Abstract
Background
Abnormalities in traditional lipids, particularly decreased high-density lipoprotein (HDL) cholesterol and increased triglycerides, precede the onset of hypertension. Whether lipoprotein particle size or subclass concentrations play a role in the development of hypertension is unknown.
Methods
17,527 initially healthy women without baseline hypertension were followed prospectively for 8 years. At baseline, traditional lipids and hypertension risk factors were obtained, and lipoprotein size and subclass concentrations were measured by nuclear magnetic resonance spectroscopy
Results
Baseline lipoprotein size and subclass concentrations were significantly associated with incident hypertension. While low density-lipoprotein (LDL) cholesterol was not associated with hypertension (odds ratio [OR] for quintile 5 vs 1: 1.08 [95% CI 0.96–1.20]), the concentration of LDL particles was associated with greater risk (OR 1.73 [1.54–1.95]), especially small LDL particles (OR 1.62 [1.45–1.83]). Increased HDL cholesterol was associated with lower risk of hypertension (OR for quintile 5 vs 1: 0.79 [0.70–0.89]). By contrast, increased concentration of HDL particles had greater risk (OR 1.48 [1.32–1.67]), especially small HDL particles (OR 1.36 [1.22–1.53]), while large HDL particles had lower risk (OR 0.80 [0.71–0.90]). Triglycerides and triglyceride-rich very-low-density lipoprotein (VLDL) particles were positively associated with hypertension, with large VLDL particles associated with greater risk (OR 1.68 [1.50–1.89]. Adding particle subclasses improved discrimination over a model with traditional lipids and risk factors (c-statistic 0.671 to 0.676, p-value <0.001).
Conclusion
In this study of initially healthy women, lipoprotein particle size and subclass concentrations were associated with incident hypertension and provided additive information to traditional lipids and risk factors.
Keywords: HDL particle size, hypertension, lipoproteins, LDL particle size, women
INTRODUCTION
Hypertension is a major risk factor for cardiovascular disease, affecting approximately 1 in 3 adults in the United States, with direct and indirect costs estimated at $43.5 billion in 2007.(1) Hypertension often clusters with dyslipidemia, especially among individuals with insulin resistance.(2) Previous studies found an increased risk of hypertension with lower concentration of high-density lipoprotein (HDL) cholesterol and higher concentrations of low-density lipoprotein (LDL) cholesterol and triglycerides.(3–6)
Lipoprotein abnormalities, particularly smaller LDL size or increased concentrations of triglyceride-rich particles, may contribute to high blood pressure by impairing endothelial function and promoting insulin resistance and vascular inflammation.(7–10) It has been recognized that a key feature of insulin resistance is the occurrence of a particular pattern of abnormalities in lipoprotein subclass distributions which is not detected by traditional lipid testing but which can be assessed using advanced lipoprotein testing such as nuclear magnetic resonance (NMR) spectroscopy of plasma.(11) Nuclear magnetic resonance (NMR) spectroscopy allows the simultaneous determination of both the concentration and size of lipoprotein particles. NMR measured lipoproteins have been shown to associate with the risk of cardiovascular disease(12–14), insulin resistance (8), and diabetes(15).
We hypothesized that lipoprotein particle size or subclass abnormalities that are associated with insulin resistance (i.e. smaller size of LDL and HDL particles, and increased concentration of triglyceride-rich particles) would predict incident hypertension. Therefore, in a large prospective cohort of initially healthy women, we evaluated the relationship between incident hypertension and baseline lipoprotein subclass size and concentration of LDL, HDL and very-low-density lipoprotein (VLDL) particles, and how they compared with traditional lipids or apolipoproteins. We further examined whether the association could be attenuated by biomarkers of inflammation/endothelial function, hyperglycemia, or other risk factors.
MATERIALS AND METHODS
Study Population
Study participants were from the Women’s Health Study (WHS), a trial begun in 1992 to study the primary prevention of cardiovascular disease and cancer in initially healthy U.S. female health professionals aged 45 years or older randomized to take vitamin E and aspirin.(16, 17) All WHS participants provided written informed consent, and the study was approved by the institutional review board of the Brigham and Women’s Hospital (Boston, Massachusetts).
A total of 28,345 (71%) WHS participants provided baseline blood samples. For this study, we excluded 7165 women who were hypertensive at baseline, defined as those who reported a systolic blood pressure greater than 140 mmHg, a diastolic blood pressure greater than 90 mmHg, any history of use of blood pressure medications, or any history of a physician diagnosis of hypertension. We further excluded women who were missing information on any lipid or lipoprotein measurements or other covariates, resulting in 17,527 women for this analysis.
Lipids and lipoprotein measures
EDTA blood samples were obtained at the time of enrollment into the WHS and stored in vapor phase liquid nitrogen (−170° C). The frozen plasma specimens were thawed and lipoprotein particle concentrations were measured by proton NMR spectroscopy (LipoScience, Inc., Raleigh, NC).(18, 19) NMR signal amplitudes of the spectroscopically distinct lipid methyl group for each lipoprotein were used to calculate concentrations for the different lipoproteins.(18) The lipoprotein particles obtained included total HDL, further subdivided into large, medium and small particles; total LDL, further subdivided into large and small LDL particles and intermediate-density lipoprotein (IDL) particles; and total very low density lipoprotein (VLDL), further subdivided into large, medium and small particles. The IDL particles, sized between VLDL and LDL particles, were categorized with the LDL particles as they exhibit similar properties. Relative mass percentages were multiplied by the diameter of each subclass to obtain weighted average size for each lipoprotein particle. (18, 19) The NMR lipoprotein variables that we examined are those that are provided when ordering a commercially-available NMR lipoprotein profile.(20) Particle diameters and coefficients of variation (CVs) have been previously published for the NMR measures, with between-run CVs 7.1% or below for all particles except IDL (13%).(20)
All other plasma measurements were analyzed in a core laboratory facility certified by the National Heart, Lung and Blood Institute/Centers for Disease Control and Prevention Lipid Standardization Program. Traditional lipid measures used in this study (total, HDL, and LDL cholesterol and tryglicerides) were all measured directly with a Hitachi 917 analyzer using reagents from Roche Diagnostics (Indianapolis, IN) with between-run CVs less than 3%. Apolipoproteins B100 (apoB) and A-1 (apoA-1) were measured using immunoturbidimetric assays (DiaSorin, Stillwater, Minn), with between-run CVs of 5% and 3%, respectively.
Hypertension
Baseline self-reported blood pressure (in mmHg categories of <110, 110–119, 120–129, 130–139, 140–149, 150–159, 160–169, 170–179, and ≥180 for systolic blood pressure and &x0003C;65, 65–74, 75–84, 85–89, 90–94, 95–104, and ≥105 for diastolic blood pressure), history of treatment for high blood pressure, and physician diagnosis of hypertension were assessed by questionnaire. Incident hypertension was ascertained by annual questionnaire using methods previously described in detail.(3) Briefly, participants were classified as hypertensive after reporting either a new physician diagnosis at year 1, 3, or annually thereafter; a new hypertensive treatment at year 1, 3, or 4; a systolic blood pressure of 140 mmHg or greater at year 1 or 4; or a diastolic blood pressure of 90 mmHg or greater at year 1 or 4. The reproducibility of self-reported hypertension status in these female health professionals was assessed in a sub-sample of participants using medical records, with high rates of agreement (96% confirmation rate for reports of hypertension and 90% confirmation rate for reports of no hypertension).(21)
Covariates
Baseline age, race, diabetes, alcohol use, exercise frequency, treatment for high cholesterol, postmenopausal hormone use, diet, education, smoking, and menopausal status were collected from self-reported questionnaires. Body mass index (BMI) was calculated from self-reported height and weight at baseline. Other markers relating to inflammation and endothelial function including C-reactive protein, fibrinogen, homocysteine, and soluble intercellular adhesion molecule-1, as well as hemoglobin A1c levels, were also measured as previously described.(22)
Statistical Methods
Logistic models with an outcome of incident hypertension at 8 years were chosen as the primary modeling strategy. Lipid measurements were divided into quintiles and were analyzed both categorically and for linear trend across quintiles using quintile number. The primary adjustment for confounding included non-lipid risk factors (baseline values of age, smoking, fasting status, use of cholesterol lowering medication, trial treatment assignment, hormone use, menopausal status, race, exercise, alcohol use, BMI, diabetes, education, vegetable, fruit, sodium, and total grain intake). The increase in the likelihood ratio obtained by adding the lipid measurement to the non-lipid risk factors was also derived.
To assess the independent impact of each of the NMR lipoprotein sizes, a fully adjusted model including all 9 lipoprotein subclasses was examined. A fully adjusted model was also examined using mean size and particle concentration for each lipoprotein plus non-lipid risk factors. C-statistics were used to compare the addition of NMR measures to models with non-lipid risk factors and traditional lipids.(23) A similar analysis was also performed with standard lipids and apolipoprotein measures plus non lipid risk factors.
In order to assess potential mediators, we examined models with additional adjustment for inflammatory/endothelial function markers (C-reactive protein, fibrinogen, homocysteine, and soluble intercellular adhesion molecule-1), hemoglobin A1c levels, and baseline blood pressure.
All analyses were also redone in designated subgroups and tested for interaction: 1) BMI divided into obese (BMI greater than or equal to 30), overweight (BMI of 25 to 30) and normal (BMI of less than 25)(24); 2) blood pressure less than 120 / 70 mmHg; 3) metabolic syndrome categories previously used in the Women’s Health Study(25) with and without the blood pressure criterion of ≥ 130/85 mmHg; 4) non-users of lipid lowering medication.
All analyses were done using R version 2.10.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
During 8 years of follow-up, 4714 cases (27%) of incident hypertension occurred. As shown in Table 1, women who developed hypertension were older at baseline with higher baseline blood pressures, along with a higher prevalence of hormone use, diabetes, and postmenopausal status. They also exercised less, had a higher BMI, and were more likely to be Black or Hispanic.
Table 1.
Baseline Characteristics of 17,572 Women Initially Free of Hypertension
| Incident hypertension at 8 years (N = 4714) |
No hypertension at 8 years (N = 12858) |
P-value* | |
|---|---|---|---|
| Age, years | 53.6 (49.4,59.6) | 51.6 (48.2,56.6) | < 0.001 |
| Systolic blood pressure, mm Hg | 125 (115,135) | 115 (105,125) | < 0.001 |
| Diastolic blood pressure, mm Hg | 80 (70,80) | 70 (70,80) | < 0.001 |
| Current hormone use, % | 46.0 | 42.8 | < 0.001 |
| Diabetes, % | 2.7 | 0.9 | < 0.001 |
| Postmenopausal, % | 56.5 | 48.4 | < 0.001 |
| Black or Hispanic, % | 2.9 | 1.9 | < 0.001 |
| Current cigarette smoker, % | 11.5 | 11.4 | 0.88 |
| Alcohol use, % | < 0.001 | ||
| Rarely/never | 45.3 | 40.5 | |
| 1–3 drinks/month | 13.2 | 13.5 | |
| 1–6 drinks/week | 31.2 | 35.4 | |
| 1+ drinks/day | 10.2 | 10.6 | |
| Exercise frequency, % | < 0.001 | ||
| Rarely/never | 39.1 | 33.4 | |
| <1 time/week | 19.7 | 19.9 | |
| 1–3 times/week | 31.0 | 33.9 | |
| 4+ times/week | 10.2 | 12.8 | |
| Body mass index, kg/m2 | 25.7 (23.1,29.2) | 23.7 (21.8,26.6) | < 0.001 |
| Current cholesterol treatment, % | 2.9 | 1.9 | < 0.001 |
| Total cholesterol, mg/dL | 210 (186,238) | 204 (181,231) | < 0.001 |
| LDL Measures | |||
| LDL cholesterol, mg/dL | 122.6 (101.6,146.1) | 118.2 (98.2,140.9) | < 0.001 |
| Apolipoprotein B, mg/dL | 105.1 (86.9,124.1) | 95.3 (79.9,115.1) | < 0.001 |
| NMR LDL particle concentration, nmol/L | |||
| Total | 1350 (1091,1679) | 1191 (970,1476) | < 0.001 |
| IDL | 38 (14,74) | 26 (8,59) | < 0.001 |
| Large | 535 (388,686) | 556 (426,692) | < 0.001 |
| Small | 730 (450,1108) | 566 (340,867) | < 0.001 |
| Average NMR LDL particle size, nm | 21.3 (20.7,21.8) | 21.5 (21,22) | < 0.001 |
| HDL Measures | |||
| HDL cholesterol, mg/dL | 50.7 (42.3,61.1) | 54.2 (45.3,64.6) | < 0.001 |
| Apolipoprotein A-I, mg/dL | 149.2 (132.2,167.9) | 150.3 (134,168.5) | 0.007 |
| NMR HDL particle concentration, μmol/L | |||
| Total | 35.5 (31.5,40) | 34.6 (30.8,38.9) | < 0.001 |
| Large | 7.1 (4.6,10) | 8.2 (5.6,10.8) | < 0.001 |
| Medium | 3.1 (0.9,6.5) | 2.6 (0.7,5.8) | < 0.001 |
| Small | 24.1 (20.5,27.7) | 22.9 (19.3,26.5) | < 0.001 |
| Average NMR HDL particle size, nm | 8.9 (8.6,9.3) | 9.1 (8.8,9.4) | < 0.001 |
| VLDL Measures | |||
| Triglycerides, mg/dL | 129 (90,187) | 106 (75,153) | < 0.001 |
| NMR VLDL particle concentration, nmol/L | |||
| Total | 70.9 (51.3,93.2) | 65.3 (46.7,86.7) | < 0.001 |
| Large | 1.9 (0.5,4.2) | 1.0 (0.2,2.9) | < 0.001 |
| Medium | 22.0 (12.0,33.4) | 19.6 (10.3,30.6) | < 0.001 |
| Small | 45.9 (32.9,58.6) | 43.1 (31.1,56.4) | < 0.001 |
| Average NMR VLDL particle size, nm | 47.6 (42.9,53) | 45.5 (41.6,50.7) | < 0.001 |
Values shown are median (25 percentile, 75 percentile) unless otherwise indicated
Kruskal-Wallis for continuous variables, Chi-squared for categorical
Median baseline lipid and lipoprotein measures significantly differed in women who went on to develop hypertension. HDL cholesterol, apoA-1, the concentration of large LDL and HDL particles, and the average LDL and HDL particle size were lower in women who developed hypertension. All other lipid and lipoprotein measures were higher in women who developed hypertension. Correlations between the NMR and traditional lipid measures were similar to previously published values for the WHS (12), with low correlations between the average sizes and total particle numbers and low to moderate correlations among the 9 particle subclasses.
LDL Measures
In unadjusted analysis by quintile of each measure, all LDL measures were associated with hypertension, as shown in Table 2. After adjustment for non-lipid risk factors (Model 1: age, smoking, fasting status, use of cholesterol lowering medication, trial treatment assignment, hormone use, menopausal status, race, exercise, alcohol use, BMI, diabetes, education, vegetable, fruit, sodium, and total grain intake), LDL cholesterol was no longer associated. By contrast, apoB and all of the LDL NMR measures remained significantly associated with incident hypertension. Large LDL particle concentration and average LDL particle size were inversely associated with risk of hypertension, while small LDL, IDL, and hence total concentration of LDL particles were associated with increased risk. Of note, the largest odds ratios and likelihood ratios for comparing quintile 5 to quintile 1 were for total LDL particle concentration (OR 1.73, LR χ2 105.61) and small LDL particles (OR 1.62, LR χ2 96.32).
Table 2.
Association of LDL Measures with Incident Hypertension
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | LR Chi2 |
P for trend |
|
|---|---|---|---|---|---|---|---|
| LDL Cholesterol mg/dL | 23.7 – 94.6 | 94.7 – 111.6 | 111.7 – 127.7 | 127.8 – 148.6 | 148.7 – 335.4 | ||
| Unadjusted | 1 | 1.06 (0.95,1.18) | 1.21 (1.08,1.34) | 1.28 (1.15,1.42) | 1.45 (1.30,1.61) | 59.37 | <0.001 |
| Model 1 | 1 | 0.97 (0.87,1.09) | 1.04 (0.93,1.16) | 1.03 (0.92,1.15) | 1.08 (0.96,1.20) | 3.58 | 0.11 |
| Model 2 | 1 | 0.97 (0.87,1.09) | 1.04 (0.93,1.16) | 1.03 (0.92,1.15) | 1.07 (0.96,1.2) | 3.32 | 0.13 |
| Apolipoprotein B, mg/dL | 21.8 – 78 | 78.0 – 91.2 | 91.3 – 106.2 | 106.3 – 122.8 | 122.9 – 257.4 | ||
| Unadjusted | 1 | 1.12 (1.00,1.26) | 1.45 (1.30,1.63) | 1.69 (1.51,1.88) | 2.23 (2.00,2.48) | 282.03 | <0.001 |
| Model 1 | 1 | 0.99 (0.88,1.11) | 1.14 (1.01,1.28) | 1.23 (1.10,1.38) | 1.44 (1.28,1.61) | 57.68 | <0.001 |
| Model 2 | 1 | 0.99 (0.88,1.11) | 1.13 (1.00,1.26) | 1.21 (1.08,1.36) | 1.39 (1.24,1.56) | 46.52 | <0.001 |
| NMR particle concentration, nmol/L | |||||||
| Total LDL Particles | 303 – 947 | 948 –1135 | 1136 – 1335 | 1336 – 1624 | 1625 – 4405 | ||
| Unadjusted | 1 | 1.29 (1.15,1.45) | 1.59 (1.42,1.78) | 2.09 (1.87,2.34) | 2.82 (2.53,3.15) | 444.67 | <0.001 |
| Model 1 | 1 | 1.13 (1.00,1.27) | 1.23 (1.09,1.39) | 1.46 (1.30,1.64) | 1.73 (1.54,1.95) | 105.61 | <0.001 |
| Model 2 | 1 | 1.11 (0.99,1.26) | 1.20 (1.07,1.36) | 1.41 (1.26,1.59) | 1.63 (1.45,1.84) | 82.09 | <0.001 |
| IDL Particles | 0 – 5 | 6 – 20 | 21 – 40 | 41 – 73 | 74 – 339 | ||
| Unadjusted | 1 | 1.12 (1.00,1.25) | 1.36 (1.22,1.52) | 1.67 (1.50,1.86) | 1.95 (1.75,2.17) | 209.03 | <0.001 |
| Model 1 | 1 | 1.02 (0.91,1.15) | 1.16 (1.03,1.30) | 1.3 (1.16,1.46) | 1.35 (1.20,1.51) | 44.52 | <0.001 |
| Model 2 | 1 | 1.01 (0.9,1.14) | 1.14 (1.01,1.28) | 1.25 (1.12,1.41) | 1.29 (1.15,1.44) | 32.10 | <0.001 |
| Large LDL Particles | 0 – 384 | 385 – 500 | 501 – 603 | 604 – 731 | 732 – 2917 | ||
| Unadjusted | 1 | 0.72 (0.65,0.80) | 0.70 (0.64,0.78) | 0.64 (0.57,0.71) | 0.74 (0.66,0.82) | 82.85 | <0.001 |
| Model 1 | 1 | 0.82 (0.73,0.91) | 0.83 (0.74,0.92) | 0.75 (0.67,0.83) | 0.82 (0.74,0.92) | 29.49 | <0.001 |
| Model 2 | 1 | 0.84 (0.75,0.93) | 0.85 (0.77,0.95) | 0.77 (0.69,0.86) | 0.85 (0.76,0.95) | 22.94 | 0.001 |
| Small LDL Particles | 0 – 313 | 314 – 507 | 508 – 712 | 713 – 1040 | 1041 – 3457 | ||
| Unadjusted | 1 | 1.13 (1.01,1.27) | 1.37 (1.22,1.53) | 1.91 (1.71,2.13) | 2.51 (2.25,2.79) | 398.83 | <0.001 |
| Model 1 | 1 | 1.06 (0.94,1.20) | 1.17 (1.04,1.32) | 1.44 (1.28,1.61) | 1.62 (1.45,1.83) | 96.32 | <0.001 |
| Model 2 | 1 | 1.05 (0.93,1.19) | 1.15 (1.02,1.29) | 1.39 (1.24,1.56) | 1.53 (1.36,1.73) | 72.90 | <0.001 |
| Average NMR LDL Size, nm | 19.0 – 20.8 | 20.9 – 21.2 | 21.3 – 21.6 | 21.7 – 22 | 22.1 – 23.0 | ||
| Unadjusted | 1 | 0.72 (0.65,0.80) | 0.61 (0.55,0.67) | 0.51 (0.46,0.57) | 0.44 (0.39,0.48) | 301.70 | <0.001 |
| Model 1 | 1 | 0.86 (0.77,0.96) | 0.78 (0.70,0.86) | 0.71 (0.64,0.79) | 0.64 (0.57,0.72) | 70.82 | <0.001 |
| Model 2 | 1 | 0.88 (0.79,0.98) | 0.81 (0.73,0.89) | 0.75 (0.67,0.83) | 0.68 (0.6,0.76) | 51.50 | <0.001 |
Ranges (minimum – maximum) and odds ratios with 95% confidence intervals are given for each quintile.
Model 1 includes age, smoking, fasting status, use of cholesterol lowering medication, trial treatment assignment, hormone use, menopausal status, race, exercise, alcohol use, body mass index, diabetes, education, vegetable, fruit, sodium, and total grain intake.
Model 2 adds C-reactive protein, homocysteine, fibrinogen, soluble inter-cellular adhesion molecule 1, and hemoglobin A1C to Model 1
The results were essentially unchanged after additional adjustments for baseline inflammatory/endothelial biomarkers (C-reactive protein, fibrinogen, homocysteine, and soluble intercellular adhesion molecule 1) and hemoglobin A1c (Model 2 results, Table 2).
HDL Measures
All HDL measures were associated with incident hypertension in unadjusted analyses (Table 3). After adjustment for non lipid risk factors (Model 1), apoA-1 was no longer independently associated, while standard HDL cholesterol and all HDL NMR measures remained significantly associated. The total concentration of HDL particles, specifically the medium and small HDL particles which make up most of the total concentration of HDL, were associated with increased risk, while large HDL particles were inversely associated with risk of hypertension. Accordingly, larger average HDL particle size was also associated with decreased incidence of hypertension. The largest odds ratios and likelihood ratios for comparison of quintile 5 to quintile 1 were for HDL particle size (OR 0.66, LR χ2 66.16) and total HDL particle concentration (OR 1.48, LR χ2 54.81). Further adjustment for inflammatory/endothelial biomarkers and hemoglobin A1c did not alter the magnitude of association (Model 2 results, Table 3).
Table 3.
Association of HDL Measures with Incident Hypertension
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | LR Chi2 |
P for trend |
|
|---|---|---|---|---|---|---|---|
| HDL Cholesterol, mg/dL | 15.9 – 42.4 | 42.5 – 49.7 | 49.9 – 56.9 | 57.0 – 66.5 | 66.6 –173.0 | ||
| Unadjusted | 1 | 0.79 (0.72,0.88) | 0.66 (0.59,0.73) | 0.58 (0.52,0.64) | 0.54 (0.49,0.60) | 174.83 | <0.001 |
| Model 1 | 1 | 0.94 (0.84,1.04) | 0.85 (0.76,0.94) | 0.78 (0.70,0.88) | 0.79 (0.70,0.89) | 25.25 | <0.001 |
| Model 2 | 1 | 0.96 (0.86,1.06) | 0.87 (0.78,0.97) | 0.8 (0.72,0.9) | 0.81 (0.71,0.91) | 20.73 | <0.001 |
| Apolipoprotein A-I, mg/dL | 49.8 –129 | 129.6 –143 | 143.4 –156 | 156.6 –172.9 | 173 – 249 | ||
| Unadjusted | 1 | 0.88 (0.80,0.98) | 0.84 (0.76,0.94) | 0.88 (0.80,0.98) | 0.85 (0.77,0.95) | 12.70 | 0.008 |
| Model 1 | 1 | 0.96 (0.86,1.07) | 0.98 (0.87,1.09) | 1.07 (0.96,1.20) | 1.03 (0.91,1.17) | 4.63 | 0.23 |
| Model 2 | 1 | 0.97 (0.87,1.09) | 0.99 (0.88,1.11) | 1.07 (0.96,1.21) | 1.01 (0.89,1.14) | 3.46 | 0.41 |
| NMR particle concentration, μmol/L | |||||||
| Total HDL Particles | 12.1 – 30.2 | 30.3 – 33.3 | 33.4 – 36.4 | 36.5 – 40.3 | 40.4 – 67.9 | ||
| Unadjusted | 1 | 1.02 (0.92,1.14) | 1.13 (1.01,1.26) | 1.24 (1.12,1.38) | 1.47 (1.32,1.63) | 69.19 | <0.001 |
| Model 1 | 1 | 1.02 (0.91,1.15) | 1.12 (1.00,1.25) | 1.23 (1.09,1.37) | 1.48 (1.32,1.67) | 54.81 | <0.001 |
| Model 2 | 1 | 1.02 (0.91,1.15) | 1.12 (1.00,1.25) | 1.21 (1.08,1.36) | 1.43 (1.26,1.61) | 41.88 | <0.001 |
| Large HDL Particles | 0 – 4.7 | 4.8 – 6.9 | 7.0 – 8.9 | 9.0 –11.3 | 11.4 –25.3 | ||
| Unadjusted | 1 | 0.74 (0.67,0.82) | 0.62 (0.56,0.69) | 0.51 (0.46,0.57) | 0.56 (0.50,0.62) | 200.89 | <0.001 |
| Model 1 | 1 | 0.88 (0.79,0.98) | 0.83 (0.75,0.93) | 0.73 (0.65,0.82) | 0.80 (0.71,0.9) | 30.21 | <0.001 |
| Model 2 | 1 | 0.90 (0.81,1.00) | 0.86 (0.77,0.96) | 0.76 (0.67,0.85) | 0.81 (0.72,0.91) | 24.29 | <0.001 |
| Medium HDL Particles | 0 – 0.5 | 0.6 – 1.8 | 1.9 – 3.8 | 3.9 – 7.0 | 7.1 – 30.4 | ||
| Unadjusted | 1 | 1.12 (1.01,1.25) | 1.15 (1.03,1.28) | 1.27 (1.14,1.41) | 1.43 (1.29,1.59) | 52.12 | <0.001 |
| Model 1 | 1 | 1.06 (0.95,1.18) | 1.06 (0.95,1.18) | 1.17 (1.05,1.30) | 1.31 (1.17,1.46) | 28.06 | <0.001 |
| Model 2 | 1 | 1.05 (0.94,1.18) | 1.04 (0.93,1.17) | 1.14 (1.02,1.27) | 1.26 (1.12,1.41) | 18.878 | <0.001 |
| Small HDL Particles | 0 – 18.7 | 18.8 – 21.9 | 22.0 – 24.5 | 24.6 – 27.7 | 27.8 – 49.9 | ||
| Unadjusted | 1 | 1.07 (0.96,1.19) | 1.27 (1.14,1.42) | 1.46 (1.31,1.63) | 1.77 (1.59,1.97) | 151.12 | <0.001 |
| Model 1 | 1 | 0.98 (0.87,1.10) | 1.11 (0.99,1.25) | 1.17 (1.04,1.30) | 1.36 (1.22,1.53) | 45.35 | <0.001 |
| Model 2 | 1 | 0.99 (0.88,1.11) | 1.12 (1.00,1.25) | 1.16 (1.04,1.30) | 1.34 (1.20,1.50) | 38.18 | <0.001 |
| Average NMR HDL Size, nm | 8.0 – 8.6 | 8.7 – 8.9 | 9.0 – 9.2 | 9.3 – 9.5 | 9.6 – 10.8 | ||
| Unadjusted | 1 | 0.73 (0.66,0.80) | 0.57 (0.51,0.62) | 0.49 (0.44,0.55) | 0.40 (0.35,0.45) | 338.06 | <0.001 |
| Model 1 | 1 | 0.85 (0.77,0.94) | 0.75 (0.68,0.84) | 0.72 (0.64,0.80) | 0.62 (0.55,0.7) | 66.16 | <0.001 |
| Model 2 | 1 | 0.86 (0.78,0.95) | 0.77 (0.70,0.86) | 0.74 (0.66,0.83) | 0.65 (0.57,0.73) | 52.73 | <0.001 |
Ranges (minimum – maximum) and odds ratios with 95% confidence intervals are given for each quintile.
Model 1 includes age, smoking, fasting status, use of cholesterol lowering medication, trial treatment assignment, hormone use, menopausal status, race, exercise, alcohol use, body mass index, diabetes, education, vegetable, fruit, sodium, and total grain intake.
Model 2 adds C-reactive protein, homocysteine, fibrinogen, soluble inter-cellular adhesion molecule 1, and hemoglobin A1C to Model 1
VLDL and Triglyceride Measures
The VLDL and triglyceride measures (Table 4) were all associated with hypertension prior to adjustment and all except small VLDL particle concentration remained associated after adjustment for non lipid risk factors (Model 1). In contrast with the association of smaller size of LDL and HDL with hypertension, larger VLDL size and large VLDL particle concentration were associated with increased risk of hypertension. The largest odds ratios and likelihood ratios for comparison of quintile 5 to quintile 1 were for triglycerides (OR 1.65, LR χ2 91.63) and large VLDL particles (OR 1.68, LR χ2 90.73). Similar results were obtained after additionally adjusting for the inflammatory/endothelial biomarkers and hemoglobin A1c (Model 2 results, Table 4).
Table 4.
Association of VLDL and Triglyceride Measures with Incident Hypertension
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | LR Chi2 |
P for trend |
|
|---|---|---|---|---|---|---|---|
| Triglycerides, mg/dL | 16 – 73 | 74 – 97 | 98 – 129 | 130 – 178 | 179 – 954 | ||
| Unadjusted | 1 | 1.22 (1.09,1.38) | 1.60 (1.43,1.79) | 1.93 (1.73,2.16) | 2.63 (2.36,2.93) | 395.09 | <0.001 |
| Model 1 | 1 | 1.06 (0.94,1.20) | 1.27 (1.13,1.43) | 1.37 (1.22,1.54) | 1.65 (1.47,1.86) | 91.63 | <0.001 |
| Model 2 | 1 | 1.04 (0.92,1.17) | 1.23 (1.09,1.38) | 1.30 (1.15,1.47) | 1.53 (1.35,1.73) | 61.79 | <0.001 |
| NMR particle concentration, nmol/L | |||||||
| Total VLDL Particles | 0.1 – 43.5 | 43.6 – 59.3 | 59.4 – 74.4 | 74.5 – 94.6 | 94.7 – 258.6 | ||
| Unadjusted | 1 | 1.06 (0.94,1.18) | 1.26 (1.13,1.40) | 1.39 (1.25,1.54) | 1.57 (1.41,1.74) | 96.21 | <0.001 |
| Model 1 | 1 | 0.96 (0.86,1.08) | 1.06 (0.95,1.19) | 1.09 (0.97,1.22) | 1.16 (1.04,1.30) | 13.54 | <0.001 |
| Model 2 | 1 | 0.96 (0.85,1.07) | 1.05 (0.94,1.18) | 1.07 (0.96,1.20) | 1.14 (1.01,1.27) | 10.57 | 0.004 |
| Large VLDL Particles | 0 – 0.2 | 0.3 – 0.7 | 0.8 – 1.9 | 2.0 – 4.0 | 4.1 – 35.8 | ||
| Unadjusted | 1 | 1.25 (1.12,1.41) | 1.55 (1.38,1.73) | 2.01 (1.80,2.23) | 2.57 (2.32,2.86) | 388.79 | <0.001 |
| Model 1 | 1 | 1.16 (1.03,1.31) | 1.28 (1.14,1.43) | 1.44 (1.28,1.61) | 1.68 (1.50,1.89) | 90.73 | <0.001 |
| Model 2 | 1 | 1.16 (1.03,1.30) | 1.25 (1.11,1.40) | 1.38 (1.23,1.55) | 1.59 (1.41,1.79) | 65.95 | <0.001 |
| Medium VLDL Particles | 0 – 8.8 | 8.9 – 16.4 | 16.5 – 24.1 | 24.2 – 34.4 | 34.5 – 138.1 | ||
| Unadjusted | 1 | 1.10 (0.99,1.23) | 1.28 (1.15,1.42) | 1.29 (1.15,1.43) | 1.53 (1.37,1.70) | 72.79 | <0.001 |
| Model 1 | 1 | 1.00 (0.90,1.12) | 1.10 (0.98,1.23) | 1.08 (0.96,1.21) | 1.19 (1.06,1.33) | 13.03 | 0.001 |
| Model 2 | 1 | 0.99 (0.88,1.11) | 1.08 (0.96,1.21) | 1.05 (0.94,1.18) | 1.15 (1.03,1.29) | 9.30 | 0.007 |
| Small VLDL Particles | 0 – 28.7 | 28.8 – 39.2 | 39.3 – 48.7 | 48.8 – 60.6 | 60.7 – 157.8 | ||
| Unadjusted | 1 | 1.04 (0.93,1.16) | 1.10 (0.99,1.22) | 1.33 (1.20,1.48) | 1.33 (1.20,1.48) | 51.28 | <0.001 |
| Model 1 | 1 | 0.95 (0.85,1.07) | 0.95 (0.85,1.06) | 1.10 (0.98,1.23) | 1.04 (0.93,1.17) | 9.53 | 0.07 |
| Model 2 | 1 | 0.95 (0.85,1.06) | 0.95 (0.85,1.06) | 1.09 (0.98,1.22) | 1.04 (0.93,1.16) | 9.28 | 0.09 |
| Average NMR VLDL Size, nm | 31.8 – 41.0 | 41.1 – 44.4 | 44.5 – 48.0 | 48.1 – 52.9 | 53.0 – 131.2 | ||
| Unadjusted | 1 | 0.99 (0.88,1.11) | 1.26 (1.13,1.40) | 1.58 (1.42,1.76) | 1.84 (1.66,2.05) | 209.57 | <0.001 |
| Model 1 | 1 | 0.97 (0.86,1.09) | 1.12 (1.00,1.25) | 1.30 (1.16,1.45) | 1.42 (1.27,1.59) | 63.54 | <0.001 |
| Model 2 | 1 | 0.97 (0.86,1.09) | 1.11 (0.99,1.24) | 1.26 (1.13,1.42) | 1.36 (1.21,1.53) | 46.22 | <0.001 |
Ranges (minimum – maximum) and odds ratios with 95% confidence intervals are given for each quintile.
Model 1 includes age, smoking, fasting status, use of cholesterol lowering medication, trial treatment assignment, hormone use, menopausal status, race, exercise, alcohol use, body mass index, diabetes, education, vegetable, fruit, sodium, and total grain intake.
Model 2 adds C-reactive protein, homocysteine, fibrinogen, soluble inter-cellular adhesion molecule 1, and hemoglobin A1C to Model 1
Mutually-adjusted Effects of Lipoprotein Particle Subclasses
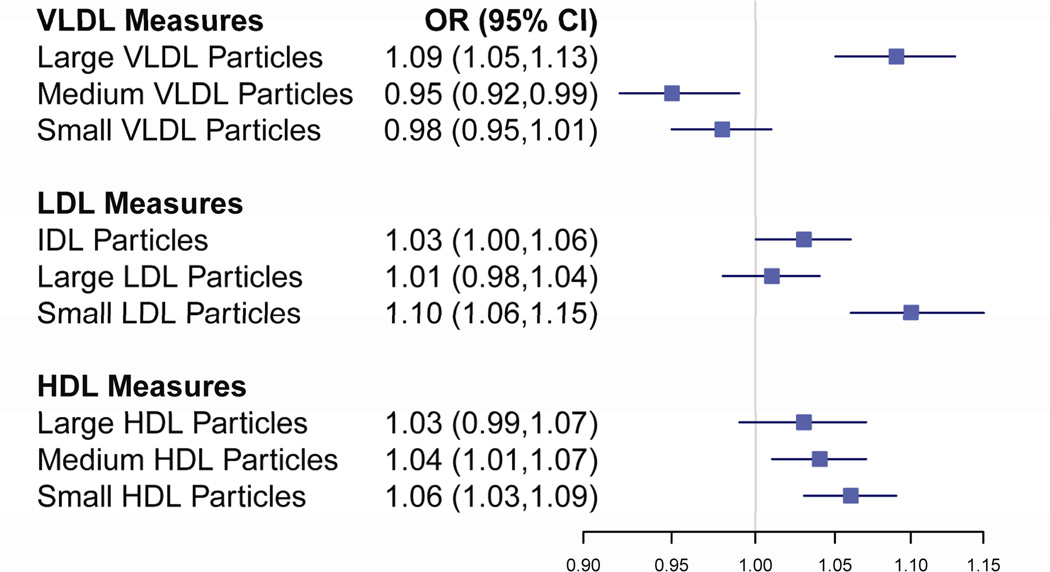
In a model including quintiles of NMR particle concentrations for the 9 non-overlapping particle subclasses and non-lipid risk factors, the medium and small HDL particles, the IDL and small LDL particles, and the medium and large VLDL particles remained independently associated with hypertension risk (Figure 1). Consistent with the previous results, these particle subclasses, with the exception of medium VLDL particles, were associated with an increased risk of hypertension.
Figure 1.
Mutually Adjusted Effects Per Quintile for Individual NMR Lipoprotein Subclasses on Incident Hypertension. All odds ratios are from a single model including NMR subclasses and non-lipid risk factors. Statistically significant p-values were noted for IDL (0.03), small LDL (<0.001), medium HDL (0.005), small HDL (<0.001), large VLDL (<0.001) and medium VLDL (0.005).
Mutually-adjusted Effects of Lipoprotein Particle Concentration versus Size
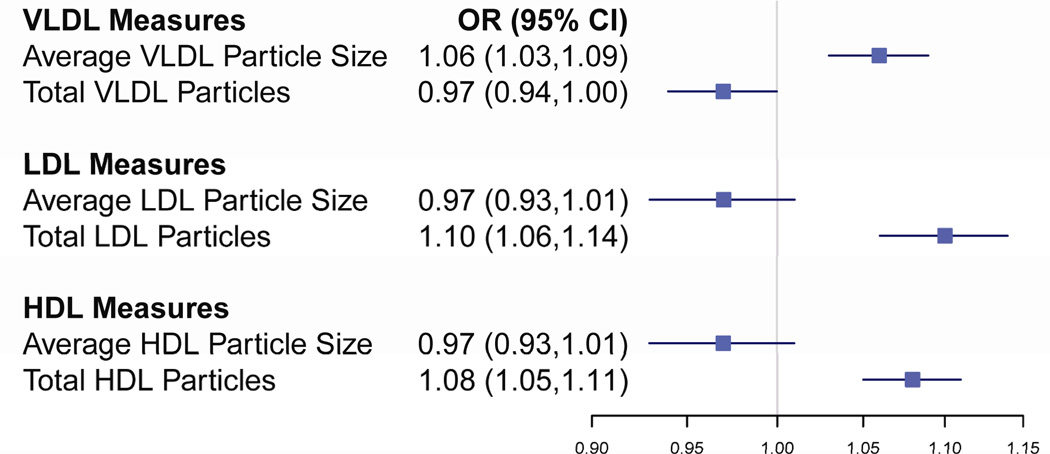
When the total particle concentration and average particle size for each lipoprotein type were combined into one model with non-lipid risk factors, the total concentration of LDL and HDL particles and average VLDL particle size were each independently associated with increased risk of hypertension. (Figure 2) These results are consistent with the finding that of the VLDL particles, the large particles were associated with the greatest increase in risk.
Figure 2.
Mutually Adjusted Effects Per Quintile for NMR Lipoprotein Size and Total Concentration on Incident Hypertension. All odds ratios are from a single model including NMR measures and non-lipid risk factors. Statistically significant p-values were noted for total LDL particles (<0.001), total HDL particles (<0.001), average VLDL particle size (<0.001) and total VLDL particles (0.033).
Adding lipoproteins to traditional lipids
Addition of the nine particle subclasses improved discrimination over a base model with non-lipid factors and traditional lipids, increasing the c-statistic from 0.671 to 0.676 (p < 0.001). In a separate analysis, adding the total particle concentration and average particle size for each lipoprotein type over a base model with non-lipid risk factors and traditional lipids also improved the c-statistic to 0.677 (p = 0.001 for comparison to the base model). Addition of apoA-1 and apoB to the base model did not statistically significantly improve the c-statistic (0.673, p=0.7), although both apolipoproteins remained independently associated with incident hypertension.
Subgroup Analyses
Results were similar to the main study results in each of the pre-specified subgroup analysis (BMI categories, low baseline blood pressure, presence of metabolic syndrome, and non-users of cholesterol lowering medications). Additionally there was no evidence of interactions with any subgroup. The BMI subgroup results are shown in Supplemental Table 1. Our results also remained similar after additional adjustments for baseline blood pressure.
DISCUSSION
This study involving 17,527 initially healthy women followed prospectively for 8 years is the first to document that the pattern of lipoprotein subclass abnormalities that predicted incident hypertension are the same pattern that has been previously found to be characteristic of insulin resistance and diabetes (i.e. higher concentrations of small LDL, small HDL, and large VLDL particles).(11, 15) Additionally, we found that the total concentration of LDL, HDL and VLDL particles was significantly associated with increased risk and provided additional information to the traditional lipid panel and other risk factors for hypertension.
Postulated mechanisms for the relationship between lipoprotein patterns and incident hypertension include the common pathways of insulin resistance, inflammation and endothelial dysfunction by increasing the endothelial oxidative burden.(7–10, 26) We explored additional adjustment for inflammation/endothelial markers including Creactive protein, fibrinogen, homocysteine, and soluble intercellular adhesion molecule-1, since some of these biomarkers have been related to incident hypertension.(27) Further adjustment for inflammation/endothelial markers did not affect the associations of lipoprotein size and concentrations with hypertension. Similarly, adjustment for hemoglobin A1c did not alter the results. These findings suggest that the mechanisms of increased hypertension risk associated with lipoprotein abnormalities are unlikely to be mediated by these biomarkers of inflammation/endothelial function or dysglycemia.
Traditional lipid measures have been shown to be associated with increased risk of hypertension in this and other cohorts.(3–6) In particular, lower HDL cholesterol and higher triglycerides (and in some studies, LDL cholesterol) have been associated with increased risk. Triglycerides and apoB were also shown to be positively associated with an increased risk of hypertension in middle-aged Finnish men.(6) Our study confirms these findings for HDL cholesterol, apoB, and triglycerides in this middle-aged and older cohort of women, although we did not find LDL cholesterol to be independently related to incident hypertension.
Previous studies in both this cohort (12, 15) and others (13, 14) have linked NMR lipoprotein measures to cardiovascular disease and diabetes. Consistent with our results, increased concentration of LDL particles, specifically small particles, has been shown to increase cardiovascular risk as well as risk of incident type 2 diabetes, as has increased concentration of small HDL particles. These patterns are consistent with a shared insulin resistance pathway. We did not have a specific measure for insulin resistance in our study, although adjustment for BMI, triglycerides, hemoglobin A1c and inflammatory biomarkers did not substantially change the results.
The study benefits from a large sample size with well-characterized subjects and a long follow-up (8 years). However, since our study is limited to women, the generalizability of our results to men remains unclear, though traditional lipids have been found to associate with hypertension in both groups. Additionally, our measure of hypertension was self-reported. While this may introduce variability into the outcome measure, we believe this variability is unlikely to be related to lipoprotein measures and is therefore unlikely to affect the direction of our results. The large sample size of the study is also helpful in providing sufficient power to observe associations despite measurement variability. Self-reported hypertension has been shown to be a valid and reliable measure in this(28), as well as other cohorts of health professionals.(29)
In summary, we found that among initially healthy women, lipoprotein particle size and subclass concentrations were associated with incident hypertension and provided additive information to traditional lipids and risk factors. Greater risk of hypertension was associated with higher total concentrations of LDL and HDL particles, especially small particles, and higher total concentration of VLDL, especially large particles. Further, our findings suggest that the concentrations and size of lipoprotein particles affect the risk of incident hypertension years before the clinical onset of hypertension, even in women with initially normal blood pressure. Further research is necessary to determine whether treatment based on lipoprotein profiles would reduce incident hypertension. However, the possibility of identification of a subgroup at increased risk for incident hypertension using lipoprotein measures assessed years before the onset of clinical hypertension may be of use for patients and clinicians. This additional information beyond traditional lipid measures may be useful both in understanding the etiology and in complementing the use of traditional risk factors for predicting the risk of incident hypertension.
Supplementary Material
Acknowledgments
Funding/Support
The analysis for this article was supported by research grants to Dr. Mora from the NHLBI (K08 HL094375). WHS is supported by grants HL-43851 and CA-47988 from the NHLBI and NCI, and by the Donald W. Reynolds Foundation, Leducq Foundation, and Doris Duke Charitable Foundation.
Abbreviations
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- OR
odds ratio
- VLDL
very-low-density lipoprotein
- NMR
nuclear magnetic resonance
- WHS
Women’s Health Study
- IDL
intermediate-density lipoprotein
- CVs
coefficients of variation
- apoB
apolipoprotein B100
- apoA-1
apolipoprotein A-1
- BMI
body mass index
- LR
likelihood ratio
Footnotes
Presentations: A preliminary version of these results were presented at the American Heart Association Scientific Sessions in 2008
Disclosures
Dr Otvos is employed by, is a stockholder of, and serves on the board of directors of LipoScience, Inc., a diagnostic laboratory company that performed the lipoprotein subclass analyses described in the manuscript. The other authors have no financial disclosures.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: A report from the american heart association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 3.Sesso HD, Buring JE, Chown MJ, Ridker PM, Gaziano JM. A prospective study of plasma lipid levels and hypertension in women. Archives of Internal Medicine. 2005;165:2420–2427. doi: 10.1001/archinte.165.20.2420. [DOI] [PubMed] [Google Scholar]
- 4.Halperin RO, Sesso HD, Ma J, Buring JE, Stampfer MJ, Michael Gaziano J. Dyslipidemia and the risk of incident hypertension in men. Hypertension. 2006;47:45–50. doi: 10.1161/01.HYP.0000196306.42418.0e. [DOI] [PubMed] [Google Scholar]
- 5.de Simone G, Devereux RB, Chinali M, Roman MJ, Best LG, Welty TK, et al. Risk factors for arterial hypertension in adults with initial optimal blood pressure: The strong heart study. Hypertension. 2006;47:162–167. doi: 10.1161/01.HYP.0000199103.40105.b5. [DOI] [PubMed] [Google Scholar]
- 6.Laaksonen DE, Niskanen L, Nyyssonen K, Lakka TA, Laukkanen JA, Salonen JT. Dyslipidaemia as a predictor of hypertension in middle-aged men. Eur Heart J. 2008 doi: 10.1093/eurheartj/ehn061. ehn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han SH, Quon MJ, Koh KK. Reciprocal relationships between abnormal metabolic parameters and endothelial dysfunction. Curr Opin Lipidol. 2007;18:58–65. doi: 10.1097/MOL.0b013e328012b627. [DOI] [PubMed] [Google Scholar]
- 8.Sacks FM, Campos H. Clinical review 163: Cardiovascular endocrinology: Low-density lipoprotein size and cardiovascular disease: A reappraisal. J Clin Endocrinol Metab. 2003;88:4525–4532. doi: 10.1210/jc.2003-030636. [DOI] [PubMed] [Google Scholar]
- 9.Urbina EM, Srinivasan SR, Kieltyka RL, Tang R, Bond MG, Chen W, Berenson GS. Correlates of carotid artery stiffness in young adults: The bogalusa heart study. Atherosclerosis. 2004;176:157–164. doi: 10.1016/j.atherosclerosis.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA: The Journal of the American Medical Association. 2003;290:2945–2951. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 11.Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52:453–462. doi: 10.2337/diabetes.52.2.453. [DOI] [PubMed] [Google Scholar]
- 12.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931–939. doi: 10.1161/CIRCULATIONAHA.108.816181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ip S, Lichtenstein AH, Chung M, Lau J, Balk EM. Systematic review: Association of low-density lipoprotein subfractions with cardiovascular outcomes. Ann Intern Med. 2009;150:474–484. doi: 10.7326/0003-4819-150-7-200904070-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kathiresan S, Otvos JD, Sullivan LM, Keyes MJ, Schaefer EJ, Wilson PW, et al. Increased small low-density lipoprotein particle number: A prominent feature of the metabolic syndrome in the framingham heart study. Circulation. 2006;113:20–29. doi: 10.1161/CIRCULATIONAHA.105.567107. [DOI] [PubMed] [Google Scholar]
- 15.Mora S, Otvos JD, Rosenson RS, Pradhan A, Buring JE, Ridker PM. Lipoprotein particle size and concentration by nuclear magnetic resonance and incident type 2 diabetes in women. Diabetes. 2010;59:1153–1160. doi: 10.2337/db09-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rexrode KM, Lee I, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the women's health study. Journal of Women's Health & Gender-Based Medicine. 2000;9:19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. The New England Journal of Medicine. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 18.Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin Lab. 2002;48:171–180. [PubMed] [Google Scholar]
- 19.Otvos JD, Jeyarajah EJ, Cromwell WC. Measurement issues related to lipoprotein heterogeneity. Am J Cardiol. 2002;90:22i–29i. doi: 10.1016/s0002-9149(02)02632-2. [DOI] [PubMed] [Google Scholar]
- 20.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Sesso HD, Wang L, Bowman TS, Buring JE, Gaziano JM. The accuracy of self-reported hypertension in middle-aged and older women and men [abstract] Circulation. 2007;115:e254. [Google Scholar]
- 22.Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006;295:1412–1419. doi: 10.1001/jama.295.12.1412. [DOI] [PubMed] [Google Scholar]
- 23.Rosner B, Glynn RJ. Power and sample size estimation for the wilcoxon rank sum test with application to comparisons of c statistics from alternative prediction models. Biometrics. 2009;65:188–197. doi: 10.1111/j.1541-0420.2008.01062.x. [DOI] [PubMed] [Google Scholar]
- 24.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: Executive summary. Expert panel on the identification, evaluation, and treatment of overweight in adults. Am J Clin Nutr. 1998;68:899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 25.Conen D, Rexrode KM, Creager MA, Ridker PM, Pradhan AD. Metabolic syndrome, inflammation, and risk of symptomatic peripheral artery disease in women: A prospective study. Circulation. 2009;120:1041–1047. doi: 10.1161/CIRCULATIONAHA.109.863092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sattar N, Petrie JR, Jaap AJ. The atherogenic lipoprotein phenotype and vascular endothelial dysfunction. Atherosclerosis. 1998;138:229–235. doi: 10.1016/s0021-9150(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 27.Wang TJ, Gona P, Larson MG, Levy D, Benjamin EJ, Tofler GH, et al. Multiple biomarkers and the risk of incident hypertension. Hypertension. 2007;49:432–438. doi: 10.1161/01.HYP.0000256956.61872.aa. [DOI] [PubMed] [Google Scholar]
- 28.Conen D, Ridker PM, Buring JE, Glynn RJ. Risk of cardiovascular events among women with high normal blood pressure or blood pressure progression: Prospective cohort study. Bmj. 2007;335:432. doi: 10.1136/bmj.39269.672188.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.