Abstract
Invadopodia are specialized actin-rich protrusions of metastatic tumor and transformed cells with crucial functions in ECM degradation and invasion. Although early electron microscopy studies described invadopodia as long filament-like protrusions of the cell membrane adherent to the matrix, fluorescence microscopy studies have focused on invadopodia as actin-cortactin aggregates localized to areas of ECM degradation. The absence of a clear conceptual integration of these two descriptions of invadopodial structure has impeded understanding of the regulatory mechanisms that govern invadopodia. To determine the relationship between the membrane filaments identified by electron microscopy and the actin-cortactin aggregates of invadopodia, we applied rapid live-cell high-resolution TIRF microscopy to examine cell membrane dynamics at the cortactin core of the invadopodia of human carcinoma cells. We found that cortactin docking to the cell membrane adherent to 2D fibronectin matrix initiates invadopodium assembly associated with the formation of a invadopodial membrane process that extends from a ventral cell membrane lacuna toward the ECM. The tip of the invadopodial process flattens as it interacts with the 2D matrix, and it undergoes constant rapid ruffling and dynamic formation of filament-like protrusions as the invadopodium matures. To describe this newly discovered dynamic relationship between the actin-cortactin core and invadopodial membranes, we propose a model of the invadopodial complex. Using TIRF microscopy, we also established that – in striking contrast to the invadopodium – membrane at the podosome of a macrophage fails to form any process- or filament-like membrane protrusions. Thus, the undulation and ruffling of the invadopodial membrane together with the formation of dynamic filament-like extensions from the invadopodial cortactin core defines invadopodia as invasive superstructures that are distinct from the podosomes.
Keywords: invadopodia, podosomes, cortactin, focal adhesions, invasion
INTRODUCTION
Transformed and metastatic tumor cells use invadopodia – specialized proteolytically-active, actin-rich cell protrusions – to degrade and invade surrounding extracellular matrix (ECM). Initially, invadopodia were identified as stable actin-rich protrusions emanating from the ventral cell membrane of transformed cells invading fluorescent two-dimensional (2D) matrix, which were shown to localize to the areas of matrix degradation (Chen, 1989). Culturing transformed cells on thin 2D layers of purified ECM proteins revealed that invadopodia degrade fibronectin, collagen type I, collagen type IV, and laminin (Kelly et al, 1994). Electron microscopy studies of transformed cells invading these thin 2D matrices described invadopodia as regions of the ventral membrane protruding toward the ECM substrate with a central electron-dense cytoplasmic core or with the central core extending into long, fine filament-like protrusions containing a meshwork of microfilaments (Kelly et al., 1994, Chen, 1989). Subsequent electron microscopy studies of invasive tumor cells grown on cross-linked gelatin beads revealed that cancer cells degrade the gelatin surface of the bead and insert invadopodia into the bead as long protrusions of the ventral cell membrane (Bowden et al., 1999). Measurements of the central electron-dense core in electron micrographs indicate that its diameter can range from 0.1 to 0.8 μm. The individual filament-like membrane extensions originating from the invadopodial core can be more than 2 μm in length. Recent ultrastructural analysis of the invadopodia of melanoma cells using a correlative confocal light and electron microscopy approach identified invadopodia as thin filament-like protrusions originating from a ventral cell membrane invagination averaging 8 μm wide and 2 μm deep (Baldassarre et al., 2003). Multiple filament-like invadopodia were shown to originate from a large ventral cell membrane invagination, indicating that invadopodia are part of an invasion superstructure.
In contrast, other research over the past two decades has characterized the invadopodium with fluorescence microscopy as a dot-like actin aggregate localized at the area of proteolytic degradation of a 2D fluorescent matrix. Functional studies identified the actin-binding protein cortactin as indispensable for invadopodial actin core formation and proposed to use cortactin as an invadopodial molecular marker in addition to actin (Artym et al., 2006). Using these invadopodia identification criteria, multiple proteins have been shown to co-localize to actin-cortactin cores of the invadopodia (Weaver, 2006, Poincloux et al., 2009, Buccione et al., 2009, Linder, 2007, Mueller et al., 2008). These invadopodia-associated proteins can be divided into four categories: (1) cell adhesion molecules, such as integrins; (2) actin and actin-associated proteins; (3) signaling proteins that include tyrosine kinases and small GTPases; and (4) soluble and membrane-bound proteases. The broad spectrum of proteins that localize to invadopodia and the function of invadopodia in ECM degradation involving directed targeting of proteases to invadopodia defines the invadopodium as a unique cellular structure characterized by coordinated interaction and interplay of cell adhesion, actin nucleation and polymerization, directed protease trafficking, endocytosis, and exocytosis. However, the identification and characterization of invadopodia structures as actin-cortactin aggregates has tended to oversimplify our understanding of invadopodia. In addition, comparisons of invadopodia to podosomes based on the similarity of their actin-cortactin cores can lead to the perception that invadopodia are the same as podosomes. Podosomes are dot-like adhesion structures of monocyte-derived cells, such as osteoclasts (Marchisio et al., 1984) and macrophages (Marchisio et al., 1987) that can also degrade ECM (Gil-Henn et al., 2007, Luxenburg et al., 2006). According to fluorescence microscopy studies, podosomes have an actin-cortactin rich core 0.5 to 1 μm in diameter. This dense actin-cortactin core is surrounded by a ring of adhesion molecules and a cloud of unpolymerized actin (Gimona et al., 2008, Gimona and Buccione, 2006). Conventional electron microscopy studies of chemically fixed cells and ultrastructural studies of frozen and freeze-substituted cells have visualized the typical podosome to be densely packed with short, highly branched F-actin filaments that are surrounded by loose, long actin filaments radiating from the podosome F-actin core and connecting it with neighboring podosomes (Akisaka et al., 2008, Marchisio et al., 1984, Luxenburg et al., 2007). The entire podosome structure measures 1 to 2 μm in diameter. Electron microscopy of podosome sections cut perpendicularly to the ECM visualize podosomes as very short dot-like membrane protrusions; the ventral cell membrane between two podosomes is elevated above the ECM by 10-50 nm (Marchisio et al., 1984); alternatively, the ventral cell membrane can be in close apposition to the ECM separated by a uniform gap of 10 nm (Akisaka et al., 2008).
To reconcile the discrepancies between descriptions of the invadopodium as a filament-like protrusion as established by electron microscopy studies versus the invadopodium as an actin-cortactin aggregate visualized by fluorescence microscopy, we examined cell membrane dynamics at the invadopodial actin-cortactin core using total internal reflection (TIRF) microscopy. TIRF microscopy is a technique that permits observation of an approximately100-150 nm thick region of the ventral cell surface that comes into direct contact with the surface of a glass coverslip coated with a thin layer of 2D ECM. In this study, rapid, live-cell, high-resolution TIRF imaging of human breast carcinoma cells adherent to a 2D fibronectin (FN) matrix revealed that the cell membrane at the invadopodial actin-cortactin core undergoes rapid dynamic remodeling, and it defined the actin-cortactin core to be a part of an invasive superstructure. We suggest that for clarity, the term “invadopodial complex” be used for the invadopodial invasion superstructure that includes both the actin-cortactin core and dynamic membrane structures. We found that the initial stages of invadopodial superstructure assembly involve the formation of a primary invadopodial membrane process that extends from the ventral cell membrane lacuna toward the ECM. The tip of the invadopodial process flattens as it interacts with the 2D matrix, and it undergoes constant rapid ruffling. As the invadopodial complex matures, filament-like invadopodia form from the invadopodial process.
Our live-cell TIRF microscopy studies describe for the first time the relationship between the cortactin core and cell membrane at the invadopodia, visualize morphological changes of the cell membrane at invadopodial superstructures, and reveal the highly dynamic nature of invadopodia. In addition, parallel TIRF microscopy studies of macrophage podosomes compared to invadopodia established that, contrary to the invadopodium, the podosome membrane does not form any process- or filament-like membrane extensions or protrusions in cells on the 2D ECM. Thus, the relationship between the actin-cortactin core and the membrane in the invadopodial superstructure is different from that at the podosome. The undulation and ruffling of the invadopodial membrane together with this formation of dynamic filament-like invadopodia defines the invadopodial complex as a highly dynamic invasive superstructure that is distinct from podosomes.
MATERIALS AND METHODS
Materials
Human plasma fibronectin (HPFN) was prepared as described (Akiyama, 2001). Bovine serum albumin (BSA) was purchased from MP Biomedicals (Solon, OH). Phalloidin-AlexaFluor and AlexaFluor protein labeling dyes were from Invitrogen (Carlsbad, CA). Anti-vinculin antibody was from Sigma-Aldrich (St. Louis, MO), and secondary antibodies conjugated to Cy5 were from Jackson ImmunoResearch (West Grove, PA). Rat tail collagen type I was purchased from BD Biosciences (San Jose, CA).
Cell lines and transfections
A stable line of MDA-MB-231 cells transfected with wild-type c-Src (wt c-Src MDA-MB-231) was a generous gift from Dr. Toshiyuki Yoneda (Myoui et al., 2003). Cells were maintained in high glucose-DMEM (HyClone, Logan, UT) supplemented with 10% fetal bovine serum (HyClone), 2 mM L-glutamine, 10 U/ml penicillin, and 10 μg/ml streptomycin. The mouse macrophage IC-21 (TIB-186) cell line was obtained from the American Type Culture Collection (Rockville, MD). This macrophage line was cultured in RPMI-1640 (HyClone, Logan, UT) supplemented with 10% fetal bovine serum, 10 mM HEPES, 1.5 g/l sodium bicarbonate, and 4.5 g/l glucose. Human foreskin fibroblasts (HFF) were cultured in high glucose-DMEM supplemented with 10% fetal bovine serum, 10 U/ml penicillin, and 10 μg/ml streptomycin.
All cell lines were transiently co-transfected with GFP and mCherry cDNA vectors, and fluorescence of exogenous proteins was imaged at 24 or 48 hours post-transfection. MDA-MB-231 cells were transfected with Lipofectamine 2000 (Invitrogen), IC-21 cells were transfected using AMAXA (Walkersville, MD), and HFF were transfected with PolyJet (SignaGen Laboratories, Ijamsville, MD) according to the manufacturers’ instructions.
cDNA vectors
Human cortactin cDNA was purchased from Invitrogen. A twelve-amino acid segment missing from the actin-binding domain compared to the original sequence (GenBank) was restored by site-directed mutagenesis (Stratagene, La Jolla, CA). The full-length cortactin cDNA was cloned into Bam H1 and Xba1 sites of pGZ21XdZ (Dr. Shin-ichi Aota, NIDCR, NIH) to produce pGFP-Cortactin. The cortactin sequence was confirmed by sequencing.
The plasmid pEIL2RNBC1 was constructed by partially replacing the multiple-cloning site (MCS) of pEGFP-C1 (Clontech, Mountain View, CA) to introduce new MCS sites by inserting the appropriate double-strand oligonucleotides using Bgl11 and Sal1 sites of the parent Clontech pEGFP-C1 plasmid, respectively, after mutating the Bam H1 site in the original MCS using a site-directed mutagenesis kit. The EGFP was then deleted from the construct using Nhe1 and Bgl11 sites and replaced by the extracellular and transmembrane domains of the α subunit of the human IL-2 receptor with deletion of the cytoplasmic domain, which was generated by PCR from pRSVIL2R (Drs. Bruce Howard and Tony Giordano, NICHD) plus addition of a Kozak sequence at the 5‘-end. Fluorescent mCherry flanked by Hind111 and Xba1 sites was produced by PCR from pRSET-B mCherry (a kind gift from Dr. Roger Tsien) and inserted into Hind 111 and Xba1 sites of pEIL2RNBC1 to create pEIL2R-mCherry. The IL-2R and mCherry sequences were confirmed by sequencing.
To construct GFP-Vinculin, chicken vinculin cDNA (a kind gift from Dr. Benjamin Geiger) was inserted in Hind111 and Xba1 sites of pGZ21XdZ.
Live-cell total internal reflection fluorescence microscopy and image processing
Live cells expressing eGFP and mCherry protein chimeras were imaged with an Olympus TIRF three-channel imaging system: an Olympus CS-71 microscope was equipped with a Photometrics Cascade II:1024 EM-CCD camera (Photometrics, Tucson, AZ) and iFLEX-Mustang 488 nm, 561 nm, and 630 nm lasers (Quoptiq, Hamble, UK), and operated by MetaMorph software (Molecular Devices, Downingtown, PA). Images were acquired with an Olympus 150X/1.4 N.A. oil objective. An environmental chamber mounted on the microscope maintained constant 37°C temperature, CO2 level, and humidity. For live-cell fluorescence microscopy, cells were seeded in dishes with glass bottoms (MatTek, Ashland, MA) coated with 5 μg/ml HPFN and blocked with 1% heat-denatured BSA. For live-cell time-lapse microscopy, images were acquired with a 2 sec frame delay, i.e., every 2 sec. The acquired raw images were processed using the FFT/Blur filter with sensitivity set to 50 (MetaMorph).
Confocal fluorescence microscopy
Cells transiently expressing eGFP-cortactin and IL2R-mCherry were polymerized between two layers of 3 mg/ml rat tail collagen type I fluorescently labeled with AlexaFluor-647. After overnight incubation, live cells expressing eGFP and mCherry protein chimeras were imaged with a Zeiss Axiovert 200M microscope equipped with a Zeiss Plan-Apochromat 63X/1.4 N.A. oil objective, using a CSU-21 confocal live cell imager (Yokogawa Electric Corporation) and 488nm, 561 nm, and 633 nm lasers lines, all controlled by MetaMorph.
Fluorescent-gelatin degradation assay
Glass coverslips were coated with a thin layer of gelatin fluorescently labeled with AlexaFluor dye as described elsewhere (Artym et al., 2009, Artym et al., 2006). The fluorescent gelatin layer was coated with 10 μg/ml human plasma FN by incubating for 1 h at room temperature and blocked with 1% heat-denatured bovine serum albumin (BSA) for 1 h at room temperature. To assess the ability of cells to form invadopodia and degrade matrix, cells were plated on coverslips coated with fluorescent gelatin matrix at 4×103 cells/ml and incubated at 37°C. Cells were fixed with 4% paraformaldehyde/5% sucrose in PBS for 20 min and permeabilized for 10 min with 0.5% Triton X-100. Cells were immunolabeled for cortactin and actin. Confocal images were collected using a Zeiss510-NLO laser scanning confocal microscope (Carl Zeiss, Thornwood, NY) with a Zeiss Plan-Apochromat 63X/1.4 N.A. oil objective. Images were analyzed with MetaMorph.
Immunocytochemistry
Cells transfected with pEIL2R-mCherry were allowed to adhere to FN-coated glass-bottom dishes. After overnight incubation, cells were fixed with 3.7% formaldehyde for 20 min and then permeabilized with 0.5%Triton X-100 in PBS for 10 min at room temperature. The cells were washed with PBS and labeled with 10 μg/ml primary antibodies for 1 hour followed by labeling with secondary fluorochrome-conjugated antibodies for 30 min. Immuno-stained cells were maintained in PBS and imaged immediately after labeling using TIRF microscopy.
Statistical analysis
The data are presented as the mean value from three pooled independent trials with the corresponding standard deviation.
RESULTS
To study membrane dynamics at invadopodia and to compare directly the membrane dynamics at invadopodia, podosomes and focal adhesions, we employed TIRF microscopy. This procedure permits visualization of the ventral surface of the cell adherent to a thin layer of 2D ECM. In TIRF, excitation light is reflected at a critical angle from the surface of the coverslip, creating an evanescent field that only excites chromophores in a narrow zone extending approximately 100-150 nm from the coverslip. The strength of the evanescent field decreases exponentially with increasing distance from the coverslip – this feature provides conditions that eliminate fluorescence from the remainder of the cell located further from the coverslip, thereby permitting collection of images representing only the ventral cell membrane and cytoplasm in very close proximity to the cell membrane. Human plasma FN at a concentration of 5 μg/ml was chosen as the ECM ligand for the 2D matrix based on its ability to promote invadopodia formation by adhering breast carcinoma MDA-MB-231 cells; it also sustains formation of podosomes by IC-21 macrophages, as well as adhesion formation by HFFs. To study membrane dynamics at the invadopodia, we have chosen breast carcinoma MDA-MB-231 cells stably expressing wild-type c-Src (wt c-Src). As we have previously shown, expression of wt c-Src increases the number of invadopodia-producing cells and increases the number of invadopodia formed per cell (Artym et al., 2006). The use of this cell line expressing increased number of invadopodia greatly facilitates live-cell imaging of invadopodia.
To study membrane dynamics, cell membranes were fluorescently labeled by transiently expressing pEIL2R-mCherry as a membrane marker, where mCherry is attached to N-terminal end of the non-signaling interleukin-2 receptor α subunit (IL-2R) with a deleted cytoplasmic domain to ensure its inability to signal or cluster cytoplasmic proteins. IL-2R is primarily expressed in T-lymphocytes, making it a convenient experimental membrane marker for cells of other origins (Rochman et al., 2009). In addition, to confirm our results obtained using expression of pEIL2R-mCherry, myristoylated-GFP was used as an alternative marker of cell membranes. Both pEIL2R-mCherry and myristoylated-GFP (data not shown) yielded comparable results for membrane dynamic behavior at invadopodia, podosomes, and focal adhesions. Transiently expressed GFP-cortactin was chosen as a marker for actin cores of invadopodia and podosomes, whereas GFP-vinculin was used as a marker for focal adhesions.
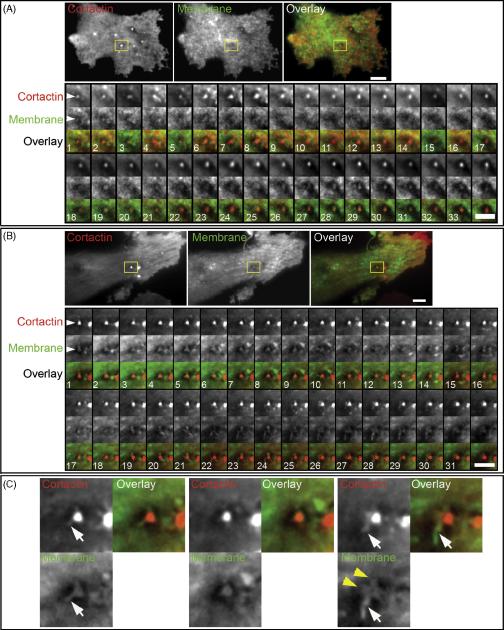
Cortactin arrival to the ventral cell membrane of carcinoma MDA-MB-231 cells adherent to 2D ECM results in the formation of the actin-cortactin structural core of the invadopodium (Artym et al., 2006). The incubation of wt c-Src MDA-MD-231cells on fluorescent gelatin matrix coated with 10 μg/ml FN and blocked with 1% heat-denatured BSA results in the formation of actin-cortactin-rich aggregates, indicative of invadopodia, at the cell membrane adherent to the fluorescent matrix (Supplemental Figure 1, A and B). An average of ~30% of these aggregates are localized to the foci of degraded matrix (Supplemental Figure 1, A and B), indicating that about a third of the membrane-associated actin-cortactin aggregates mature into matrix-degrading mature invadopodia when wt c-Src MDA-MB-231 cells are adherent to FN. This finding confirms that FN matrix is able to induce mature invadopodia capable of ECM degradation and justifies the use of FN as a matrix for invadopodia studies with TIRF microscopy. We examined the dynamics of the cell membrane at the invadopodium of wt c-Src MDA-MB-231 cells at early time points after cortactin arrival to the cell membrane, which indicates formation of the early invadopodium (Figure 1, A; Supplementary Movie 1). We also characterized membrane dynamics at cortactin aggregates associated with the cell membrane for longer periods of time, representing assembly of the mature invadopodium (Figure 1, B). We found that the onset of assembly of the actin-cortactin core of the invadopodium, which occurs upon the arrival of cortactin to the cell membrane at site of cell-ECM interaction, is accompanied by the extension of tiny cell membrane process toward the ECM (Figure 1, A). The production of this membrane extension occurs concomitantly with the formation of an invagination of the cell membrane around the invadopodial process, as detected by the appearance of a dark region surrounding the invadopodial process (which indicates that the membrane is sufficiently far from the coverslip that the TIRF evanescent field cannot produce fluorescence). We have termed the cell membrane invagination that contains invadopodial membrane extensions the membrane lacuna. Formation of the lacuna and extension of the invadopodial processes are very dynamic and interrelated events. Disassembly of an invadopodial process and narrowing of the ventral cell membrane space near the cortactin aggregate can repeat several times during the early phase of invadopodia formation. However, the invadopodial process eventually becomes well-organized, the size of the tip interacting with the ECM increases, and the tip begins to form a small ruffle.
Figure 1.
Cell membrane dynamics at invadopodia. Human breast carcinoma wt c-Src MDA-MB-231 cells expressing GFP-Cortactin and membrane marker IL2R-mCherry were cultured on a layer of FN. A. Initiation of invadopodium formation. An invadopodium in early stages of formation is shown selected with a region-of-interest (ROI) frame. The ROI montage shows every 6th frame, and the total duration of the time-lapse image acquisition used for the montage is 6.9 min. Scale bar indicates 3 μm. B. Mature invadopodium. This stage is characterized by a high degree of cell membrane morphological changes as exemplified by the invadopodial complex analyzed in the ROI. The montage of ROIs shows every 5th frame, and the total duration of the time-lapse image acquisition used for the montage is 5.3 min. Scale bar indicates 3 μm. C. Enlarged views of three frames from the ROI montage shown in B. White arrows point to filament-like membrane extensions that originate from the cortactin core; they also indicate the furthest extent of cortactin along the membrane filament-like protrusion. Yellow arrowheads point to membrane ruffles.
In well-established, mature invadopodia of wt c-Src MDA-MB-231 cells, examination of membrane at the long-lived cortactin cores revealed that the cell membrane undergoes continual dynamic morphological changes, with the extension of transient, filament-like protrusions from the initial primary invadopodial process (Figure 1, B; Supplementary Movie 2). The consecutive time frames in Fig. 1 document the active elongation of filament-like extensions and ruffling of the cell membrane from various sides of the invadopodial process (Figure 1, B and C). The diameter of these filament-like extensions measured 0.23 ± 0.05 μm, and they achieve lengths of 0.88 ± 0.30 μm (Table 1). The main invadopodial process with extending filament-like protrusions is located inside of the cell membrane lacuna, which has an average diameter of 1.35 ± 0.27 μm (Table 1). It is important to stress that cortactin does not extend the entire distance to the tips of the membrane filament-like extensions (Figure 1, C). Multiple cortactin jetting vesicles can be seen to dock to and to dissipate from the cortactin core during life span of the invadopodia (Supplementary Movie 2).
Table 1.
Dimensions of invadopodial ECM invasive structures.
| Structural component |
Parameter | Average size1, μm | I2 | N3 |
|---|---|---|---|---|
| Cortactin aggregate |
Diameter | 0.62 ± 0.17 | 12 | 4 |
| Lacuna | Diameter | 1.35 ± 0.27 | 8 | 4 |
| Filopodia-like extension |
Length | 0.88 ± 0.30 | 8 | 3 |
| Filopodia-like extension |
Diameter | 0.23 ± 0.05 | 8 | 3 |
Average size in micrometers ± SD
number of invadopodial structures examined
number of repeats performed
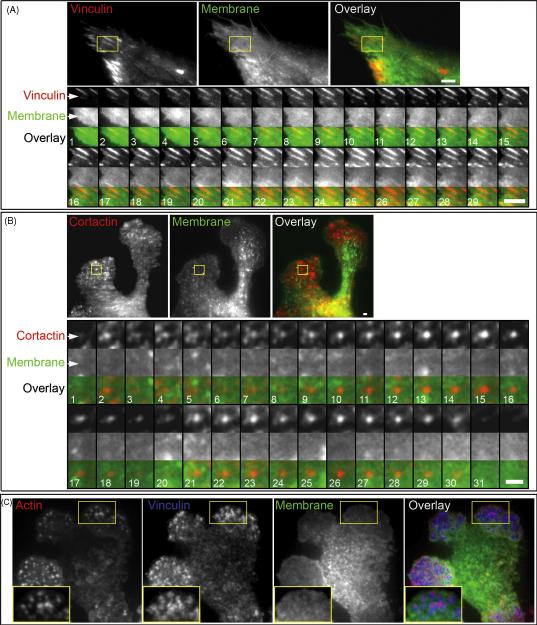
To exclude the possibility that the expression of the exogenous membrane marker pEIL2R-mCherry can artificially stimulate the formation of micro-spikes or microfilaments on the cell surface, we used HFF as a control and examined membrane dynamics at the focal adhesions of this fibroblast. Electron microscopy studies of the fibroblast ventral surface adherent to the ECM have shown no membrane micro-spikes or filament-like extensions formed at the regions of focal adhesions (Abercrombie et al., 1971, Heath and Dunn, 1978). Transient co-expression of GFP-Vinculin and pEIL2R-mCherry in HFFs visualized focal adhesion initiation and growth. In contrast to invadopodia, they detected only a morphologically homogeneous and smooth appearance of the cell membrane near the adhesion (Figure 2, A; Supplementary Movie 3). No filament-like extensions could be detected at the focal adhesions. Instead, broad retrograde membrane flow was visible near the focal adhesions at the leading front of the migrating HFF cells.
Figure 2.
A. Dynamics of the cell membrane at the focal adhesion of HFF transiently expressing GFP-Vinculin and membrane marker IL2R-mCherry. HFF were cultured on a 2D FN matrix. A representative focal adhesion is highlighted by the ROI. The ROI montage shows every 10th frame of the time lapse of focal adhesion extension; the total duration of time-lapse image acquisition used for the montage is 12.7 min. Scale bar indicates 3 μm. B. Dynamics of the cell membrane at a podosome of IC-21 macrophage transiently expressing GFP-Cortactin and membrane marker IL2R-mCherry. Transfected macrophages were cultured on a 2D FN matrix. The ROI montage shows every 10th frame, and the total duration of the time-lapse image acquisition used for the montage is 5.2 min. Scale bar indicates 1 μm. C. TIRF microscopy of IC-21 macrophage expressing IL2R-mCherry and immuno-labeled with phalloidin-Alexa488 and anti-vinculin Cy-5-conjugated antibody. Enlargements of the ROI are shown as insets.
Examination of membrane dynamics at the podosomes of IC-21 macrophages in cells transiently co-expressing GFP-Cortactin and pEIL2R-mCherry revealed that the cell membrane adjacent to the actin-cortactin core of the podosome does not show formation of any membrane processes or protrusions (Figure 2, B; Supplementary Movie 4). Time-lapse analyses of the membrane at the cortactin core of the podosome throughout its history from initial docking of the cortactin aggregate to the cell membrane adherent to the 2D FN matrix to the disassembly of the cortactin aggregate showed no morphological changes of the cell membrane during the lifespan of the podosome. To confirm the formation of podosomes in cells expressing pEIL2R-mCherry, podosomes were immuno-labeled for actin and vinculin and examined with TIRF microscopy (Figure 2, C). In macrophages, podosomes were detected as actin cores with a surrounding vinculin ring that occurred in “structurally flat” areas of the cell membrane, i.e., the cell membrane was relatively uniform in intensity with no evidence for membrane processes or filament-like extensions, or a lacuna.
DISCUSSION
Using live-cell, high-resolution TIRF microscopy, we have characterized and compared cell membrane dynamics at the invadopodia of MDA-MB-231 carcinoma cells, podosomes of IC-21 macrophages, and focal adhesions of HFF cells, each adhering to a thin 2D FN matrix. To our knowledge, we are the first to demonstrate that among these three cell adhesion types, invadopodia stand out as the only cell membrane structure where the membrane undergoes rapid, dramatic morphological changes during invadopodial assembly and life-span.
We found that the initiation of invadopodium formation upon cortactin arrival to the cell membrane adherent to the FN matrix results in the formation of fine membrane processes or protrusions that extend from the lacuna invagination of the ventral cell membrane; the fine protrusions interact with the FN matrix. Maturation of the invadopodium is accompanied by morphological changes of the membrane at the tip of the primary invadopodial process: the tip of the process widens and begins to ruffle. In addition in mature invadopodia, the primary invadopodial process can rapidly change its shape and start to emanate filament-like invadopodial protrusions. Multiple filament-like invadopodia can extend from a single invadopodial core.
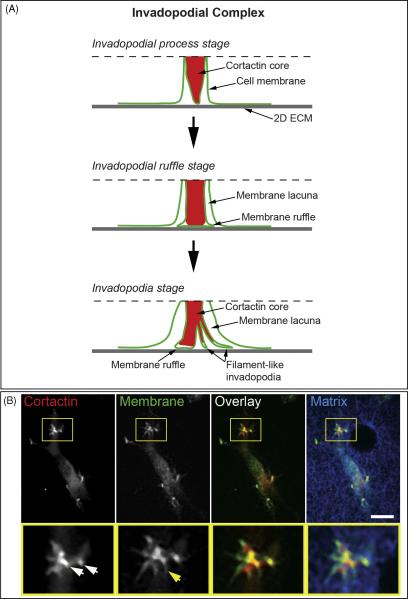
Early electron microscopy (EM) studies described invadopodia as long filament-like protrusions of the ventral cell membrane adherent to the ECM that insert into degraded ECM. The filament-like protrusions originating from the invadopodial core process that we have detected with TIRF microscopy correspond in size to the filamentous invadopodia identified by EM. These findings raise a question about the proper naming of the filament-like membrane extensions identified by TIRF relative to the actin-cortactin invadopodial cores that are used as a marker for invadopodia in fluorescence microscopy studies. More studies are required to confirm whether the filament-like invadopodial extensions or the invadopodial actin-cortactin cores directly degrade the ECM, as well as to clarify the relationship between the filament-like invadopodia and filopodia of non-invasive cells. However, our TIRF studies clearly indicate that the fluorescently-labeled actin-cortactin core of the invadopodium represents part of a complex invasive structure that we suggest should be described as the invadopodial complex, reserving the term “invadopodia” for the filament-like protrusions (Figure 3). Consequently, we suggest referring collectively to the actin-cortactin core, invadopodial membranes, and membrane lacuna together as the invadopodial complex. Recently, the term “invadopodial complex” was proposed to include co-localized cortactin and phospho-tyrosine at a punctuate localization at the plasma membrane associated with matrix degradation (Bowden et al., 2006). Here, we further clarify the term invadopodial complex as encompassing a dynamic invasion superstructure that can exist in several stages.
Figure 3.
A, Model of the invadopodial complex. Dashed line indicates the upper boundary of the membrane-cytoplasmic zone visualized by TIRF microscopy. B, Invadopodial complex formation in wt c-Src MDA-MB-231 cells invading 3D matrix of 3 mg/ml rat tail collagen type I fluorescently labeled with Alexa647. MDA-MB-231 cells transiently expressing GFP-Cortactin and IL2R-mCherry were polymerized between two layers of rat tail collagen and allowed to invade 3D collagen matrix overnight. White arrows point to cortactin-rich invadopodial cores and yellow arrow points to filament-like invadopodia. Scale bar indicates 10 μm.
Depending on the dynamic status of the invadopodial membrane, it can exist at the invadopodial process stage, invadopodial ruffle stage, or invadopodia stage. We summarize the dynamic nature of the invadopodial complex in Figure 3 by presenting schematic diagrams of each of these stages of the invadopodial complex. In the diagrams, the space between the surface of 2D ECM and the dotted line represents an optical slice of the cell by TIRF microscopy that indicates the depth of the evanescent field of about 100-150 nm, which represents the zone within which fluorescent molecules can be excited and imaged. Due to the intrinsic shallow depth of TIRF microscopy, we could not visualize the parts of the actin-cortactin core of the invadopodial complex deeper in the cytoplasm (farther from the coverslip and beyond the evanescent field). A previous study using wide-field fluorescence microscopy identified mature invadopodia of A375 melanoma cells as actin comet-based structures because of the dynamic nature of the actin at the proximal side of the invadopodial actin core (Baldassarre et al., 2006). More studies are needed in the future using correlative wide-field and TIRF microscopy in conjunction with electron tomography to examine this phenomenon for different stages of the invadopodial complex in other tumor cells. From our time-lapse studies, we have established that cortactin enters the filament-like invadopodia and elongates together with the protruding membrane filament of invadopodia. However, we find that cortactin does not extend completely to the tip of the filament-like invadopodial protrusions or to the edge of the membrane ruffles. These various dynamics of cortactin might be linked to, or possibly even regulate, the dynamic actin polymerization that underlies ruffling and filament-like invadopodial protrusion formation at the invadopodial complex. These cortactin dynamics may also suggest that cortactin could play a role in stabilizing a proximal part of the invadopodial complex as a micro-scale invasive leading edge of the cell that degrades and penetrates ECM locally enough to allow the distal part of the invadopodial complex freedom to ruffle and protrude filamentous invadopodia that interact with and locally degrade ECM. The formation of the invadopodial process inside of the membrane lacuna could also imply that actin polymerization at the invadopodial actin core can generate enough force to push against the ECM to protrude outside the cell and into the matrix. However, in the case of the thin 2D matrix on glass, polymerization of actin at the actin-cortactin core of the invadopodial complex likely pushes the invadopodial core into the cell as the membrane-associated part of invadopodial complex presses against the rigid ECM substrate. This membrane rearrangement would promote cell membrane invagination to form the lacuna. To test this hypothesis and to confirm the existence of the invadopodial complex in cells invading a 3D matrix permissive for invadopodia extension, we examined invasion of wt c-Src MDA-MB-231 cells transiently expressing GFP-cortactin and IL2R-Cherry into 3D collagen type I matrix (Figure 3, B). We find that cortactin-rich invadopodial processes protrude into this 3D collagen matrix, and some of them extend filament-like invadopodia of different lengths. This result demonstrates that cells have the capacity to insert invadopodial processes into 3D matrix, and that the tip of this invadopodial process can extend dynamic filament-like invadopodia to interact with the matrix. Since the invadopodial process protrudes from the cell and becomes embedded into the 3D matrix, the membrane lacuna is replaced by extracellular space. Thus, the invadopodial complex model remains valid for cells invading 3D matrices permissive for invasion if we substitute extracellular space for the membrane lacuna.
Comparison of the membrane dynamics at the actin-cortactin cores of the invadopodial complex versus podosomes indicates that the invadopodial complex and podosomes differ substantially. Although both of these structures have similar actin-cortactin cores as detected by immunofluorescence, and both are known to be involved in ECM degradation, podosomes do not exhibit any central membrane protrusion and filament-like extension formation in macrophages cultured on 2D FN matrix. The region of macrophage cell membrane adherent to the 2D ECM is a region rich in podosomes visualized as actin cores surrounded by vinculin rings, but which appears to lack any of the membrane extensions characteristic of invadopodial complexes. TIRF microscopy shows that the membrane in regions rich in podosomes has a sheet-like appearance when it overlays ECM. We cannot exclude the possibility of cell membrane forming small and shallow nanometer-high arches between podosomes by slightly lifting off the ECM that cannot be resolved by TIRF. This hypothesis is consistent with previously published EM studies. Thus, based on the striking differences in the behavior of the cell membrane at the invadopodial complex and podosomes, we suggest that the relationship between the actin-cortactin cores and cell membrane at these two cell adhesion structures are based on distinct mechanisms. Perhaps mechanisms controlling actin polymerization at the membrane near the actin-cortactin structural cores are different.
Supplementary Material
Movie 1. Membrane dynamics at the early invadopodium stage of the wt c-Src MDA-MB-231carcinoma cell. Upper image is cortactin, middle image is the membrane marker, and lower image is an overlay of cortactin in red and membrane marker in green. TIRF images of the cortactin and membrane marker were collected every 2 sec. Total duration of the original data acquisition was 4.6 min, and the movie is played at a speed of 1 frame/30th of a second.
Movie 2. Membrane dynamics at the mature invadopodium of the carcinoma wt c-Src MDA-MB-231 cell. Upper image is cortactin, middle image is membrane marker, and lower image is the overlay of cortactin in red and membrane marker in green. TIRF images of the cortactin and membrane marker were collected every 2 sec. Total acquisition time was 8.5 min, and the movie is played at 30 frames/second.
Movie 3. Membrane dynamics at the focal adhesion of HFF. Upper image is vinculin, middle image is membrane marker, and lower image is an overlay of vinculin in red and membrane marker in green. TIRF images of the vinculin and the membrane marker were collected every 2 sec. Total duration was 10 min, and the movie is played at 30 frames/second.
Movie 4. Membrane dynamics at podosome of IC-21 macrophage. Upper image is cortactin, middle image is membrane marker, and lower image is an overlay of cortactin in red and membrane marker in green. TIRF images of cortactin and the membrane marker were collected every 2 sec. Total duration was 10.4 min, and the movie is played at 30 frames/second.
Supplemental Figure 1. Invadopodia of wt c-Src MDA-MB-231 cells and their matrix-degrading potential. Wt c-Src MDA-MB-231 cells were cultured on 10 μg/ml FN coated on a thin layer of fluorescent gelatin and blocked with 1% heat-denatured BSA. After overnight incubation, cells were fixed and immunostained for actin and cortactin and imaged with confocal microscopy. A, Fluorescent images were analyzed with MetaMorph software to calculate the mean total number ofactin-cortactin aggregates at the ventral cell membrane that form upon cell adhesion to FN (white bar), as well as the mean number of actin-cortactin aggregates localized to areas of matrix degradation (black bar). B, Representative confocal micrograph of actin cores of invadopodia of wt c-Src MDA-MB-231. Mature invadopodia are identified as actin aggregates that localize to areas of degraded matrix. Scale bar indicates 10 μm.
Acknowledgements
This work was supported by Pathway to Independence Award K99CA129205 from NCI (V.V.A.), NIH/NIDCR Intramural project DE 000719 (K.M.Y.), and NIH R01 CA112673 (S.C.M.).
Abbreviations used in this paper
- ECM
extracellular matrix
- TIRF microscopy
total internal reflection fluorescence microscopy
- 2D
two-dimensional
- 3D
three-dimensional
- HFF
human foreskin fibroblast
- HPFN
human plasma fibronectin
- FN
fibronectin
Literature cited
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp Cell Res. 1971;67:359–367. doi: 10.1016/0014-4827(71)90420-4. [DOI] [PubMed] [Google Scholar]
- Akisaka T, Yoshida H, Suzuki R, Takama K. Adhesion structures and their cytoskeleton-membrane interactions at podosomes of osteoclasts in culture. Cell Tissue Res. 2008;331:625–641. doi: 10.1007/s00441-007-0552-x. [DOI] [PubMed] [Google Scholar]
- Akiyama SK. Curr Protoc Cell Biol. 2001. Purification of fibronectin; p. 15. Chapter 10, Unit 10. [DOI] [PubMed] [Google Scholar]
- Artym VV, Yamada KM, Mueller SC. ECM degradation assays for analyzing local cell invasion. Methods Mol Biol. 2009;522:211–219. doi: 10.1007/978-1-59745-413-1_15. [DOI] [PubMed] [Google Scholar]
- Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–3043. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- Baldassarre M, Ayala I, Beznoussenko G, Giacchetti G, Machesky LM, Luini A, Buccione R. Actin dynamics at sites of extracellular matrix degradation. Eur J Cell Biol. 2006;85:1217–1231. doi: 10.1016/j.ejcb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Baldassarre M, Pompeo A, Beznoussenko G, Castaldi C, Cortellino S, McNiven MA, Luini A, Buccione R. Dynamin participates in focal extracellular matrix degradation by invasive cells. Mol Biol Cell. 2003;14:1074–1084. doi: 10.1091/mbc.E02-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–4449. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- Bowden ET, Onikoyi E, Slack R, Myoui A, Yoneda T, Yamada KM, Mueller SC. Co-localization of cortactin and phosphotyrosine identifies active invadopodia in human breast cancer cells. Exp Cell Res. 2006;312:1240–1253. doi: 10.1016/j.yexcr.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Buccione R, Caldieri G, Ayala I. Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev. 2009;28:137–149. doi: 10.1007/s10555-008-9176-1. [DOI] [PubMed] [Google Scholar]
- Chen WT. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J Exp Zool. 1989;251:167–185. doi: 10.1002/jez.1402510206. [DOI] [PubMed] [Google Scholar]
- Gil-Henn H, Destaing O, Sims NA, Aoki K, Alles N, Neff L, Sanjay A, Bruzzaniti A, De Camilli P, Baron R, Schlessinger J. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2(−/−) mice. J Cell Biol. 2007;178:1053–1064. doi: 10.1083/jcb.200701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimona M, Buccione R. Adhesions that mediate invasion. Int J Biochem Cell Biol. 2006;38:1875–1892. doi: 10.1016/j.biocel.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol. 2008;20:235–241. doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Heath JP, Dunn GA. Cell to substratum contacts of chick fibroblasts and their relation to the microfilament system. A correlated interference-reflexion and high-voltage electron-microscope study. J Cell Sci. 1978;29:197–212. doi: 10.1242/jcs.29.1.197. [DOI] [PubMed] [Google Scholar]
- Kelly T, Mueller SC, Yeh Y, Chen WT. Invadopodia promote proteolysis of a wide variety of extracellular matrix proteins. J Cell Physiol. 1994;158:299–308. doi: 10.1002/jcp.1041580212. [DOI] [PubMed] [Google Scholar]
- Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Luxenburg C, Geblinger D, Klein E, Anderson K, Hanein D, Geiger B, Addadi L. The architecture of the adhesive apparatus of cultured osteoclasts: from podosome formation to sealing zone assembly. PLoS One. 2007;2:e179. doi: 10.1371/journal.pone.0000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxenburg C, Parsons JT, Addadi L, Geiger B. Involvement of the Src-cortactin pathway in podosome formation and turnover during polarization of cultured osteoclasts. J Cell Sci. 2006;119:4878–4888. doi: 10.1242/jcs.03271. [DOI] [PubMed] [Google Scholar]
- Marchisio PC, Cirillo D, Naldini L, Primavera MV, Teti A, Zambonin-Zallone A. Cell-substratum interaction of cultured avian osteoclasts is mediated by specific adhesion structures. J Cell Biol. 1984;99:1696–1705. doi: 10.1083/jcb.99.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchisio PC, Cirillo D, Teti A, Zambonin-Zallone A, Tarone G. Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp Cell Res. 1987;169:202–214. doi: 10.1016/0014-4827(87)90238-2. [DOI] [PubMed] [Google Scholar]
- Mueller SM, Artym VV, Kelly TJ. Invadopodia: Interface for Invasion. In: Hoyer-Hansen G, Blasi F, Sloane BF, editors. The Cancer Degradome-Proteases and Cancer Biology. Springer; New York: 2008. pp. 401–429. [Google Scholar]
- Myoui A, Nishimura R, Williams PJ, Hiraga T, Tamura D, Michigami T, Mundy GR, Yoneda T. C-SRC tyrosine kinase activity is associated with tumor colonization in bone and lung in an animal model of human breast cancer metastasis. Cancer Res. 2003;63:5028–5033. [PubMed] [Google Scholar]
- Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009;122:3015–3024. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clin Exp Metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie 1. Membrane dynamics at the early invadopodium stage of the wt c-Src MDA-MB-231carcinoma cell. Upper image is cortactin, middle image is the membrane marker, and lower image is an overlay of cortactin in red and membrane marker in green. TIRF images of the cortactin and membrane marker were collected every 2 sec. Total duration of the original data acquisition was 4.6 min, and the movie is played at a speed of 1 frame/30th of a second.
Movie 2. Membrane dynamics at the mature invadopodium of the carcinoma wt c-Src MDA-MB-231 cell. Upper image is cortactin, middle image is membrane marker, and lower image is the overlay of cortactin in red and membrane marker in green. TIRF images of the cortactin and membrane marker were collected every 2 sec. Total acquisition time was 8.5 min, and the movie is played at 30 frames/second.
Movie 3. Membrane dynamics at the focal adhesion of HFF. Upper image is vinculin, middle image is membrane marker, and lower image is an overlay of vinculin in red and membrane marker in green. TIRF images of the vinculin and the membrane marker were collected every 2 sec. Total duration was 10 min, and the movie is played at 30 frames/second.
Movie 4. Membrane dynamics at podosome of IC-21 macrophage. Upper image is cortactin, middle image is membrane marker, and lower image is an overlay of cortactin in red and membrane marker in green. TIRF images of cortactin and the membrane marker were collected every 2 sec. Total duration was 10.4 min, and the movie is played at 30 frames/second.
Supplemental Figure 1. Invadopodia of wt c-Src MDA-MB-231 cells and their matrix-degrading potential. Wt c-Src MDA-MB-231 cells were cultured on 10 μg/ml FN coated on a thin layer of fluorescent gelatin and blocked with 1% heat-denatured BSA. After overnight incubation, cells were fixed and immunostained for actin and cortactin and imaged with confocal microscopy. A, Fluorescent images were analyzed with MetaMorph software to calculate the mean total number ofactin-cortactin aggregates at the ventral cell membrane that form upon cell adhesion to FN (white bar), as well as the mean number of actin-cortactin aggregates localized to areas of matrix degradation (black bar). B, Representative confocal micrograph of actin cores of invadopodia of wt c-Src MDA-MB-231. Mature invadopodia are identified as actin aggregates that localize to areas of degraded matrix. Scale bar indicates 10 μm.