Abstract
The neuroanatomical substrate of seizures induced by picomolar amounts of corticotropin-releasing hormone in infant rats was investigated. Electrographic and behavioral phenomena were monitored in 42 rat pups aged 5 to 22 days. Rat pups carried bipolar electrodes implanted in subcortical limbic structures, as well as cortical electrodes and intracerebroventricular cannulae. The administration of corticotropin-releasing hormone produced age-specific seizures within minutes, which correlated with rhythmic amygdala discharges. Paroxysmal hippocampal and cortical discharges developed subsequently in some rats. Corticotropin-releasing hormone-induced electrographic and behavioral seizures originate in the amygdala.
Corticotropin-releasing hormone (CRH) is a 41-amino acid neuropeptide, isolated originally from the mammalian hypothalamus [1], It has since been shown to be distributed nonrandomly in the central nervous system, and to perform roles other than the control of secretion of ACTH and endorphins from the anterior pituitary [2]. Specifically, central transduction of stress, anxiety, depression, and anorexia have been demonstrated [2, 3].
CRH activates neurons both in vivo and in vitro [4–9]. The peptide increases both spontaneous and evoked spike discharge from locus ceruleus neurons in vivo [4], CRH induces neuronal depolarization in CA1 and CA3 hippocampal pyramidal cells in the slice preparation in vitro [5]. CRH administered into the cerebral ventricles of adult rats causes epileptiform discharges in the amygdala after a 1- to 3-hour delay, which spread to the dorsal hippocampus [6]. These discharges progress over 3 to 7 hours to behavioral and electrographic seizures. The doses needed for frank seizure generation in adult rats axe 1–5 to 3–75 × 10−9 mol (0.75–1.88 × 10−9 mol/gm of brain weight) [6–8].
We have previously shown that CRH is a far more rapid and potent convulsant in the neonatal rat [10]. Seizures occur with a latency of as little as 2 minutes and with CRH doses as low as 7.5 × 10−12 mo! or 0.05 × 10−9 mol/gm of brain weight [10]. The present study was designed to define the neurobiological matrix of the behavioral and electrographic effects of the neuropeptide. We used infant rats, starting on postnatal day 5.
Materials and Methods
Animals
Timed-pregnancy Sprague–Dawley–derived rats were obtained from Zivic-Miller (Zelionplc, PA). They were housed under a 12-hour light/dark cycle and fed ad libitum. Delivery times were monitored and were accurate to within 12 hours. The day of birth was considered day zero. The pups were kept with the mothers, and litters were culled to 12 pups. Infant rats were subjected to surgery 24 hours before recording and returned to their mothers. CRH was always administered between 9 and 10:30 AM, to minimize diurnal variations in seizure susceptibility [11] and in endogenous CRH levels [12].
Surgical Procedure
Electrodes were implanted under halothane anesthesia, using an infant rat stereotaxic apparatus as previously described [10, 13]. Two or four cortical stainless-steel electrodes were placed over the frontal and posteroparietal cortex. Bipolar twisted wire electrodes, enameled except for the tip, were used. Electrodes were inserted through a burr hole into the amygdala or hippocampus (wire diameter, 0.1–0.15 mm; vertical intertip distance, 0.5–1.0 mm). Electrodes were anchored to the skull with an acrylic cement “cap” attached also to two screws. Age-specific basal-lateral amygdala coordinates, with reference to bregma, were adapted for our strain [14] and are shown in Table 1.
Table 1.
Age-Specific Basal-Lateral Amygdala Coordinates
| Age (days) | AP | Lat | V |
|---|---|---|---|
| 4–5 | −1.5 | 3.5 | 6.0 |
| 7–9 | −1.5 | 3.6 | 8.0 |
| 10–12 | −1.5 | 3.6 | 8.0 |
| 15–17 | −1.5 | 3.6 | 8.5 |
AP = anteroposterior; Lat = lateral; V = ventral.
In each age group, several rat pups carried bipolar hippocampal electrodes (Table 2). These were aimed at the dorsal or ventral hippocampus, either simultaneously with those in the amygdala or with a larger array of cortical electrodes. Age-specific coordinates for hippocampal electrodes are shown in Table 2. CRH was injected via a cannula placed stereotaxically in the lateral ventricle [10].
Table 2.
Age-Specific Coordinates for Hippocampal Electrodes
| Dorsal Hippocampus
|
Ventral Hippocampus
|
|||||
|---|---|---|---|---|---|---|
| Age (days) | AP | Lat | V | AP | Lat | V |
| 5–7 | −1.5 | 1.6 | 2.8 | −2.8 | 3.5 | 6.5 |
| 9–12 | −1.5 | 1.7 | 3.0 | −3.0 | 4.2 | 7.0 |
| 13–18 | −2.0 | 2.0 | 3.0 | −3.3 | 4.5 | 7.0 |
AP = anteroposterior; Lat = lateral; V = ventral.
Electrophysiology and Behavior Monitoring
Recordings were performed in heated, shielded Plexiglas chambers. A Grass 78E Polygraph (Grass Instrument Co, Quincy, MA) was connected via long, flexible wires to freely moving rat pups, which were continuously observed throughout the recording. To ascertain the cerebral origin of recorded discharges, two maneuvers were undertaken. At least 1 rat pup per age was restrained (using medical tape) in an infant-rat stereotaxic apparatus. This prevented all motion of electrodes and wires. Electrographic recordings were compared with those of unrestrained peers, and with those obtained without restraint from the same pup. In addition, a motion-detecting bipolar electrode (“EMG”), was attached to the angle of the jaw or the chest and connected to the Polygraph.
After a 30-minute habituation and baseline recording period, CRH or saline was administered intracerebroventricularly in 1 to 4 μl (depending on dose and animal age) using a microinfusion pump. CRH doses used were 0.0375 to 0.3 nmol. These doses have been found to elicit behavioral epileptic phenomena in infant rats [10]. Electrographic recording and observation of behavior were supplemented by intermittent video recording. These procedures were performed for a minimum of 4 hours, or for at least 1 hour subsequent to cessation of abnormal activity. At the end of each experiment, electrolytic lesions were created at the bipolar electrode tips. Rat pups were killed and brains frozen on dry ice. Brains were cut into 20-μm sections and stained with cresyl violet for verification of electrode placement.
Seizure definition has been discussed in detail elsewhere [10]. Briefly, behavioral phenomena were considered epileptic if (1) they were not seen before CRH administration [10], and (2) they were recognized as epileptic in infant [15–17] and adult rats [18–20]. Though no formal grading system was used, observed behaviors conformed to the infantile scale of kindling-induced phenomena [15, 17]. The correlation of specific behaviors and electrographic discharges, the goat of this study, is discussed in the following section.
Results
The ages of rats, doses of CRH administered, and times to onset of CRH-induced seizure are shown in Table 3. Age-specific electrographic and behavioral phenomena are summarized in Table 4. The detailed sequence of age- and dose-dependent CRH-induced behavioral events has been described elsewhere [10].
Table 3.
CRH-induced Seizures: Latency, Peptide Doses, and Age of Rat Pups
| Age (days) | n | CRH (nmol) | n | Latency (min) | Duration (hr) | Electrode Location |
|---|---|---|---|---|---|---|
| 5–6 | 10 | 0.038 | 2 | 9.0 ± 1.0a | >5;2 | A;A |
| 0.075 | 2 | 5.5 ± 0.7a | 5;>5 | A;A | ||
| 0.15 | 6 | 4.9 ± 1.5a | 2 to >5 | 2A;2DH;2VH | ||
| 8–10 | 13 | 0.15 | 11 | 5.9 ± 1.5a | all >3 | 6A;2VH;1DH;2 (A + VH) |
| 0.30 | 2 | 3;5 | >4 | A + VH;A | ||
| 11–15 | 13 | 0.08 | 1 | N/Ab | 3 | A |
| 0.15 | 10 | 3.8 ± 0.5a | 1 to >4 | 4A;2(A + DH); 1VH;3(A + VH) | ||
| 0.22 | 1 | N/Ab | >4 | A | ||
| 0.30 | 1 | <2 | 3 | A | ||
| 16–22 | 6 | 0.15 | 1 | 16 | 2 | A |
| 0.22 | 1 | 10 | <2 | A + VH | ||
| 0.3 | 3 | 4.0 ± 1.7a | 2;2;3 | DH;A;A | ||
| 0.75 | 1 | 2 | 2.7 | DH |
The mean plus the standard error of the mean.
Not available, animal restrained to control for motion artifact (see Materials and Methods)
CRH = corticotropin-releasing hormone; n = number of rats; A = amygdala; DH = dorsal hippocampus; VH = ventral hippocampus; A + VH, A + DH = simultaneous amygdala and hippocampal electrodes.
Table 4.
Behavioral and EEG Characteristics of CRH-induced Seizures: Age Dependence
| Age (days) | Behavior | Electrographic Correlates |
|---|---|---|
| 5–6 | JM; forepaw grooming and clonus; tonus; LOB | Amygdala; semirhythmic spikes (4/6); fast activity (2/6) |
| 8–10 | JM; semirhythmic fore paw clonus (run); LOB | Rhythmic slow wave or spike wave; rare cortical discharge |
| 11–14 | JM; grooming, scratching; “swimming”; LOB | Amygdala rhythmic spike wave; rare H or cortex sharp waves |
| 16–22 | JM; grooming; rare LOB or “major” seizures | Similar to 11- to 14-day-old group; higher voltage |
EEG = electroencephalography, CRH = corticotropin-releasing hormone, JM = jaw myoclonus manifesting as vigorous, persistent, and unstoppable licking and chewing; LOB = loss of balance; H = hippocampus.
At the end of the first postnatal week, minimal effective CRH doses (0.038 nm or 38 pm) resulted in stereotyped behaviors (see Table 4). These were accompanied, in most rat pups carrying amygdala electrodes (5 of 6 pups), by abnormal discharges. Semirhythmic spike waves (Fig 1B, D), the appearance of low-amplitude fast (13–15 Hz) activity in the amygdala leads (Fig 1C), or both preceded the onset of jaw myoclonus in 3 of 6 rat pups. In 2 additional pups, amygdala discharges followed the onset of behavioral events. These discharged were confined to the bipolar amygdala leads and to those connected co both amygdala and homologous cortex.. No paroxysmal discharges were recorded from either ventral or dorsal hippocampus (not shown).
Fig 1.
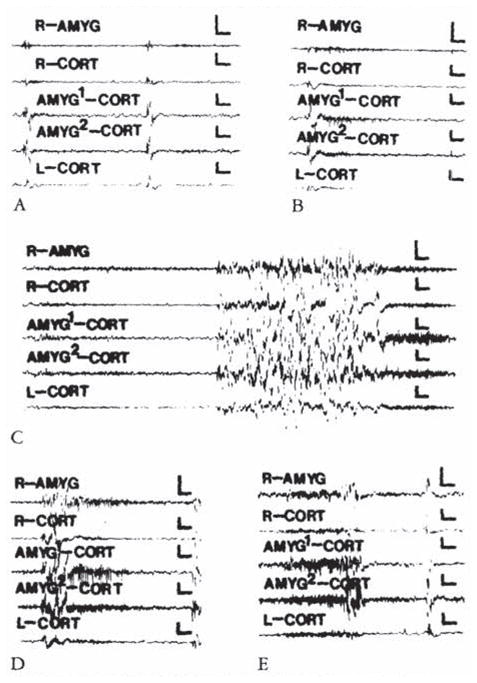
Electroencephalograms of a 5-day-old rat. (A) Before corticotropin-releasing hormone (CRH) infusion. (B, C, D, and E) Two, 11.5, 20, 59. and 85 minutes after infusion of 0.15 nmol of CRH into the cerebral ventricle. AMYC = amygdala; CORT = cortex; AMYG1/AMYG2 =one of the wires of the bipolar amygdala electrode. R/L = right / left. Vertical bar = 50 μV; horizontal bar = 1 second.
CRH-induced amygdala discharges in 8- to 10-day-old infant rats are shown in Figure 2. The baseline electroencephalogram (EEG) at this age was more developed than in younger pups (see Fig 2A). Figure 2B, 9 minutes subsequent to administration of CRH, shows rhythmic slow wave discharges in the amygdala leads, which did not correlate with chest motion or with the rhythm of jaw myoclonus (“EMG”). The latency of paroxysmal amygdala discharges and accompanying behaviors (see Table 4) was dose dependent. Using 015 nm of CRH, frequent electrographic and behavioral seizures persisted for 4 to 5 hours. The first two panels of Figure 3 illustrate the electrographic correlates of CRH-induced seizures in a pup carrying both amygdala and ventral hippocampus (VH) electrodes. By the end of the second postnatal week (days 11–14), CRH induced repetitive, well-formed sharp wave discharges confined to amygdala leads (Fig 4B–D). No paroxysmal discharges were present in simultaneously recorded dorsal hippocampus and cortex. Rare sharp hippocampal and cortical spikes (see Fig 4D) could not be distinguished with certainty from motion artifacts. In a different rat pup, persistent jaw myoclonus was not associated with abnormal discharges in VH leads (see Fig 3C, D).
Fig 2.
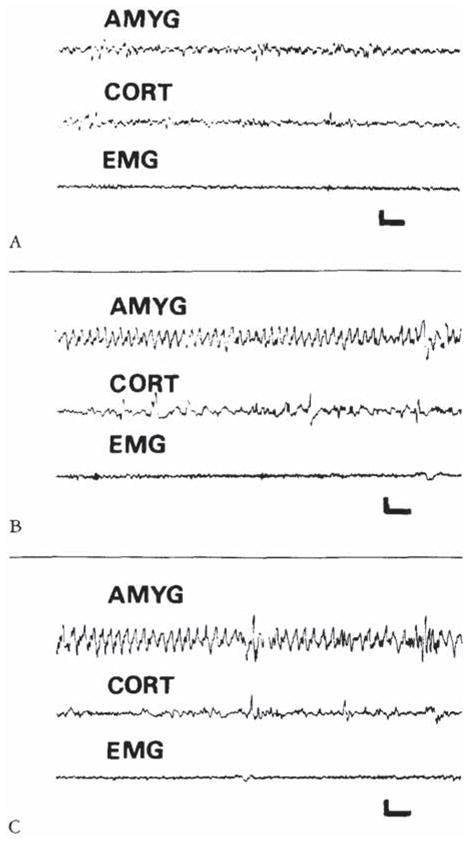
Electroencephalograms of a 9-day-old rat before corticotropin-releasing hormone (CRH) administration (A), and 9 and 120 minutes after intracerebroventricular infusion of 015 nm of CRH (B, C). The onset of semirhythmic slow wave discharges, confined to amygdala leads (AMYG). is evident in B. They persisted intermittently for several hours (C). CORT = cortical lead; EMG = motion-detecting electrodes placed over the angle of the jaw. Vertical bar = 50 μV; horizontal bar = 1 second.
Fig 3.
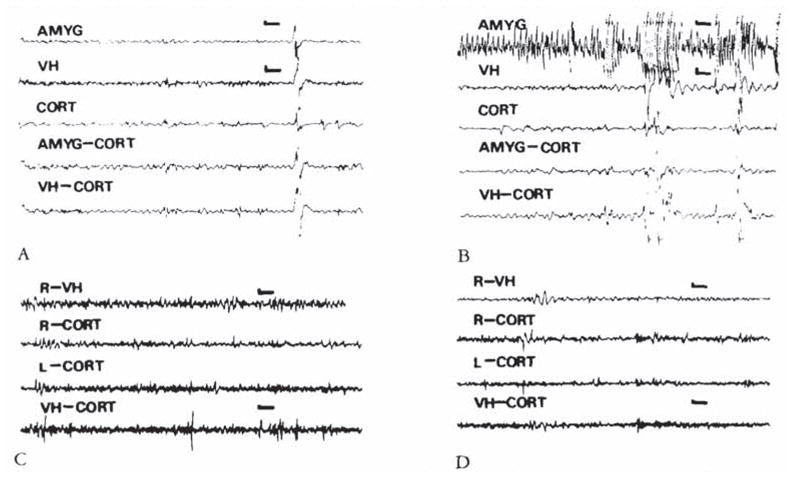
(A, B) Electroencephalograms (EEGs) of an 8-day-old infant rat. carrying both bipolar amygdala (AMYG) and ventral hippocampus (VH) electrodes, before administration of corticotropin-releasing hormone (CRH) (A) and 8 minutes after administration of 0.15 nm of the peptide (B). Paroxysmal discharges originate in the amygdala leads. Movement artifacts are later seen in all leads. (C, D) EEGs of an 11-day-old rat before (C). and 26 minutes subsequent to the administration of 0.15 nm of CRH. Though the pup displayed jaw myoclonus, only attenuation of the theta rhythm is seen in the ventral hippocampus (VH). CORT = cortex; AMYG-CORT/VH-CORT = a lead combining one of the subcortical electrodes to the cortical one. Vertical bar = 50 μV; horizontal bar = 1 second.
Fig 4.
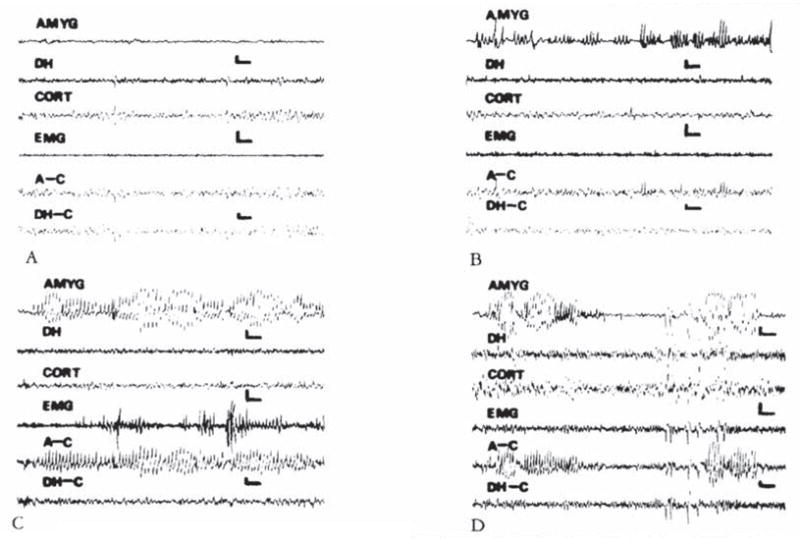
Electroencephalograms of a 13-day-old rat. (A) Baseline. (B. C. and D) Two. 35. and 174 minutes after intracerebro-ventricular administration of 0.15 nm of corticotropin-releasing hormone. High-voltage, repetitive discharges are present exclusively in bipolar amygdala (AMYG) and amygdala-cortex (A–C) leads, and not in bipolar hippocampal (DH). DH–cortex (DH–C), or the motion-detecting (EMG) leads. Vertical bar = 50 μV; horizontal bar = 1 second.
During the third postnatal week, larger closes of CRH (0.15 nm or more) were needed to elicit seizures. Behavior was limited to persistent, uncontrollable jaw myoclonus accompanied by grooming, scratching, and so on. Loss of balance was observed in only one 16-day-old pup (given 0.3 nm of CRH), and clonus was not common. CRH (0.15–0.75 × 10 −9 mol) induced spike wave discharges in amygdala leads (Fig 5B). Despite recording periods of up to 9 hours, propagation to hippocampal or conical leads was only rarely observed (not shown). Placement of electrodes in amygdala and dorsal and ventral hippocampus is seen in Figure 6.
Fig 5.
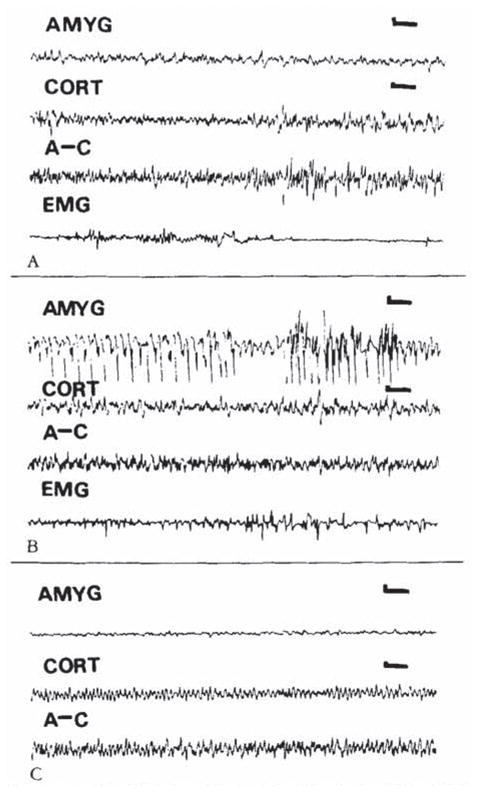
Electroencephalograms (EEGs) of an 18-day-old rat. (A) Baseline. (B. C) Nine and 143 minutes after intracerebroventrkular administration of cortkotropin-releasing hormone (0.3 nm). High-voltage spike and slow wave discharges confined to the amygdala (latency. 4 minutes) (B). Two hours later, amygdala EEG has normalized (C). AMYG = amygdala; CORT = cortex; A–C = amygdala–cortex lead; EMG = motion-detecting electrode (see text). Vertical bar = 50 μV; horizontal bar = 1 second.
Fig 6.
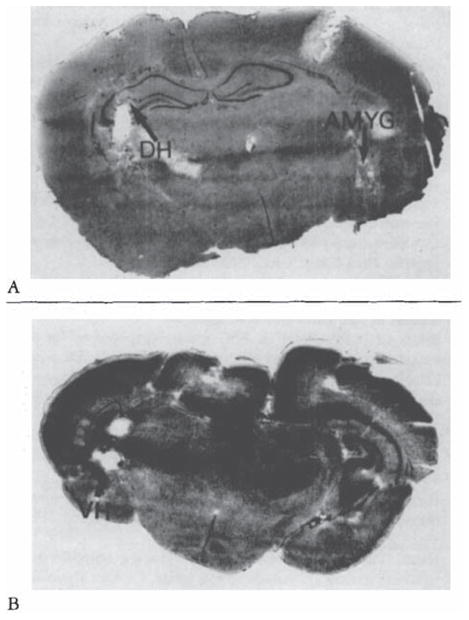
Histological verification of electrode placement in subcortical structures of the infant rat, Cresyl blue–stained. 20-μm-thick coronal sections. (A) Amygdala (AMYG) and dorsal hippocampus (DH) (B) Ventral hippocampus (VH).
Discussion
CRH induces convulsions in adult rats [6–8, 21], though the time course and origin of paroxysmal discharges remain unresolved, Ehlers [7] and Ehlers and colleagues [6] demonstrated the onset of amygdala spikes 1 to 3 hours after the administration of CRH. Behavioral seizures, most commonly jaw myoclonus, developed 3 to 7 hours later. These were accompanied by the spread of paroxysmal discharges to the hippocampus and cortex [6]. Other investigators [8, 21, 22] have described CRH-induced epileptiform discharges localized to the hippocampal leads in both rats and rabbits. Minimal latency to hippocampal spikes was 35 to 39 minutes [22].
We have found CRH to be a more rapid and potent convulsant in the neonatal and infant rat compared with the adult rat [10]. Jaw myoclonus developed in less than 2 minutes, and effective peptide doses were as low as 0.0075 nmol (50 × 10−12 mol/gm of brain weight). Reported convulsant doses of CRH in the adult were 1.5 to 3.75 nmol, or 750 × 10−12 mol/gm of brain weight [6] The underlying mechanisms for this age-dependent potency and rapidity of action of CRH remain unclear. CRH gene expression increases markedly at the end of the first postnatal week [23, 24]. That, coupled with a maximal number of CRH receptors present at that time [25], may partially explain this phenomenon.
The neuroanatomical substrate of CRH-induced seizures in the infant rat has not been elucidated. Cortical electrographic correlates of behavioral phenomena have been inconsistent [10], and data regarding adult rodents are in disagreement [6–8, 21, 22]. Jaw myoclonus as an epileptic phenomenon has been extensively described in both adult [6, 20] and infant rats [16, 26, 27]. Some authors ascribe the origin of jaw myoclonus exclusively to the amygdaloid complex [19]. After kainic acid administration, jaw myoclonus has been found as a manifestation of limbic seizures originating in the hippocampus and spreading to the amygdala [16, 18, 20].
Electrographic recording from discrete structures in the neonatal and infant rat is fraught with difficulties due to the small size of brain regions, the low voltage of EEG discharges, and the presence of motion artifacts. Localization was achieved using bipolar amygdala electrodes and validated in smaller animals via amygdala–cortex leads. Not uncommonly, paroxysmal discharges were confined to bipolar amygdala and only one amygdala–cortex lead. Simultaneous recording was obtained from amygdala and either dorsal or ventral hippocampus. Paroxysmal discharges originated from the amygdala (see Figs 3, 4).
Movement artifacts were avoided by several maneuvers. EEGs of completely restrained rat pups were compared with those of free-moving pups. Rat pups were observed constantly, and a motion-detecting electrode was placed at the angle of the jaw or around the chest. The latter detected the increased ventilatory rate and amplitude induced by CRH [28]. Curarization [29] was avoided because it has been reported to alter EEGs in adult rats [30].
The assignment of the amygdala as the origin of CRH-induced paroxysmal discharges is compatible with the observed behavioral phenomena. Furthermore, the amygdala is quite rich in CRH. The central amygdaloid nucleus has the highest concentration of the neuropeptide in the brain [31, 32]. CRH receptors have been demonstrated in the central amygdaloid nucleus and basolateral amygdala [33].
In conclusion, we have demonstrated that age-specific CRH-induced seizures originate in the amygdala. The role of this neuropeptide as an endogenous convulsant in the immature brain, as well as its potential role in the enhanced susceptibility of the neonatal brain to epilepsy, remain speculative [10, 34].
Acknowledgments
This work was supported by NS01307 and by a grant from the Epilepsy Foundation of America (T.Z.B.).
We are thankful to Ms Shana Friedman for her assistance with the manuscript.
References
- 1.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 2.Vale W, Rivier C, Brown MR, et al. Chemical and biological characterization of corticotropin releasing factor. Recent Prog Horm Res. 1983;83:245–270. doi: 10.1016/b978-0-12-571139-5.50010-0. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF, Britton KT. Behavioral effects of corticotropin-releasing factor. In: De Souza EB, Nemeroff CB, editors. Corticotropin-releasing factor: basic and clinical studies of a neuropeptide. Boca Raton: CRC; 1990. pp. 253–265. [Google Scholar]
- 4.Valentino RJ, Foote SL, Aston-Jones G. Corticotropin releasing factor activates neurons of the locus coeruleus. Brain Res. 1983;270:363–367. doi: 10.1016/0006-8993(83)90615-7. [DOI] [PubMed] [Google Scholar]
- 5.Siggins GR. Electrophysiology of corticotropin-releasing factor in nervous tissue. In: De Souza EB, Nemeroff CB, editors. Corticotropin-releasing factor: basic and clinical studies of a neuropeptide. Boca Raton: CRC; 1990. pp. 205–214. [Google Scholar]
- 6.Ehlers CL, Henriksen SJ, Wang M, et al. Corticotropin releasing factor produces increases in brain excitability and convulsive seizures in rats. Brain Res. 1983;278:332–336. doi: 10.1016/0006-8993(83)90266-4. [DOI] [PubMed] [Google Scholar]
- 7.Ehlers CL. CRF effects on EEG activity: implications for the modulation of normal and abnormal brain states. In: De Souza EB, Nemeroff CB, editors. Corticotropin-releasing factor: basic and clinical studies of a neuropeptide. Boca Raton: CRC; 1990. pp. 233–248. [Google Scholar]
- 8.Marrosu F, Fratta W, Carcangiu P, et al. Localized epileptiform activity induced by murine CRF in rats. Epilepsia. 1988;29:369–373. doi: 10.1111/j.1528-1157.1988.tb03733.x. [DOI] [PubMed] [Google Scholar]
- 9.Baram TZ, Citron M, Schultz L. CRH in frog retina—quantitative and detailed anatomical analysis. Soc Neurosci. 1988;19:13. (Abstract) [Google Scholar]
- 10.Baram TZ, Schultz L. Corticotropin-releasing hormone is a rapid and potent convulsanr in the infant rar. Dcv Brain Res. 1991;61:97–101. doi: 10.1016/0165-3806(91)90118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliverio A, Castellano C, Puglisi-Allegra S, Renzi P. Diurnal variations in electroconvulsive shock induced seizures involvement of endogenous opioids. Neurosci Lett. 1985;57:237–240. doi: 10.1016/0304-3940(85)90497-5. [DOI] [PubMed] [Google Scholar]
- 12.Watts AG, Swansoo LW. Diurnal variation in the content of preprocorticotropin releasing hormone mRNA in the hypothalamic paraventricular nucleus of rats of both sexes as measured by in situ hybridization. Endocrinology. 1989;125:1734–1738. doi: 10.1210/endo-125-3-1734. [DOI] [PubMed] [Google Scholar]
- 13.Baram TZ, Snead OC., III The ontogeny of bicuculline induced seizures in infant rats—behavioral and electrocortical phenomena. Dev Brain Res. 1990;57:291–295. doi: 10.1016/0165-3806(90)90055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherwood NM, Timiras PS. A stereotaxic atlas of the developing rat brain. Berkeley: University of California; 1970. [Google Scholar]
- 15.Moshe SL. The kindling phenomenon and its possible relevance to febrile seizures. In: Nelson KB, Ellenberg JH, editors. Febrile seizures, long term management of children with fever associated seizures. New York: Raven; 1981. pp. 59–63. [Google Scholar]
- 16.Cherubini E, DeFeo MR, Mecarelli O, Ricci GG. Behavioral and electrographic patterns induced by systemic administration of kainic acid in developing rats. Dev Brain Res. 1983;9:69–77. doi: 10.1016/0165-3806(83)90110-4. [DOI] [PubMed] [Google Scholar]
- 17.Albala BJ, Moshe SL, Okada R. Kainic-acid–induced seizures: a developmental study. Dev Brain Res. 1984;13:139–148. doi: 10.1016/0165-3806(84)90085-3. [DOI] [PubMed] [Google Scholar]
- 18.Lothman EW, Collins RC. Kainic acid induced limbic seizures: metabolic, behavioral, electroencephalographic and neuropatho-logical correlates. Brain Res. 1981;218:299–318. doi: 10.1016/0006-8993(81)91308-1. [DOI] [PubMed] [Google Scholar]
- 19.Ikonomidou-Turski C, Cavalhciro EA, Turski WA, et al. Con vulsant action of morphine, [D-ALA2,D-LEU6]-enkephalin and naloxone in the rat amygdala; electroencephalographic, morphological and behavioural sequelae. Neuroscience. 1987;20:671– 686. doi: 10.1016/0306-4522(87)90118-7. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Ari Y, Tremblay E, Riche D, et al. Electrographic, clinical and pathological alterations following systemic administration of kainic acid, bicuculline or pentetrazole: metabolic mapping using the deoxyglucose method with special reference to the pathology of epilepsy. Neuroscience. 1981;6:1361–1391. doi: 10.1016/0306-4522(81)90193-7. [DOI] [PubMed] [Google Scholar]
- 21.Ortolani E, Di Giannuario A, Ncrozzi D, et al. Some endorphin derivatives and hydrocortisone prevent EEG limbic seizures induced by CRF in rabbits. Epilepsia. 1990;31:702–707. doi: 10.1111/j.1528-1157.1990.tb05509.x. [DOI] [PubMed] [Google Scholar]
- 22.Marrosu F, Argiolas A, Carcangiu P, et al. Neonatal monosodium glutamate abolishes corticotropin releasing factor-induced epileptic activity in rats. Epilepsia. 1990;31:708–712. doi: 10.1111/j.1528-1157.1990.tb05510.x. [DOI] [PubMed] [Google Scholar]
- 23.Grino MW, Young WS, III, Burgunder JM. Ontogeny of the expression of the CRF gene in the hypothalamic paraventricular nucleus and of the proopiomelanocortin gene in rat pituitary. Endocrinology. 1989;124:60–68. doi: 10.1210/endo-124-1-60. [DOI] [PubMed] [Google Scholar]
- 24.Baram TZ, Lerner SP. Corticotropin releasing hormone— ontogeny of gene expression in rat hypothalamus. Int J Dev Neurosci. 1991;9:473–478. doi: 10.1016/0736-5748(91)90033-i. [DOI] [PubMed] [Google Scholar]
- 25.Insel TR, Battaglia G, Fairbanks DW. De Souza EB The ontogeny of brain receptors for corticotropin-releasing factor and the development of their functional association with adenylate cyclase. J Neurosci. 1988;8:4151–4158. doi: 10.1523/JNEUROSCI.08-11-04151.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilbert ME, Cain DP. A developmental study of kindling in the rat. Dev Brain Res. 1982;2:321–328. doi: 10.1016/0165-3806(81)90041-9. [DOI] [PubMed] [Google Scholar]
- 27.Holmes GL, Thompson JL. Effects of kainic acid on seizure susceptibility in the developing brain. Dev Brain Res. 1988;39:51–59. doi: 10.1016/0165-3806(88)90066-1. [DOI] [PubMed] [Google Scholar]
- 28.Bohmer G, Schmid K, Ramsbott M. Effects of corticotropin releasing factor on central respiratory activity. Eur J Pharmacol. 1990;182:405–411. doi: 10.1016/0014-2999(90)90037-7. [DOI] [PubMed] [Google Scholar]
- 29.Cavalheiro EA, Delrio FS, Turski WA, et al. The susceptibility of rats to pilocarpine-induced seizures is age dependent. Dev Brain Res. 1987;37:43–58. doi: 10.1016/0165-3806(87)90227-6. [DOI] [PubMed] [Google Scholar]
- 30.Pokorny J, Sterc J. Effect of D-tubocurarine immobilization on the resting electroencephalogram in the rat. Electroencephalogr Clin Neurophysiol. 1980;48:242–245. doi: 10.1016/0013-4694(80)90311-9. [DOI] [PubMed] [Google Scholar]
- 31.Palkovits M, Brownstein MJ, Vale W. Distribution of CRF in the rat brain. Fed Proc. 1985;44:215–219. [PubMed] [Google Scholar]
- 32.Gray TS. The organization and possible function of amygdaloid corticotropin-releasing factor pathways. In: De Souza EB, Nemeroff CB, editors. Corticotropin-releasing factor: basic and clinical studies of a neuropeptide. Boca Raton: CRC; 1990. pp. 53–69. [Google Scholar]
- 33.De Souza EB, Insel TR, Perrin MH, et al. Corticotropin-releasing factor receptors are widely distributed within the rat central nervous system: an autoradiographic study. J Neurosci. 1985;5:3189–3199. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baram TZ, Mitchell WG, Snead OC, III, et al. Neurology. 1992. Brain adrenal axis hormones are altered in the CSF of infants with massive infantile spasms. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]


