Abstract
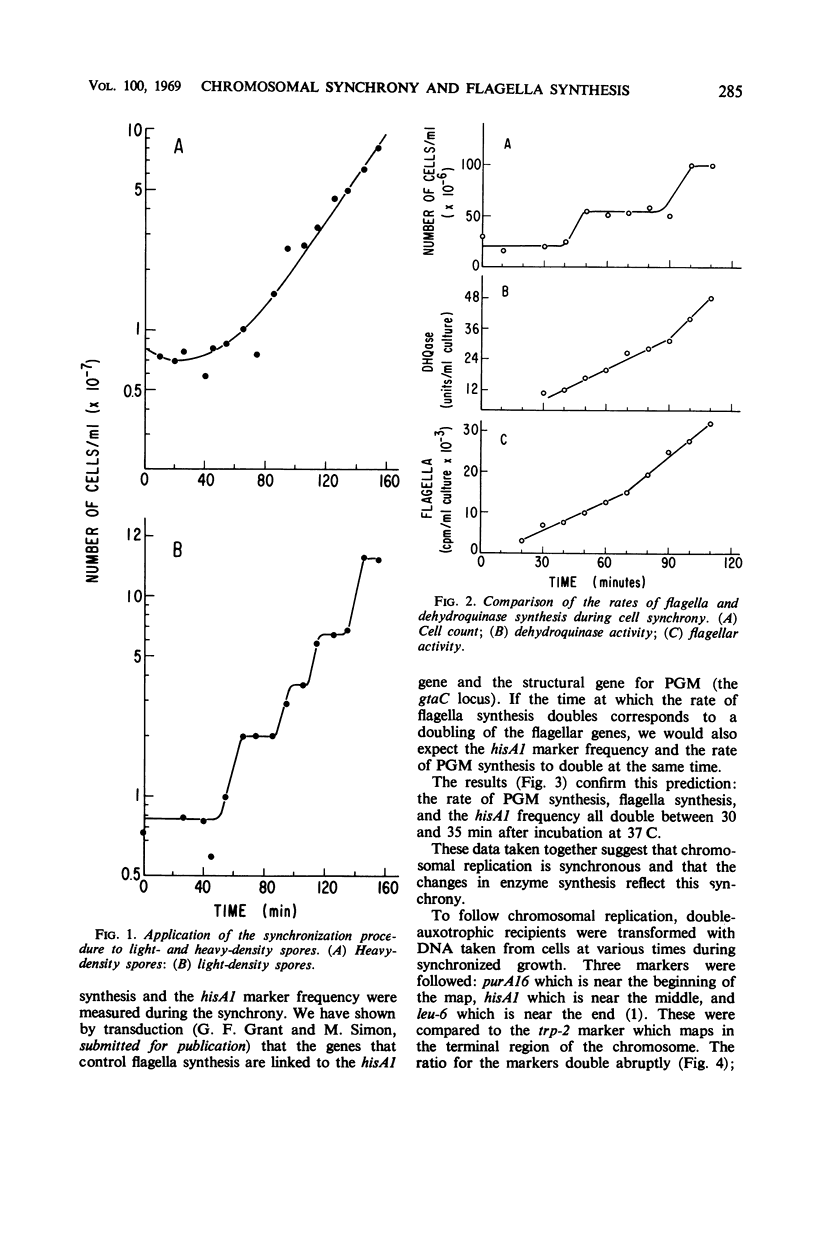
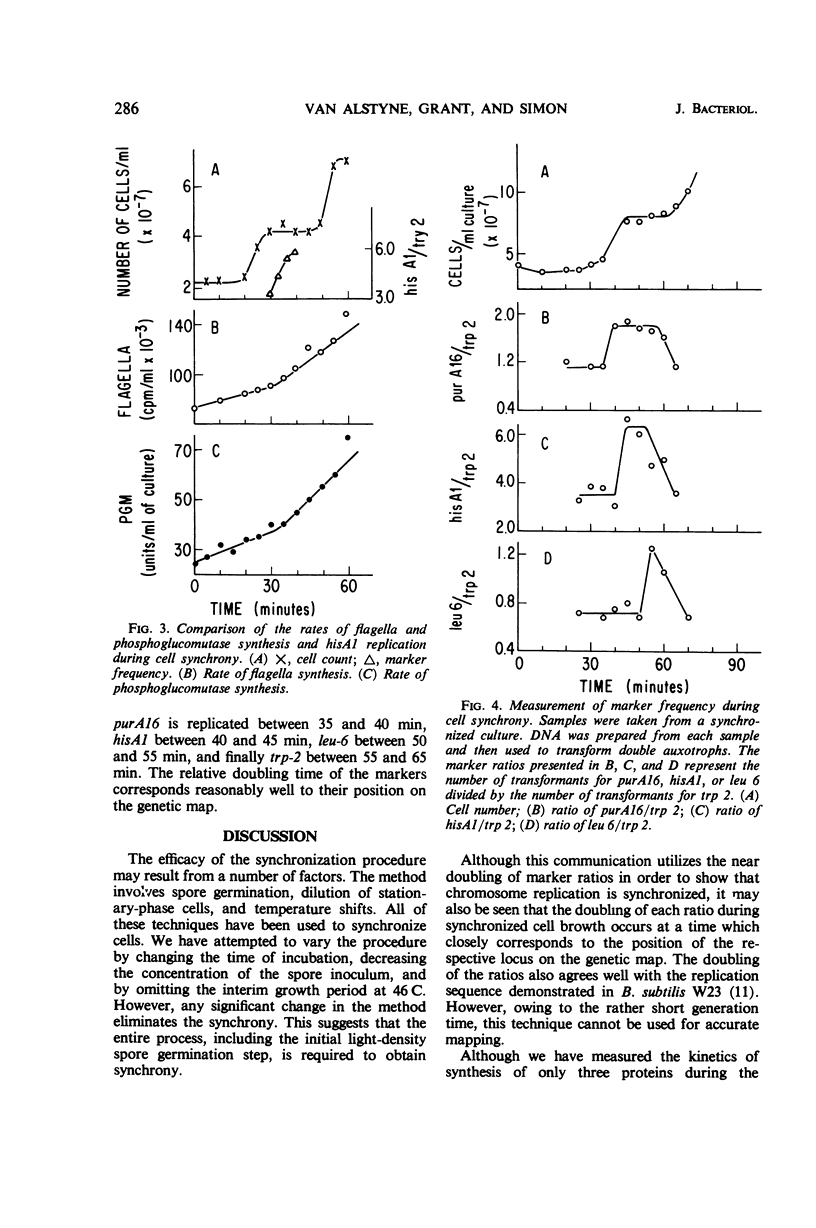
Synchronous cultures of Bacillus subtilis 168 M were obtained from light-density spores germinated at 46 C and grown at 37 C. This procedure synchronizes both cell division and chromosome replication. The chromosome synchrony was demonstrated by using transformation to measure changes in marker frequency during the cell cycle. The synthesis of two enzymes and of bacterial flagellar protein was also followed. All of the proteins were found to be synthesized continuously with an abrupt doubling in the rate of synthesis at a specific time in the cell cycle. The time at which the doubling occurred for each enzyme corresponded to the time at which the structural gene for the enzyme was replicated. The doubling of the rate of flagella synthesis corresponded to the time of replication of the hisA1 gene. We conclude that the genetic locus for the factors involved in the rate-limiting steps in flagella synthesis are located on the genetic map near the hisA1 locus.
Full text
PDF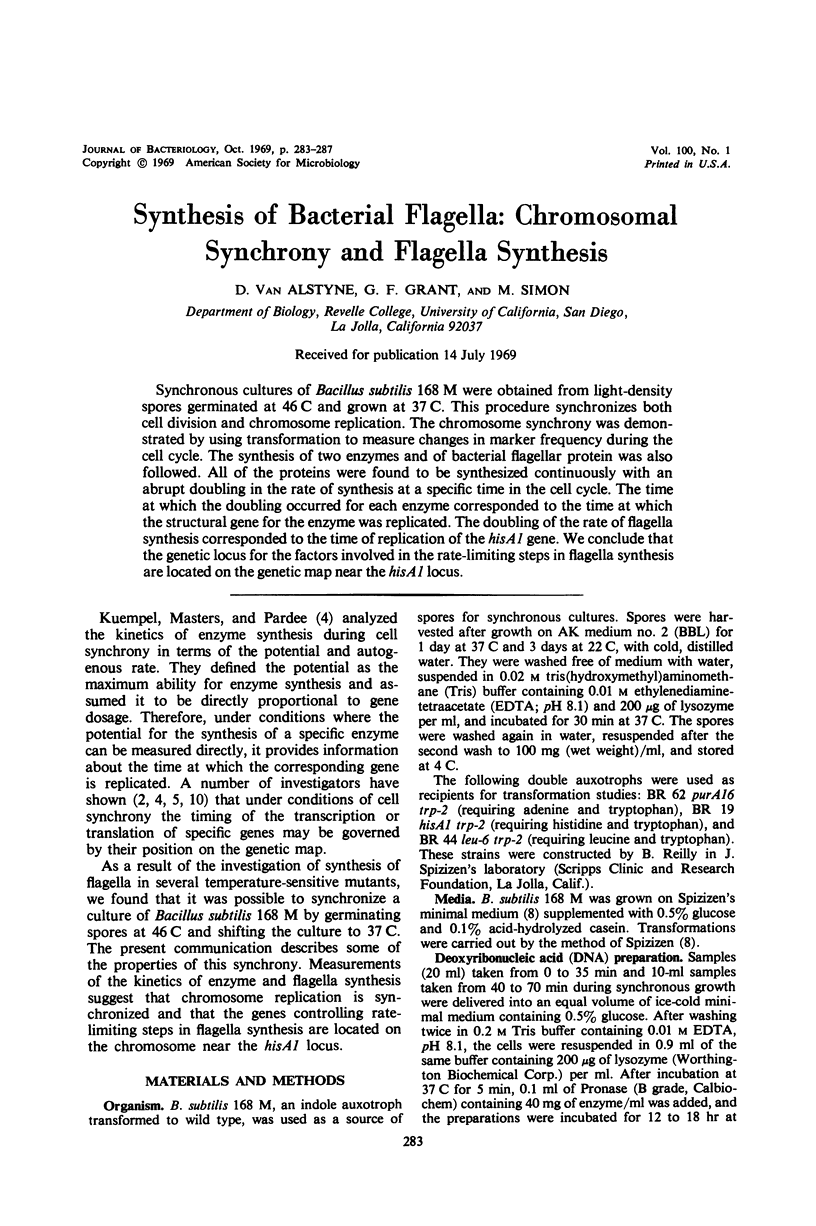


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dubnau D., Goldthwaite C., Smith I., Marmur J. Genetic mapping in Bacillus subtilis. J Mol Biol. 1967 Jul 14;27(1):163–185. doi: 10.1016/0022-2836(67)90358-0. [DOI] [PubMed] [Google Scholar]
- Ferretti J. J., Gray E. D. Enzyme and nucleic acid formation during synchronous growth of Rhodopseudomonas spheroides. J Bacteriol. 1968 Apr;95(4):1400–1406. doi: 10.1128/jb.95.4.1400-1406.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant G. F., Simon M. Use of radioactive antibodies for characterizing antigens and application to the study of flagella synthesis. J Bacteriol. 1968 Jan;95(1):81–86. doi: 10.1128/jb.95.1.81-86.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITSUHASHI S., DAVIS B. D. Aromatic biosynthesis. XII. Conversion of 5-dehydroquinic acid to 5-dehydroshikimic acid dy 5-dehydroquinase. Biochim Biophys Acta. 1954 Sep;15(1):54–61. doi: 10.1016/0006-3002(54)90093-1. [DOI] [PubMed] [Google Scholar]
- Masters M., Pardee A. B. Sequence of enzyme synthesis and gene replication during the cell cycle of Bacillus subtilis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):64–70. doi: 10.1073/pnas.54.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir H., Gilvarg C. Density gradient centrifugation for the separation of sporulating forms of bacteria. J Biol Chem. 1966 Mar 10;241(5):1085–1090. [PubMed] [Google Scholar]
- Tauro P., Halvorson H. O. Effect of gene position on the timing of enzyme synthesis in synchronous cultures of yeast. J Bacteriol. 1966 Sep;92(3):652–661. doi: 10.1128/jb.92.3.652-661.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIKAWA H., SUEOKA N. Sequential replication of the Bacillus subtilis chromosome. II. Isotopic transfer experiments. Proc Natl Acad Sci U S A. 1963 Jun;49:806–813. doi: 10.1073/pnas.49.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]


