent of granulation in afferent arterioles (1). As has been documented previously the high contrast regions usually seen at the end of afferent arterioles represent clusters of renin-positive cells (1). It is evident from the examples shown in Fig. 2 that the extent of granulation is clearly augmented in the vascular trees from NKCC1−/− kidneys. Furthermore, vessels from NKCC1-deficient mice show high contrast areas in upstream regions of the afferent arterioles representing recruitment of renin-producing cells outside the juxtaglomerular location. In vessel trees from wild type mice, quantification in 4 vessel trees with a total of 110 arterioles showed that 52.9 ± 2% of all vessels had renin positivity at the terminal ends of the arterioles while 47.1 ± 2% were negative. In contrast, analysis of 4 vessel trees from NKCC1−/− kidneys with 163 identifiable arterioles showed recruitment to upstream regions in 73.1 ± 3.9% of the vessels while exclusive “classical” JG positivity was seen in 22.4 ± 5% with only 4.5 ± 1.5% of arterioles being negative.
Fig. 2.
Upper: Visualization of renin granules after acid maceration of microdissected renal arterial vessel trees from a NKCC1+/+ (left) and a NKCC1−/− mouse. High contrast regions at the end of the arterioles represent clusters of renin granules (glomeruli are sheared off in the microdissection process). Lower: Count of negative, JG positive and JG positive afferent arterioles with upstream recruitment.
Renin mRNA
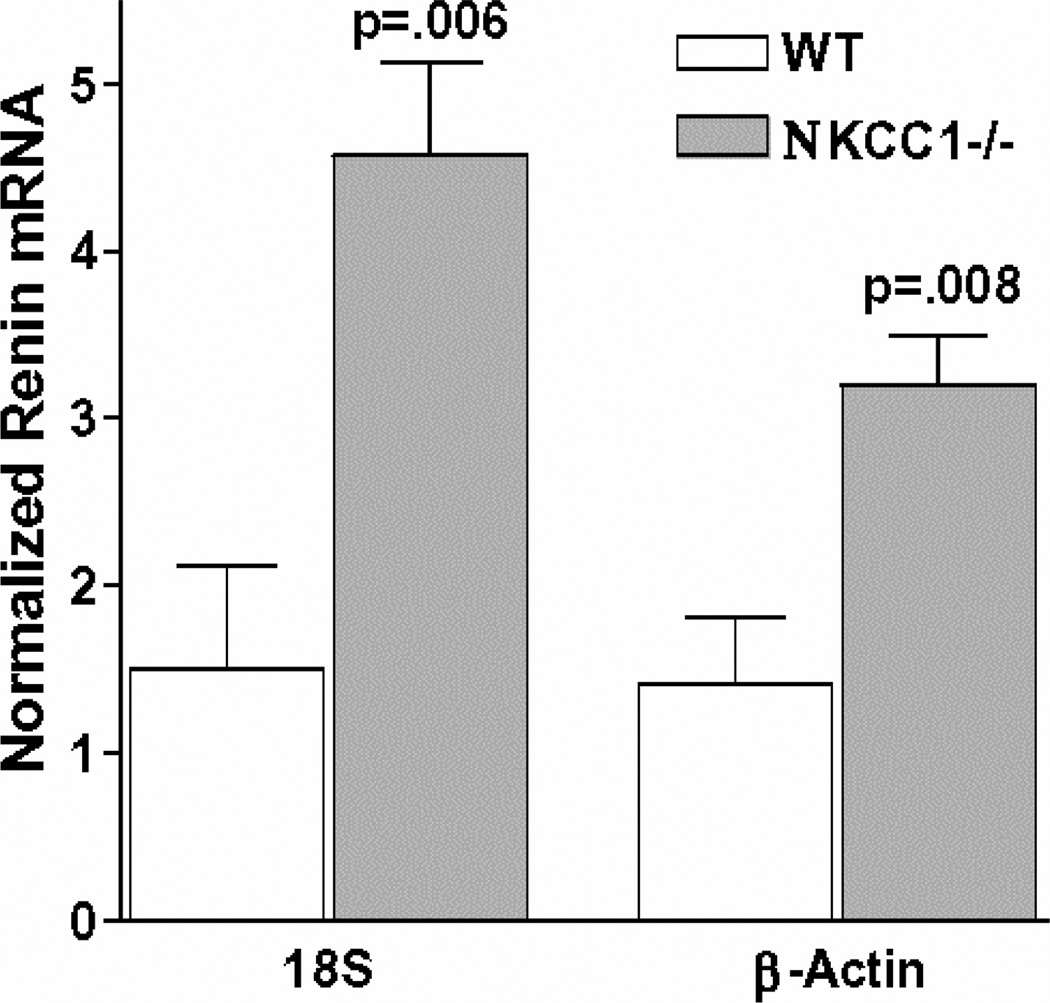
Renin mRNA was determined by real time PCR in 5 kidneys from wild type mice and in 5 kidneys from NKCC1−/− mice. Relative amounts of renin mRNA in wild type mice, expressed as 2−ddCT values calculated from threshold cycle numbers (CT), averaged 1.51 ± 0.6 when corrected for 18S rRNA and 1.42 ± 0.4 when corrected for β-actin. As shown in Fig. 3, renin mRNA levels in kidneys of NKCC1−/− mice were 3 times higher than in control when corrected for 18S rRNA (4.57 ± 0.57; p=.0006 compared to wild type), and 2.2 times higher than in control when corrected for β-actin (3.2 ± 0.3; p=.004 compared to wild type).
Fig. 3.
Basal renin mRNA from kidneys of NKCC1+/+ and −/− mice (n=5 each) determined by real time RT-PCR. Values are given after normalization with β-actin or 18S rRNA, respectively.
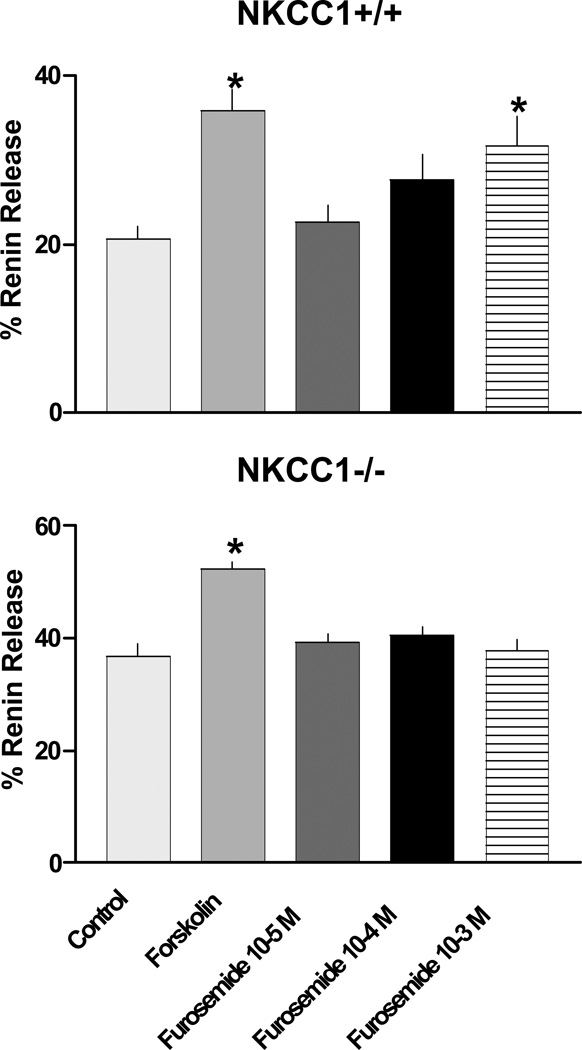
Effect of furosemide on renin secretion from isolated juxtaglomerular (JG) cells in primary culture. Experiments were performed in isolated JG cells to investigate a possible direct effect of furosemide on renin secretion. In 4 independent batches of JG cells harvested from wild type and NKCC1−/− mice fractional renin release under baseline conditions averaged 20.6 ± 1.6% (n=5) in wild type compared to 36.9 ± 2.2% (n=4) in NKCC1−/− mice. After incubation with furosemide mean fractional renin release was 22.6 ± 2.1%, 27.7 ± 3.0%, 31.7 ± 3.4% for 10−5, 10−4 and 10−3 M of furosemide, respectively, with differences reaching the 5% level of significance at a furosemide concentration of 10−3 M in wild type mice (Fig. 4). In this preparation, the well-known stimulator of renin release forskolin at a concentration of 10−5 M increased fractional renin release to 35.9 ± 2.4%. As can also be seen in Fig. 4, furosemide had no effect on mean fractional renin release in cells from NKCC1−/− mice, averaging 39.3 ± 1.6%, 40.4 ± 1.6%, 37.7 ± 2.0% for 10−5, 10−4 and 10−3 M of furosemide. Forskolin increased renin release to 52.2 ± 1.4% in NKCC1−/− mice.
Fig. 4.
Fractional renin release in preparations of isolated JG cells in primary culture. Basal renin release is compared to the effect of forskolin (10−5 M) and to that of increasing concentrations of furosemide for JG cells from wild type (upper) and NKCC1-deficient mice (lower). * p<.05 vs. control.
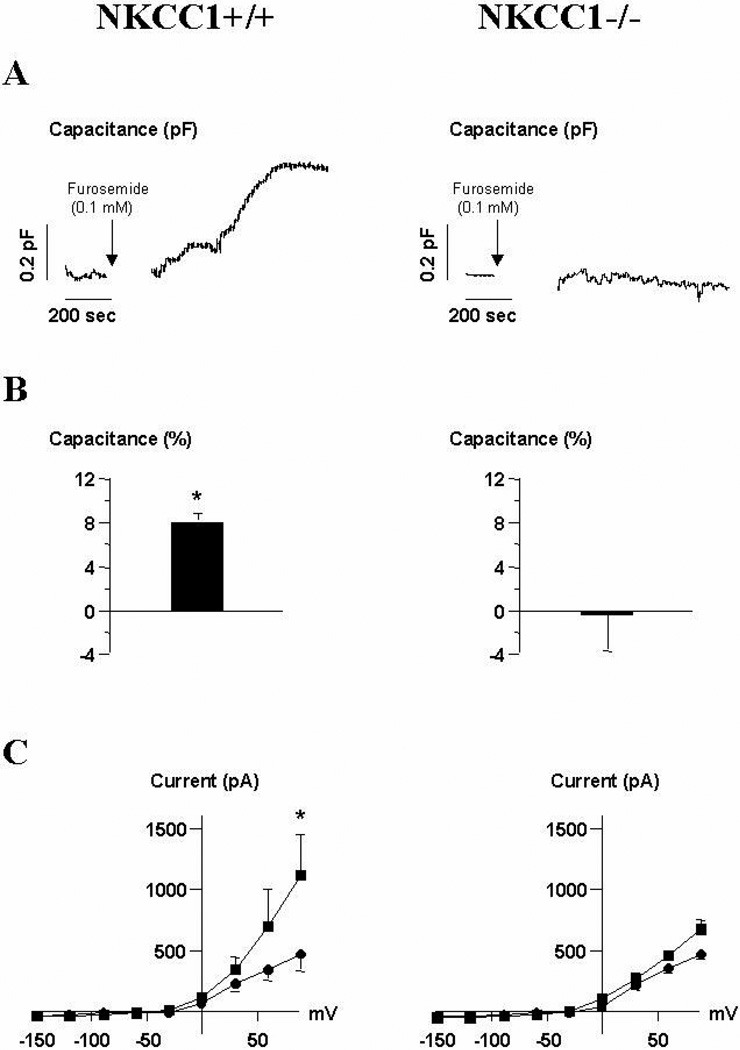
Patch-clamp experiments
Since primary cultures of JG cells isolated by gradient centrifugation are to some extent contaminated by other cell types, we measured the change in membrane capacitance (Cm) of single JG cells from wild type and NKCC1-deficient mice in response to furosemide by patch-clamp in the whole cell configuration as a measure for secretion. As shown in Fig. 5A for a single trace, in JG cells from wild type mice furosemide (10−4 M) led to a gradual increase in Cm over a period of 1200 seconds corresponding to secretory activity. As can be seen in Fig. 5B, the average change in Cm after 1200 seconds equaled + 8.1 ± 0.9 % (p<.05, n=5) in JG cells from wild type and −0.53 ± 3.3 % in JG cells from NKCC1−/− mice (p=.28, n=4). Fig. 5C shows the I-V curves for JG cells from wild type and NKCC1−/− mice recorded before and after superfusion with furosemide. Nine pulses were applied from −150 mV to +90 mV in 30 mV steps for 200 milliseconds from a holding potential of −30 mV. The current- voltage relationship was measured immediately after the whole-cell configuration was obtained (circle) and after 20 minutes of capacitance recording (square) in the presence of furosemide (10−4 M) during the last 14 minutes. A large increase in the outward current during superfusion with furosemide in JG cells from wild type mice can be noted. The maximal outward current at + 90 mV amounted to 469 ± 139 pA, at the beginning of the experiment without furosemide, and 1118 ± 329 pA after 14 minutes of superfusion with furosemide (10−4 M) for JG cells from NKCC1+/+ (p<.05; n=5), and 466 ± 65 and 512 ± 82 pA, respectively, for JG cells from NKCC1−/− mice (p=.09; n=4). The increase in outward current in wild type JG cells is also reflected as a hyperpolarization of the membrane potential amounting to −14.2 ± 6.6 mV (p<.05, n=5). In a control experiment, intracellular application of cAMP increased both Cm and outward current in JG cells from both NKCC1+/+ and NKCC1−/− mice. The outward current was probably carried by K+ through BKCa channels of the ZERO-splice variant (10).
Fig. 5.
A: Original recording of membrane capacitance in a single mouse juxtaglomerular cell from NKCC1+/+ (left) and NKCC1−/− mice (right). The pipette contained control internal solution and the cell was bathed in control external solution supplemented with furosemide (10−4M) at the time indicated by the arrow (see method section). B: Mean relative change of Cm for JG cells from NKCC1+/+ (left) and NKCC1−/− mice (right). C: Whole-cell currents were measured in response to 9 pulses from −150 mV to +90 mV in 30 mV steps for 200 ms from a holding potential of −30 mV. These pulses were applied before and after superfusion with furosemide (10−4M). Mean steady-state I-V curves from 5 independent experiments before (circle) and after (square) 20 minutes of capacitance measurements are given. * p<.05 vs. control.
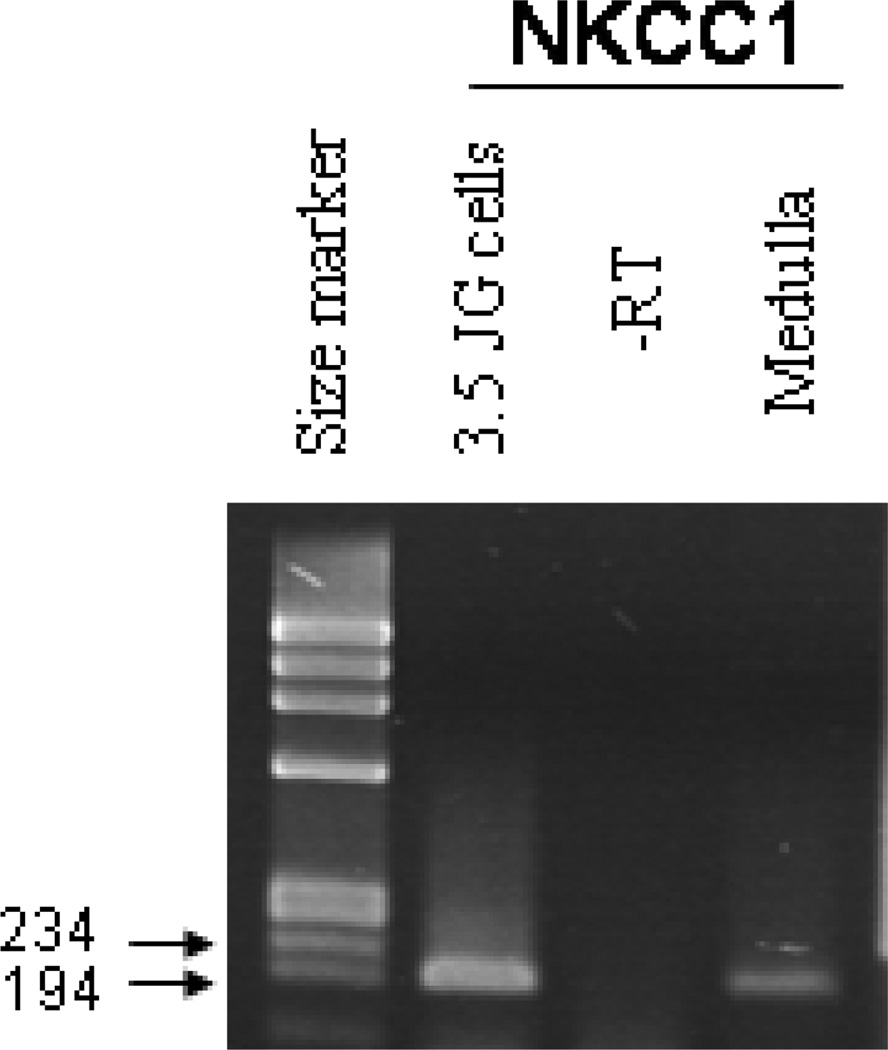
NKCC1 expression in single JG cells
In cDNA prepared from single isolated JG cells, PCR amplification with primers specific for NKCC1 consistently yielded a PCR product of the expected size (194 bp). An example is shown in Fig. 6. A band of this size was also detected in cDNA from kidney medulla used as positive control whereas no band was obtained in the negative RT minus and water controls.
Fig. 6.
RT-PCR on RNA prepared from single isolated juxtaglomerular granular cells. cDNA from the renal medulla was used as positive control.
Blood pressure and heart rate in NKCC1+/+ and NKCC1−/− mice
In view of the substantial basal activation of the renin system in NKCC1-deficient mice, blood pressure was determined in NKCC1+/+ and −/− mice by tail cuff manometry. Systolic blood pressure averaged 122 ± 3.4 in wild type (n=6) and 114 ± 3.3 mm Hg in NKCC1−/− mice (n=5; p=.13). Heart rate was 588 ± 17 in wild type and 537 ± 29 min−1 in knockout mice (p=.17).
Discussion
Furosemide-stimulated renin secretion is widely thought to affect renin-secreting granular cells indirectly through modulation of NKCC2-dependent NaCl transport in MD cells (29, 31). The present studies were performed with the specific aim to examine whether the stimulatory effect of loop diuretics on renin release includes a component that may be mediated by a direct interaction with JG cells and may therefore be due to inhibition of NKCC1. Since specific inhibitors of the two NKCC isoforms are not available, we made an attempt to examine the effect of loop diuretics in experimental models in which only one of the two isoforms was present.
The first goal of these studies was to compare the effect of furosemide on plasma renin concentration in wild type and NKCC1-deficient mice (6). Our data show that basal PRC of NKCC1−/− mice is significantly elevated, by a factor of about 3.2, compared to wild type controls, and that this increase in PRC is paralleled by a marked elevation of renal renin mRNA and protein expression. These observations suggest that the net effect of a functional NKCC1 is to tonically suppress renin synthesis and renin release. It is appreciated that these in vivo experiments do not permit a clear distinction between direct and indirect consequences of the NKCC1 null mutation on the renin-angiotensin system. In particular, the possibility needs to be considered that the stimulation of renin is the result of the reduced arterial blood pressure that has been observed in NKCC1−/− mice (6, 23). However, in another strain of NKCC1 null mutant mice blood pressure tended to be lower in the null animals, but this change did not reach statistical significance (28). Measurements of blood pressure with the tail cuff method in our laboratory also showed a tendency towards lower systolic blood pressures in NKCC1-deficient mice, but again this difference was not significant statistically. In view of these variable findings even in the same strain of NKCC1 mice, we assume that differences in blood pressure observed by different groups might not reflect actual differences in baseline blood pressure, but may rather be related to differential blood pressure responses to stressful interventions in these animals. The 3.2 fold difference in basal PRC between wild type and NKCC1−/− mice observed in our study seems somewhat disproportionate to a systolic blood pressure reduction which appears to be only marginally significant. Thus, while a reduction in blood pressure may be a contributory factor to the elevated PRC in NKCC1−/− mice, we consider it unlikely that it is the main reason. In addition to the absence of a significant change of blood pressure in these studies, the in vitro evidence discussed below clearly supports the likelihood of a direct, pressure-independent effect of NKCC1.
There is little reason to assume that the increase in PRC in the NKCC1−/− mice could be the result of an activation of the MD pathway. NKCC1−/− mice do not appear to be volume-depleted as judged from normal plasma aldosterone levels and lack of effect of a low Na diet on arterial blood pressure (23, 28). It is therefore unlikely that NaCl concentration in the MD region is markedly reduced. Absence of volume depletion also suggests that sympathetic input to the kidney is probably unaltered in NKCC1−/− mice. Finally, while NKCC1 has been found to be expressed in a cell line with MD characteristics, there is little evidence that native MD cells express NKCC1 at detectable levels (14). Thus, the stimulation of the renin system in NKCC1−/− mice does probably not result from a specific deficiency of the transporter in MD cells. In view of our observations in isolated JG cells we believe that the increase in PRC in NKCC1−/− mice reflects disinhibition of renin release from tonic suppression exerted by NKCC1 at the level of the JG cells. It is of note that chronic NKCC1−/− deficiency is associated with a change in the expression pattern of renin that is characterized by a remarkable recruitment of renin-expressing cells outside the juxtaglomerular region. It is possible therefore that NKCC1 transport activity is related to suppression of renin expression in more proximal vascular cells and to the restriction of renin expression to the JG region. Release of renin by recruited cells may contribute to the higher levels of basal plasma renin in NKCC1−/− mice.
Direct evidence for an MD-independent renin-stimulatory effect of NKCC1 inhibition was derived from the effect of loop diuretics in primary cultures of JG cells isolated by gradient centrifugation methods. Basal renin release was higher in cells from NKCC1−/− than wild type mice. In addition, furosemide caused a significant increase of renin release in wild type mice whereas it had no effect in NKCC1−/− mice. Compared to the reported IC50 values of furosemide inhibition of NKCC1 or NKCC2 the concentrations of furosemide required to cause significant stimulation of renin release in the isolated JG cell preparation were relatively high. While this finding may suggest a non-specific action of the diuretic it is also possible that the inhibitory constant of furosemide is to some extent cell- specific (13). Furthermore, the affinity of the diuretic to inhibit NKCC1 appears to depend upon the activation state of the transporter. For example, an about 100fold increase in the IC50 concentration for furosemide has been observed in rat thymocytes under non- hypertonic conditions where the transporter activity is markedly reduced (13). Direct evidence for a specific effect of furosemide on NKCC1-mediated transport in our preparations is furnished by the finding that the diuretic did not alter renin secretion in JG cells from NKCC1-deficient mice.
Since JG cell preparations are usually somewhat contaminated with other cell types we also assessed the effect of furosemide on renin secretion in single JG cells. Increases in membrane capacitance of single juxtaglomerular granular cells have previously been shown to occur with the administration of intracellular cAMP and extracellular isoproterenol as well as with cell swelling (7, 8). As previously demonstrated, cell membrane capacitance measured by patch clamp techniques is a reflection of the secretory activity of a cell because exocytosis requires fusion events that increase membrane area and capacitance (24). Thus, membrane capacitance changes in granular cells are very likely an expression of exocytosis of renin granules (8). Our data permit the conclusion that furosemide causes a significant increase in renin secretion in JG cells from wild type mice that is comparable in magnitude to that produced by forskolin (7). In contrast to its effect in JG cells from wild type mice, furosemide did not affect the capacitance of JG cells isolated from NKCC1-deficient mice. These observations provide clear evidence for the notion that furosemide stimulates the renin system by interacting with NKCC1 expressed in the membrane of JG cells and thereby eliminating a renin-suppressing effect of the NaCl transporter. Previous transporter localization studies have consistently failed to show NKCC2 or its mRNA outside the epithelium of the thick ascending limb (25, 26). On the other hand, the presence of NKCC1 in renin-positive cells of afferent arterioles has previously been suggested by immunohistochemistry (20). In addition, we could detect NKCC1 mRNA by RT-PCR in RNA isolated from single granular cells. On the basis of these observations we believe that the effect of furosemide in vivo is to some extent the result of a direct interaction of the loop diuretic with NKCC1 in granular cells.
The patch clamp studies also provide some insights into the mechanisms underlying the increase in renin secretion during blockade of NKCC1 activity. These experiments have shown that furosemide induced an increase in an outward current that was carried by potassium and caused a marked cell hyperpolarization. Previous studies in isolated JG cells have shown that activation of the cAMP-sensitive ZERO splice variant of BKCa is the likely cause for the hyperpolarization (10). However, it is unclear how furosemide causes an increase in cAMP that could explain its effect on membrane potential and renin release. Alternatively, a decreased intracellular chloride concentration caused by inhibition of NKCC1-dependent chloride transport might either inhibit voltage-dependent calcium channels (27) directly or indirectly by driving HCO3− out of the cell via the Cl−/HCO3− exchanger (33). Thus, a resulting reduction in cytosolic Ca in granular cells may be another pathway through which loop diuretics stimulate renin release (12). However, inactivation of L type Ca channels may be an unlikely mechanism of action in view of the recent finding that these channels are essentially inactive at the membrane voltages observed in JG cells (9). Regardless of the exact mechanism our studies in isolated JG cells indicate that NKCC1 transport activity suppresses basal renin synthesis and secretion and that this transport activity resides in the JG cells themselves.
A second goal of this study was to assess the possibility that the acute renin-stimulatory response to furosemide in vivo may be mediated by inhibition of both NKCC isoforms. Such dual actions of loop diuretics on renin secretion are suggested by earlier evidence. For example, in the isolated perfused JGA of the rabbit stimulation of renin secretion has been observed when loop diuretics were administered into the tubular lumen, a situation where inhibition of extratubular NKCC1 may be unlikely (15, 17, 22). However, this increase of renin release amounted to only about 3 fold (22), much less than the approximately 6–8 fold increase caused by loop diuretics in this and several other in vivo studies. Furthermore, an action of furosemide on renin secretion through a macula densa-, and therefore presumably NKCC2-independent pathway has been suggested in non- filtering kidneys (4, 32). Our results show that acute in vivo administration of furosemide increased PRC levels of wild type mice by a factor of 9.9 while the relative increase over control was only 4.4-fold in NKCC1−/− mice. However, this reduction in the relative increase of PRC in NKCC1−/− mice appears to be due to the elevated baseline levels since the absolute increase in PRC caused by furosemide was not significantly different between wild type and NKCC1−/− mice (8112 vs. 11118 ng AngI/ml hr; p=.23). Thus, it appears that furosemide stimulates renin release to a comparable extent with or without NKCC1 suggesting that the acute effect of furosemide is largely the result of NKCC2 inhibition. It is conceivable that absence of a demonstrable effect of furosemide on NKCC1 results from the fact that because of tubular secretion furosemide concentrations at any given plasma level will be higher at the MD so that NKCC2 is exposed to higher furosemide concentrations than NKCC1. However, at the dose of 50 mg/kg used in these studies one would expect all NKCC isoforms to be at least transiently inhibited although it is not certain that inhibition was sustained for the entire 45 minute period. Nevertheless, since the effect of furosemide on PRC was comparable between wild type and NKCC1-deficient mice, inhibition of NKCC1 does not appear to significantly contribute to the acute renin-stimulatory effect of furosemide. The newly recruited renin-producing cells that may be the main cause for the elevated basal renin levels of NKCC1−/− mice are not expected to respond to furosemide since they are presumably not under MD control and therefore not affected by NKCC2 inhibition.
In conclusion, our results show that basal plasma renin concentration is markedly elevated in NKCC1−/− compared to wild type mice suggesting tonic suppression of renin synthesis and secretion by NKCC1 transport activity. Patch clamp studies in isolated granular cells have demonstrated the capacity of furosemide to stimulate renin exocytosis. Thus, NKCC1 exerts its renin- inhibiting actions at least in part through a direct effect at the level of the JG cells.
Acknowledgements
This work was supported by intramural funds from the National Institute of Diabetes and Digestive and Kidney Diseases (JS), and by grant DK057552 (JL). Hayo Castrop was the recipient of a Visiting Fellowship Grant from the Deutsche Forschungsgemeinschaft (CA278/3-1). Drs. Hansen and Oppermann are the recipients of Visiting Fellowships of NIDDK. The authors thank Dr. Susan Wall from Emory University for generously supplying additional wild type and NKCC1−/− mice.
References
- 1.Casellas D, Dupont M, Kaskel FJ, Inagami T, Moore LC. Direct visualization of renin-cell distribution in preglomerular vascular trees dissected from rat kidney. Am J Physiol Renal Physiol. 1993;265:F151–F156. doi: 10.1152/ajprenal.1993.265.1.F151. [DOI] [PubMed] [Google Scholar]
- 2.Castrop H, Schweda F, Mizel D, Huang Y, Briggs J, Kurtz A, Schnermann J. Permissive role of nitric oxide in macula densa control of renin secretion. Am J Physiol Renal Physiol. 2004 doi: 10.1152/ajprenal.00272.2003. in press. [DOI] [PubMed] [Google Scholar]
- 3.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 4.Corsini WA, Hook JB, Bailie MD. Control of renin secretion in the dog. Effects of furosemide on the vascular and macula densa receptors. Circ Res. 1975;37:464–470. doi: 10.1161/01.res.37.4.464. [DOI] [PubMed] [Google Scholar]
- 5.Delpire E, Lu J, England R, Dull C, Thorne T. Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat Genet. 1999;22:192–195. doi: 10.1038/9713. [DOI] [PubMed] [Google Scholar]
- 6.Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, Gawenis LR, Kramer J, Duffy JJ, Doetschman T, Lorenz JN, Yamoah EN, Cardell EL, Shull GE. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem. 1999;274:26946–26955. doi: 10.1074/jbc.274.38.26946. [DOI] [PubMed] [Google Scholar]
- 7.Friis UG, Jensen BL, Aas JK, Skott O. Direct demonstration of exocytosis and endocytosis in single mouse juxtaglomerular cells. Circ. Res. 1999;84:929–936. doi: 10.1161/01.res.84.8.929. [DOI] [PubMed] [Google Scholar]
- 8.Friis UG, Jensen BL, Sethi S, Andreasen D, Hansen PB, Skott O. Control of renin secretion from rat juxtaglomerular cells by cAMP-specific phosphodiesterases. Circ Res. 2002;90:996–1003. doi: 10.1161/01.res.0000017622.25365.71. [DOI] [PubMed] [Google Scholar]
- 9.Friis UG, Jorgensen F, Andreasen D, Jensen BL, Skott O. Membrane potential and cation channels in rat juxtaglomerular cells. Acta Physiol Scand. 2004;181:391–396. doi: 10.1111/j.1365-201X.2004.01310.x. [DOI] [PubMed] [Google Scholar]
- 10.Friis UG, Jorgensen F, Andreasen D, Jensen BL, Skott O. Molecular and functional identification of cyclic AMP-sensitive BKCa potassium channels (ZERO variant) and L-type voltage-dependent calcium channels in single rat juxtaglomerular cells. Circ Res. 2003;93:213–220. doi: 10.1161/01.RES.0000085041.70276.3D. [DOI] [PubMed] [Google Scholar]
- 11.Haas M, Forbush B., 3rd The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol. 2000;62:515–534. doi: 10.1146/annurev.physiol.62.1.515. [DOI] [PubMed] [Google Scholar]
- 12.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 1990;70:1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 13.Hannaert P, Alvarez-Guerra M, Pirot D, Nazaret C, Garay RP. Rat NKCC2/NKCC1 cotransporter selectivity for loop diuretic drugs. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:193–199. doi: 10.1007/s00210-001-0521-y. [DOI] [PubMed] [Google Scholar]
- 14.He H, Podymow T, Zimpelmann J, Burns KD. NO inhibits Na+-K+-2Cl-cotransport via a cytochrome P-450-dependent pathway in renal epithelial cells (MMDD1) Am J Physiol Renal Physiol. 2003;284:F1235–F1244. doi: 10.1152/ajprenal.00192.2002. [DOI] [PubMed] [Google Scholar]
- 15.He XR, Greenberg SG, Briggs JP, Schnermann J. Effects of furosemide and verapamil on the NaCl dependency of macula densa- mediated renin secretion. Hypertension. 1995;26:137–142. doi: 10.1161/01.hyp.26.1.137. [DOI] [PubMed] [Google Scholar]
- 16.Ikebe M, Nonoguchi H, Nakayama Y, Tashima Y, Tomita K. Upregulation of the secretory-type Na(+)/K(+)/2Cl(−)-cotransporter in the kidney by metabolic acidosis and dehydration in rats. J Am Soc Nephrol. 2001;12:423–430. doi: 10.1681/ASN.V123423. [DOI] [PubMed] [Google Scholar]
- 17.Itoh S, Carretero OA. Role of the macula densa in renin release. Hypertension. 1985;7:I49–I54. doi: 10.1161/01.hyp.7.3_pt_2.i49. [DOI] [PubMed] [Google Scholar]
- 18.Jensen BL, Schmid C, Kurtz A. Prostaglandins stimulate renin secretion and renin mRNA in mouse renal juxtaglomerular cells. Am J Physiol Renal Physiol. 1996;271:F659–F669. doi: 10.1152/ajprenal.1996.271.3.F659. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan MR, Mount DB, Delpire E. Molecular mechanisms of NaCl cotransport. Annu Rev Physiol. 1996;58:649–668. doi: 10.1146/annurev.ph.58.030196.003245. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan MR, Plotkin MD, Brown D, Hebert SC, Delpire E. Expression of the mouse Na-K-2Cl cotransporter, mBSC2, in the terminal inner medullary collecting duct, the glomerular and extraglomerular mesangium, and the glomerular afferent arteriole. J Clin Invest. 1996;98:723–730. doi: 10.1172/JCI118844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lapointe JY, Bell PD, Cardinal J. Direct evidence for apical Na+:2Cl- :K+ cotransport in macula densa cells. Am J Physiol Renal Physiol. 1990;258:F1466–F1469. doi: 10.1152/ajprenal.1990.258.5.F1466. [DOI] [PubMed] [Google Scholar]
- 22.Lorenz JN, Weihprecht H, Schnermann J, Skott O, Briggs JP. Renin release from isolated juxtaglomerular apparatus depends on macula densa chloride transport. Am J Physiol Renal Physiol. 1991;260:F486–F493. doi: 10.1152/ajprenal.1991.260.4.F486. [DOI] [PubMed] [Google Scholar]
- 23.Meyer JW, Flagella M, Sutliff RL, Lorenz JN, Nieman ML, Weber CS, Paul RJ, Shull GE. Decreased blood pressure and vascular smooth muscle tone in mice lacking basolateral Na(+)-K(+)-2Cl(−) cotransporter. Am J Physiol Heart Circ Physiol. 2002;283:H1846–H1855. doi: 10.1152/ajpheart.00083.2002. [DOI] [PubMed] [Google Scholar]
- 24.Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen S, Maunsbach AB, Ecelbarger CA, Knepper MA. Ultrastructural localization of Na-K-2Cl cotransporter in thick ascending limb and macula densa of rat kidney. Am J Physiol Renal Physiol. 1998;275:F885–F893. doi: 10.1152/ajprenal.1998.275.6.F885. [DOI] [PubMed] [Google Scholar]
- 26.Obermuller N, Kunchaparty S, Ellison DH, Bachmann S. Expression of the Na-K-2Cl cotransporter by macula densa and thick ascending limb cells of rat and rabbit nephron. J Clin Invest. 1996;98:635–640. doi: 10.1172/JCI118834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okuda T, Kojima I, Ogata E, Kurokawa K. Ambient Cl− ions modify rat mesangial cell contraction by modulating cell inositol trisphosphate and Ca2+ via enhanced prostaglandin E2. J Clin Invest. 1989;84:1866–1872. doi: 10.1172/JCI114373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pace AJ, Lee E, Athirakul K, Coffman TM, O'Brien DA, Koller BH. Failure of spermatogenesis in mouse lines deficient in the Na(+)-K(+)-2Cl(−) cotransporter. J Clin Invest. 2000;105:441–450. doi: 10.1172/JCI8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlatter E, Salomonsson M, Persson AE, Greger R. Macula densa cells sense luminal NaCl concentration via furosemide sensitive Na+2Cl-K+ cotransport. Pflugers Arch. 1989;414:286–290. doi: 10.1007/BF00584628. [DOI] [PubMed] [Google Scholar]
- 30.Schnermann J, Briggs JP. Function of the juxtaglomerular apparatus: control of glomerular hemodynamics and renin secretion. In: Seldin DW, Giebisch G, editors. The Kidney Physiology and Pathophysiology. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 945–980. [Google Scholar]
- 31.Vander AJ. Control of renin release. Physiol Rev. 1967;47:359–382. doi: 10.1152/physrev.1967.47.3.359. [DOI] [PubMed] [Google Scholar]
- 32.Vandongen R. Intrarenal stimulation of renin secretion by frusemide in the isolated kidney of the rat. Br J Pharmacol. 1977;60:73–76. doi: 10.1111/j.1476-5381.1977.tb16749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamakage M, Lindeman KS, Hirshman CA, Croxton TL. Intracellular pH regulates voltage-dependent Ca2+ channels in porcine tracheal smooth muscle cells. Am J Physiol. 1995;268:L642–L646. doi: 10.1152/ajplung.1995.268.4.L642. [DOI] [PubMed] [Google Scholar]
- 34.Yang T, Huang YG, Singh I, Schnermann J, Briggs JP. Localization of bumetanide- and thiazide-sensitive Na-K-Cl cotransporters along the rat nephron. Am J Physiol. 1996;271:F931–F939. doi: 10.1152/ajprenal.1996.271.4.F931. [DOI] [PubMed] [Google Scholar]
- 35.Yang T, Park JM, Arend L, Huang Y, Topaloglu R, Pasumarthy A, Praetorius H, Spring K, Briggs JP, Schnermann J. Low chloride stimulation of prostaglandin E2 release and cyclooxygenase-2 expression in a mouse macula densa cell line. J Biol Chem. 2000;275:37922–37929. doi: 10.1074/jbc.M006218200. [DOI] [PubMed] [Google Scholar]