Abstract
Hepatitis C virus (HCV) infections are treated with interferon α plus ribavirin, but it is unknown how ribavirin works against HCV. Ribavirin is a guanosine analog that can be a substrate for the viral RNA polymerase. HCV is genetically variable, and this genetic variation could affect the polymerase’s use of ribavirin triphosphate. Thirteen patients infected with HCV who failed interferon α monotherapy and were retreated with interferon α plus ribavirin were identified; seven were responders and six were nonresponders to combination therapy. The consensus sequences encoding the 13 polymerases plus seven sequences from treatment-naive controls were determined. The responder sequences were more genetically variable than the nonresponders and controls, the amino acid variations unique to responders had lower BLOSUM90 scores than variations in responders and controls, and the amino acid variations correlated with response to therapy clustered around the RNA-binding channel of the polymerase. These data imply that that the responder enzymes were probably more functionally variable than the nonresponder enzymes. Enzymatic activity was measured for ten recombinant polymerases; RNA synthesis activity varied by over 7-fold and polymerases from two of the responders used GTP much better than UTP, but technical limitations prevented direct measurement of ribavirin triphosphate use. Because response to combination therapy in these patients was primarily due to addition of ribavirin to the treatment regimen, these data imply that genetic variation in the polymerase may have affected the efficiency of ribavirin incorporation into the viral genome and hence may have modulated ribavirin’s efficacy against HCV.
Keywords: Hepatitis C virus, ribavirin, RNA polymerase, genetic variation
Hepatitis C virus (HCV) causes chronic hepatitis, cirrhosis, liver failure, and hepatocellular carcinoma. HCV infections are treated with pegylated interferon α plus ribavirin (1–4). Ribavirin is ineffective alone, but it roughly doubles the effectiveness of interferon (3;5). Ribavirin is a guanosine analogue with multiple effects on cells, including altering the TH1/TH2 profile of the immune response and depleting GTP pools (6–8). Ribavirin is phosphorylated to ribavirin triphosphate and is a substrate for many viral RNA-directed RNA polymerases (RdRps) (9;10). Incorporation of ribavirin into viral genomes may have direct antiviral effects because it is a mutagen that base-pairs with both uracil and cytosine (9), and in poliovirus and GB Virus B, ribavirin can raise the mutation rate and induce error catastrophe (11;12). Alternatively, ribavirin may act as a template poison by inhibiting extension of RNA when it is in the template strand (9). While these effects have been shown in cultured cells (6), the antiviral mechanism(s) of ribavirin effective against HCV in humans are not known (7).
The HCV genome is highly variable, with six genotypes that are less than 72% identical at the nucleotide level (13–16). Within the genotypes, subtypes with nucleotide identities of 75–86% may occur. Individual isolates of a subtype typically differ by ~8–10%, and as HCV replicates as quasispecies, multiple variants differing by up to a few percent exist even within individual patients.
HCV’s genetic variation results from errors by the viral RdRp encoded in the NS5B region of the genome. The RdRp crystal structure has been solved (17–19), and like all nucleic acid polymerases can be represented as a right hand with fingers, palm, and thumb domains (18). Unlike other RdRps, the HCV RdRp has a connection from the fingers to the thumb that forms a loop around the active site (17;18).
Genetic variation within the NS5B gene would be predicted to alter the enzyme’s activity, potentially including its ability to employ ribavirin triphosphate as a substrate. Therefore, we hypothesized that ribavirin acts against HCV at least in part through the HCV RdRp, and that genetic variation in the RdRp modulates ribavirin’s effectiveness. To test this hypothesis, we analyzed HCV NS5B sequences and RdRp activity from 13 HCV infected people who were nonresponders to interferon α monotherapy and were subsequently retreated with interferon α plus ribavirin. These patients were chosen because response to the second round of therapy would be primarily due to the addition of ribavirin, and examining this cohort would maximize the ability to detect viral variation associated with sensitivity or resistance to ribavirin.
Materials and Methods
Patient selection
Thirteen HCV genotype 1b infected patients who failed interferon α monotherapy and were re-treated with ribavirin plus interferon α in a clinical trial (20) were studied. Seven were sustained viral responders following combination therapy, and six were nonresponders. To be eligible for re-treatment, patients must have received interferon α at a dose equivalent to 3 million units three times per week for ≥ 12 weeks. The patients received interferon α-2b (3 million units three times weekly by subcutaneous injection) and ribavirin (1000 to 1200 mg per day orally based on body weight). Patients were randomized to 24 or 48 weeks of therapy. Serum samples were obtained prior to combination therapy and from 3 to 6 months after therapy. Seven untreated, treatment-naïve HCV genotype 1b patients were examined as controls (21). All patients provided written consent for the clinical trials and for their samples to be used in subsequent studies. This work was conducted in accord with the Helsinki declarations and was approved by the Saint Louis University Institutional Review Board.
Nested RT-PCR and DNA sequencing
Viral RNA was isolated from serum with the QIAamp Viral RNA Mini Kit (Qiagen, Valencia, CA). cDNA was synthesized using random hexamer primers and Enhanced Avian Reverse Transcriptase. The NS5B gene was amplified in two overlapping segments with Taq polymerase employing genotype 1b primers (22). The NS5B consensus sequence was determined by directly sequencing both strands of the nested RT-PCR products with the DTCS CEQ Quick Start system (Beckman-Coulter, Fullerton, CA). The Genbank accession numbers for these sequences are FJ380064-FJ380089.
Derivation of the NS5B genotype 1b reference sequence
A consensus sequence was generated from 107 NS5B genotype 1b sequences in the Los Alamos HCV sequence database (http://hcv.lanl.gov). This represents an “average” NS5B gene and was used as the reference sequence in all genetic analyses.
Genetic analyses
Sequences from each treatment group were aligned to the reference HCV sequence using ClustalW and the alignments were analyzed with MutationMaster (23) employing the BLOSUM90 matrix. Multiple variations at the same position unique to a treatment group were treated as separate events. The numbers of variations between groups and the sums of the BLOSUM90 scores of variations in each sample were compared between treatment groups using the Mann-Whitney test. Variations unique to the responders or nonresponders were mapped onto the HCV RdRp crystal structure [PDB ID 1C2P (18)] using PyMOL.
RdRp expression and purification
NS5B sequences lacking the carboxy-terminal 21 codons were cloned into pTrcHisB (Invitrogen, Carlsbad, CA) and the plasmids were sequenced to confirm they represented the patient sequences. RdRps were expressed in E. coli Bl21 codon+ cells (Invitrogen) and purified by nickel-agarose affinity as described (24).
Homopolymeric-templated RNA synthesis
Two micrograms of RdRp was incubated in 5 mM MgCl2, 25 mM Tris pH 7.5, 3 µCi [α32P]GTP (10 µCi mMol−1), 1 unit µl−1 RNasein, 50 ng µl−1 poly C, 5 ng µl−1 G10 in 20 µl at 30°C for 90 minutes. RNAs were collected on nitrocellulose filters, the filters were washed five times with 2X SSC, and retained radioactivity was detected by scintillation counting. The procedure was repeated with poly A, [α32P]UTP, and U15 replacing poly C, [α32P]GTP, and G10, respectively.
RNA binding
Two micrograms of RdRp was incubated with 5 mM MgCl2, 25 mM Tris pH 7.5 and 2 pM radiolabeled poly C. RNA binding was performed as described (25). Briefly, the RdRps were incubated with radiolabeled RNAs for 30 minutes on ice and were then passed through polysulfone (Pall, East Hills, NY), Hybond-ECL nylon-backed nitrocellulose (GE Healthcare), and Hybond-N (GE Healthcare) membranes using a slot-blot apparatus. The membranes were dried and retained radioactivity was quantified with a Storm phosphorimager (GE Healthcare).
Results
Determination of the patient-derived NS5B sequences
HCV sequences can be represented by either the dominant quasispecies or the consensus sequence. The dominant quasispecies is the most common individual sequence in a patient and the consensus sequence is composed of the most common nucleotide at each position in the viral population. Because the consensus sequence represents the most-fit sequence as averaged over the viral population, we determined the consensus HCV NS5B sequences from 13 HCV infected people who were nonresponders to interferon α monotherapy and were subsequently retreated with interferon α, plus ribavirin plus seven untreated controls, by directly sequencing RT-PCR amplicons. These sequences are in Fig. 1.
Figure 1.
The RdRp sequences. The amino acid sequences for the responder pre-treatment (Rpre), nonresponder pre-treatment (NRpre), nonresponder post-treatment (NRpost), and control (C) sequences were aligned against a genotype 1b population-wide consensus sequence derived from 107 NS5B sequences available in Genbank.
Lack of conserved differences between the response groups
To assess genetic variability between isolates that may correlate with response to therapy, the deduced amino acid sequences for all isolates were aligned. No individual amino acid variations correlated highly with response to therapy, indicating that response to therapy was not a function of a simple genetic variation in NS5B. Phylogenetic analysis revealed no clustering of the samples by response groups (data not shown), indicating that variation between the samples at the whole gene level did not correlate with response to combination therapy. These results were anticipated due to the high degree of neutral variation between primary HCV isolates.
Diversity differences correlating with response to therapy
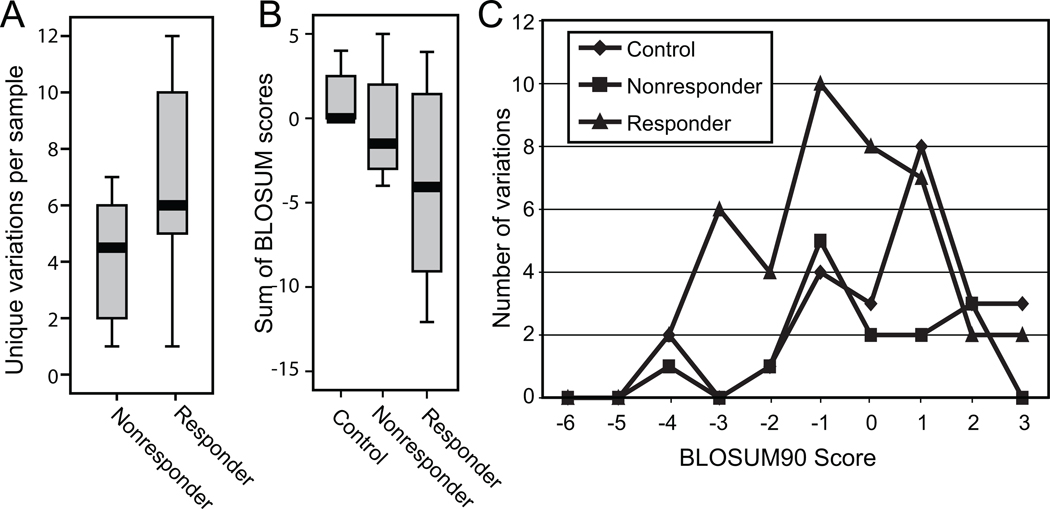
Because there were no conserved sequences that correlated with response to combination therapy, we asked if there were differences in the degree of inter-patient genetic variability between the pre-therapy nonresponder and responder sequences that may correlate with response to therapy. The responder and nonresponder sequences were aligned against the reference sequence and amino acid differences relative to the reference sequence were identified. To reduce neutral genetic variation that could obscure biologically relevant differences, we then eliminated all variations that were found in both the responders and nonresponders, leaving only variations that were unique to the two groups. We also identified a biologically irrelevant set of variations by aligning the sequences from the untreated control patients with the reference sequence, and then identifying variations that were unique to the control sequences relative to the nonresponders. Variations unique to the control samples were assumed to be neutral because these samples were not selected based on response to therapy. Comparisons of the number of variations between the two groups revealed that responders had more unique variations than nonresponders and control samples (Fig. 2A). This difference was not statistically significant, possibly due to the low number of samples. However, the proportion of unique to non-unique variations in the responder was significantly higher than in the nonresponders (p = 0.025 by the Fisher’s exact test; Table 1), whereas there was no significant difference in the proportion of unique variations between the controls and the nonresponders.
Figure 2.
Genetic variability among the NS5B genes from the responders is higher than in the nonresponders. A. Number of amino acid variations unique to the response groups. Variations relative to a population-wide genotype 1b NS5B reference sequences were determined, variations found in both the responder and nonresponder groups or the control and nonresponder groups were eliminated to reduce genetic noise, and the remaining variations unique to each response group were compared. B. Sum of BLOSUM90 scores. The BLOSUM90 scores for the unique variations in panel A were summed for each sequence, and the sums were compared between the response groups. C. Distribution of BLOSUM90 scores. The frequency of the individual BLOSUM90 scores in each response class was plotted.
Table 1.
Proportion of variations unique the nonresponder sequences relative to the responders and controls
| Response | Unique variations |
Non-unique variations |
P value1 |
|
|---|---|---|---|---|
| Nonresponder | Observed | 25 | 44 | 0.025 |
| Expected | 32.1 | 36.9 | ||
| Responder | Observed | 49 | 41 | |
| Expected | 41.9 | 48.1 | ||
| Nonresponder | Observed | 18 | 51 | 0.089 |
| Expected | 23.3 | 45.7 | ||
| Control | Observed | 35 | 53 | |
| Expected | 29.7 | 58.3 |
Fisher’s exact test
To determine if there were possible differences in the chemical nature of the amino acid residues unique to the control, nonresponder, or responder groups, the BLOSUM90 scores of unique variations in each response group were compared (Fig. 2B). BLOSUM90 scores are log-odds ratios of substitution frequencies among related proteins, with higher scores representing conservative substitutions and lower scores being nonconservative. The sums of the scores in the responders were lower than in the nonresponders and controls, but this difference was not statistically significant, again possibly due to the low sample number. The nonresponder scores were also lower than the control scores; this was expected since the variations unique to the controls were expected to be random.
The difference in the BLOSUM sums could be affected by the larger number of variations in the responders relative to the nonresponders or controls. Therefore, we plotted the frequency of each score in the controls, nonresponders, and responders (Fig. 2C). The distribution was similar for the variations unique to the nonresponder and control sequences, with most of the variations having conservative scores of zero or greater. However, the distribution for responders was shifted to the left, with more non-conservative scores ranging from -3 to -1. Therefore, variations in the responders were more likely to alter protein activity.
Genetic variation during therapy
Variation in the ability of the RdRp to employ ribavirin could either be present prior to therapy, or it could evolve in response to the strong selective pressures induced by therapy. Therefore, we identified mutations in the paired pre- and post-therapy samples from each nonresponder that accumulated in NS5B during treatment. The NR1, NR4, NR5, and NR8 RdRps had zero or one amino acid mutations between the paired samples in the 591 amino acid protein. However, NR7 had 6 changes and NR3 had 19. Therefore, NS5B in patient NR3, and perhaps NR7, may have adapted to the genetic pressures induced by therapy, whereas the other NS5B sequences remained relatively stable during treatment.
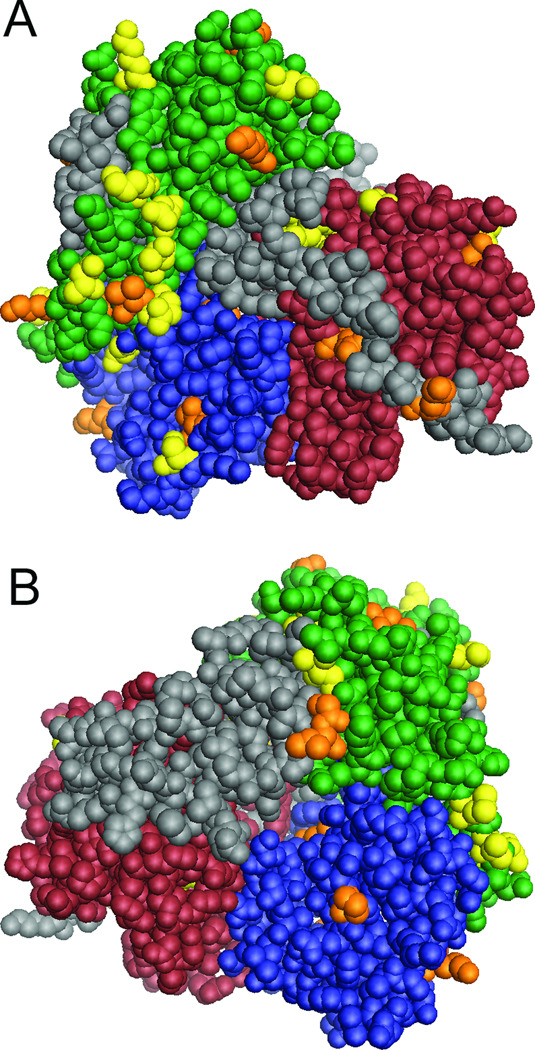
Asymmetric distribution of variability
We next mapped positions of variability associated with response to therapy onto the three-dimensional structure of the RdRp to reveal correlations that may be obvious on the folded protein, but unapparent in the linear structure of the NS5B gene. We employed the responder pre-treatment and nonresponder post-treatment sequences because to the extent that viral variation affects response to therapy, the responder pre-treatment sequences were sensitive to therapy and the sequences in the nonresponders after treatment were resistant. Variations that were unique to either group were mapped onto the RdRp structure (Fig. 3) The variations clustered asymmetrically on one face of the enzyme surrounding the RNA binding channel. Most notably, 12 amino acid positions with variations unique to the pre-treatment responder sequences were in the fingers domain and three were in the β-loop which extends into the RNA binding channel (17;18;26). This asymmetric distribution of amino acid variations associated with response to therapy suggests that they may affect a common aspect of RdRp function, such as nucleotide selectivity. If this applies to ribavirin use, the variations in the responders would be predicted to increase ribavirin use, and the variations unique to the nonresponders to reduce its use.
Figure 3.
Asymmetric clustering of amino acid differences unique to the responders and nonresponders. The positions of variations unique to the pretherapy responder and post-therapy nonresponder samples were mapped onto the RdRp structure. The domains of the enzyme are indicated: green, fingers; blue, palm; red, thumb; grey, other. Positions unique to the responders are yellow and positions unique to the nonresponders are orange. A. Front view. B. Back view.
RNA synthesis
To determine how variability in the RdRp affected its function, pre-therapy sequences from six responders, six nonresponders, and two control samples were randomly selected for biochemical analysis. The NS5B genes were cloned into the bacterial expression vector pTrcHis. To increase RdRp solubility, the C-terminal 21 amino acids were deleted; this has no effect on RdRp activity in vitro (24). To facilitate purification, a hexahistidine tag was added to the C-terminus of the protein. The genotype 1b strain J4 RdRp from the CG1b infectious clone (27) was used as a reference in all assays.
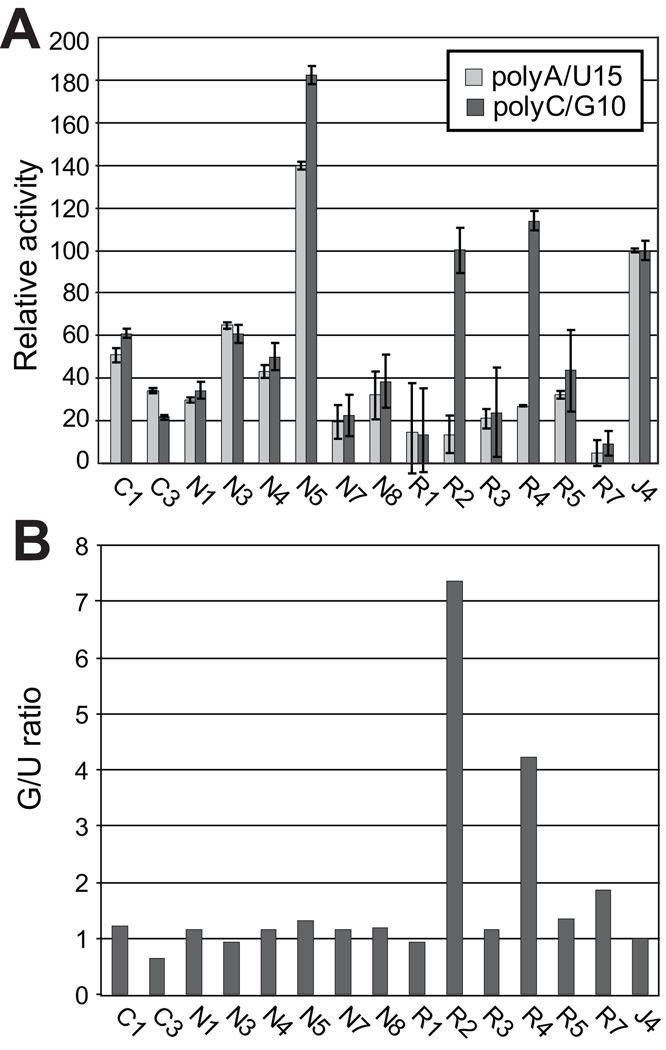
To quantitatively assess the ability of the RdRp to incorporate specific nucleotides, polymerization activities were measured using primed poly-C or poly-A templates and [α32P]GTP or [α32P]UTP, respectively. RdRps were incubated with the primer/templates and radiolabeled nucleotides for 90 minutes at 30°C, RNAs were collected on nitrocellulose filters, the filters were washed, radioactivity incorporated into newly synthesized RNA was measured by scintillation counting, and the activity was normalized to the activity of the J4 RdRp (Fig. 4A). As expected from the genetic variability of the isolates, there was a range of activities of the RdRps, from less than 25% to over 175% relative to the J4 enzyme.
Figure 4.
Enzymatic activity varies among the patient-derived RdRps. A. Activity of the RdRp on homopolymeric templates. The activities of the variant RdRps on primed poly C or poly A templates were measured and normalized relative to the activity of the genotype 1b J4 isolate. Error bars represent the standard deviation from three to five assays. C#, control samples; N#, nonresponder pretherapy samples; R#, responder pretherapy samples. B. The G/U ratios for the variant RdRps. All ratios are normalized to the J4 sample.
We next compared incorporation of GTP to that of UTP for each RdRp to determine if there were isolate-specific differences in NTP use (Fig. 4B). The J4 reference enzyme and 12 of the 14 patient-derived samples had GTP to UTP incorporation ratios (G/U ratio) close to 1.0, indicating there was little variation in their relative ability to use UTP and GTP. However, RdRps from responders R2 and R4 had 4- and 7-fold higher G/U ratios. The elevated G/U ratio in two of the six responder RdRps implies that ribavirin, a GTP analogue, may have had a greater impact on HCV in these patients than on the other isolates.
We attempted to directly measure ribavirin use by these variant RdRps in both homopolymeric RNA synthesis assays with labeled ribavirin triphosphate and by measuring extension of labeled RNA primers in the presence of unlabeled ribavirin triphosphate, but we were unsuccessful. This failure was presumably due to the very low efficiency of ribavirin use by the HCV RdRP, detection of which often requires removal of the β-loop that extends into the active site and limits the use of primer-templates by the RdRp (9).
Effect of NTP concentration on RNA synthesis
The high G/U ratios of the R2 and R4 RdRps could have been due to elevated GTP use and/or reduced UTP use. Therefore, the GTP and UTP use profiles of the R2, R4, N4 (the low ratio RdRp most similar to R2 and R4), and J4 (the reference enzyme which had a low G/U ratio) RdRps were assayed with nucleotide concentrations ranging from 6 nM to 185 nM. There was a linear increase in poly-U synthesis with increasing UTP concentrations for all samples, and the two low G/U ratio samples (J4 and N4) synthesized poly-U better than the two high G/U ratio samples (Fig. 5A). However, the high G/U ratio samples employed GTP more efficiently than the low G/U ratio samples, and there was a distinct difference in the shapes of the curves for the high and low G/U ratio samples under these conditions (Fig. 5B). The high ratio samples had nearly linear increases in GTP use over the concentration range tested (r2 > 0.95). In contrast, RNA synthesis by the low G/U ratio samples plateaued around 75–100 nM GTP.
Figure 5.
Differences in the G/U ratio are primarily due to alterations in GTP use. A. Dependence of poly U synthesis on UTP concentration. J4 and N4 are low-G/U isolates and R2 and R4 are high G/U ratio samples. B. Dependence of poly G synthesis on GTP concentration. C. Poly C binding by the variant RdRps. Binding to radiolabeled poly C was measured by a filter-binding assay and the results are expressed as the proportion of RNA retained on the filter. The error bars in all panels represent the standard deviation from three to five assays.
RNA binding
The differences in GTP use could either be due to variations in use of GTP (catalytic rate or GTP affinity) or to differences in the ability of the variant RdRps to bind the poly C template. Therefore, we used a filter-binding assay to ask if there were differences in binding of the RdRps to poly C RNA. The proteins were incubated with radiolabeled poly-C RNA under the buffer conditions that were employed in the polymerizations reactions, but the RNA concentration had to be reduced to 2 pM due to saturation of the binding. RNA binding varied roughly three-fold among the RdRps, but these variations did not correlate with response to therapy, activity of the polymerase, or elevated G/U ratios (Fig. 5C). Therefore, differences in template binding could not account for the elevated G/U ratios in the R2 and R4 RdRps.
Discussion
Ribavirin is a guanosine analog that is a substrate for viral RdRps when converted in cells to ribavirin triphosphate (9;10). Incorporation of ribavirin into the HCV genome could inhibit viral replication, either as a mutagen and/or as a template poison. We hypothesized that natural variation in the HCV NS5B gene would yield RdRps with variable ability to incorporate ribavirin into the HCV genome, and that this variation would affect ribavirin’s antiviral efficacy. To test this hypothesis, we analyzed genetic and biochemical variability of the NS5B RdRp from 13 patients who failed to respond to interferon α monotherapy and were then re-treated with interferon α plus ribavirin. Response to the second round of therapy in these patients was primarily due to the addition of ribavirin, and we reasoned that this would maximize our ability to detect viral variation associated with effectiveness of ribavirin.
HCV’s high genetic variation can mask key differences associated with response to therapy. Therefore, we focused on variations that were unique to the response classes. Importantly, variations unique to both the pre-therapy responder and nonresponder samples clustered asymmetrically on one face of the enzyme surrounding the RNA binding channel (Fig. 3), especially in the fingers domain. Residues in this region of nucleic acid polymerases are not directly involved in catalysis, but can affect nucleotide positioning and catalytic rate (28;29). Mutations causing resistance to nucleoside inhibitors by altering selectivity can be found in the analogous region of the human Immunodeficiency virus (HIV) reverse transcriptase (30–32). Therefore, the genetic differences between the responders and nonresponders could have altered the RNA replication rate and/or altered their nucleotide use profile. However, because these variations were associated with success or failure of therapy that was primarily due to addition of ribavirin, we feel that their effect was most likely to modulate use of ribavirin triphosphate. Variations unique to the responders would be predicted to increase incorporation of ribavirin, and those unique to nonresponders would be predicted to reduce its incorporation.
Unfortunately, the low efficiency with which the HCV RdRp employs ribavirin triphosphate as a substrate prevented us from directly testing this hypothesis, despite extensive attempts. However, indirect support for this hypothesis also comes from three other observations. First, there were more unique variations in the responder than the control or nonresponder RdRps (Fig. 2A and Table 1). This difference is consistent with the existence of relatively resistant sequence that discriminates against ribavirin, and that many independent variations within the RdRp can weaken this discrimination. In this context, the nonresponder patients would have been infected with variants more closely related to a master “resistant” sequence. Second, if variation unique to the response classes were genetic noise, the variations would have high BLOSUM90 scores similar to the scores of the variations unique to the control RdRps. However, the responders had lower BLOSUM90 scores than the nonresponders or controls (Figs. 2B and 2C). This indicates that variations in the responders were more likely to alter the activity of the RdRp than variations in the nonresponders or controls. Third, RdRps from patients R2 and R4 had high G/U ratios due to a better ability to use GTP as a substrate, while none of the nonresponder or control samples had high ratios (Figs. 4 and 5). Ribavirin is a guanosine analogue, so the increased ability of these RdRps to incorporate GTP implies that ribavirin incorporation may also have been elevated.
Resistance to ribavirin’s contribution to combination therapy could either be present prior to treatment or it could evolve during therapy. Therefore, we examined genetic mutations in the nonresponders over the course of combination treatment. Amino acid mutations between paired pre- and post-treatment samples were found primarily in two samples, NR3 (19 mutations) and NR7 (6 mutations). The other nonresponders had zero or one mutation each. The clustering of changes in two nonresponders indicates the dominant pretreatment sequence in these patients was suppressed and a variant that was more fit under the treatment regimen became dominant. Therefore, NR3 and NR7 showed genetic variation in NS5B consistent with emergence of resistance during therapy. The remaining four nonresponders were genetically stable, consistent with either a lack of selective pressure on NS5B or the presence of pre-existing resistant sequences. The relative stability of most of the sequences is consistent with our recent analysis of the full HCV coding region during failed therapy, in which the amino acid substitution rate outside of the hypervariable regions in E2 was 0.6% (33).
Resistance to ribavirin has been associated with alteration of RdRp position 415 from phenylalanine to tyrosine (34). The population-wide NS5B consensus sequence used as a reference in these studies had tyrosine at position 415. Tyrosine was found at this position in all of our samples except R4, which had a methionine. Hence, we found no evidence that residue 415 contributed to success or failure of combination therapy. Similar negative results were recently reported by Ward et al. (35). These discordant results may be due to differences in study design: we examined genotype 1b patients undergoing combination therapy, whereas the association of tyrosine 415 with resistance to ribavirin was observed in genotype 1a patients undergoing ribavirin monotherapy.
Ribavirin has very small (< 1 log10) effect on viral titers when used as a monotherapy (8) and it appears to contribute significantly to the decline of viral titers only when interferon effectiveness is low (8;36). At least two non-exclusive mechanisms could account for its ability to increase the effectiveness of interferon-based therapy. First, ribavirin is a mutagen and at very low titers, a small modulation of the viral mutation rate could push the viral population to extinction because the maximum mutation rate that can be tolerated increases with increasing population size (37). Such a synergistic effect of a mutagen plus a replication inhibitor has been observed to drive the HIV and Foot and Mouth Disease Virus (FMDV) to extinction (38;39). An increase of only 2- to 4-fold in the mutation rates is sufficient to induce error catastrophe in FMDV (40), and mutagenized FMDV viral genomes can reduce replication of unmutated genomes (40). Therefore, the elevation of the mutation rate due to ribavirin treatment needed to help eliminate HCV in the presence of interferon α could be consistent with the very low incorporation rate of ribavirin by the HCV RdRp (41). This could explain why genetic studies of HCV during ribavirin treatment have rarely detected higher variation in treated samples (34;42–45). The second mechanism by which incorporation of ribavirin into the HCV genome may contribute to elimination of the virus after interferon has greatly suppressed the viral titres is by acting as a template poison. In vitro studies have shown that the HCV RdRp extends RNA past a ribavirin residue in the template very inefficiently (9;46). This indirect inhibition of the RdRp during replication may be sufficient to nudge the virus into extinction after interferon has greatly suppressed the viral titres.
In summary, we observed genetic patterns in the HCV RdRp consistent with differential use of ribavirin triphosphate by responders and nonresponders to interferon α plus ribavirin therapy. Our results do not address the possibility that ribavirin may also act through other mechanisms, such as altering the Th1/Th2 profile or depleting GTP levels. These data are correlative, but they provide provocative evidence that ribavirin exerts some of its effects against HCV in humans through the RdRp.
Acknowledgements
We thank Weidong Zhong for helpful comments and I-hung Shih for technical assistance.
Declaration of funding interests
This study was funded by grants DK60345, DK5643501, and CA125321 from the NIH and by a grant from the Friends of the Saint Louis University Liver Center.
Abbreviations
- HCV
Hepatitis C virus
- RdRp
RNA-dependent RNA polymerase
- NS5B
Nonstructural gene 5B
- G/U ratio
GTP to UTP incorporation ratios
- FMDV
Foot and Mouth Disease Virus
Footnotes
Statement of personal interests
None of the authors have personal interests relevant to this research to declare.
References
- 1.Fried MW, Shiffman M, Reddy KR, Smith C, Marinos G, Goncales F, Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002 Sep 26;347(13):975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 2.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001 Sep 22;358(9286):958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 3.McHutchison JG, Gordon SC, Schiff ER, Shiffman M, Lee WM, Rustgi VK, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. New England Journal of Medicine. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 4.Teo M, Hayes P. Management of hepatitis C. Br Med Bul. 2004;70:51–69. doi: 10.1093/bmb/ldh022. [DOI] [PubMed] [Google Scholar]
- 5.Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT) Lancet. 1998 Oct 31;352(9138):1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 6.Graci JD, Cameron CE. Quasispecies, error catastrophe, and the antiviral activity of ribavirin. Virology. 2002 Jul 5;298(2):175–180. doi: 10.1006/viro.2002.1487. [DOI] [PubMed] [Google Scholar]
- 7.Lau JYN, Tam RC, Liang TJ, Hong Z. Mechanism of action of ribavirin in the RNA combination treatment of chronic HCV infection. Perspectives in Clinical Hepatology. 2002 May;35(5):1002–1009. doi: 10.1053/jhep.2002.32672. [DOI] [PubMed] [Google Scholar]
- 8.Pawlotsky JM, Dahari H, Neumann AU, Hezode C, Germanidis G, Lonjon I, et al. Antiviral action of ribavirin in chronic hepatitis C. Gastroenterology. 2004 Mar;126(3):703–714. doi: 10.1053/j.gastro.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Maag D, Castro C, Hong Z, Cameron CE. Hepatitis C virus RNA-dependent RNA polymerase (NS5B) as a mediator of the antiviral effects of ribavirin. Journal of Chemical Biology. 2001;276(49):46094–46098. doi: 10.1074/jbc.C100349200. [DOI] [PubMed] [Google Scholar]
- 10.Miller JP, Kigwana LJ, Streeter DG, Robins RK, Simon LN, Roboz J. The relationship between the metabolism of ribavirin and its proposed mechanism of action. Ann N Y Acad Sci. 1977 Mar 4;284:211–229. doi: 10.1111/j.1749-6632.1977.tb21953.x. [DOI] [PubMed] [Google Scholar]
- 11.Crotty S, Cameron CE, Andino R. RNA virus error catastrophe: direct molecular test by using ribavirin. Proc Natl Acad Sci U S A. 2001 Jun 5;98(12):6895–6900. doi: 10.1073/pnas.111085598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanford RE, Chavez D, Guerra B, Lau JYN, Hong Z, Brasky KM, et al. Ribavirin induces error-prone replication of GB virus B in primary tamarin hepatocytes. Journal of Virology. 2001;75(17):8074–8081. doi: 10.1128/JVI.75.17.8074-8081.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simmonds P, Holmes EC, Cha TA, Chan SW, McOmish F, Irvine B, et al. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. Journal of General Virology. 1993;74:2391–2399. doi: 10.1099/0022-1317-74-11-2391. [DOI] [PubMed] [Google Scholar]
- 14.Bukh J, Miller R, Purcel R. Genetic heterogeneity of hepatitis c virus: quasispecies and genotypes. Seminars in Liver Disease. 1995;15:41–63. doi: 10.1055/s-2007-1007262. [DOI] [PubMed] [Google Scholar]
- 15.Robertson B, Myers G, Howard C, Brettin T, Bukh J, Gaschen B, et al. Classification, nomenclature, and database development for hepatitis C virus (HCV) and related viruses: proposals for standardization. Archives of Virology. 1998;143:2493–2503. doi: 10.1007/s007050050479. [DOI] [PubMed] [Google Scholar]
- 16.Simmonds P. Genetic diversity and evolution of hepatitis C virus--15 years on. J Gen Viro. 2004 Nov;85(Pt 11):3173–3188. doi: 10.1099/vir.0.80401-0. [DOI] [PubMed] [Google Scholar]
- 17.Bressanelli S, Tomei L, Rousse A, Incitti I, Vitale R, Mathieu M, et al. Crystal structure of the RNA-dependent RNA polymerase of Hepatitis C virus. Proceedings of the National Academy of Sciences of the United States of America. 1999 Nov 9;96(23):13034–13039. doi: 10.1073/pnas.96.23.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lesburg CA, Cable MB, Ferrari E, Hong Z, Mannarino AF, Weber PC. Crystal structure of the RNA dependent RNA polymerase from hepatitis C virus reveals a fully encircled active site. Nature Structural Biology. 1999;6:937–943. doi: 10.1038/13305. [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Ng KKS, Cherney MM, Chan MM, Yannopoulos CG, Bedard J, et al. Non-Nucleoside Analogue Inhibitors Bind to an Allosteric Site on HCV NS5B Polymerase: Crystal Structures and Mechanism of Inhibition. Journal of Biological Chemistry. 2003;278(11):9489–9495. doi: 10.1074/jbc.M209397200. [DOI] [PubMed] [Google Scholar]
- 20.Di Bisceglie AM, Thompson J, Smith-Wilkaitis N, Brunt EM, Bacon BR. Combination of interferon and ribavirin in chronic hepatitis C: re-treatment of nonresponders to interferon. Hepatology. 2001 Mar;33(3):704–707. doi: 10.1053/jhep.2001.22346. [DOI] [PubMed] [Google Scholar]
- 21.Di Bisceglie AM, Bonkovsky H, Chopra S, Flamm S, Reddy RK, Grace N, et al. Iron reduction as an adjuvant to interferon therapy in patients with chronic hepatitis C who have previously not responded to interferon: a multicenter, prospective, randomized, controlled trial. Hepatology. 2000 Jul;32(1):135–138. doi: 10.1053/jhep.2000.8700. [DOI] [PubMed] [Google Scholar]
- 22.Yao E, Tavis JE. A general method for nested RT-PCR amplification and sequencing the complete HCV genotype 1 open reading frame. Virol J. 2005;2:88. doi: 10.1186/1743-422X-2-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walewski J, Gutierrez JA, Branch-Elliman W, Stump DD, Keller TR, Rodriguez A, et al. Mutation Master: profiles of substitutions in hepatitis C virus RNA of the core, alternate reading frame, and NS2 coding regions. RNA. 2002 May;8(5):557–571. doi: 10.1017/s1355838202029023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrari E, Wright-Minogue J, Fang JWS, Baroudy BM, Lau JYN, Hong Z. Characterization of soluble hepatitis C virus RNA dependent RNA polymerase expressed in Escherichia coli. Journal of Virology. 1999;73:1649–1654. doi: 10.1128/jvi.73.2.1649-1654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang L, Hwang J, Sharma SD, Hargittai MR, Chen Y, Arnold JJ, et al. Hepatitis C virus nonstructural protein 5A (NS5A) is an RNA-binding protein. J Biol Chem. 2005 Oct 28;280(43):36417–36428. doi: 10.1074/jbc.M508175200. [DOI] [PubMed] [Google Scholar]
- 26.Bressanelli S, Tomei L, Rey FA, De Francesco R. Structural analysis of the hepatitis C virus RNA polymerase in complex with Ribonucleotides. Journal of Virology. 2002 Apr;76(7):3482–3492. doi: 10.1128/JVI.76.7.3482-3492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomson M, Nascimbeni M, Gonzales S, Murthy KK, Rehermann B, Liang TJ. Emergence of a distinct pattern of viral mutations in chimpanzees infected with a homogeneous inoculum of hepatitis C virus. Gastroenterology. 2001 Nov;121(5):1226–1233. doi: 10.1053/gast.2001.28669. [DOI] [PubMed] [Google Scholar]
- 28.Jager J, Pata JD. Getting a grip: polymerases and their substrate complexes. Curr Opin Struct Bio. 1999 Feb;9(1):21–28. doi: 10.1016/s0959-440x(99)80004-9. [DOI] [PubMed] [Google Scholar]
- 29.Steitz TA. DNA polymerases: structural diversity and common mechanisms. J Biol Chem. 1999 Jun 18;274(25):17395–17398. doi: 10.1074/jbc.274.25.17395. [DOI] [PubMed] [Google Scholar]
- 30.Boyer P, Sarafianos SG, Arnold E, Hughes SH. Nucleoside analog resistance caused by insertions in the fingers of human immunodeficiency virus type 1 reverse transcriptase involves ATP-mediated excision. J Viro. 2002 Sep;76(18):9143–9151. doi: 10.1128/JVI.76.18.9143-9151.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarafianos SG, Das K, Ding J, Boyer P, Hughes SH, Arnold E. Touching the heart of HIV-1 drug resistance: the fingers close down on the dNTP at the polymerase active site. Chem Bio. 1999 May;6(5):R137–R146. doi: 10.1016/s1074-5521(99)80071-4. [DOI] [PubMed] [Google Scholar]
- 32.Sharma B, Kaushik N, Upadhyay A, Tripathi S, Singh K, Pandey VN. A positively charged side chain at position 154 on the beta8-alphaE loop of HIV-1 RT is required for stable ternary complex formation. Nucleic Acids Res. 2003 Sep 1;31(17):5167–5174. doi: 10.1093/nar/gkg708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cannon NA, Donlin MJ, Fan X, Aurora R, Tavis JE. Hepatitis C virus diversity and evolution in the full open-reading frame during antiviral therapy. PLoS ONE. 2008;3(5):e2123. doi: 10.1371/journal.pone.0002123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young KC, Lindsay K, Lee KJ, Liu WC, He JW, Milstein S, et al. Identification of a ribavirin-resistant NS5B mutation of hepatitis C virus during ribavirin monotherapy. Hepatology. 2003 Oct;38(4):869–878. doi: 10.1053/jhep.2003.50445. [DOI] [PubMed] [Google Scholar]
- 35.Ward C, Dev A, Rigby S, Symonds WT, Pate K, Zekry A, et al. Interferon and ribavirin therapy does not select for resistance mutations in hepatitis C virus polymerase. J Viral Hepat. 2008 Mar 24; doi: 10.1111/j.1365-2893.2008.00980.x. [DOI] [PubMed] [Google Scholar]
- 36.Dixit NM, Layden-Almer JE, Layden TJ, Perelson AS. Modelling how ribavirin improves interferon response rates in hepatitis C virus infection. Nature. 2004 Dec 16;432(7019):922–924. doi: 10.1038/nature03153. [DOI] [PubMed] [Google Scholar]
- 37.Nowak M, Schuster P. Error thresholds of replication in finite populations mutation frequencies and the onset of Muller's ratchet. J Theor Bio. 1989 Apr 20;137(4):375–395. doi: 10.1016/s0022-5193(89)80036-0. [DOI] [PubMed] [Google Scholar]
- 38.Tapia N, Fernandez G, Parera M, Gomez-Mariano G, Clotet B, Quinones-Mateu M, et al. Combination of a mutagenic agent with a reverse transcriptase inhibitor results in systematic inhibition of HIV-1 infection. Virology. 2005 Jul 20;338(1):1–8. doi: 10.1016/j.virol.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Pariente N, Sierra S, Lowenstein PR, Domingo E. Efficient virus extinction by combinations of a mutagen and antiviral inhibitors. J Viro. 2001 Oct;75(20):9723–9730. doi: 10.1128/JVI.75.20.9723-9730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez-Lopez C, Arias A, Pariente N, Gomez-Mariano G, Domingo E. Preextinction viral RNA can interfere with infectivity. J Viro. 2004 Apr;78(7):3319–3324. doi: 10.1128/JVI.78.7.3319-3324.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perelson AS, Layden TJ. Ribavirin: is it a mutagen for hepatitis C virus? Gastroenterology. 2007 May;132(5):2050–2052. doi: 10.1053/j.gastro.2007.03.077. [DOI] [PubMed] [Google Scholar]
- 42.Lutchman G, Danehower S, Song BC, Liang TJ, Hoofnagle JH, Thomson M, et al. Mutation rate of the hepatitis C virus NS5B in patients undergoing treatment with ribavirin monotherapy. Gastroenterology. 2007 May;132(5):1757–1766. doi: 10.1053/j.gastro.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 43.Gerotto M, Sullivan DG, Polyak SJ, Chemello L, Cavalletto L, Pontisso P, et al. Effect of retreatment with interferon alone or interferon plus ribavirin on hepatitis C virus quasispecies diversification in nonresponder patients with chronic hepatitis C. Journal of Virology. 1999;73:7241–7247. doi: 10.1128/jvi.73.9.7241-7247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schinke J, de J, Bruning B, van HB, Spaan WJ, Kroes AC. The potentiating effect of ribavirin on interferon in the treatment of hepatitis C: lack of evidence for ribavirin-induced viral mutagenesis. Antivir Ther. 2003 Dec;8(6):535–540. [PubMed] [Google Scholar]
- 45.Chevaliez S, Brillet R, Lazaro E, Hezode C, Pawlotsky JM. Analysis of ribavirin mutagenicity in human hepatitis C virus infection. J Viro. 2007 Jul;81(14):7732–7741. doi: 10.1128/JVI.00382-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vo NV, Young KC, Lai MM. Mutagenic and inhibitory effects of ribavirin on hepatitis C virus RNA polymerase. Biochemistry. 2003 Sep 9;42(35):10462–10471. doi: 10.1021/bi0344681. [DOI] [PubMed] [Google Scholar]