Abstract
GBM (glioblastoma multiforme) is a highly aggressive brain tumour with very poor prognosis despite multi-modalities of treatment. Furthermore, recent failure of targeted therapy for these tumours highlights the need of appropriate rodent models for preclinical studies. In this review, we highlight the most commonly used rodent models (U251, U86, GL261, C6, 9L and CNS-1) with a focus on the pathological and genetic similarities to the human disease. We end with a comprehensive review of the CNS-1 rodent model.
Keywords: U251, U87, GL261, C6, 9L, CNS-1
Abbreviations: Akt, protein kinase B; BBB, blood–brain barrier; BEHAB, brain-enriched hyaluronan-binding; CNS, central nervous system; CXCR4, CXC chemokine receptor 4; EGFR, endothelial growth factor receptor; GBM, glioblastoma multiforme; GFAP, glial fibrillary acidic protein; H/E, haematoxylin/eosin; HIF-1α, hypoxia-inducible factor-1α; IGF-1, insulin-like growth factor 1; IL-1α, interleukin-1α; MMP, matrix metalloproteinase; MNU, methylnitrosourea; MRI, magnetic resonance imaging; NCAM, neural cell adhesion molecule; PI3K, phosphoinositide 3-kinase; TNFα, tumour necrosis factor α; VEGF, vascular endothelial growth factor; PTEN, phosphatase and tensin homologue deleted on chromosome 10
INTRODUCTION
GBM (glioblastoma multiforme) is the most common and aggressive primary brain tumour, with an extremely poor prognosis and very few therapeutic advances in the last decade (Wen and Kesari, 2008). Patients have an average survival of 1–2 years after diagnosis with current available treatments (Wen and Kesari, 2008). Recently, research has advanced our understanding of the genetics and molecular pathology of the disease and has identified several therapeutic targets, such as inhibitors of VEGF (vascular endothelial growth factor) and EGFR (endothelial growth factor receptor) (Van Meir et al., 2010). Unfortunately, these targeted therapies have not shown success in clinical trials, despite strong preclinical data (Furnari et al., 2007; Krakstad and Chekenya, 2010; Van Meir et al., 2010). Although these factors developed for targeted therapy (e.g. VEGF and EGFR) are known to be overexpressed in GBM, the failure of successful clinical outcomes reveals the complex nature of the disease. The dichotomy between what should be successful therapeutic targets and the ineffectiveness of drugs directed towards them underscores the critical importance of appropriate and predictive rodent models in the study of innovative therapies for the treatment of gliomas. As researchers begin to better understand the complex interaction between glioma cells and their CNS (central nervous system)/immune microenvironment, a shift away from xenograft tumour models towards non-immunogenic models is now at the forefront of GBM research. This review will first briefly discuss the most commonly used rodent models of GBM, with a focus on the potential for these models to recapitulate the human brain neoplasm on genetic, imaging, pathophysiology and therapeutic levels. Finally, we present a comprehensive review of the non-immunogenic CNS-1 rodent model for GBM.
U251 GLIOMA MODEL
General protocol
The U251 malignant glioma cell line was originally established from a 75-year-old male with GBM by Ponten and others (Ponten, 1975; Houchens et al., 1983). This GBM cell line is known to mimic the salient features of human GBM and as such has received significant attention over the last four decades in xenogeneic mouse models of cancer (Houchens et al., 1983; Harada et al., 1994; Husain et al., 1998; Candolfi et al., 2007; Radaelli et al., 2009). The U251 cell line has been used both in subcutaneous and intracranial mouse models (Camphausen et al., 2005). For example, the intracranial model involves injections of 1×105–1×106 of U251 cells suspended in 5–25 ml (Houchens et al., 1983; Candolfi et al., 2007), approx. 2 mm anterior to the bregma and 2 mm to the left (or right) of the midline inserted 2–3 mm deep into the striatum of the brain (Houchens et al., 1983; Valdes et al., 2010) of athymic nude mice.
Pathology
This intracranial mouse model recapitulates most of the key salient features of GBMs at the histopathological level (Candolfi et al., 2007; Wen and Kesari, 2008; Radaelli et al., 2009). Under H/E (haematoxylin/eosin) staining, U251 cells show an infiltrative pattern of invasion into normal brain parenchyma, significant foci of palisading necrosis, a tortuous pattern of microvascular proliferation, hypertrophic endothelium, cellular pleomorphism, giant multinucleated cells, atypia including mitotic figures and irregular nucleoli, and foci of oedema and haemorrhage (Candolfi et al., 2007; Radaelli et al., 2009). While the invasion pattern is not strongly diffused along white matter tracks, it does present perivascular satellitosis and subpial spread. However, this rodent model lacks perineuronal satellitosis and invasion along white matter tracks. Immunohistochemical analysis of U251 xenografts shows striking similarities to GBMs, with neoplastic cells positive for GFAP (glial fibrillary acidic protein), vimentin and S100B staining (Kleihues and Cavenee, 2000; Brat and Van Meir, 2004; Homma et al., 2006; Radaelli et al., 2009). Tumour cells also show high levels of cellular proliferation, with over 50% of tumour cells staining positive for Ki-67. At regions of pseudo-palisading necrosis and perinecrotic areas, these tumours display positive caspase 3 and HIF1-α (hypoxia-inducible factor-1α) staining (Radaelli et al., 2009). Finally, in recent years, interest has arisen regarding tumour stem cells (Van Meir et al., 2010). This tumour model contains a CD133+ subpopulation of cells able to form neurosphere aggregates with self-propagating potential (Qiang et al., 2009).
Genetics
The U251 xenograft model also displays similarities at the genetic level to human GBM (Louis, 1994; Louis et al., 2001; Furnari et al., 2007; Krakstad and Chekenya, 2010; Van Meir et al., 2010), with the identification of non-functional mutant tumour suppressor p53 (Radaelli et al., 2009) as well as mutant PTEN (phosphatase and tensin homologue deleted on chromosome 10). Deletions of p14ARF and p16, two cell cycle suppressor genes whose function is as critical negative regulators of Cdks (cyclin-dependent kinases), and whose deletion leads to loss of activity of the retinoblastoma protein (Furnari et al., 2007), are observed in U251 (Fueyo et al., 1996; Ishii et al., 1999) (Table 2). The PI3K (phosphoinositide 3-kinase)/Akt (also known as protein kinase B) pathway shows up-regulation in U251 as a result of high Akt expression. This has been shown to be a contributing factor in the increased survival, proliferation, migration, angiogenesis and resistance to apoptosis observed in GBM (Koul et al., 2006; Furnari et al., 2007; Radaelli et al., 2009). A study by Camphausen et al. (2005). reported gene expression profile differences between subcutaneous and intracranial tumour models, noting the importance of different in vivo growth conditions and the role of the microenvironment in U251.
Table 2. Genetic characteristics of rodent GBM models.
The presence of a genetic mutation is indicated by (+), while overexpression is indicated by (++).
| Genetic mutation or overexpression | ||||||
|---|---|---|---|---|---|---|
| Rodent and model | p14 | p16 | PTEN | p53 | kRAS | EGFR |
| Mouse | ||||||
| U251 | + | + | + | + | + | ++ |
| U87 | + | + | + | − | + | − |
| GL261 | + | + | + | + | + | ++ |
| Rat | ||||||
| C6 | − | + | − | − | N/A | ++ |
| 9L | − | − | − | + | N/A | ++ |
| CNS-1 | N/A | N/A | N/A | N/A | N/A | N/A |
Summary
In summary, U251 recapitulates the salient histological and immunohistochemical features of human GBM. In addition, a number of genetic alterations show similarities to human GBM, including alterations in key tumour suppressors and oncogenic pathways. Further, MRI (magnetic resonance imaging) features of the U251 mouse model correlate with human GBM, including a necrotic centre, poorly demarcated, infiltrative tumour borders and an enhanced rim on T2-weighted imaging; on post-contrast T1-weighted images an intense rim is observed similar to human GBM (Radaelli et al., 2009). Brain volume is a limiting factor for imaging, as more powerful, expensive magnets are required to achieve proportionately better imaging of anatomical structures. Equivalent images of whole mice brains compared with rat brains require a more expensive, powerful MRI magnet to obtain similar resolution of anatomical structures, which may be unavailable to the investigator. Finally, this xenogeneic mouse model is criticized for not reproducing the tumour–host immune response. Future work requires full genome sequencing of U251 and comparison with GBM.
U87 GLIOMA MODEL
General protocol
The U87 GBM model was originally established by Ponten and colleagues from a female with GBM (Ponten, 1975). This tumour model shows significant dissimilarities when compared with the U251 model and human GBM, but has nevertheless received significant attention, especially for assessing tumour angiogenesis and anti-angiogenic therapies (Candolfi et al., 2007; de Vries et al., 2009; Radaelli et al., 2009). Similar to the U251 model, this model shows significant gene expression profile differences between differing in vivo growth conditions (e.g. subcutaneous and intracranial) (Camphausen et al., 2005). Similar to the U251 murine model, the intracranial model involves concentrations of 1×105–1×106 of U87 cells suspended in 5–10 ml, and injections at approx. 1 mm anterior and 3 mm lateral to the bregma inserted 3–4 mm deep into the brain (Roberts et al., 1998; Rubin et al., 2003; Moore et al., 2004; Candolfi et al., 2007) or right striatum (Roberts et al., 1998) of athymic nude mice.
Pathology
The U87 model displays key dissimilarities to the U251 model and human GBM at the histopathological level (Kleihues and Cavenee, 2000; Brat and Van Meir, 2004; Homma et al., 2006; Rong et al., 2006; Candolfi et al., 2007; de Vries et al., 2009; Radaelli et al., 2009). U87 tumours are highly cellular with atypia such as mitotic figures and irregular nucleoli, and profuse neovascularization (Candolfi et al., 2007). Unlike GBM, these tumours show a non-diffusely infiltrative growth pattern, with a well-demarcated tumour mass surrounded by reactive astrocytes (de Vries et al., 2009; Radaelli et al., 2009). Tumour vasculature shows significantly more homogeneous and leaky vessels, which means greater access by systemic drugs, in contrast with GBM (de Vries et al., 2009). Further, necrotic foci are rare in number with features differing from GBM, including no pseudo-palisading patterns and neutrophil infiltration (Candolfi et al., 2007; Radaelli et al., 2009). U87 tumour cells stain negative for GFAP and S100, but positive for vimentin and over 40% positive nuclei for Ki-67. GFAP positive staining is only observed at the reactive astrocyte border surrounding the well-demarcated tumour mass (Candolfi et al., 2007; Radaelli et al., 2009). The necrotic foci also display areas of positive caspase 3 and HIF1-α staining (Radaelli et al., 2009). Finally, similar to the U251 model, U87 tumours show a CD133+ subpopulation of cells able to form neurosphere aggregates with self-propagating potential, of interest to the tumour stem cell community (Qiang et al., 2009).
Genetics
U87 cells show several similarities as well as some key differences to human GBM (Louis, 1994; Louis et al., 2001; Furnari et al., 2007; Krakstad and Chekenya, 2010; Van Meir et al., 2010). Unlike the U251 model, U87 demonstrates a wild-type tumour suppressor p53 (Radaelli et al., 2009). U87 shows a mutant PTEN, deletion of p14ARF and p16 (Fueyo et al., 1996; Ishii et al., 1999), and PI3K/Akt pathway up-regulation as a result of high Akt expression (Koul et al., 2006; Furnari et al., 2007; Radaelli et al., 2009) (Table 2). The study by Camphausen et al. (2005) mentioned above found significant differences between U87 and U251 when grown in vitro or under subcutaneous conditions, but found that both lines displayed similar gene expression patterns when grown intracranially. A recent whole genome sequencing of U87 was performed (Clark et al., 2010). This study examined the various mutations, homo/heterozygous deletions and intra/interchromosomal events, which included over 140 genes affected by complete deletion (e.g. CDKN2A) and common mutations found in gliomas (e.g. PTEN).
Summary
Unlike the U251 xenogeneic model, U87 shows significantly less similarity to human GBM and as such caution must be used when extrapolating conclusions from studies using the U87 cell line. Further, a key feature of human GBM, which significantly contributes to its resistance to therapy and high recurrence rate, is the diffusely invasive infiltration pattern into normal brain parenchyma of tumour cells (Louis et al., 2001). In the U87 model, tumours show clear demarcation from normal parenchyma without the diffusely infiltrative pattern of GBM and lack GFAP or S100B immune staining. At the genetic level, there are key differences and similarities to human GBM, which include a wild-type p53 and aberrant PI3K/Akt respectively. Finally, MRI features of the U87 mouse model including a homogeneous and enhanced, well-demarcated tumour nodule on T2-weighted imaging, which does not correlate well with GBM (Radaelli et al., 2009). Furthermore, investigators should be aware that the xenograft models can incorporate host (non-human) glycolipids, possibly altering the biochemical and immunogenic properties of the model (Ecsedy et al., 1999). Overall, this tumour model has been used to test anti-angiogenic therapies, but limitations of extrapolating conclusions from this model system include the aforementioned dissimilarities to human GBM, as well as the usual concerns with xenogeneic models and limitations with MRI of small rodents.
GL261 GLIOMA MODEL
General protocol
GL261 is a syngeneic mouse model of GBM in C57BL/6 mice that do not require a deficient immune system. As a result this model may mimic more closely the growth and immune response of human GBM. Seligman and Shear originally developed the model in 1939 through intracranial implantation of 20 methylcholanthrene pellets into mice brains (Seligman and Shear, 1939; Newcomb and Zagzag, 2009). In general, 1×105 GL261 tumour cells are injected in a volume of 2–4 μl, 2 mm from the sagittal suture at a depth of 3 mm (Zhu et al., 2010). Several advances in experimental immunotherapy for GBM have been achieved using this model given this is an immune competent model and there are a vast number of antibodies and immune markers available for mice (Maes and Van Gool, 2011).
Pathology
Early descriptions of GL261 cells describe this tumour as displaying ependymoblastoma characteristics. Recent work further describes poorly differentiated cells with morphology similar to human GBM cells (Shapiro et al., 1970; Zagzag et al., 2000). A key feature of this model is the diffusely infiltrating and invasive characteristics of GL261 cells into normal brain. Histopathological examination demonstrates individual cell invasion several millimetres away from the tumour margin (Zagzag et al., 2003; Newcomb and Zagzag, 2009). These invasive cells display several of the 'secondary structures of Scherer': (i) perineuronal satellitosis, (ii) perivascular satellitosis, (iii) subpial spread and (iv) invasion along the white matter tracts (Burger et al., 2002). Furthermore, GL261 tumour cells express CXCR4 (CXC chemokine receptor 4), which has been linked to the invasive and migratory properties of GBM (Zagzag et al., 2008). GL261 also displays endothelial thickening based on increased CD31 expression [30]. Similar to GBM, GL261 tumours have areas of pseudo-palisading necrosis. Along with an invasive phenotype, the GL261 mouse model shares many histopathological markers with human GBM. GL261 tumours stain positive for GFAP and S100 and endothelial cells stain positive for CD31 antigen (Newcomb and Zagzag, 2009). The tumours express areas of angiogenesis and hypoxia, which correlate with expression of VEGF and HIF-1α (Zagzag et al., 2003). Both of these markers have been shown to correlate with an increase in growth and decreased survival in patients with GBM (Zagzag et al., 2006).
Genetics
Genetically, GL261 cells share many key mutations with human GBM. Point mutations in the K-ras oncogene and p53 tumour suppressor gene have been identified (Szatmari et al., 2006; Candolfi et al., 2007) (Table 2). GL261 cells also demonstrate increased activation of the PI3K pathway and phosphorylation of Akt, a serine/threonine protein kinase, leading to downstream signalling both in vitro and in vivo (Choe et al., 2003; Furnari et al., 2007). The PI3K/Akt signalling pathway is often dysregulated in human GBM tumour cells, leading to EGFR expression, increased proliferation, survival and cellular migration observed in GBM (Choe et al., 2003; Furnari et al., 2007; Van Meir et al., 2010).
Summary
Several aspects of GL261 allow for an excellent model of human GBM, particularly when compared with xenograft models (U251 and U87) in immune suppressed mice. GL261 tumour cells share several important markers and mutations with human GBM. The diffusely infiltrative property of GBM is one of the major reasons for failure of current treatment (Wen et al., 1990; DeAngelis, 2001; Furnari et al., 2007; Van Meir et al., 2010), and the GL261 model recapitulates this feature on microscopic examination. As mentioned previously, one of the major challenges in using a mouse model is the size of the brain, particularly in studies that require imaging techniques like MRI. Equivalent images of whole mice brains compared with rat brains require a more expensive, powerful MRI magnet to obtain similar resolution of anatomical structures, which may be unavailable to the investigator. In conclusion, when imaging is not a limitation, GL261 is a strong model for studying GBM therapies, particularly immune-based therapies.
C6 GLIOMA MODEL
General protocol
The C6 glioma cell line was developed in the late 1960s in Sweet's laboratory by repetitive administration of MNU (methylnitrosourea) in adult Wistar–Furth rats (Benda et al., 1968). Although originally developed in Wistar rats, C6 can be implanted in Sprague–Dawley and Long–Evans rats without rejection (Nagano et al., 1993; Whittle et al., 1998). Tumour cells are usually implanted into the fronto-parietal lobe at 1×105 cells in 5 μl, with variable rates of tumour take (Parsa et al., 2000; Branle et al., 2002). This tumour can also be implanted on the right flank for easier measurements in growth studies and associated limitations in extrapolating from subcutaneous studies.
Pathology
C6 glioma cells share several general histopathological and specific tumour markers with human GBM. C6 glioma tumours demonstrate regions of focal invasion into brain tissue when implanted in Wistar rats, similar to the diffuse infiltrating pattern seen in GBM (Chicoine and Silbergeld, 1995) At the cellular level, C6 tumours do display areas of necrosis, nuclear polymorphism and high mitotic rates (Auer et al., 1981). In terms of histopathological markers, C6 cells express S100B protein, but do not express GFAP, and variable levels of vimentin (Pfeiffer et al., 1970; Whittle et al., 1998; Chou et al., 2003).
Genetics
The genetics of the C6 cell line offer further comparison with human GBM. A common tumour suppressor gene, p16, is known to have a high mutation rate in GBM and mutations in the p16/CDKN2A/NK4A locus are frequent occurrences in C6 tumour cells (Ueki et al., 1996; Ishii et al., 1999; Schlegel et al., 1999; Furnari et al., 2007) (Table 2). The tumour suppressor, p53 is one of the most frequently mutated genes in human GBM, and the C6 cell line differs from humans in their expression of the tumour suppressor protein p53 with a wild-type p53 along with minimal PTEN expression (Asai et al., 1994).
Summary
A clear advantage in using the C6 glioma model is the extensive characterization and the literature produced using this model, including genetic analysis (Grobben et al., 2002). However, the immunogenicity of this model is an important factor that must be considered even when studying non-immune based therapies. When placed in a Sprague–Dawley or Long–Evans rats, the tumour loses its invasive characteristics and grows in an encapsulated manner, no longer resembling the diffusely infiltrative nature of GBM (San-Galli et al., 1989). For example, Parsa et al. (2000) demonstrated strong antibody responses to C6 even when grown in Wistar rats. In conclusion, any therapeutic development and testing in this model must be interpreted with caution.
9L GLIOMA MODEL
General protocol
The 9L glioma model is historically one of the most widely used glioma tumour models. It has been extensively used to study chemotherapy and radiation effects (Henderson et al., 1981; Wen et al., 1990; Kimler et al., 1992; Kruse et al., 1993). Recently, it has also been used to study tumour stem cells (Ghods et al., 2007). The 9L gliosarcoma cell line was originally developed by Brenda, Schmidek and colleagues in the 1970s through repetitive administration of MNU in Fischer 344 rats (Benda et al., 1971; Schmidek et al., 1971). Most research in this cell line has been conducted with the syngeneic Fischer rats; however, it has also been characterized in allogenic Wister rats (Stojiljkovic et al., 2003). Tumour cells are usually injected into the striatum at a volume of 1×105 cells in 5 μl of cell suspension, showing high rates of tumour take after implantation (Stojiljkovic et al., 2003).
Pathology
It is important to note that 9L tumour cells have a sarcomatous appearance under histopathological examination, differentiating this model from other glioma models (Barth, 1998). 9L cells share some histological similarities with GBM tumour cells. For example, they are S100β-positive. However, they do not express GFAP and do not show the typical GBM pattern of diffusely invasive cells (Benda et al., 1971). In Wistar rats, tumours are infiltrated by macrophages and microglia (ED1 positive) and CD4+/CD8+ T-cells after approx. 2 weeks of growth (Stojiljkovic et al., 2003). Despite evidence of a T-cell immunological response, tumour growth is not inhibited in this model when compared with other rodent models with immune responses.
Genetics
Similar to human GBM cells, 9L cells have a mutant p53 gene (Asai et al., 1994). They also over express EGFR (epithelial growth factor receptor), which has been extensively studied as a therapeutic target in human GBM (Sibenaller et al., 2005; Lo, 2010). 9L differs from humans in their expression of p16, FGFR-1 (fibroblast growth factor receptor-1) and PDGFRβ (platelet-derived growth factor receptor β; Sibenaller et al., 2005), all of which are mutated or highly expressed in GBM, but not in 9L. The 9L tumour cells also fail to express other commonly mutated tumour suppressor genes such as PTEN.
Summary
Despite the wide use of the 9L glioma model, it is important to note the significant differences when compared with human GBM. Currently, the WHO (World Health Organization) classifies gliosarcoma as a subset of grade IV gliomas (Louis et al., 2001). However, careful consideration should be implemented when using a gliosarcoma model as a general model for GBM. Recent review of the literature has identified unique histological and genetic characteristics of gliosarcoma compared with GBM (Han et al., 2010). Some of these differences include infrequency of EGFR mutations and temporal lobe predilection, an implication that this subset of tumours may respond uniquely to various therapies (Han et al., 2010). 9L is considered an immunogenic tumour, since it is possible to immunize syngeneic rats with irradiated 9L tumour cells (Denlinger et al., 1975). Furthermore, the circumscribed growth pattern serves as a poor model to study the invasive properties of gliomas.
CNS-1 GLIOMA MODEL
General protocol
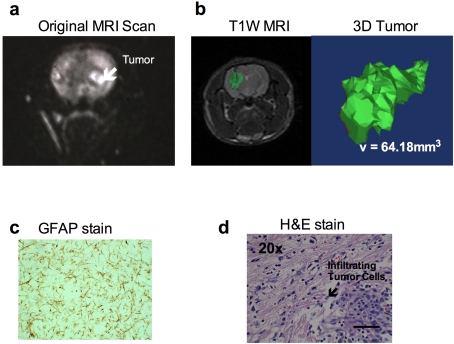
In 1990, Dr Michael C. Molleston, working in the laboratory of one of the authors (W.F.H.) sought to develop a syngeneic astrocytoma model. Each week for 36 weeks, he intravenously injected young rats with 5.0 mg/kg of MNU. Approx. 7 months after the start of treatment, rats began to die of tumours. One rat was found moribund and it was possible to obtain an MRI scan (Figure 1a). The study showed a well-developed neoplasm in the basal ganglia. From this tumour, the CNS-1 tumour line was established. CNS-1 tumour cells are usually injected into the striatum at a volume of 1×105 cells in 5 μl of cell suspension. Original studies with this cell line indicate median survival time of 30 days with high tumour take (Kruse et al., 1994).
Figure 1. Characteristics of the CNS-1 rat glioma model.
(a) MRI of the original tumour in which the CNS-1 tumour cell line was derived. (b) T1-weighted MRI of 1×105 CNS-1 cells in Lewis rat on day 15. Tumour is highlighted in green. To the right is a 3D reconstruction of the tumour using Mimics Software (Materialise, Leuven, Belgium) by summing the number of voxels that exceeded the intensity threshold in each plane on the T1-weighted subtraction images and multiplying by the appropriate spatial scaling factor (0.35 mm×0.35 mm×1.00 mm per voxel). (c) GFAP staining of the first cell line of CNS-1. (d) An H/E staining of infiltrating tumour cells into normal parenchyma of a Lewis rat brain (scale bar indicates 100 μm).
Pathology
At the time that the CNS-1 cell line was developed, the currently available GBM models failed to recapitulate many of the key characteristics of tumour growth in vivo. CNS-1 tumour cells were unique in their ability to express several glioma markers. These include positive staining for GFAP, S100Bβ, vimentin, RAR-α (retinoic acid receptor α), intracellular adhesion molecule-1 and neuron-specific enolase (Kruse et al., 1994) (Table 1). A key feature of the CNS-1 model is also the diffusely infiltrative and invasive pattern of growth in vivo. Unlike the 9L glioma model, CNS-1 cells demonstrate perivascular spread and individual tumour cell infiltration into normal parenchyma (Kruse et al., 1994). CNS-1 cells demonstrate periventricular spread and liptomeningeal spread (Kruse et al., 1994).
Table 1. Characteristic histological markers and growth of tumour cells in GBM rodent models.
Expression of histological markers and growth characteristics are indicated by (+), while failure of expression is indicated by (−).
| Rodent and model | Markers | Growth | |||
|---|---|---|---|---|---|
| GFAP | S100β | Vimentin | Invasive | Immunogenic | |
| Mouse | |||||
| U251 | + | + | + | − | + |
| U87 | − | − | + | − | + |
| GL261 | + | + | +/− | + | − |
| Rat | |||||
| C6 | − | + | N/A | +/− | + |
| 9L | − | + | N/A | − | + |
| CNS-1 | + | + | + | + | − |
The tumour microenvironment is known to promote tumour cell growth and invasion. This should be taken into consideration when choosing an appropriate animal model for therapeutic studies. Several immunological studies have been conducted with the CNS-1 model to elucidate the microenvironment's role in tumour growth (Owens et al., 1998; Regina et al., 2003; Demeule et al., 2004). In the presence of a brain tumour, endothelial cells may undergo phenotypic and functional changes, which result in promotion of tumour growth (Demeule et al., 2004). In the presence of CNS-1 tumour, endothelial cells undergo hyperplasia and display a pseudo-palisading pattern accompanying necrosis (Kerbel, 2008), both of which are indications of increased angiogenesis mimicking human GBM (Beranek, 2002).
The angiogenic nature of GBM is well established and has historically been a target for therapeutic development (Kerbel, 2008). Endothelial cells are also involved in drug resistance across the BBB (blood-brain barrier), by helping to prevent leakiness of the BBB. For example, P-glycoprotein is an efflux transporter in endothelial cells, which prevents significant accumulation of hydrophobic drugs (van Asperen et al., 1997). Histopathologic examination of human GBM shows a high expression of P-glycoprotein, possibly offering an explanation for clinical drug resistance in these tumours (Sawada et al., 1999).
In addition to endothelial cells, microglia and macrophages also support tumour growth and invasion. In human GBM, these cells accounts for as much as 10–34% of the tumour mass (Roggendorf et al., 1996). There is a strong positive correlation between the number of macrophages and microglia and the grade of tumour (Kielian et al., 2002). Similar to human GBM, CNS-1 brain tumours demonstrate infiltration of macrophages and microglia, constituting a major proportion of the tumour cell mass (Kielian et al., 2002). As a result, the CNS-1 model has been used to investigate the relationship between tumour cells, microglia and macrophages. MCP-1 (monocyte chemoattractant protein-1) was identified as a chemoattractant secreted by tumour cells, promoting the migration of microglia and macrophages to the tumour site (Kielian et al., 2002). Several tumour-promoting cytokines have been detected at the tumour site in vivo, but not by CNS-1 cells in vitro, further supporting the role of immune cells in the tumour microenvironment (Kielian et al., 2002). These cytokines include IL-1α, IL-1β, IL-10, TNFα (tumour necrosis factor α), TNFβ and IFNγ (interferon-γ; Kielian et al., 2002).
As previously mentioned, the CNS-1 model may be used to study the invasive properties of tumour cells. Plasminogen activators are traditionally involved in the breakdown of blood clots. However, they are also highly expressed in GBM and involved in degradation of surrounding tissue, promoting tumour invasion (Demeule et al., 2004). Endothelial cells surrounding CNS-1 tumours up-regulate urokinase plasminogen activators [tPA (tissue plasminogen activator), PA (plasminogen activator), PAI-1 (plasminogen-activator inhibitor) and uPAR (urokinase-type plasminogen activator receptor)] (Demeule et al., 2004). MMPs (matrix metalloproteinases) are another class of enzymes involved in degradation and tumour invasion. MMPs have been implicated as a crucial target in GBM cell invasion and as a result have been the focus for therapeutic development (Furnari et al., 2007). CNS-1 cells express MMP-2, but do not express MMP-9. However, expression of MMP-9 has been detected in endothelial cells and microglia found within the tumour microenvironment (Regina et al., 2003).
Unlike some of the other tumour models, the genetics of CNS-1 have not been extensively studied. Here, we briefly discuss the molecular work that has been undertaken and molecular players identified in the CNS-1 cell line. The karyotype of CNS-1 cells demonstrates one or more trisomies often of chromosomes 11 and 13 (Kruse et al., 1994). In terms of protein expression, CNS-1 cells were used to correlate decreased activity of L-isoaspartyl methyltransferase (a repair enzyme) in gliomas (Lapointe et al., 2005). Most of the research surrounding CNS-1 proteins has focused on its invasive phenotype. BEHAB (brain-enriched hyaluronan-binding) protein is a chondroitin sulfate proteoglycan found in the ECM (extracellular matrix). BEHAB expression is increased in gliomas and has been associated with glioma cell migration and proliferation (Jaworski et al., 1996). BEHAB/brevin cDNA was transfected into CNS-1 cells and implanted into Lewis rats. Tumours with BEHAB/brevin cDNA had a decreased survival and increased invasion and vasculature on histology (Nutt et al., 2001). The CNS-1 rodent model was then used to identify ADAMTS5 (a disintegrin and metalloproteinase with thrombospondin motifs 5) as the MMP required for proteolytic cleavage of BAHAB/brevin (Viapiano et al., 2008). Another protein characterized in CNS-1 involved in migration and invasion is the NCAM (neural cell adhesion molecule; Owens et al., 1998). CNS-1 cells transfected with NCAM demonstrated increased invasion in vitro. However, under in vivo conditions, NCAM increases local infiltration but decreases long-range invasion and migration. These results help to differentiate the mechanisms and signalling involved in glioma invasion. Many of the therapeutic studies investigated using the CNS-1 cell model involve gene therapy. For a summary of therapeutic studies in the CNS-1, we refer readers to Table 3.
Table 3. Therapeutic studies conducted in the CNS-1 GBM rodent model.
| Category | Year | Therapy | References |
|---|---|---|---|
| Virus | 2004, 2008 and 2010 | Adenovirus vectors encoding Flt3L adenovirus vector expressing HSV1-TK (Herpes simplex virus type I thymidine kinase) and FLT3L | (Ali et al., 2004; King et al., 2008; Puntel et al., 2010) |
| Vectors | 2004 | Block TGFβ with transfected decorin gene | (Biglari et al., 2004) |
| Gene therapy | 2000 and 2003 | CpG oligodeoxynucleotides blockage of IGF-1 (insulin-like growth factor 1) with antisense and triple helix IGF-1 expression | (Carpentier et al., 2000; Trojan et al., 2003) |
| Imaging | 2002 and 2007 | BOLD imaging of O2 detection PPIX fluorescence | (Dunn et al., 2002; Bisland et al., 2007) |
| Small molecule | 2011 | Propentofylline | VL Jacobs, RP Landry, Y Liu, EA Romero-Sandoval and SA De Leo, unpublished work |
Summary
The CNS-1 rodent model shares several key features with human GBM, including histological markers and growth pattern. The role of the immune environment has also been extensively investigated in this model. Although there has been some work, an extensive understanding of the genetics in CNS-1 cells is lacking. Despite this, CNS-1 cells share several histological features and diffuse growth pattern with human GBM and is one of the few models that also recapitulates the tumour microenvironment.
CONCLUSION
Rodent models of GBM have been available for decades; however, very few new therapies have successfully translated into the clinic during this time (Louis et al., 2001). The extent to which these models recapitulate the human disease remains debatable. GBM is one of the most challenging tumours to treat with approx. 1 year median survival following diagnosis. Despite aggressive surgery, chemotherapy and radiation treatments, the tumours virtually always relapse (Van Meir et al., 2010). An ideal model should recapitulate the key histopathological, genetic and imaging features encountered in GBM's aggressive growth as well as being a reproducible, reliable model.
As ongoing research continues to shed light on the genetic and cellular mechanisms involved in GBM, these new advances must be taken into consideration when choosing an appropriate model. Currently available rodent models can be classified into spontaneous oncogenesis, allograft or xenograft. For this review, we focused on engrafted models. However, we direct the reader to Sonabend et al. (2007) for a more extensive review on the several GBM genetic mouse models available. Also, our review provides only a general overview on the histopathological comparison of the rodent tumour cells to human GBM, since this has been reported previously (Radaelli et al., 2009).
We summarize herein some of the most commonly used rodent models of GBM with a focus on the CNS-1 rat model. Two of the most commonly used mouse models presented herein, U251 and U87 are xenograft models used in athymic nude mice. Comparing these cell lines, U251 is histologically most similar to human GBM. While these models enable the use of human GBM tumour cell lines, they are in an immune-compromised rodent, which does not allow for adequate study of the tumour–CNS/immune microenvironment. The mouse GL261 model, however, is a syngeneic model in C57BL/6 mice. This murine model demonstrates several of the critical GBM characteristics such as secondary structures of Scherer and tumour suppressor gene mutations. It is important to mention a more recent mouse model of GBM that has not been extensively reported in the literature but does share several key phenotypes with GBM. The VM-M3 model is derived from a spontaneous astrocytoma in the VM/dK strain of mice. These tumour cells are highly infiltrative, demonstrating all four secondary structures of Scherer and expression of CXCR4 (Shelton et al., 2010). Since this rodent model was only recently introduced, there are no extensive data pertaining to its histological and genetic markers. It is known that they do not express GFAP. However, this presents yet another promising model for the study of glioma invasion. Both 9L and C6 in rats (when grown in Sprague–Dawley rats) as well as U87 in mice demonstrate well-circumscribed tumour growth patterns in vivo. C6 can grow invasively in Wistar–Furth rats. However, rats do produce strong immune responses to C6 tumour cells. The CNS-1, U251 and GL261 models, in comparison, share several histological surface markers with human GBM and grow in a very similar pattern (i.e. diffusely infiltrative) in vivo. An advantage of using a rat rodent model compared with mouse is the ease in performing imaging studies in a rat due to its larger size.
Advancements have been made in the understanding of how GBM tumour cells invade and interact with their immune environment. As we move towards developing new therapeutics and testing in animal models, researchers must ensure rodent models are incorporating our new understandings of GBM. Failure to do this will only elicit further therapeutic developments that will fail in clinical trials. Both the CNS-1 model in rats and the GL261 model in mice have been used to investigate the role of the tumour microenvironment. This review presented the most common rodent glioma models, highlighting their differences when compared with the human disease. In addition, we presented the first comprehensive review of the CNS-1 glioma model. An understanding of how glioma models recapitulate the disease will enable scientists to choose the best model for preclinical studies, hopefully translating to more successful clinical trials in the future.
Footnotes
This work was supported by the National Institutes of Health/National Institute on Drug Abuse [grant numbers 1RO1DA025211, T32 A107363].
REFERENCES
- Ali S, Curtin JF, Zirger JM, Xiong W, King GD, Barcia C, Liu C, Puntel M, Goverdhana S, Lowenstein PR, Castro MG. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004;10:1071–1084. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai A, Miyagi Y, Sugiyama A, Gamanuma M, Hong SH, Takamoto S, Nomura K, Matsutani M, Takakura K, Kuchino Y. Negative effects of wild-type p53 and s-Myc on cellular growth and tumorigenicity of glioma cells. Implication of the tumor suppressor genes for gene therapy. J Neurooncol. 1994;19:259–268. doi: 10.1007/BF01053280. [DOI] [PubMed] [Google Scholar]
- Auer RN, Del Maestro RF, Anderson R. A simple and reproducible experimental in vivo glioma model. Can J Neurol Sci. 1981;8:325–331. doi: 10.1017/s0317167100043468. [DOI] [PubMed] [Google Scholar]
- Barth RF. Rat brain tumor models in experimental neuro-oncology: the 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas. J Neurooncol. 1998;36:91–102. doi: 10.1023/a:1005805203044. [DOI] [PubMed] [Google Scholar]
- Benda P, Lightbody J, Sato G, Levine L, Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968;161:370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- Benda P, Someda K, Messer J, Sweet WH. Morphological and immunochemical studies of rat glial tumors and clonal strains propagated in culture. J Neurosurg. 1971;34:310–323. doi: 10.3171/jns.1971.34.3.0310. [DOI] [PubMed] [Google Scholar]
- Beranek JT. Endothelial hyperplasia: an important indicator of actual angiogenesis. Br J Cancer. 2002;86:658. doi: 10.1038/sj.bjc.6600055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biglari A, Bataille D, Naumann U, Weller M, Zirger J, Castro MG, Lowenstein PR. Effects of ectopic decorin in modulating intracranial glioma progression in vivo, in a rat syngeneic model. Cancer Gene Ther. 2004;11:721–732. doi: 10.1038/sj.cgt.7700783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisland SK, Goebel EA, Hassanali NS, Johnson C, Wilson BC. Increased expression of mitochondrial benzodiazepine receptors following low-level light treatment facilitates enhanced protoporphyrin IX production in glioma-derived cells in vitro. Lasers Surg Med. 2007;39:678–684. doi: 10.1002/lsm.20544. [DOI] [PubMed] [Google Scholar]
- Branle F, Lefranc F, Camby I, Jeuken J, Geurts-Moespot A, Sprenger S, Sweep F, Kiss R, Salmon I. Evaluation of the efficiency of chemotherapy in in vivo orthotopic models of human glioma cells with and without 1p19q deletions and in C6 rat orthotopic allografts serving for the evaluation of surgery combined with chemotherapy. Cancer. 2002;95:641–655. doi: 10.1002/cncr.10710. [DOI] [PubMed] [Google Scholar]
- Brat DJ, Van Meir EG. Vaso-occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis, and accelerated growth in glioblastoma. Lab Invest. 2004;84:397–405. doi: 10.1038/labinvest.3700070. [DOI] [PubMed] [Google Scholar]
- Burger PC, Scheithauer BW, Vogel FS. New York: Churchill Livingstone; 2002. Surgical Pathology of the Nervous System and its Coverings; p. 687. [Google Scholar]
- Camphausen K, Purow B, Sproull M, Scott T, Ozawa T, Deen DF, Tofilon PJ. Influence of in vivo growth on human glioma cell line gene expression: convergent profiles under orthotopic conditions. Proc Natl Acad Sci USA. 2005;102:8287–8292. doi: 10.1073/pnas.0502887102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candolfi M, Curtin JF, Nichols WS, Muhammad AG, King GD, Pluhar GE, McNiel EA, Ohlfest JR, Freese AB, Moore PF, Lerner J, Lowenstein PR, Castro MG. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol. 2007;85:133–148. doi: 10.1007/s11060-007-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier AF, Xie J, Mokhtari K, Delattre JY. Successful treatment of intracranial gliomas in rat by oligodeoxynucleotides containing CpG motifs. Clin Cancer Res. 2000;6:2469–2473. [PubMed] [Google Scholar]
- Chicoine MR, Silbergeld DL. Invading C6 glioma cells maintaining tumorigenicity. J Neurosurg. 1995;83:665–671. doi: 10.3171/jns.1995.83.4.0665. [DOI] [PubMed] [Google Scholar]
- Choe G, Horvath S, Cloughesy TF, Crosby K, Seligson D, Palotie A, Inge L, Smith BL, Sawyers CL, Mischel PS. Analysis of the phosphatidylinositol 3′-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742–2746. [PubMed] [Google Scholar]
- Chou YH, Khuon S, Herrmann H, Goldman RD. Nestin promotes the phosphorylation-dependent disassembly of vimentin intermediate filaments during mitosis. Mol Biol Cell. 2003;14:1468–478. doi: 10.1091/mbc.E02-08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MJ, Homer N, O'Connor BD, Chen Z, Eskin A, Lee H, Merriman B, Nelson SF. U87MG decoded: the genomic sequence of a cytogenetically aberrant human cancer cell line. PLoS Genet. 2010;6:e1000832. doi: 10.1371/journal.pgen.1000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries NA, Beijnen JH, van Tellingen O. High-grade glioma mouse models and their applicability for preclinical testing. Cancer Treat Rev. 2009;35:714–723. doi: 10.1016/j.ctrv.2009.08.011. [DOI] [PubMed] [Google Scholar]
- DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- Demeule M, Regina A, Annabi B, Bertrand Y, Bojanowski MW, Beliveau R. Brain endothelial cells as pharmacological targets in brain tumors. Mol Neurobiol. 2004;30:157–183. doi: 10.1385/MN:30:2:157. [DOI] [PubMed] [Google Scholar]
- Denlinger RH, Axler DA, Koestner A, Liss L. Tumor-specific transplantation immunity to intracerebral challenge with cells from a methylnitrosourea-induced brain tumor. J Med. 1975;6:249–259. [PubMed] [Google Scholar]
- Dunn JF, O'Hara JA, Zaim-Wadghiri Y, Lei H, Meyerand ME, Grinberg OY, Hou H, Hoopes PJ, Demidenko E, Swartz HM. Changes in oxygenation of intracranial tumors with carbogen: a BOLD MRI and EPR oximetry study. J Magn Reson Imaging. 2002;16:511–521. doi: 10.1002/jmri.10192. [DOI] [PubMed] [Google Scholar]
- Ecsedy JA, Holthaus KA, Yohe HC, Seyfried TN. Expression of mouse sialic acid on gangliosides of a human glioma grown as a xenograft in SCID mice. J Neurochem. 1999;73:254–259. doi: 10.1046/j.1471-4159.1999.0730254.x. [DOI] [PubMed] [Google Scholar]
- Fueyo J, Gomez-Manzano C, Yung WK, Clayman GL, Liu TJ, Bruner J, Levin VA, Kyritsis AP. Adenovirus-mediated p16/CDKN2 gene transfer induces growth arrest and modifies the transformed phenotype of glioma cells. Oncogene. 1996;12:103–110. [PubMed] [Google Scholar]
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- Ghods AJ, Irvin D, Liu G, Yuan X, Abdulkadir IR, Tunici P, Konda B, Wachsmann-Hogiu S, Black KL, Yu JS. Spheres isolated from 9L gliosarcoma rat cell line possess chemoresistant and aggressive cancer stem-like cells. Stem Cells. 2007;25:1645–1653. doi: 10.1634/stemcells.2006-0624. [DOI] [PubMed] [Google Scholar]
- Grobben B, De Deyn PP, Slegers H. Rat C6 glioma as experimental model system for the study of glioblastoma growth and invasion. Cell Tissue Res. 2002;310:257–270. doi: 10.1007/s00441-002-0651-7. [DOI] [PubMed] [Google Scholar]
- Han SJ, Yang I, Tihan T, Prados MD, Parsa AT. Primary gliosarcoma: key clinical and pathologic distinctions from glioblastoma with implications as a unique oncologic entity. J Neurooncol. 2010;96:313–320. doi: 10.1007/s11060-009-9973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K, Yoshida J, Mizuno M, Sugita K, Kurisu K, Uozumi T. Growth inhibition of subcutaneously transplanted human glioma by transfection-induced tumor necrosis factor-alpha and augmentation of the effect by gamma-interferon. J Neurooncol. 1994;22:221–225. doi: 10.1007/BF01052922. [DOI] [PubMed] [Google Scholar]
- Henderson SD, Kimler BF, Morantz RA. Radiation therapy of 9L rat brain tumors. Int J Radiat Oncol Biol Phys. 1981;7:497–502. doi: 10.1016/0360-3016(81)90136-x. [DOI] [PubMed] [Google Scholar]
- Homma T, Fukushima T, Vaccarella S, Yonekawa Y, Di Patre PL, Franceschi S, Ohgaki H. Correlation among pathology, genotype, and patient outcomes in glioblastoma. J Neuropathol Exp Neurol. 2006;65:846–854. doi: 10.1097/01.jnen.0000235118.75182.94. [DOI] [PubMed] [Google Scholar]
- Houchens DP, Ovejera AA, Riblet SM, Slagel DE. Human brain tumor xenografts in nude mice as a chemotherapy model. Eur J Cancer Clin Oncol. 1983;19:799–805. doi: 10.1016/0277-5379(83)90012-3. [DOI] [PubMed] [Google Scholar]
- Husain SR, Behari N, Kreitman RJ, Pastan I, Puri RK. Complete regression of established human glioblastoma tumor xenograft by interleukin-4 toxin therapy. Cancer Res. 1998;58:3649–3653. [PubMed] [Google Scholar]
- Ishii N, Maier D, Merlo A, Tada M, Sawamura Y, Diserens AC, Van Meir EG. Frequent co-alterations of TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human glioma cell lines. Brain Pathol. 1999;9:469–479. doi: 10.1111/j.1750-3639.1999.tb00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski DM, Kelly GM, Piepmeier JM, Hockfield S. BEHAB (brain enriched hyaluronan binding) is expressed in surgical samples of glioma and in intracranial grafts of invasive glioma cell lines. Cancer Res. 1996;56:2293–2298. [PubMed] [Google Scholar]
- Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, van Rooijen N, Hickey WF. MCP-1 expression in CNS-1 astrocytoma cells: implications for macrophage infiltration into tumors in vivo. J Neurooncol. 2002;56:1–12. doi: 10.1023/a:1014495613455. [DOI] [PubMed] [Google Scholar]
- Kimler BF, Liu C, Evans RG, Morantz RA. Intracerebral chemotherapy in the 9L rat brain tumor model. J Neurooncol. 1992;14:191–200. doi: 10.1007/BF00172594. [DOI] [PubMed] [Google Scholar]
- King GD, Muhammad AK, Curtin JF, Barcia C, Puntel M, Liu C, Honig SB, Candolfi M, Mondkar S, Lowenstein PR, Castro MG. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro Oncol. 2008;10:19–31. doi: 10.1215/15228517-2007-045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleihues P, Cavenee WK International Agency for Research on Cancer. Lyon: IARC Press; 2000. Pathology and Genetics of Tumours of the Nervous System; p. 314. [Google Scholar]
- Koul D, Shen R, Bergh S, Sheng X, Shishodia S, Lafortune TA, Lu Y, de Groot JF, Mills GB, Yung WK. Inhibition of Akt survival pathway by a small-molecule inhibitor in human glioblastoma. Mol Cancer Ther. 2006;5:637–644. doi: 10.1158/1535-7163.MCT-05-0453. [DOI] [PubMed] [Google Scholar]
- Krakstad C, Chekenya M. Survival signalling and apoptosis resistance in glioblastomas: opportunities for targeted therapeutics. Mol Cancer. 2010;9:135. doi: 10.1186/1476-4598-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse CA, Mitchell DH, Kleinschmidt-DeMasters BK, Bellgrau D, Eule JM, Parra JR, Kong Q, Lillehei KO. Systemic chemotherapy combined with local adoptive immunotherapy cures rats bearing 9L gliosarcoma. J Neurooncol. 1993;15:97–112. doi: 10.1007/BF01053931. [DOI] [PubMed] [Google Scholar]
- Kruse CA, Molleston MC, Parks EP, Schiltz PM, Kleinschmidt-DeMasters BK, Hickey WF. A rat glioma model, CNS-1, with invasive characteristics similar to those of human gliomas: a comparison to 9L gliosarcoma. J Neurooncol. 1994;22:191–200. doi: 10.1007/BF01052919. [DOI] [PubMed] [Google Scholar]
- Lapointe M, Lanthier J, Moumdjian R, Regina A, Desrosiers RR. Expression and activity of l-isoaspartyl methyltransferase decrease in stage progression of human astrocytic tumors. Brain Res Mol Brain Res. 2005;135:93–103. doi: 10.1016/j.molbrainres.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Lo HW. EGFR-targeted therapy in malignant glioma: novel aspects and mechanisms of drug resistance. Curr Mol Pharmacol. 2010;3:37–52. doi: 10.2174/1874467211003010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DN. The p53 gene and protein in human brain tumors. J Neuropathol Exp Neurol. 1994;53:11–21. doi: 10.1097/00005072-199401000-00002. [DOI] [PubMed] [Google Scholar]
- Louis DN, Holland EC, Cairncross JG. Glioma classification: a molecular reappraisal. Am J Pathol. 2001;159:779–786. doi: 10.1016/S0002-9440(10)61750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes W, Van Gool SW. Experimental immunotherapy for malignant glioma: lessons from two decades of research in the GL261 model. Cancer Immunol Immunother. 2011;60:153–160. doi: 10.1007/s00262-010-0946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore XL, Lu J, Sun L, Zhu CJ, Tan P, Wong MC. Endothelial progenitor cells' 'homing' specificity to brain tumors. Gene Ther. 2004;11:811–818. doi: 10.1038/sj.gt.3302151. [DOI] [PubMed] [Google Scholar]
- Nagano N, Sasaki H, Aoyagi M, Hirakawa K. Invasion of experimental rat brain tumor: early morphological changes following microinjection of C6 glioma cells. Acta Neuropathol. 1993;86:117–125. doi: 10.1007/BF00334878. [DOI] [PubMed] [Google Scholar]
- Newcomb EW, Zagzag D. The murine GL261 glioma experimental model to assess novel brain tumor treatments. In: Meir EG, editor. In CNS Cancer: Models, Markers, Prognostic Factors, Targets and Therapeutic Approaches. Dordrecht: Springer; 2009. pp. 227–241. [Google Scholar]
- Nutt CL, Zerillo CA, Kelly GM, Hockfield S. Brain enriched hyaluronan binding (BEHAB)/brevican increases aggressiveness of CNS-1 gliomas in Lewis rats. Cancer Res. 2001;61:7056–7059. [PubMed] [Google Scholar]
- Owens GC, Orr EA, DeMasters BK, Muschel RJ, Berens ME, Kruse CA. Overexpression of a transmembrane isoform of neural cell adhesion molecule alters the invasiveness of rat CNS-1 glioma. Cancer Res. 1998;58:2020–2028. [PubMed] [Google Scholar]
- Parsa AT, Chakrabarti I, Hurley PT, Chi JH, Hall JS, Kaiser MG, Bruce JN. Limitations of the C6/Wistar rat intracerebral glioma model: implications for evaluating immunotherapy. Neurosurgery. 2000;47:993–999. doi: 10.1097/00006123-200010000-00050. [DOI] [PubMed] [Google Scholar]
- Pfeiffer SE, Herschman HR, Lightbody J, Sato G. Synthesis by a clonal line of rat glial cells of a protein unique to the nervous system. J Cell Physiol. 1970;75:329–339. doi: 10.1002/jcp.1040750309. [DOI] [PubMed] [Google Scholar]
- Ponten J. Neoplastic human glia cells in culture. In: Fogh J, editor. In Human Tumor Cells in Vitro. New York: Plenum Press; 1975. pp. 175–185. [Google Scholar]
- Puntel M, Muhammad AK, Candolfi M, Salem A, Yagiz K, Farrokhi C, Kroeger KM, Xiong W, Curtin JF, Liu C, Bondale NS, Lerner J, Pechnick RN, Palmer D, Ng P, Lowenstein PR, Castro MG. A novel bicistronic high-capacity gutless adenovirus vector that drives constitutive expression of herpes simplex virus type 1 thymidine kinase and tet-inducible expression of Flt3L for glioma therapeutics. J Virol. 2010;84:6007–6017. doi: 10.1128/JVI.00398-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Yang Y, Ma YJ, Chen FH, Zhang LB, Liu W, Qi Q, Lu N, Tao L, Wang XT, You QD, Guo QL. Isolation and characterization of cancer stem like cells in human glioblastoma cell lines. Cancer Lett. 2009;279:13–21. doi: 10.1016/j.canlet.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Radaelli E, Ceruti R, Patton V, Russo M, Degrassi A, Croci V, Caprera F, Stortini G, Scanziani E, Pesenti E, Alzani R. Immunohistopathological and neuroimaging characterization of murine orthotopic xenograft models of glioblastoma multiforme recapitulating the most salient features of human disease. Histol Histopathol. 2009;24:879–891. doi: 10.14670/HH-24.879. [DOI] [PubMed] [Google Scholar]
- Regina A, Demeule M, Berube A, Moumdjian R, Berthelet F, Beliveau R. Differences in multidrug resistance phenotype and matrix metalloproteinases activity between endothelial cells from normal brain and glioma. J Neurochem. 2003;84:316–324. doi: 10.1046/j.1471-4159.2003.01521.x. [DOI] [PubMed] [Google Scholar]
- Roberts WG, Delaat J, Nagane M, Huang S, Cavenee WK, Palade GE. Host microvasculature influence on tumor vascular morphology and endothelial gene expression. Am J Pathol. 1998;153:1239–1248. doi: 10.1016/s0002-9440(10)65668-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggendorf W, Strupp S, Paulus W. Distribution and characterization of microglia/macrophages in human brain tumors. Acta Neuropathol. 1996;92:288–293. doi: 10.1007/s004010050520. [DOI] [PubMed] [Google Scholar]
- Rong Y, Durden DL, Van Meir EG, Brat DJ. ‘Pseudopalisading’ necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J Neuropathol Exp Neurol. 2006;65:529–539. doi: 10.1097/00005072-200606000-00001. [DOI] [PubMed] [Google Scholar]
- Rubin JB, Kung AL, Klein RS, Chan JA, Sun Y, Schmidt K, Kieran MW, Luster AD, Segal RA. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci USA. 2003;100:13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San-Galli F, Vrignaud P, Robert J, Coindre JM, Cohadon F. Assessment of the experimental model of transplanted C6 glioblastoma in Wistar rats. J Neurooncol. 1989;7:299–304. doi: 10.1007/BF00172924. [DOI] [PubMed] [Google Scholar]
- Sawada T, Kato Y, Sakayori N, Takekawa Y, Kobayashi M. Expression of the multidrug-resistance P-glycoprotein (Pgp, MDR-1) by endothelial cells of the neovasculature in central nervous system tumors. Brain Tumor Pathol. 1999;16:23–27. doi: 10.1007/BF02478898. [DOI] [PubMed] [Google Scholar]
- Schlegel J, Piontek G, Kersting M, Schuermann M, Kappler R, Scherthan H, Weghorst C, Buzard G, Mennel H. The p16/Cdkn2a/Ink4a gene is frequently deleted in nitrosourea-induced rat glial tumors. Pathobiology. 1999;67:202–206. doi: 10.1159/000028073. [DOI] [PubMed] [Google Scholar]
- Schmidek HH, Nielsen SL, Schiller AL, Messer J. Morphological studies of rat brain tumors induced by N-nitrosomethylurea. J Neurosurg. 1971;34:335–340. doi: 10.3171/jns.1971.34.3.0335. [DOI] [PubMed] [Google Scholar]
- Seligman AM, Shear MJ. Experimental production of brain tumors in mice with methylcholanthrene. Am J Cancer. 1939;37:364–395. [Google Scholar]
- Shapiro WR, Ausman JI, Rall DP. Studies on the chemotherapy of experimental brain tumors: evaluation of 1,3-bis(2-chloroethyl)-l-nitrosourea, cyclophosphamide, mithramycin, and methotrexate. Cancer Res. 1970;30:2401–2413. [PubMed] [Google Scholar]
- Shelton LM, Mukherjee P, Huysentruyt LC, Urits I, Rosenberg JA, Seyfried TN. A novel pre-clinical in vivo mouse model for malignant brain tumor growth and invasion. J Neurooncol. 2010;99:165–176. doi: 10.1007/s11060-010-0115-y. [DOI] [PubMed] [Google Scholar]
- Sibenaller ZA, Etame AB, Ali MM, Barua M, Braun TA, Casavant TL, Ryken TC. Genetic characterization of commonly used glioma cell lines in the rat animal model system. Neurosurg Focus. 2005;19:E1. doi: 10.3171/foc.2005.19.4.2. [DOI] [PubMed] [Google Scholar]
- Sonabend AM, Ulasov IV, Lesniak MS. Emerging role of new transgenic mouse models in glioma research. Expert Rev Anticancer Ther. 2007;7:S7–S13. doi: 10.1586/14737140.7.12s.S7. [DOI] [PubMed] [Google Scholar]
- Stojiljkovic M, Piperski V, Dacevic M, Rakic L, Ruzdijic S, Kanazir S. Characterization of 9L glioma model of the Wistar rat. J Neurooncol. 2003;63:1–7. doi: 10.1023/a:1023732619651. [DOI] [PubMed] [Google Scholar]
- Szatmari T, Lumniczky K, Desaknai S, Trajcevski S, Hidvegi EJ, Hamada H, Safrany G. Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer Sci. 2006;97:546–553. doi: 10.1111/j.1349-7006.2006.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojan LA, Kopinski P, Mazurek A, Chyczewski L, Ly A, Jarocki P, Niklinski J, Shevelev A, Trzos R, Pan Y, Gitis DJ, Bierwagen M, Czapiewska JL, Wei MX, Michalkiewicz J, Henin D, Popiela T, Evrard F, Kasprzak H, Anthony D, Trojan J. IGF-I triple helix gene therapy of rat and human gliomas. Rocz Akad Med Bialymst. 2003;48:18–27. [PubMed] [Google Scholar]
- Ueki K, Ono Y, Henson JW, Efird JT, von Deimling A, Louis DN. CDKN2/p16 or RB alterations occur in the majority of glioblastomas and are inversely correlated. Cancer Res. 1996;56:150–153. [PubMed] [Google Scholar]
- Valdes PA, Samkoe K, O'Hara JA, Roberts DW, Paulsen KD, Pogue BW. Deferoxamine iron chelation increases delta-aminolevulinic acid induced protoporphyrin IX in xenograft glioma model. Photochem Photobiol. 2010;86:471–5. doi: 10.1111/j.1751-1097.2009.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asperen J, Mayer U, van Tellingen O, Beijnen JH. The functional role of P-glycoprotein in the blood-brain barrier. J Pharm Sci. 1997;86:881–884. doi: 10.1021/js9701364. [DOI] [PubMed] [Google Scholar]
- Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viapiano MS, Hockfield S, Matthews RT. BEHAB/brevican requires ADAMTS-mediated proteolytic cleavage to promote glioma invasion. J Neurooncol. 2008;88:261–272. doi: 10.1007/s11060-008-9575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen P, Loeffler JS, Morris JH, Lampson LA. The effects of irradiation on major histocompatibility complex expression and lymphocytic infiltration in the normal rat brain and the 9L gliosarcoma brain tumor model. J Neuroimmunol. 1990;27:239–244. doi: 10.1016/0165-5728(90)90074-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- Whittle IR, Macarthur DC, Malcolm GP, Li M, Washington K, Ironside JW. Can experimental models of rodent implantation glioma be improved? A study of pure and mixed glioma cell line tumours. J Neurooncol. 1998;36:231–242. doi: 10.1023/a:1005831111337. [DOI] [PubMed] [Google Scholar]
- Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL. Expression of hypoxia-inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer. 2000;88:2606–2618. [PubMed] [Google Scholar]
- Zagzag D, Lukyanov Y, Lan L, Ali MA, Esencay M, Mendez O, Yee H, Voura EB, Newcomb EW. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Invest. 2006;86:1221–1232. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- Zagzag D, Esencay M, Mendez O, Yee H, Smirnova I, Huang Y, Chiriboga L, Lukyanov E, Liu M, Newcomb EW. Hypoxia- and vascular endothelial growth factor-induced stromal cell-derived factor-1alpha/CXCR4 expression in glioblastomas: one plausible explanation of Scherer's structures. Am J Pathol. 2008;173:545–560. doi: 10.2353/ajpath.2008.071197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagzag D, Miller DC, Chiriboga L, Yee H, Newcomb EW. Green fluorescent protein immunohistochemistry as a novel experimental tool for the detection of glioma cell invasion in vivo. Brain Pathol. 2003;13:34–37. doi: 10.1111/j.1750-3639.2003.tb00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Fujita M, Snyder LA, Okada H. Systemic delivery of neutralizing antibody targeting CCL2 for glioma therapy. J Neurooncol, in the press. 2010 doi: 10.1007/s11060-010-0473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]