Abstract
Although high doses of sodium salicylate impair cochlear function, it paradoxically enhances sound-evoked activity in the auditory cortex (AC) and augments acoustic startle reflex responses, neural and behavioral metrics associated with hyperexcitability and hyperacusis. To explore the neural mechanisms underlying salicylate-induced hyperexcitability and “increased central gain”, we examined the effects of γ-aminobutyric acid (GABA) receptor agonists and antagonists on salicylate-induced hyperexcitability in the AC and startle reflex responses. Consistent with our previous findings, local or systemic application of salicylate significantly increased the amplitude of sound-evoked AC neural activity, but generally reduced spontaneous activity in the AC. Systemic injection of salicylate also significantly increased the acoustic startle reflex. S-baclofen or R-baclofen, GABA-B agonists, which suppressed sound-evoked AC neural firing rate and local field potentials, also suppressed the salicylate-induced enhancement of the AC field potential and the acoustic startle reflex. Local application of vigabatrin, which enhances GABA concentration in the brain, suppressed the salicylate-induced enhancement of AC firing rate. Systemic injection of vigabatrin also reduced the salicylate-induced enhancement of acoustic startle reflex. Collectively, these results suggest that the sound-evoked behavioral and neural hyperactivity induced by salicylate may arise from a salicylate-induced suppression GABAergic inhibition in the AC.
Keywords: salicylate, hyperacusis, GABA, auditory cortex, startle reflex, baclofen
Introduction
Sodium salicylate, the active ingredient of aspirin, is the most widely used anti-inflammatory drug for treating chronic pain and fever. High doses of salicylate are toxic and can cause temporary hearing loss and tinnitus and even death (Proudfoot, 1983, Wood et al., 2005, Minns et al., 2010). Moreover, high doses and prolonged use of salicylate can cause permanent hearing impairment and spiral ganglion degeneration (Zheng and Gao, 1996, Chen et al., 2010, Wei et al., 2010a). Approximately 45% of patients who regularly took salicylate to treat rheumatoid arthritis developed tinnitus or sensorineural hearing loss; ~25% had blood salicylate concentrations lower than 1.4 mM (Halla and Hardin, 1988). Elderly patients taking salicylate were more likely to develop tinnitus than younger peers (Grigor et al., 1987). Since sodium salicylate reliably induces tinnitus in humans, it is has become a standard pharmacological tool for inducing transient tinnitus in animal models (Jastreboff et al., 1988, Lobarinas et al., 2004, Yang et al., 2007).
Accumulating evidence indicates that high doses of salicylate affect both the peripheral and central auditory systems. Very high doses of salicylate reversibly block the electromotility of outer hair cells which in turn may contribute to salicylate-induced transient sensorineural hearing loss (Kakehata and Santos-Sacchi, 1996, Ermilov et al., 2005). The effects of high doses of salicylate on auditory activity have been mixed. Some reports indicate that salicylate increases auditory nerve fiber spontaneous rates while other reports suggest that it has no effect or reduces spontaneous discharge rates (Evans et al., 1981, Stypulkowski, 1990, Muller et al., 2003). Salicylate enhances glutamate-induced N-Methyl-D-aspartic acid (NMDA) receptor currents in spiral ganglion neurons presumably by increasing the level of arachidonic acid (Peng et al., 2003, Ruel et al., 2008). Salicylate also exerts effects on the central auditory system; some of these effects appear to originate in the central auditory pathway while others are relayed centrally from the cochlea or more peripheral loci (Eggermont and Kenmochi, 1998, Wang et al., 2006, Wang et al., 2008, Sun et al., 2009). Physiological studies indicate that systemic injection of salicylate elevates the discharge rates of neurons in the inferior colliculus (IC) and the auditory cortex (AC); however, the mechanisms leading to these increases are poorly understood (Jastreboff and Sasaki, 1986, Chen and Jastreboff, 1995, Ochi and Eggermont, 1996). There is growing evidence that salicylate affects γ-aminobutyric acid (GABA) inhibition in the central auditory system (Gong et al., 2008). Salicylate reduces the miniature inhibitory postsynaptic currents in the IC to about 60% (Wang et al., 2006). Vigabatrin, an anti-seizure drug that increases GABA concentration, suppresses noise-induced tinnitus suggesting that diminished GABA inhibition contributes to tinnitus (Brozoski et al., 2007).
In a previous study we showed that 250 mg/kg of salicylate, a dose that reliably induces tinnitus in rats, decreased the sound-evoked compound action potential from the cochlea. Paradoxically, salicylate increased the amplitude of the sound-evoked field potential from the AC of awake-rats and increased the amplitude of acoustic startle reflex (Sun et al., 2009), (Newman et al., 1994, Henry et al., 2005). Isoflurane, which enhances GABA-medicated inhibition, blocked the salicylate-induced increase in AC response amplitude presumably by suppressing GABA-mediated inhibition. To evaluate the role of GABA mediated inhibition in salicylate-induced AC hyperactivity, we applied salicylate directly on the AC in the presence of a GABA receptor agonist. To determine if the salicylate-induced enhancement of the acoustic startle amplitude was due to the loss of GABA mediated inhibition we measured the startle amplitude before and after the administration of salicylate with or without GABA receptor agonists.
Materials and Methods
Animals
Adult male Sprague Dawley rats (3-5 months, 200-400 g) were used in the acute and chronic electrophysiological experiments and behavioral tests. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University at Buffalo and were consistent with the guidelines issued by the National Institutes of Health.
Acute AC response recordings
Rats were anesthetized with ketamine (50 mg/kg) and acepromazine (0.5 mg/kg). The head was fixed in a stereotaxic frame (World Precision Instrument, Sarasota, FL) and the skin and muscle on the right temporal bone were carefully removed to expose the right AC. The anatomical landmarks on the skull and the blood vessels on the surface of the cortex were used to identify the AC as noted previously (Horikawa et al., 1988, Sally and Kelly, 1988, Polley et al., 2007). The dura mater was excised and a 16 channel electrode array (NeuroNexus, A4x4-3mm-100-177, Ann Arbor, Michigan) mounted on a hydraulic manipulator was advanced into the AC. A stainless steel electrode was inserted into the muscle adjacent to the opening and used as a ground electrode. Body temperature was measured and maintained at 37 °C using a thermally regulated heating pad system (Homeothermic Blanket Control Unit, Harvard Apparatus, MA). The AC responses were recorded before and after local delivery of salicylate alone or in conjunction with a GABA receptor agonist applied on the surface of the AC (100 μl).
AC electrode implantation for chronic recording
Rats were anesthetized with ketamine (50 mg/kg) and acepromazine (0.5 mg/kg). Then the head was fixed in a stereotaxic frame (WPI) and the surface of the parietal bone and part of the frontal bone was exposed. A 1-2 mm diameter hole was opened in the skull over the AC (5-6 mm posterior to bregma on the internal side of the suture between the parietal and the temporal bones (Polley et al., 2007). A high impedance, Teflon coated electrode (~5-6 MΩ, 75 μm diameter, FHC, ME) mounted on the manipulator of the stereotaxic frame was gently advanced through the opening in the skull using a hydraulic manipulator. A sweep tone was presented as the electrode was advanced into the AC. The AC response was amplified using Tucker Davis Technologies (TDT, Alachua, FL, USA) System-3 hardware and software and the response was monitored on a computer screen and a monitor speaker. Once a synchronized AC response was evoked by sound, the electrode was fixed to the skull using dental cement. Six small stainless steel screws (Small Parts Inc.) were inserted into the skull to provide additional anchoring for the dental cement. A custom threaded rod (1/2” long with ¼” diameter) was attached to the skull with dental cement. The rod was later used to maintain the rat’s head in a relatively stable position during the recording. A stainless steel ground electrode was implanted under the dura and fixed on the surface of the parietal bone using dental cement. The wound was sutured around the electrode connector and the animal was allowed to recover for 3-5 days before recording.
Stimuli and recordings
Stimuli were generated with TDT System-3 hardware and software (SigGen, TDT) and presented through a high frequency speaker (Fostex FT28D, Madisound Speaker Components Inc., Middleton, WI). Tone-bursts (50 ms duration, 1 ms rise/fall time) at the characteristic frequency of the AC neuron were used to elicit responses; sound intensity was varied from 10 to 100 dB SPL. The sound driven spike rate was calculated by using the average firing rate in a 50 ms window when the sound stimulus was presented minus the average baseline spike rate measured in a 50 ms window prior to stimulus onset. The spontaneous rate was the averaged spike rate calculated in a separate 5 minute interval either before or after drug treatment. Stimuli were calibrated using a sound level meter (824, Larson Davis, Depew, NY) and ½”condenser microphone (Larson Davis).
The output of the AC response was connected to a 16 channel preamplifier (RA16PA, TDT) using a flexible, low noise cable. The output of the preamplifier was delivered to a digital signal processing module (RX5-2, Pentusa Base Station, TDT) connected to a computer. Peri-stimulus time histograms (PSTH) were constructed from spike discharges (100-3000 Hz) using Brainware software (TDT).
Acoustic startle reflex test
Our startle reflex procedures have been described previously (Yang et al., 2007, Sun et al., 2009). Each animal was placed in an acoustically transparent, wire mesh cage (7 × 5.5 ×18 cm) mounted on a Plexiglass base which rested on a sensitive piezoelectric transducer (Radio Shack). The output of the piezo transducer was connected to an A/D converter on an RP2 Real-time Processor (TDT). The startle reflex was low-pass filtered (1000 Hz, LPF-300, World Precision Instruments, Sarasota, FL) and the root mean square (rms) of the response (100 ms window) was measured using custom software. Sound stimuli were presented using a high frequency speaker (Fostex FT28D) located approximately 28 cm above the rat’s head. Sound signals were generated by an RP2 Real-time Processor (TDT) controlled by custom software. The startle eliciting stimulus consisted of a broadband noise burst presented at intensities from 50 to 100 dB SPL (10 dB step). The stimulus was presented 10 times at each intensity in a pseudo-random order. The inter-trial interval was randomly varied from 18 to 22 seconds. The amplitude of the acoustic startle response (rms voltage) was measured in a 100 ms window after the onset of the startle stimulus.
Chemicals
R-baclofen (R-Bac; also D-baclofen or (+) baclofen) and S-baclofen (S-Bac; also L-baclofen, (-)-baclofen) were purchased from Douglas Pharmaceuticals (Auckland, New Zealand). R-Bac and S-Bac are enantiomers of baclofen, a GABA-b receptor agonist. R-Bac is more potent as a muscle relaxant than S-Bac. Vigabatrin (Sabril) was purchased from Global Drugs (Surrey, BC Canada). Sodium salicylate (S2679) was purchased from Sigma. For local application, all drugs were dissolved in artificial cerebral spinal fluid (ACSF) containing 142 mM NaCl, 5 mM KCl, 10 mM glucose, 10 mM HEPES, 3.1 mM Ca2+ and 1.3 mM Mg2+. The pH was adjusted to 7.4 using HCl or NaOH. For systemic injections, drugs were dissolved in saline.
Statistic Data Analysis
Graphs and statistic analyses were generated using GraphPad Prism (Version 5, GraphPad Software, San Diego, CA, USA). Results are presented as mean ± standard error of the mean.
Results
Salicylate Effect on AC Response in Awake Rats
The effects of salicylate (250 mg/kg, i.p.) on the AC neural responses were first tested in awake-rats using a chronically implanted electrode in the AC. In order to reduce noise generated by animal movements, rats were placed in the testing cage for 1-2 h per day (acoustic stimuli were presented) for at least three days prior to data collection to allow the animals to habituate (Sun et al., 2009). After the rats acclimated to the testing apparatus and sound stimuli, sound driven and spontaneous activity were recorded from the AC at least two times on different days until a stable response was collected. The rats were then injected with 250 mg/kg salicylate (intraperitoneal injection, i.p.) and the AC neural responses were recorded 1 h, 1 day and 2 days post-treatment. The typical PSTH of AC responses to tone bursts (50 ms duration, 1 ms rise/fall time, at the characteristic frequency, CF) are shown in Figure 1A-B. Most (~75%) AC neurons (17 units from 15 rats) showed a robust onset response followed by a relative small offset response (Figure 1A) while a small proportion showed a sustained response (Figure 1B, ~25%). The mean (n=8) sound driven rate-intensity functions are shown in Figure 1C before and 1 h after salicylate treatment. There was no obvious threshold change of the AC spikes and the suprathreshold firing rates 1 h post-salicylate were substantially higher than pre-treatment firing rates. The sound driven firing rates between 70 and 100 dB SPL increased by approximately 100% 1 h after salicylate treatment; these changes were statistically significant (Figure 1C, Two-way ANOVA, F(1, 50) = 51.37, p<0.0001). Recordings were obtained from the chronically implanted electrodes on the 2nd and 3rd day after salicylate treatment (Figure 1D). The average sound driven firing rates obtained at 90 dB SPL are shown in Figure 1D. The average firing rate increased by approximately 100% 1 h after salicylate treatment and was essentially back to normal on the second and the third day (Figure 1D, One-way ANOVA, p < 0.05, n = 8). The spontaneous firing rate (averaged rate, 5 min recording window) of most AC neurons showed only slight increases or decreases 1 h after salicylate application as illustrated by the pre-post scatter plot in Figure 1E. The average spontaneous firing rate of the sample was 15.7 ± 2 Hz before salicylate injection and 14.5 ± 2 Hz 1 h after salicylate injection (Figure 1F, paired Student’s t-test, p > 0.05, n = 17).
Figure 1.
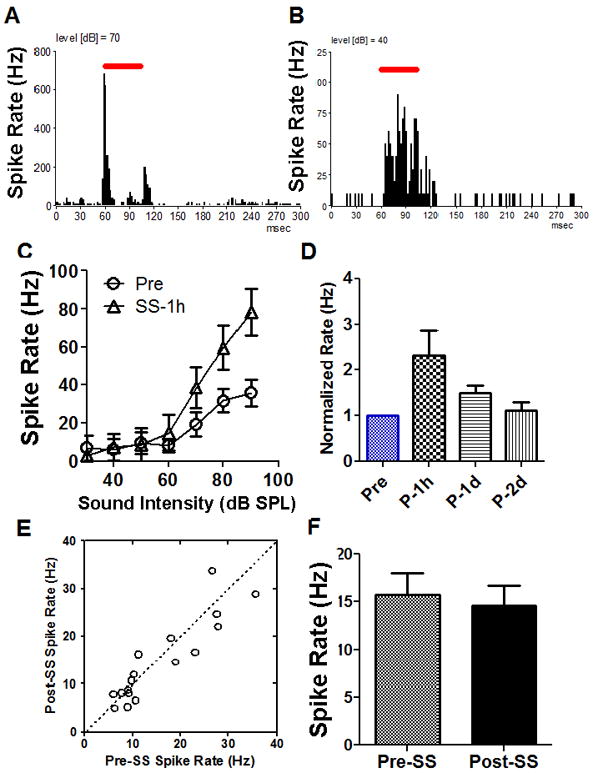
Effect of systemic salicylate (SS, 250 mg/kg, i.p.) on AC responses recorded from awake rats. (A) Typical peri-stimulus time histograms (PSTH) of an AC multiunit cluster to tone burst stimuli (70 dB SPL, tone duration indicated by the horizontal line). (B) PSTH of an AC multiunit cluster with a sustained neural response (40 dB SPL). (C) Mean (n = 8, SEM) sound driven rate-intensity function before and 1 h after salicylate treatment (250 mg/kg); note large increase in suprathreshold firing rate (Two-way ANOVA, F(1, 50) = 51.37, p<0.0001). (D) Mean (SEM) AC firing rate at 90 dB SPL showed a significant enhancement 1 h after salicylate application and totally recovered 1-2 days salicylate washout (One-way ANOVA, p < 0.05, n = 8). (E) Salicylate-induced both increases and decreases in spontaneous firing rates of AC neurons (n = 17). (F) The average (n = 17, SEM) spontaneous rate showed a slight decline 1h after salicylate treatment that was not significant.
Salicylate effects on AC response in anesthetized rats
Recordings were also obtained from the AC of anesthetized (ketamine) rats using a 16 channel electrode array. Measurements were obtained before and after applying salicylate (2 mM, 100 μl) on the AC. Local application of salicylate enhanced the amplitude of the AC local field potential (Figure 2A-B) consistent with our previous results (Figure 1) (Sun et al., 2009). Typical PSTH (tone burst at CF, 90 dB SPL) recorded from the AC before and after salicylate are shown in Figure 2C-D. The PSTH showed a sharp onset response to tone bursts presented at CF. The firing rates increased within 1 min after local application of salicylate and both the peak and width of the PSTH increased.
Figure 2.
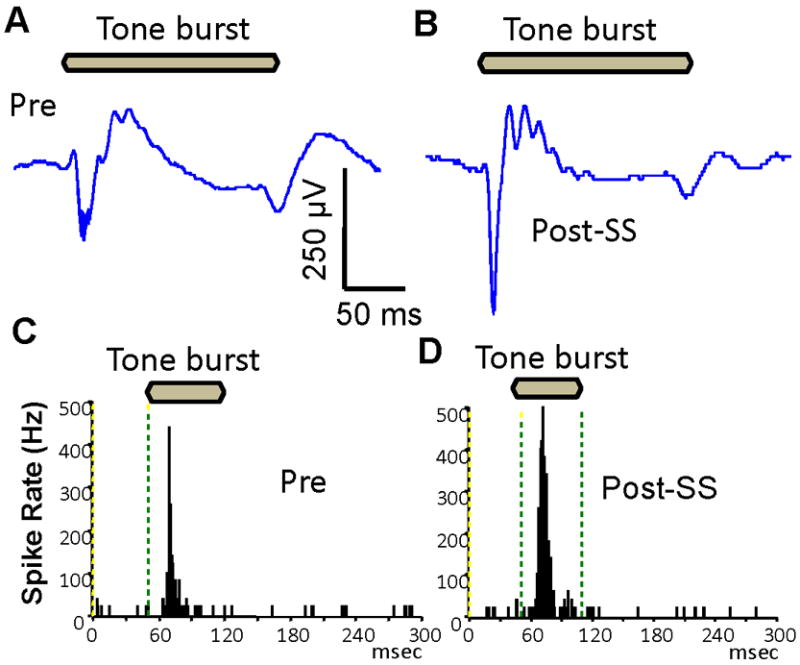
Local application of salicylate (2 mM) on the surface of AC increased the local AC field potential and firing rate in anesthetized rats. (A-B) Local field potential to tone burst (200 ms, 90 dB SPL) almost doubled 1 minute after local salicylate application. (C-D) Peri-stimulus time histograms (PSTH) of AC multi-unit cluster showed that the peak and width of the PSTH increased after local salicylate application.
Figure 3A shows an example of a spike rate-intensity function of a typical multiunit cluster in the AC before and 1 min after salicylate application. There was no change in the threshold after salicylate was applied to the AC since local AC application has no adverse effects on the cochlea. However, the suprathreshold spike rate increased by two to three fold in this case. Sound driven rate-intensity functions were recorded pre and post-SS from six multiunit clusters. The mean rate-intensity functions (data not shown) increased significantly after SS application (Two-way ANOVA, F (1, 50) = 51.37, p<0.0001, n = 6, CF was from 8 to 32 kHz). The average sound driven spike rate at 50 dB SPL increased by approximately 120% shortly after salicylate application (paired t-test, p < 0.001, n = 6, Figure 3B). Despite the increase in sound evoked spike rate, the spontaneous firing rates of most AC neurons decreased after salicylate treatment. Figure 3C is a scatter-plot showing the spontaneous rate before and shortly after salicylate application (21 units from 9 rats). The spontaneous rate (5 min recording window) of all but one AC neurons decreased after salicylate application. The average spontaneous rates decreased from 21 ± 9.4 Hz to 15 ± 7.7 Hz (paired t-test, p = 0001, n = 21, Figure 3D).
Figure 3.
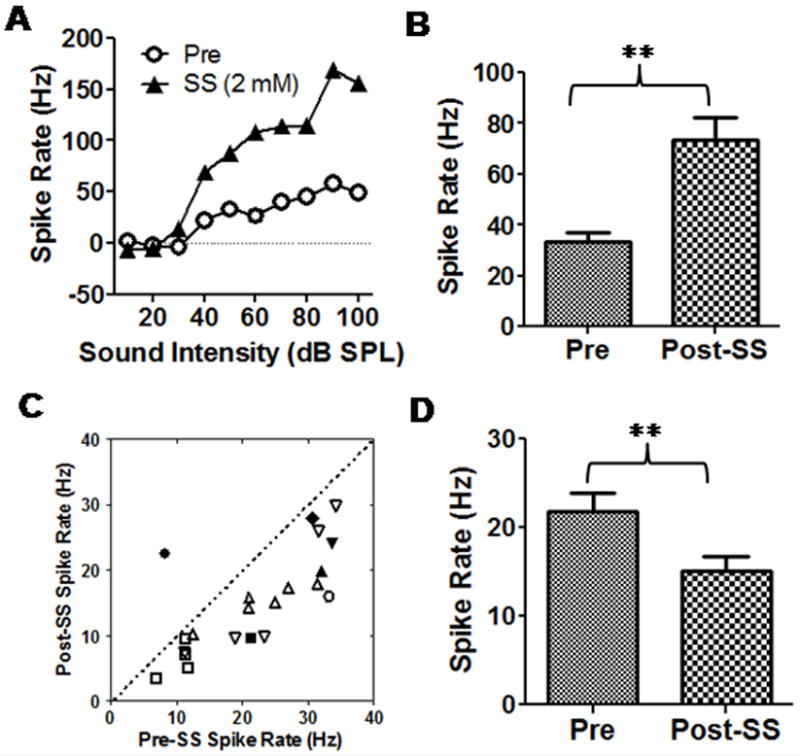
Effects of local AC application of salicylate (SS, 2 mM) on AC sound driven activity and spontaneous activity. (A) Salicylate significantly enhanced AC firing rate. (B) The averaged firing rate at 50 dB SPL showed a significant enhancement 1 min after salicylate application. (paired t-test, p < 0.001, n = 6). (C) Scatter-plot showing the spontaneous rates of AC neurons before and after local salicylate treatment; spontaneous rates decreased in most neurons. (D) Mean (+SEM) spontaneous rate pre and post-salicylate. Spontaneous rate decreased significantly (paired t-test, p = 0001, n = 21).
Effects of Baclofen on AC response
Baclofen is a potent GABA-B receptor agonist that can suppress sound evoked neural activity (Szczepaniak and Moller, 1995, 1996). We evaluated the local effects of S-baclofen on spontaneous and sound driven firing rate in the AC. A small drop (100 μl) of ACSF (control condition), salicylate (2 mM, 37 °C), S-baclofen (1 mg/ml) or the combination of salicylate plus S-baclofen was applied to the surface of the AC. Figure 4A shows an example of a spike rate-intensity function of a multiunit cluster from the AC before and after salicylate treatment with and without S-baclofen. Salicylate alone increased suprathreshold firing rates relative to the pre-treatment baseline obtained with ACSF. In contrast, the firing rates with salicylate plus S-baclofen were lower than pre-treatment firing rates and slightly higher than S-baclofen alone. Figure 4B shows the effect of salicylate, S-baclofen and salicylate plus S-baclofen on the average (n = 6) firing rates at 50 dB SPL. Salicylate increased the mean firing rate from it pre-treatment baseline of 33 ± 4 Hz to 73 ± 9 Hz (~ 120% increase) whereas S-baclofen mixed with salicylate decreased the firing rate to 25 ± 4 Hz (~25% below the baseline firing rate). The average firing rate with S-baclofen alone was 27 ± 7 Hz. The effect of treatment was statistically significant (One-way ANOVA, F = 15.14, p < 0.0001, n = 6). A Tukey’s post-hoc test showed that salicylate caused a significant increase in firing rate relative to pre-treatment baseline (p < 0.001). The firing rates with S-baclofen plus salicylate or S-baclofen alone were significantly lower than salicylate alone (p < 0.001). There was no difference in firing rate between pre-treatment baseline and S-baclofen plus salicylate treatment. Thus, S-baclofen (1 mg/ml) blocked the salicylate-induced enhancement of sound-evoked firing rate. The effect of S-baclofen (1 mg/ml) on the spontaneous activity of AC neurons is shown in Figure 4C-D. S-baclofen caused an increase in spontaneous rate in 4 cases and a decrease in 2 cases; the mean spontaneous rate after S-baclofen treatment did not differ significantly from the pre-treatment rate (Figure 4C-D). When salicylate (2 mM) was applied alone, there was a reduction in mean firing rate (Figure 4D). When salicylate and S-baclofen (1 mg/ml) were applied together, the mean firing rate was not significantly different from salicylate alone (Figure 4D, One-way ANOVA, P>0.05).
Figure 4.
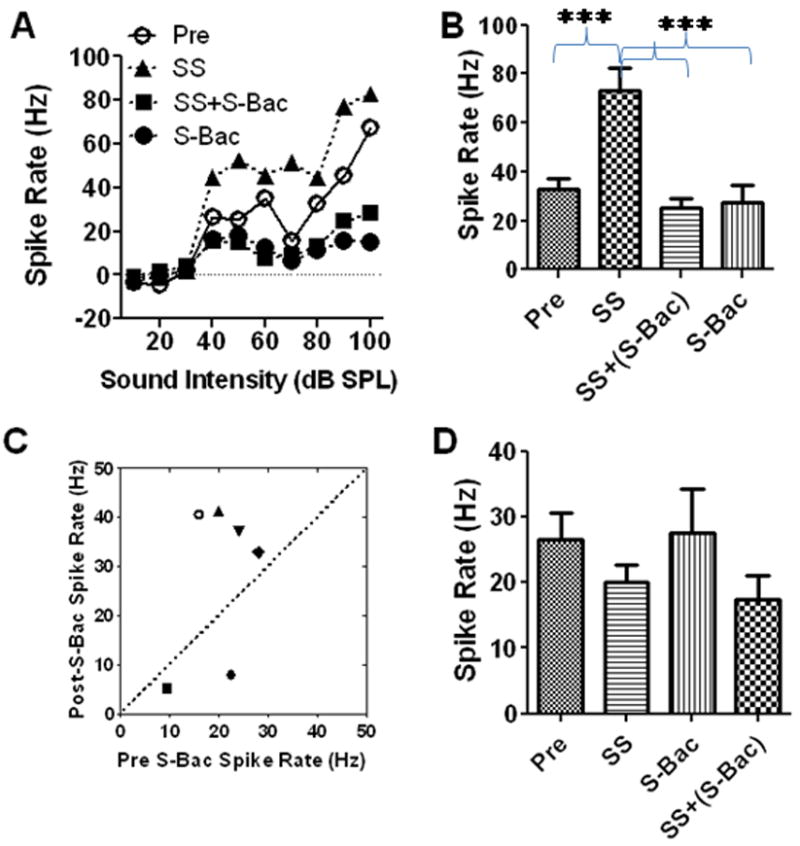
(A) Spike rate-intensity function of multiunit cluster. Local application of salicylate (SS, 2 mM) enhanced sound-driven firing rate. Local application of S-baclofen (S-Bac, 1 mg/ml) plus salicylate and S-Bac alone reduced sound driven firing rate relative to pre-treatment. (B) Mean (+ SEM, n=6) sound evoked firing rate at 50 dB SPL before (Pre) and after local salicylate (SS, 2mM), SS+S-Bac and S-Bac alone (1 mg/ml). Average (SEM) firing rate increased significantly by salicylate (2 mM); firing rate with SS+S-Bac and S-Bac alone significantly less than SS alone (One-way ANOVA, F = 16.14, p = 0.0007, n = 6). (C) Scatter-plot showing spontaneous rates post S-Bac versus pre S-Bac. (D) Mean (+SEM, n=6) spontaneous rates (+SEM) pre-treatment and after local treatment with SS (2 mM), S-Bac (1 mg/ml) or SS+S-Bac.
The effects of salicylate (2 mM), R-baclofen (1 mg/ml), and R-baclofen plus salicylate on the mean (n=6) spike-rate intensity functions are shown in Figure 5A. R-baclofen (1 mg/ml) strongly suppressed the firing rate at all intensities compared to baseline (ACSF). In contrast, SS increased the firing rate at suprathreshold levels, but decreased the rate at low intensities. When R-baclofen was administered with salicylate (2 mM), the combination strongly suppressed the firing rate and produced effects similar to those seen with R-baclofen alone. A statistical analysis showed a significant interaction effect of treatment and intensity (Two-way ANOVA, F(27, 210) = 5.52, p<0.0001, n = 8). The effects of salicylate alone and R-baclofen alone were also statistically significant (F(3, 210) = 67.17, p <0.0001). Bonferroni post-tests showed that R-baclofen and R-baclofen plus salicylate caused a significant decrease in firing rates from 30 to 90 dB SPL compared to the pre-test baseline (p < 0.001). In this particular case, salicylate alone increased the firing rates at high intensities (90-100 dB SPL, p < 0.05), but decreased the firing rates at low intensities (30-70 dB, p < 0.05) compared to pre-treatment baseline. Figure 5B shows the average firing rate at 90 dB SPL (n = 6) before and after R-baclofen alone, SS alone and SS plus R-baclofen (One-way ANOVA, F(3, 31) = 11.6, p = 0.0001, n = 8). The AC firing rate increased from 96 ± 12 Hz to 146 ± 27 Hz after salicylate application (~50% increase, p < 0.05, Tukey’s test). The firing rate decreased to 35 ± 14 Hz after R-baclofen application (~70% reduction, p < 0.05, Tukey’s test). The firing rate remained significantly depressed at 51 ± 17 Hz (n = 4) after the combined treatment of salicylate (2 mM) plus R-baclofen. Thus, R-baclofen greatly reduced AC firing rates and blocked the salicylate-induced increase in AC firing. Figure 5C is a scatter-plot comparing the spontaneous rate before and after R-baclofen (1 mg/ml). R-baclofen significantly decreased the spontaneous firing rate of all AC neurons (n = 8, Figure 5C). Figure 5D shows the mean (n = 8) spontaneous spike rate before and after salicylate, R-baclofen and salicylate plus R-baclofen. All three treatments caused a significant decrease in spontaneous activity (One-way ANOVA, F = 56.9, p<0.0001). The averaged spontaneous firing rate (n = 8) dropped from approximately 21 Hz pre-treatment to 1.4 Hz after R-baclofen, to 1.3 Hz after R-baclofen plus salicylate treatment and to 1.3 Hz after salicylate alone (One-way ANOVA, F = 56.9, p<0.0001).
Figure 5.
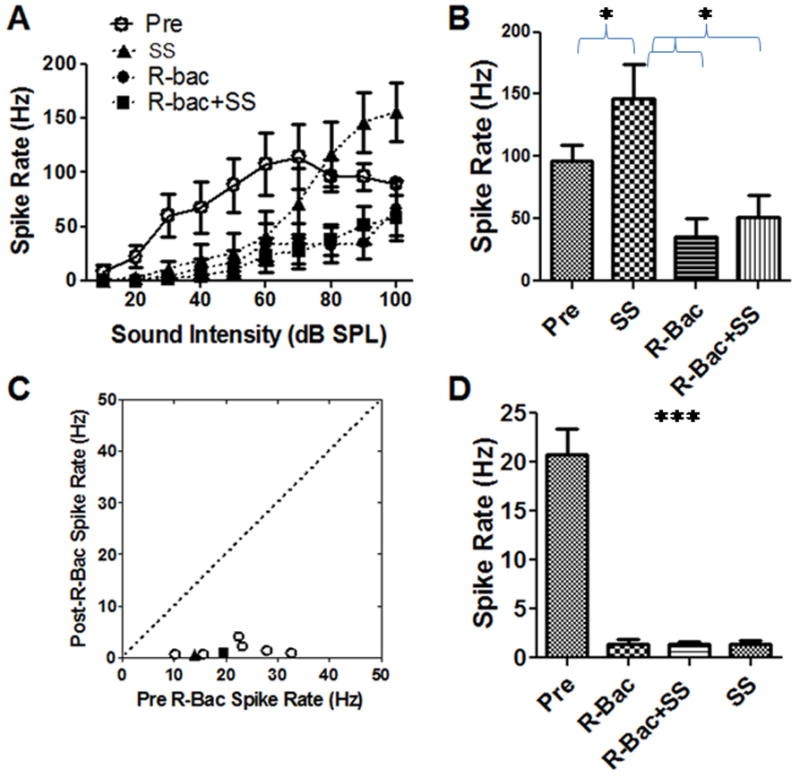
(A) Mean (SEM, n=6) spike rate-intensity function measured before and after local salicylate (SS, 2 mM) and during local treatment with R-baclofen (R-Bac, 1 mg/ml) or salicylate plus R-Bac. Firing rates with R-Bac or R-Bac plus salicylate caused a large reduction of sound driven firing rate. (B) The averaged firing rate decreased significantly after application of R-baclofen mixed with or without salicylate (One-way ANOVA, F = 16.14, p = 0.0007, n = 6). (C) R-balcofen significantly decreased the spontaneous firing rate of AC neurons. (D) R-Bac, salicylate and R-Bac plus salicylate caused a significant decrease in mean (SEM) spontaneous activity (one-way ANOVA, F = 56.9, p<0.0001, n = 8).
Effect of Vigabatrin on AC response
Vigabatrin is an irreversible inhibitor of gamma-aminobutyric acid transaminase which can block the catabolism of GABA and increase GABA levels in the brain. We tested the effects of local application of vigabatrin on sound evoked and spontaneous firing rates in the AC. Mean (±SEM, n = 15) rate-intensity functions obtained before and after treatment with salicylate (2 mM), vigabatrin (1 mg/ml, 100 μl) or the combination of vigabatrin plus salicylate are shown in Figure 6. Local application of salicylate (2 mM) increased the suprathreshold firing rates of AC neurons (Figure 6A). In contrast, vigabatrin caused a slight decrease in AC firing rate; this effect was most pronounced at high intensities. When vigabatrin was applied with salicylate, it suppressed the salicylate-induced enhancement of AC firing rate. There was a significant effect of treatment (Two-way ANOVA, F (3, 420) = 76.53, p < 0.001). Mean (±SEM, n = 15) spike rates measured at 90 dB SPL before and after treatment with salicylate (2 mM), vigabatrin (1 mg/ml), or vigabatrin plus salicylate are shown in Figure 6B. The average spike rate increased from 48 ± 6 Hz (n = 15) pre-treatment to 81 ± 11 Hz (n = 15) after salicylate application and then dropped to 28 ± 4 Hz and 28 ± 4 Hz (n = 15) after vigabatrin or vigabatrin plus salicylate treatment. The effect of treatment was statistically significant (One-way ANOVA, F (3, 59) = 12.81, Figure 6B). A Tukey’s post-hoc analysis showed that the sound-evoked spike rate during salicylate was significantly higher (p < 0.001) than pre-treatment. In addition, firing rate during salicylate treatment was significantly higher than with vigabatrin (p < 0.001) or vigabatrin plus salicylate (p < 0.001).
Figure 6.
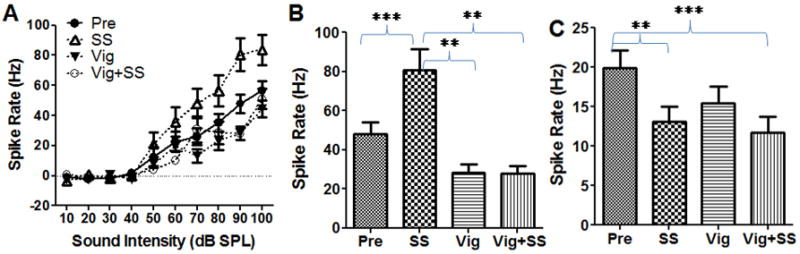
(A) Mean (SEM, n =15) rate-intensity function from multiunit clusters in the AC obtained pre-treatment and after local application of salicylate (2 mM), vigabatrin (Vig, 1 mg/ml) and vigabatrin plus salicylate (SS). (B) Mean (SEM, n = 15) firing rate at 90 dB SPL measured pre-treatment and after local treatment with salicylate (2 mM), vigabatrin (1 mg/ml) and vigabatrin plus salicylate. Firing rates during salicylate significantly higher (p < 0.001) than pre-treatment or during treatment with salicylate plus vigabatrin or vigabatrin alone (one-way ANOVA). (C) Mean (SEM, n = 15) spontaneous spike rate measured pre-treatment and during treatment with salicylate (2 mM), vigabatrin (1 mg/ml) and salicylate plus vigabatrin. Spontaneous rates during salicylate or vigabatrin plus salicylate were significantly less than pre-treatment (One-way ANOVA, F (3, 59) = 7.82, P<0.0001). Tukey’s test showed there was a significant difference between pre-treatment with salicylate alone and vigabatrin plus salicylate application (p<0.01 and p<0.001 respectively) (** p<0.01, *** p<0.001).
Vigabatrin and salicylate also induced a significant change in spontaneous firing rate (Figure 6C, One-way ANOVA, F (3, 59) = 7.82, P<0.0001). A Tukey’s post-hoc analysis showed there was a significant decrease in spike rate between pre-treatment versus salicylate or vigabatrin plus salicylate (p<0.01 and p<0.001 respectively). Although the average spike rate dropped from 19.9 ± 8.7 Hz before vigabatrin treatment to 15.5 ± 8.2 Hz after vigabatrin treatment, this decrease was not significant (P > 0.05) (Figure 6C). Thus, vigabatrin diminished the salicylate-induced enhancement of sound driven firing rates, but did not completely block the salicylate-induced decrease in spontaneous activity.
Effect of Baclofen and Vigabatrin on Startle Reflex Amplitude
In addition to increasing the amplitude of the AC response, sodium salicylate also increased the amplitude of the acoustically evoked startle reflex (Sun et al., 2009). To determine if baclofen would suppress the salicylate-induced enhancement of the startle reflex, we measured the startle reflex input/output function before and after treatment with salicylate, saline, baclofen and baclofen plus salicylate. Testing was conducted on separate days with a 2-3 day washout period between tests. During baseline testing, the mean (n = 9) startle amplitude gradually increased from 70 to 95 dB SPL with a plateau at 100 to 115 dB SPL (Figure 7). Mean startle amplitudes measured 1-2 h after salicylate treatment (250 mg/kg, i.p.) were substantially larger than baseline. For example, after salicylate treatment (i.p., 250 mg/kg) the startle amplitude at 115 dB SPL was nearly 40% greater than baseline. Mean startle amplitude after treatment with S-baclofen (10 mg/kg, i.p.) was smaller than normal at intermediate intensities, but at higher intensities the maximum amplitude was comparable to baseline. When S-baclofen was co-administered with salicylate, the startle amplitude input/output function was nearly identical to baseline. Mean startle response amplitudes from each intensity was compared across the four treatments (One-way repeated measure ANOVA, F(3, 27) = 53.8, p< 0.0001). Mean startle amplitude with salicylate alone was significantly greater than during baseline or during salicylate plus S-baclofen treatment whereas mean startle amplitude during S-baclofen was significantly smaller than baseline. Importantly, mean startle amplitude during S-baclofen plus salicylate was not different from baseline suggesting that S-baclofen blocks salicylate-induced startle enhancement. R-baclofen, a more potent muscle relaxant than S-baclofen, showed a strong suppression of the acoustic startle reflex even in a very low dose (1 mg/kg, i.p., data not show). Therefore we did not test its effect on salicylate.
Figure 7.
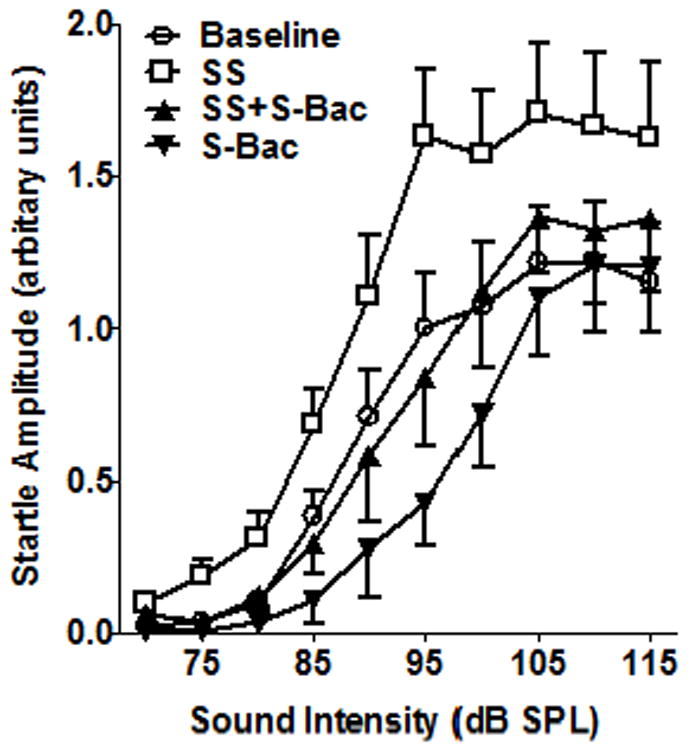
Mean (SEM, n=9) startle reflex amplitude (arbitrary voltage units) versus stimulus intensity. Salicylate (SS, i.p., 250 mg/kg) consistently increased the startle amplitude. S-baclofen (S-Bac, 10 mg/kg, i.p.) alone reduced the startle amplitude at intermediate intensities, but did not suppress the maximum startle amplitude at high intensities. The startle amplitude-intensity function obtained with S-Bac (10 mg/kg) plus salicylate was nearly identical to baseline.
To determine if vigabatrin would also suppress the salicylate-induce startle enhancement, a separate group of rats (n=4) were treated rats with vigabatrin, salicylate, or vigabatrin plus salicylate. Each drug treatment was separated by a 4-5 days washout period. Prior to drug treatment, a baseline acoustic startle reflex input/output function was obtained from each animal. Approximately 4 h later, subjects were treated with salicylate, vigabatrin or the combination of salicylate plus vigabatrin. As expected, salicylate (250 mg/kg, i.p.) alone increased the amplitude of the startle reflex (Figure 8A); the mean (±SEM) increase was on the order of 34 ± 24% between 80 and 110 dB SPL (Figure 8D). In contrast, vigabatrin alone (250 mg/kg, i.p.) significantly reduced the amplitude of the startle response (Figure 8B, n = 4, two-way ANOVA, F(1, 36) = 8.9, p < 0.01); the mean (±SEM) amplitude reduction was 28 ± 5% between 80 and 110 dB SPL (Figure 8D). When salicylate (250 mg/kg, i.p.) was co-administrated with vigabatrin (250 mg/kg), the startle amplitude showed a significant decrease (Figure 8C, two-way ANOVA, F(1, 18) = 7.36, p < 0.05); the mean (±SEM) amplitude decrease was 19 ± 17% (Figure 8D).
Figure 8.
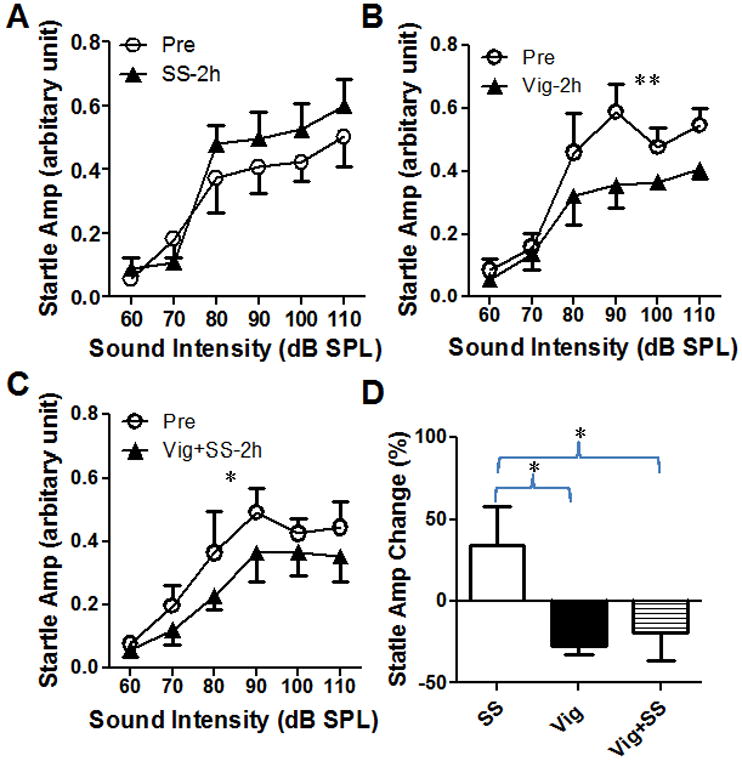
The amplitude of acoustic startle reflex was affected by vigabatrin and salicylate. (A) Salicylate (SS, i.p., 250 mg/kg) increased the startle amplitude. (B) Vigabatrin (Vig, 250 mg/kg, i.p.) reduced the startle amplitude by 28 ± 5%. (C) Vigabatrin plus salicylate (Vig+SS) reduced the startle amplitude by 19 ± 17%. (D) The average change in startle amplitude (80-110 dB SPL) caused by salicylate, vigabatrin and salicylate plus vigabatrin (* p<0.05, ** p<0.01).
Discussion
Our results show for the first time that local application of salicylate on to the AC enhances the amplitude of sound-evoked AC field potentials and suprathreshold firing rates of AC neurons (Figures 2-6), but reduces the spontaneous activity of most AC neurons (Figure 2). Thus, salicylate has a direct effect on the functional properties of the AC. Importantly, local application of baclofen, a GABA-B agonist, suppressed the salicylate-induced increase in sound evoked AC activity; these suppressive effects were substantially greater for R-baclofen than for S-baclofen (Figures 3-4). Additionally, local application of vigabatrin, which increases GABA levels, also suppressed the salicylate-induced enhancement of AC activity. In terms of its behavioral effects, salicylate enhanced the amplitude of the startle reflex. The salicylate-induced enhancement of the startle reflex was suppressed by S-baclofen.
Central Effects of Salicylate
Converging evidence from several different species and labs indicates that systemic and local administration of salicylate enhances sound evoked activity in the AC (Lobarinas et al., 2006, Yang et al., 2007, Sun et al., 2009, Norena et al., 2010, Zhang et al., 2011). The mechanisms that lead to enhanced AC activity are poorly understood and somewhat paradoxical. On the one hand, systemic high-dose salicylate treatment suppresses the neural output of the cochlea (Stypulkowski, 1990, Cazals, 2000, Ruel et al., 2008, Yu et al., 2008, Ralli et al., 2010), but enhances sound evoked activity from AC (Sun et al., 2009). However, when salicylate is applied directly to the cochlea, it suppresses sound evoked activity in the inner ear as well as in the IC and the AC (Sun et al., 2009). These results suggest that the hyperactivity observed in the AC following systemic drug administration originates centrally in the auditory pathway. Our results support this interpretation, since application of salicylate to the AC enhanced the amplitude of sound-evoked field potentials and suprathreshold firing rates of AC neurons (Figure 2).
Salicylate and GABA
GABA is the major inhibitory neurotransmitter in the central nervous system (Cox et al., 1992). In the AC, GABA release sharpens the frequency-response receptive fields and improves temporal processing (Jones, 1993, Schulze and Langner, 1999, Wang et al., 2000, Liu et al., 2007). Application of GABA-A or GABA-B receptor antagonists on the AC can decrease sound-evoked thresholds and enhance the onset response (Foeller et al., 2001, Wang et al., 2002, Kurt et al., 2006). Previous in vitro studies indicate that salicylate suppresses GABA mediated inhibition in the AC as well as other regions of the central nervous system (Wang et al., 2006, Gong et al., 2008, Wang et al., 2008). Salicylate can also block the serotonin-induced enhancement of GABAergic activity in the IC leading to increased excitation (Wang et al., 2008). Consistent with these in vitro results, we found that the salicylate-induced hyperactivity in the AC was suppressed by local administration of baclofen (Figure 4-5), a GABA-B receptor agonist, as well as by vigabatrin (Figure 6), which enhances GABA. Collectively, these results suggest that the salicylate-induced hyperactivity in the AC arises from a reduction in GABA mediated inhibition (Wang et al., 2006). Several additional lines of evidence support this interpretation. First, isoflurane anesthesia which enhances GABA mediated inhibition blocks the salicylate-induced enhancement of AC activity (Sun et al., 2009). Second, in AC brain slice preparations, salicylate suppresses the firing rate of fast spiking, GABAergic interneurons (Su et al., 2009); reducing the activity of these inhibitory interneurons would presumably enhance the sound evoked activity of the AC. Based on these results, we propose that salicylate-induced hyperactivity in the AC arises from a loss of GABA-medicated inhibition.
Salicylate and Spontaneous Activity
Although systemic salicylate administration consistently enhanced sound evoked activity in the AC, it decreased spontaneous activity in most AC neurons. These results are consistent with previous reports showing that systemic salicylate treatment decreases spontaneous activity in the primary AC (Eggermont and Kenmochi, 1998, Yang et al., 2007, Zhang et al., 2011). Our new results show that local application of salicylate also suppresses spontaneous activity in most AC neurons (Figure 3). The salicylate-induced suppression of AC spontaneous activity is similar to the suppression we observed in vitro in fusiform cells from the dorsal cochlear nucleus after salicylate treatment (Wei et al., 2010b). It seems unlikely that the salicylate-induced suppression of spontaneous activity is due to an increase in inhibitory postsynaptic currents because baclofen and vigabatrin did not reverse the spontaneous hypoactivity induced by salicylate (Figure 4-6). Previous in vitro studies also have shown that salicylate decreased the frequency and amplitude of the spontaneous inhibitory postsynaptic currents in AC pyramidal neurons (Wang et al., 2006). One possibility is that the salicylate-induced decrease in spontaneous activity is mediated by other neurotransmitter receptors in the AC such as acetylcholine, serotonin or dopamine (Campbell et al., 1987, Stark and Scheich, 1997). Salicylate could have an effect on the intrinsic membrane properties of AC neurons; however, no changes of this type were observed in recent brain slice studies of the AC (Wang et al., 2006, Su et al., 2009).
While a number of clear trends were evident in the results, some variability was seen in sound evoked spiking rate and spontaneous activity. The source of this variability may be due to the relatively small sample of neurons recorded at different CFs. Another factor may be that the various subtypes of neurons in the AC are likely to responds differently to salicylate, baclofen and vigabatrin properties (Wang et al., 2006, Su et al., 2009).
Salicylate Enhanced Startle Reflex
We previously reported that high doses of salicylate significantly enhanced the acoustic startle reflex (Sun et al., 2009). The exaggerated acoustic startle response may be linked to the enhanced auditory evoked potentials seen with cochlear hearing loss (Salvi et al., 2000, Ison et al., 2007). As suggested previously, the exaggerated startle responses could conceivably represent a behavioral correlate of collapsed sound tolerance or hyperacusis (Ison et al., 2007, Turner and Parrish, 2008, Sun et al., 2009). Hyperacusis, which is characterized by an abnormally strong reaction to loud sounds (Anari et al., 1999), is commonly seen in individuals with sensorineural hearing loss and tinnitus (Jansen et al., 2009)(Dauman and Bouscau-Faure, 2005, Schmuziger et al., 2006). The mechanisms that give rise to this exaggerated startle response are poorly understood. In addition, it would be useful to determine if the exaggerated startle responses are seen in humans with clinical manifestations of hyperacusis (Kumari et al, 1996). It has been suggested that the exaggerated response results from a loss of descending inhibition on the brainstem sensory-motor circuits that directly mediate the acoustic startle response (Swerdlow et al., 2001, Ison et al., 2007). Consistent with this view, bilateral lesions of the AC temporarily increase the amplitude of the startle reflex response evoked by high intensity sounds providing evidence that the AC can modulate brainstem circuits mediating the startle response (Hunter and Willott, 1993). To determine if the salicylate-induced increase in startle amplitude was due to a loss of GABA mediated inhibition, we administered S-baclofen or vigabatrin to rats that had been treated with a high doses of salicylate. Systemic administration of S-baclofen or vigabatrin blocked the salicylate-induced enhancement of the acoustic startle reflex. Acoustic startle reflex amplitudes are also enhanced in patients with spinal cord injury (Kumru et al., 2009). Importantly, intrathecal administration of baclofen reversed these effects and brought reflex amplitudes back into the normal range. Taken together, the physiological and behavioral results suggest that exaggerated acoustic startle reflexes may result from a salicylate-induced loss of GABA mediated inhibition.
Research Highlights.
Salicylate increases the amplitude of sound-evoked auditory cortex neural activity
Baclofen suppresses sound-evoked auditory cortex neural firing rate and local field potentials
Baclofen suppresses salicylate-induced enhancement of the auditory cortex response
Vigabatrin suppresses the salicylate-induced enhancement of auditory cortex firing rate
Acknowledgments
This work was supported in part by Royal National Institute for Deaf People (RNID), Tinnitus Research Initiative (TRI) and NIH (DC008685 and DC009091).
List of abbreviations
- ABR
auditory brainstem response
- AC
auditory cortex
- ACSF
artificial cerebral spinal fluid
- CF
characteristic frequency
- GABA
γ-Aminobutyric acid
- IC
inferior colliculus
- i.p.
intraperitoneal injection
- mM
millimole
- NMDA
N-Methyl-D-aspartic acid
- PSTH
Peri-stimulus time histograms
- R-Bac
R-baclofen
- rms
the root mean square
- S-Bac
S-baclofen
- SPL
sound pressure level
- SS
salicylate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anari M, Axelsson A, Eliasson A, Magnusson L. Hypersensitivity to sound--questionnaire data, audiometry and classification. Scand Audiol. 1999;28:219–230. doi: 10.1080/010503999424653. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Spires TJ, Bauer CA. Vigabatrin, a GABA transaminase inhibitor, reversibly eliminates tinnitus in an animal model. J Assoc Res Otolaryngol. 2007;8:105–118. doi: 10.1007/s10162-006-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MJ, Lewis DA, Foote SL, Morrison JH. Distribution of choline acetyltransferase-, serotonin-, dopamine-beta- hydroxylase-, tyrosine hydroxylase-immunoreactive fibers in monkey primary auditory cortex. J Comp Neurol. 1987;261:209–220. doi: 10.1002/cne.902610204. [DOI] [PubMed] [Google Scholar]
- Cazals Y. Auditory sensori-neural alterations induced by salicylate. Prog Neurobiol. 2000;62:583–631. doi: 10.1016/s0301-0082(00)00027-7. [DOI] [PubMed] [Google Scholar]
- Chen GD, Jastreboff PJ. Salicylate-induced abnormal activity in the inferior colliculus of rats. Hear Res. 1995;82:158–178. doi: 10.1016/0378-5955(94)00174-o. [DOI] [PubMed] [Google Scholar]
- Chen GD, Kermany MH, D’Elia A, Ralli M, Tanaka C, Bielefeld EC, Ding D, Henderson D, Salvi R. Too much of a good thing: long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hear Res. 2010;265:63–69. doi: 10.1016/j.heares.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Metherate R, Weinberger NM, Ashe JH. Synaptic potentials and effects of amino acid antagonists in the auditory cortex. Brain Res Bull. 1992;28:401–410. doi: 10.1016/0361-9230(92)90039-z. [DOI] [PubMed] [Google Scholar]
- Dauman R, Bouscau-Faure F. Assessment and amelioration of hyperacusis in tinnitus patients. Acta Otolaryngol. 2005;125:503–509. doi: 10.1080/00016480510027565. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Kenmochi M. Salicylate and quinine selectively increase spontaneous firing rates in secondary auditory cortex. Hear Res. 1998;117:149–160. doi: 10.1016/s0378-5955(98)00008-2. [DOI] [PubMed] [Google Scholar]
- Ermilov SA, Murdock DR, El-Daye D, Brownell WE, Anvari B. Effects of salicylate on plasma membrane mechanics. J Neurophysiol. 2005;94:2105–2110. doi: 10.1152/jn.00414.2005. [DOI] [PubMed] [Google Scholar]
- Evans EF, Wilson JP, Borerwe TA. Animal models of tinnitus. Ciba Found Symp. 1981;85:108–138. doi: 10.1002/9780470720677.ch7. [DOI] [PubMed] [Google Scholar]
- Foeller E, Vater M, Kossl M. Laminar analysis of inhibition in the gerbil primary auditory cortex. J Assoc Res Otolaryngol. 2001;2:279–296. doi: 10.1007/s101620010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong N, Zhang M, Zhang XB, Chen L, Sun GC, Xu TL. The aspirin metabolite salicylate enhances neuronal excitation in rat hippocampal CA1 area through reducing GABAergic inhibition. Neuropharmacology. 2008;54:454–463. doi: 10.1016/j.neuropharm.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Grigor RR, Spitz PW, Furst DE. Salicylate toxicity in elderly patients with rheumatoid arthritis. Journal of Rheumatology. 1987;14:60–66. [PubMed] [Google Scholar]
- Halla JT, Hardin JG. Salicylate ototoxicity in patients with rheumatoid arthritis: a controlled study. Annals of the Rheumatic Diseases. 1988;47:134–137. doi: 10.1136/ard.47.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JA, Zaugg TL, Schechter MA. Clinical guide for audiologic tinnitus management II: Treatment. Am J Audiol. 2005;14:49–70. doi: 10.1044/1059-0889(2005/005). [DOI] [PubMed] [Google Scholar]
- Horikawa J, Ito S, Hosokawa Y, Homma T, Murata K. Tonotopic representation in the rat auditory cortex. Proc Japan Acad Ser B. 1988;64:260–263. [Google Scholar]
- Hunter KP, Willott JF. Effects of bilateral lesions of auditory cortex in mice on the acoustic startle response. Physiol Behav. 1993;54:1133–1139. doi: 10.1016/0031-9384(93)90337-f. [DOI] [PubMed] [Google Scholar]
- Ison JR, Allen PD, O’Neill WE. Age-Related Hearing Loss in C57BL/6J Mice has both Frequency-Specific and Non-Frequency-Specific Components that Produce a Hyperacusis-Like Exaggeration of the Acoustic Startle Reflex. J Assoc Res Otolaryngol. 2007;8:539–550. doi: 10.1007/s10162-007-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen EJ, Helleman HW, Dreschler WA, de Laat JA. Noise induced hearing loss and other hearing complaints among musicians of symphony orchestras. Int Arch Occup Environ Health. 2009;82:153–164. doi: 10.1007/s00420-008-0317-1. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Brennan JF, Sasaki CT. An animal model for tinnitus. Laryngoscope. 1988;98:280–286. doi: 10.1288/00005537-198803000-00008. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Sasaki CT. Salicylate-induced changes in spontaneous activity of single units in the inferior colliculus of the guinea pig. Journal of the Acoustical Society of America. 1986;80:1384–1391. doi: 10.1121/1.394391. [DOI] [PubMed] [Google Scholar]
- Jones EG. GABAergic neurons and their role in cortical plasticity in primates. Cereb Cortex. 1993;3:361–372. doi: 10.1093/cercor/3.5.361-a. [DOI] [PubMed] [Google Scholar]
- Kakehata S, Santos-Sacchi J. Effects of salicylate and lanthanides on outer hair cell motility and associated gating charge. J Neurosci. 1996;16:4881–4889. doi: 10.1523/JNEUROSCI.16-16-04881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumru H, Kofler M, Valls-Sole J, Portell E, Vidal J. Brainstem reflexes are enhanced following severe spinal cord injury and reduced by continuous intrathecal baclofen. Neurorehabil Neural Repair. 2009;23:921–927. doi: 10.1177/1545968309335979. [DOI] [PubMed] [Google Scholar]
- Kurt S, Crook JM, Ohl FW, Scheich H, Schulze H. Differential effects of iontophoretic in vivo application of the GABA(A)-antagonists bicuculline and gabazine in sensory cortex. Hear Res. 2006;212:224–235. doi: 10.1016/j.heares.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Liu BH, Wu GK, Arbuckle R, Tao HW, Zhang LI. Defining cortical frequency tuning with recurrent excitatory circuitry. Nat Neurosci. 2007;10:1594–1600. doi: 10.1038/nn2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Sun W, Cushing R, Salvi R. A novel behavioral paradigm for assessing tinnitus using schedule-induced polydipsia avoidance conditioning (SIP-AC) Hear Res. 2004;190:109–114. doi: 10.1016/S0378-5955(04)00019-X. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Yang G, Sun W, Ding D, Mirza N, Dalby-Brown W, Hilczmayer E, Fitzgerald S, Zhang L, Salvi R. Salicylate- and quinine-induced tinnitus and effects of memantine. Acta Otolaryngol Suppl. 2006:13–19. doi: 10.1080/03655230600895408. [DOI] [PubMed] [Google Scholar]
- Minns AB, Cantrell FL, Clark RF. Death Due to Acute Salicylate Intoxication despite Dialysis. J Emerg Med. 2010 doi: 10.1016/j.jemermed.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Muller M, Klinke R, Arnold W, Oestreicher E. Auditory nerve fibre responses to salicylate revisited. Hear Res. 2003;183:37–43. doi: 10.1016/s0378-5955(03)00217-x. [DOI] [PubMed] [Google Scholar]
- Newman CW, Wharton JA, Shivapuja BG, Jacobson GP. Relationships among psychoacoustic judgments, speech understanding ability and self-perceived handicap in tinnitus subjects. Audiology. 1994;33:47–60. doi: 10.3109/00206099409072954. [DOI] [PubMed] [Google Scholar]
- Norena AJ, Moffat G, Blanc JL, Pezard L, Cazals Y. Neural changes in the auditory cortex of awake guinea pigs after two tinnitus inducers: salicylate and acoustic trauma. Neuroscience. 2010;166:1194–1209. doi: 10.1016/j.neuroscience.2009.12.063. [DOI] [PubMed] [Google Scholar]
- Ochi K, Eggermont JJ. Effects of salicylate on neural activity in cat primary auditory cortex. Hear Res. 1996;95:63–76. doi: 10.1016/0378-5955(96)00019-6. [DOI] [PubMed] [Google Scholar]
- Peng BG, Chen S, Lin X. Aspirin selectively augmented N-methyl-D-aspartate types of glutamate responses in cultured spiral ganglion neurons of mice. Neurosci Lett. 2003;343:21–24. doi: 10.1016/s0304-3940(03)00296-9. [DOI] [PubMed] [Google Scholar]
- Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in the albino rat. J Neurophysiol. 2007;97:3621–3638. doi: 10.1152/jn.01298.2006. [DOI] [PubMed] [Google Scholar]
- Proudfoot AT. Toxicity of salicylates. Am J Med. 1983;75:99–103. doi: 10.1016/0002-9343(83)90239-5. [DOI] [PubMed] [Google Scholar]
- Ralli M, Lobarinas E, Fetoni AR, Stolzberg D, Paludetti G, Salvi R. Comparison of Salicylate- and Quinine-Induced Tinnitus in Rats: Development, Time Course, and Evaluation of Audiologic Correlates. Otol Neurotol. 2010 doi: 10.1097/MAO.0b013e3181de4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel J, Chabbert C, Nouvian R, Bendris R, Eybalin M, Leger CL, Bourien J, Mersel M, Puel JL. Salicylate enables cochlear arachidonic-acid-sensitive NMDA receptor responses. J Neurosci. 2008;28:7313–7323. doi: 10.1523/JNEUROSCI.5335-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sally SL, Kelly JB. Organization of auditory cortex in the albino rat: sound frequency. J Neurophysiol. 1988;59:1627–1638. doi: 10.1152/jn.1988.59.5.1627. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–274. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Schmuziger N, Patscheke J, Probst R. Hearing in nonprofessional pop/rock musicians. Ear Hear. 2006;27:321–330. doi: 10.1097/01.aud.0000224737.34907.5e. [DOI] [PubMed] [Google Scholar]
- Schulze H, Langner G. Auditory cortical responses to amplitude modulations with spectra above frequency receptive fields: evidence for wide spectral integration. J Comp Physiol A. 1999;185:493–508. doi: 10.1007/s003590050410. [DOI] [PubMed] [Google Scholar]
- Stark H, Scheich H. Dopaminergic and serotonergic neurotransmission systems are differentially involved in auditory cortex learning: a long-term microdialysis study of metabolites. J Neurochem. 1997;68:691–697. doi: 10.1046/j.1471-4159.1997.68020691.x. [DOI] [PubMed] [Google Scholar]
- Stypulkowski PH. Mechanisms of salicylate ototoxicity. Hear Res. 1990;46:113–145. doi: 10.1016/0378-5955(90)90144-e. [DOI] [PubMed] [Google Scholar]
- Su YY, Luo B, Wang HT, Chen L. Differential effects of sodium salicylate on current-evoked firing of pyramidal neurons and fast-spiking interneurons in slices of rat auditory cortex. Hear Res. 2009;253:60–66. doi: 10.1016/j.heares.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Sun W, Lu J, Stolzberg D, Gray L, Deng A, Lobarinas E, Salvi RJ. Salicylate increases the gain of the central auditory system. Neuroscience. 2009;159:325–334. doi: 10.1016/j.neuroscience.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Szczepaniak WS, Moller AR. Evidence of decreased GABAergic influence on temporal integration in the inferior colliculus following acute noise exposure: a study of evoked potentials in the rat. Neuroscience Letters. 1995;196:77–80. doi: 10.1016/0304-3940(95)11851-m. [DOI] [PubMed] [Google Scholar]
- Szczepaniak WS, Moller AR. Effects of (-)-baclofen, clonazepam, and diazepam on tone exposure-induced hyperexcitability of the inferior colliculus in the rat: possible therapeutic implications for pharmacological management of tinnitus and hyperacusis. Hear Res. 1996;97:46–53. [PubMed] [Google Scholar]
- Turner JG, Parrish J. Gap detection methods for assessing salicylate-induced tinnitus and hyperacusis in rats. Am J Audiol. 2008;17:S185–192. doi: 10.1044/1059-0889(2008/08-0006). [DOI] [PubMed] [Google Scholar]
- Wang HT, Luo B, Huang YN, Zhou KQ, Chen L. Sodium salicylate suppresses serotonin-induced enhancement of GABAergic spontaneous inhibitory postsynaptic currents in rat inferior colliculus in vitro. Hear Res. 2008;236:42–51. doi: 10.1016/j.heares.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Wang HT, Luo B, Zhou KQ, Xu TL, Chen L. Sodium salicylate reduces inhibitory postsynaptic currents in neurons of rat auditory cortex. Hear Res. 2006;215:77–83. doi: 10.1016/j.heares.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Wang J, Caspary D, Salvi RJ. GABA-A antagonist causes dramatic expansion of tuning in primary auditory cortex. Neuroreport. 2000;11:1137–1140. doi: 10.1097/00001756-200004070-00045. In Process Citation. [DOI] [PubMed] [Google Scholar]
- Wang J, McFadden SL, Caspary D, Salvi R. Gamma-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res. 2002;944:219–231. doi: 10.1016/s0006-8993(02)02926-8. [DOI] [PubMed] [Google Scholar]
- Wei L, Ding D, Salvi R. Salicylate-induced degeneration of cochlea spiral ganglion neurons-apoptosis signaling. Neuroscience. 2010a;168:288–299. doi: 10.1016/j.neuroscience.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Ding D, Sun W, Xu-Friedman MA, Salvi R. Effects of sodium salicylate on spontaneous and evoked spike rate in the dorsal cochlear nucleus. Hear Res. 2010b doi: 10.1016/j.heares.2010.03.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DM, Dargan PI, Jones AL. Measuring plasma salicylate concentrations in all patients with drug overdose or altered consciousness: is it necessary? Emerg Med J. 2005;22:401–403. doi: 10.1136/emj.2003.010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, Sun W. Salicylate induced tinnitus: Behavioral measures and neural activity in auditory cortex of awake rats. Hear Res. 2007;226:244–253. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Yu N, Zhu ML, Johnson B, Liu YP, Jones RO, Zhao HB. Prestin up-regulation in chronic salicylate (aspirin) administration: an implication of functional dependence of prestin expression. Cell Mol Life Sci. 2008;65:2407–2418. doi: 10.1007/s00018-008-8195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yang P, Cao Y, Qin L, Sato Y. Salicylate induced neural changes in the primary auditory cortex of awake cats. Neuroscience. 2011;172:232–245. doi: 10.1016/j.neuroscience.2010.10.073. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Differential damage to auditory neurons and hair cells by ototoxins and neuroprotection by specific neurotrophins in rat cochlear organotypic cultures. Eur J Neurosci. 1996;8:1897–1905. doi: 10.1111/j.1460-9568.1996.tb01333.x. [DOI] [PubMed] [Google Scholar]


