Abstract
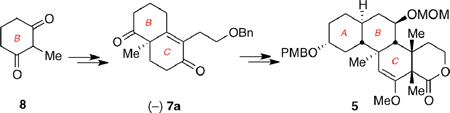
An enantioselective strategy for the synthesis of tetracyclic motif 5, representing the northern fragment of norzoanthamine, is presented. Key to the strategy is the use of two asymmetric Robinson annulation reactions that produce the tricyclic ABC ring system with excellent stereoselectivity. Further functionalization at the periphery of the C ring produces compound 5 containing six contiguous stereocenters of the natural product.
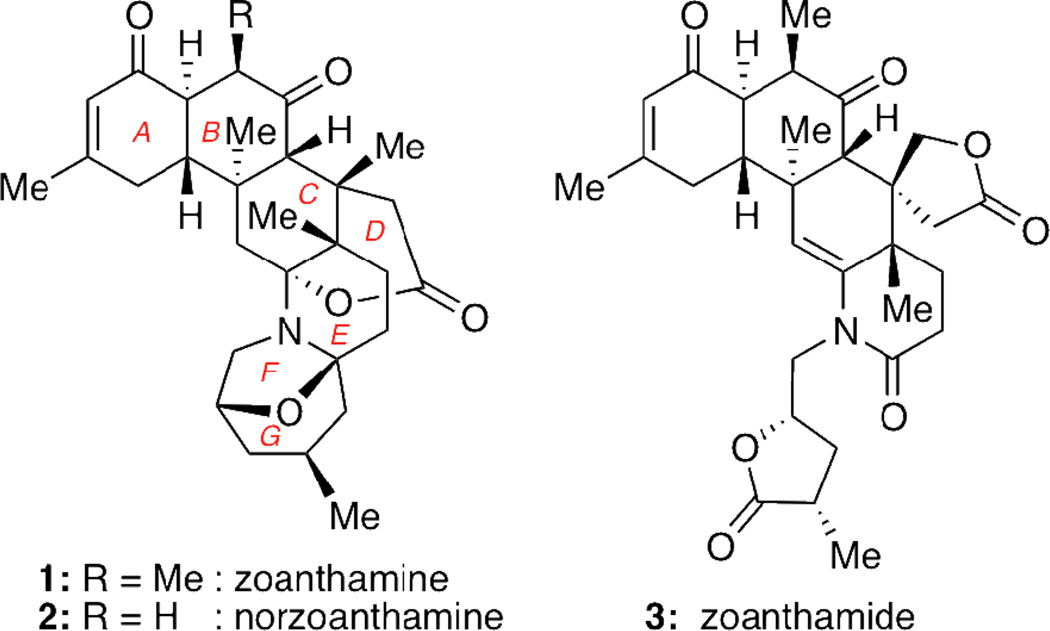
The zoanthamine alkaloids constitute a class of marine natural products that have been isolated from colonial zoanthids of the genus Zoanthus sp.1 These compounds are structurally defined by a densely functionalized and stereochemically rich scaffold, as shown with the structures of zoanthamine (1),2 norzoanthamine (2)3 and zoanthamide (3)4 (Figure 1).
Figure 1.
Structures of selected zoanthamine natural products.
In addition to their impressive chemical architecture, the zoanthamines also display an attractive spectrum of biological activities. For instance, several family members were found to inhibit thrombin-, collagen- and arachidonic acid-induced inflammation of platelets,5 and exhibit potent cytotoxicity against a number of cancer cell lines.4,6, In addition, 1 and 3 inhibit inflammation induced by myristate acetate in mouse ears,4,6a while 2, the demethylated version of 1, has been found to exhibit promising antiosteoporotic properties in vivo in ovariectomized mice.1,7
The combination of challenging structures and potent bioactivities has prompted the design of various synthetic approaches by several groups.8,9 These efforts led to two total syntheses of norzoanthamine by the Miyashita10 and Kobayashi11 groups. More recently, Miyashita and co-workers also reported the total synthesis of zoanthenol via oxidation of norzoanthamine hydrochloride.12
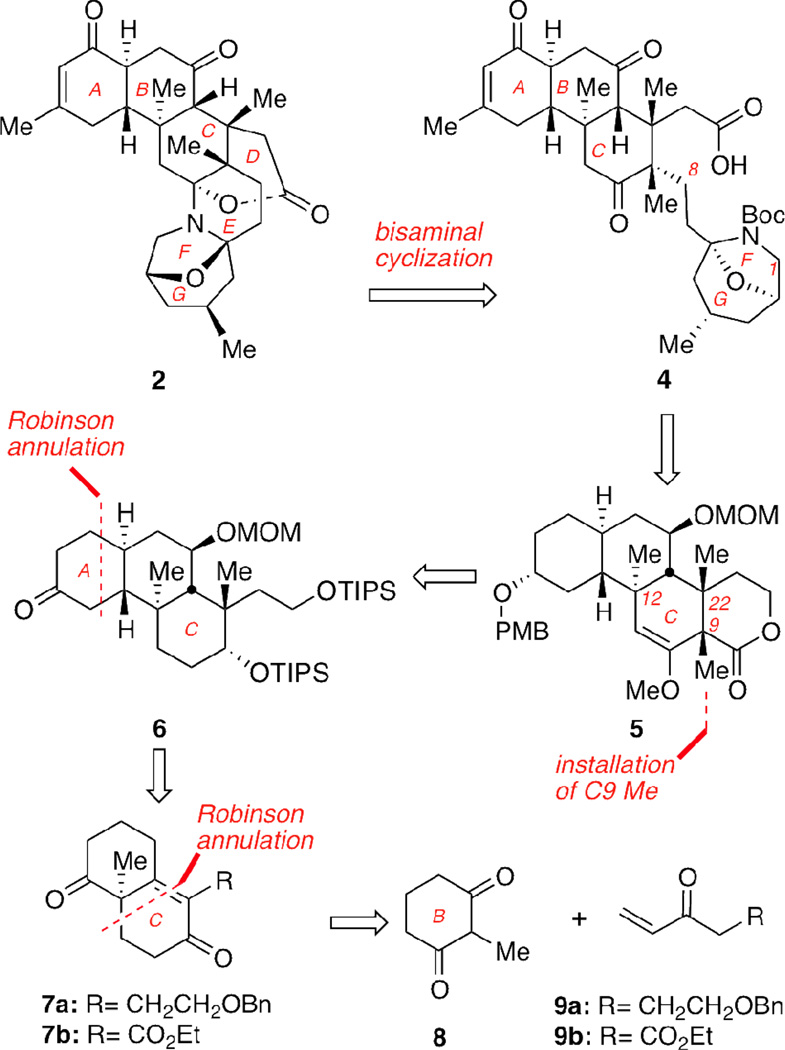
Inspection of the polycyclic norzoanthamine framework suggests that this compound can be dissected in two main fragments: a rigid tricyclic ABC core and a bisaminal motif that forms the DEFG part. Arguably, the most significant challenges for the synthesis of 2 are the construction of the AB trans decalin system and the functionalization of the stereochemically rich C ring. In fact, both Miyashita and Kobayashi have shown that the synthesis of 2 could be achieved from condensation of compound 4 in which a partially folded C1–C8 side chain has been attached to a fully functionalized ABC motif (Figure 2). With this in mind, we focused our efforts on the development of an enantioselective synthesis of the ABC ring fragment, represented here by structure 5. In the retrosynthetic direction, 5 can derive from 6 after functionalization at the periphery of the C ring and stereocontrolled installation of the C9 methyl group. Construction of the carbon backbone of 6 could be accomplished with two Robinson annulation reactions that would form rings A and C. Along these lines, reaction between 8 and 9a promoted by a chiral reagent would produce bicyclic motif 7a enantioselectively.13 It should be noted that, in a previous study we attempted the synthesis of the ABC ring fragment of 1 using bicyclic motif 7b that was readily available from condensation of 8 with 9b.14 However, implementation of this strategy to an enantioselective synthesis of 7b proved to be lengthy and inefficient.
Figure 2.
Retrosynthetic analysis of norzoanthamine (2).
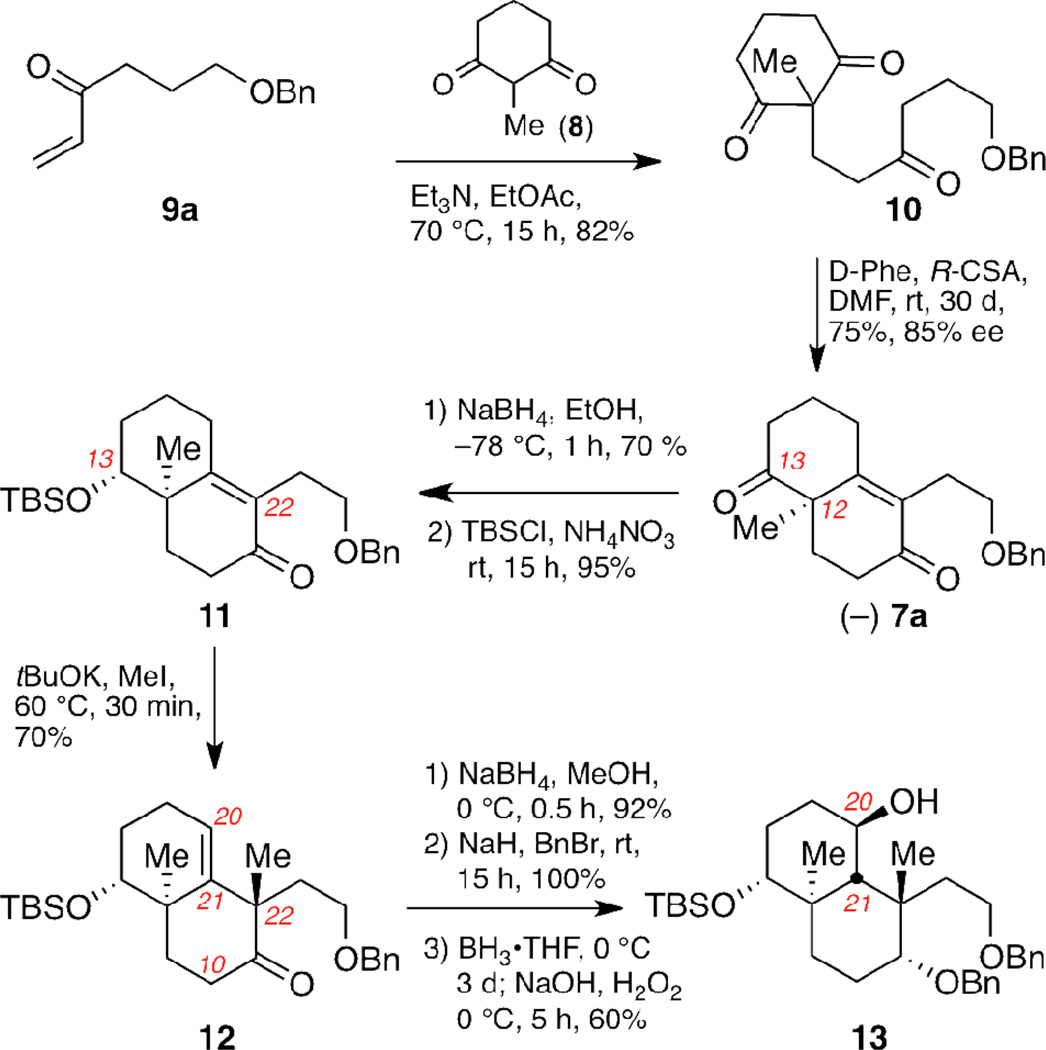
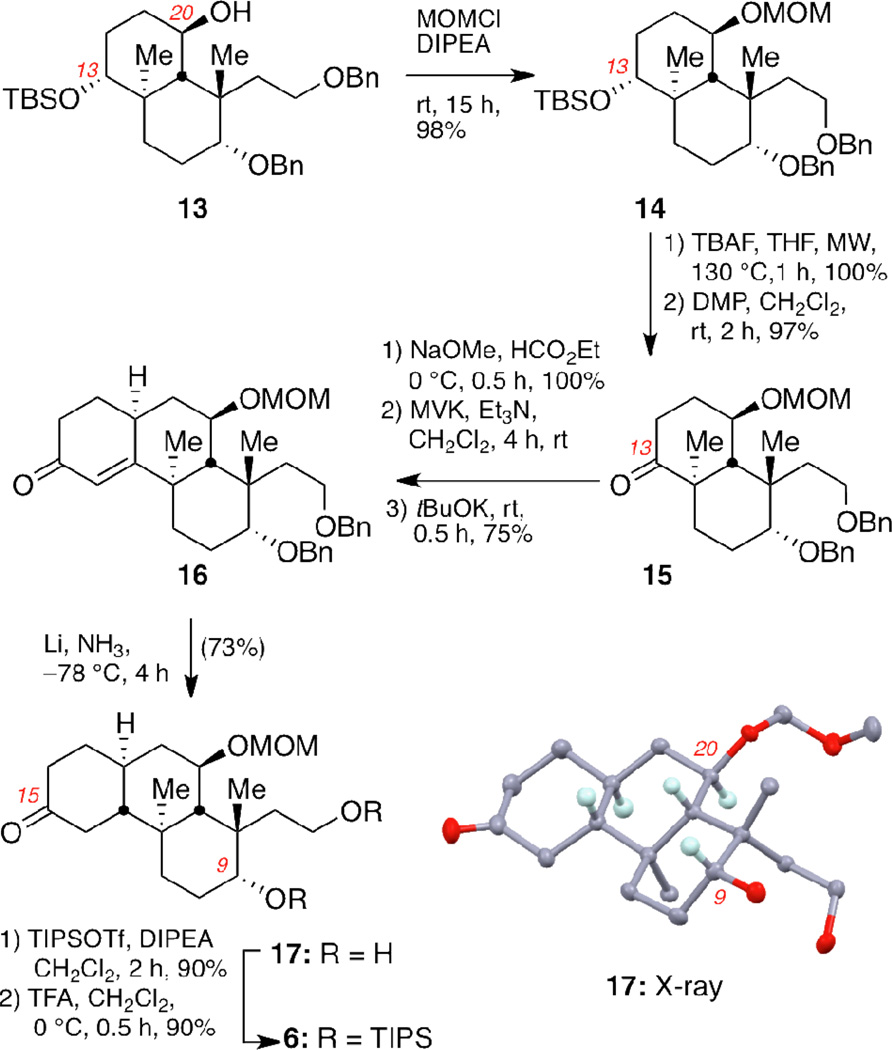
The synthesis of the BC ring system of 2 is shown in Scheme 1. Compound 10, the required precursor for the asymmetric Robinson annulation, was produced in high yield after Michael addition of 2-methyl-1,3-cyclohexadione (8) to enone 9a. The latter was synthesized from butane-1,4-diol according to a reported protocol.16 Treatment of 10 with D-Phe and R-CSA in DMF17 gave the annulated product 7a, containing the C12 quaternary center, in 75% yield. The enantioselectivity of this reaction was 85% ee as determined by the chiral shift agent Eu(hfc)3.18 The C13 carbonyl group of 7a was selectively reduced with sodium borohydride and the resulting alcohol was protected as a silyl ether to afford enone 11 (67% overall yield). The second quaternary center at C22 was then installed. Methylation of 11 with tBuOK produced 12 as a single stereoisomer. Reduction of the carbonyl group of 12 gave an alcohol, which was protected as a benzyl ether. Stereoselective hydroboration of the C20–21 alkene occurred at 0 °C over the course of 72 hours to give alcohol 13 (55% yield over 3 steps).
Scheme 1.
Enantioselective synthesis of fragment 13.a
With the fully functionalized BC ring system in hand, we shifted our attention to the stereoselective construction of the trans-anti-trans-fused perhydrophenanthrene skeleton of norzoanthamine (Scheme 2). Alcohol 13 was protected as the corresponding MOM ether in 98% yield. The C13 silyl ether of 14 was then deprotected using TBAF under microwave (MW) radiation at 130 °C for 1 hour. It is worth noting that conventional heating in THF under reflux gave only a 50% conversion after 5 days. The resulting C13 alcohol was oxidized using Dess-Martin Periodinane (DMP) to provide compound 15 in 97% yield over 2 steps. Our initial attempts to react 15 with methyl vinyl ketone (MVK) using a strong base led to a complex mixture of products. To circumvent this problem, we converted 15 to a β-keto aldehyde, that underwent a smooth Michael addition with MVK and triethylamine.19 The Robinson cyclization proceeded cleanly with tBuOK to provide the tricyclic motif 16 in 75% yield over three steps. The final trans-anti-trans decalin 17 was synthesized after stereoselective reduction of 16 using lithium-ammonia conditions. Under these reductive conditions, both benzyl ethers were cleaved, producing diol 17. The chemical structure and stereochemistry of compound 17 was unambiguously confirmed via a single crystal X-ray analysis.20
Scheme 2.
Stereoselective synthesis of trans-anti-trans-fused perhydrophenanthrene fragment 6.a
Conversion of diol 17 to the corresponding di-TIPS ether 6 was achieved in two steps: (a) exhaustive silylation of both ketone and diol functionalities of 17 and (b) deprotection of the silyl enol ether using TFA (80% yield overall). This two-step strategy was necessary because the C15 ketone was shown to be more reactive over the C9 secondary alcohol.
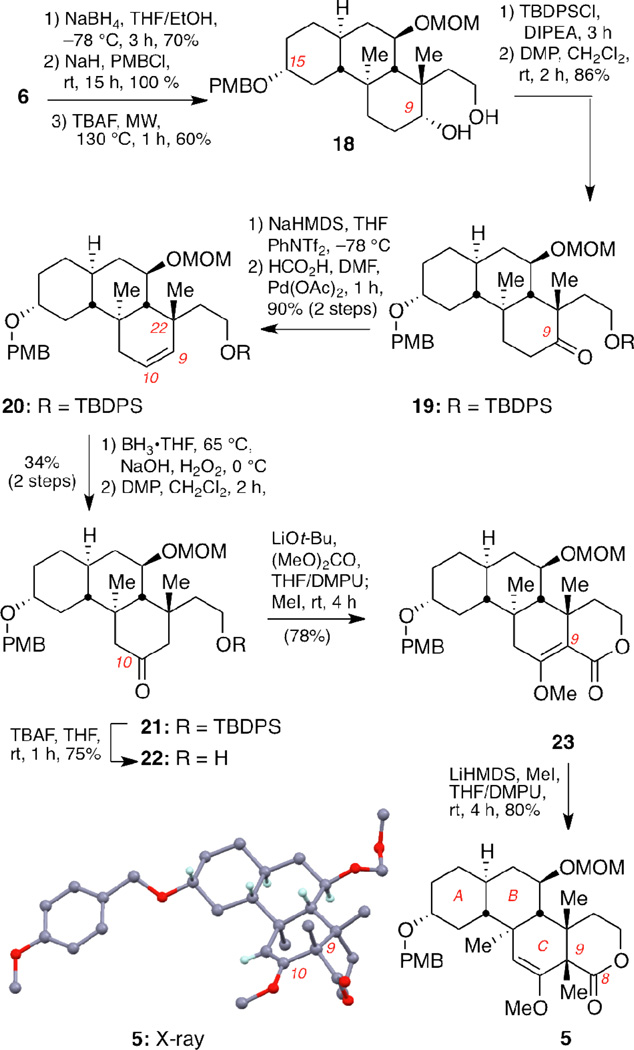
The next task was the complete functionalization of the C ring including the installation of the last quaternary center at C9 (Scheme 3). To this end, the C15 carbonyl group of 6 was stereoselectively reduced at −78 °C21 and the resulting equatorial alcohol was protected as the p-methoxybenzyl ether. The two silyl ether groups were then removed using TBAF to provide diol 18 (42% yield over 3 steps). Selective TBDPS monoprotection of the primary alcohol followed by DMP oxidation of the C9 hydroxyl group afforded ketone 19 in excellent yield (82% yield over 2 steps).
Scheme 3.
Stereoselective synthesis of the ABC ring system 5.a
Conversion of 19 to ketone 22 was accomplished via a sequence of five steps that included: (a) triflation of the C9 ketone; (b) reduction of the resulting vinyl triflate using formic acid and palladium(II) acetate to give alkene 20; (c) hydroboration/oxidation of the C9–C10 alkene; (d) oxidation of the resulting alcohol to ketone 21 and (e) deprotection of the primary silyl ether (23% yield over 5 steps). We anticipated that, by virtue of steric hindrance, the C22 quaternary carbon center would lead to a regioselective hydroboration reaction in favor of the desired product (hydroxylation at C10). Indeed, this hydroxylation produced, after further oxidation, a 2:1 mixture of ketones 21 and 19 (34% and 17% yield respectively). The undesired ketone 19 can be recycled to yield the desired product 21. Treatment of 22 with tBuOLi as base, dimethyl carbonate and iodomethane yielded methyl enol ether 23 that, upon alkylation with LiHMDS and iodomethane formed the desired compound 5 (2 steps, 62% yield).10a The absolute stereochemistry of 5 was confirmed by single crystal X-ray analysis.20
In conclusion, we have described an enantioselective approach to the fully functionalized ABC ring system of norzoanthamine, represented by compound 5. Key to the strategy is a double Robinson annulation reaction that installs the C and A rings of this motif. The initial Robinson annulation (formation of the BC ring) proceeds with excellent enantioselectivity, setting the desired stereochemistry at the C12 quaternary group. A sequence of stereoselective transformations was then developed to install the five remaining stereocenters. The C8 lactone functionality could serve as an attachment point for the remaining side chain of norzoanthamine.
Supplementary Material
Acknowledgment
We gratefully acknowledge the National Institutes of Health (NIH) for financial support of this work through Grant Number R01 GM081484. We thank the National Science Foundation for instrumentation grants CHE9709183 and CHE0741968. We also thank Dr. Anthony Mrse (UCSD NMR Facility); Dr. Yongxuan Su (UCSD MS Facility) and Dr. Arnold L. Rheingold and Dr. Curtis E. Moore (UCSD X-Ray facility). We also thank Sanne Bouwman and Weng K. Chang (UCSD) for technical assistance.
Footnotes
Supporting Information Available: Experimental procedures, characterization data and copies of the 1H and 13C NMR of all new compounds, as well as X-ray data of compounds 5 and 17. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For selected reviews on this topic, see: Rahman AU, Choudhary MI. Alkaloids. Vol 52. New York: Academic Press; 1999. pp. 233–260. Kuramoto M, Yamaguchi K, Tsuji T, Uemura D. Zoanthamines, Antiosteoporotic Alkaloids. In: Fusetani N, editor. Drugs from the Sea. Basel: Karger; 2000. pp. 98–106. Yamada K, Kuramoto M, Uemura D. Rec. Res. Devel. Pure Appl. Chem. 1999;3:245–254. Fernandez JJ, Souto ML, Daranas AH, Norte M. Curr. Topics Phytochem. 2000;4:105–119.
- 2.Rao CB, Anjaneyula ASR, Sarma NS, Venkatateswarlu Y, Rosser RM, Faulkner DJ, Chen MHM, Clardy J. J. Am. Chem. Soc. 1984;106:7983–7984. [Google Scholar]
- 3.a) Fukuzawa S, Hayashi Y, Uemura D, Nagatsu A, Yamada K, Ijuin Y. Heterocycl. Commun. 1995;1:207–214. [Google Scholar]; b) Kuramoto M, Hayashi K, Fujitani Y, Yamaguchi K, Tsuji T, Yamada K, Ijuin Y, Uemura D. Tetrahedron Lett. 1997;38:5683–5686. [Google Scholar]
- 4.Rao CB, Anjaneyulu ASR, Sarma NS, Venkatateswarlu Y, Rosser RM, Faulkner DJ. J. Org. Chem. 1985;50:3757–3760. [Google Scholar]
- 5.Villar RM, Gil-Longo J, Daranas AH, Souto ML, Fernandez JJ, Peixinho S, Barral MA, Santafe G, Rodriguez J, Jimenez C. Bioorg. Med. Chem. 2003;11:2301–2306. doi: 10.1016/s0968-0896(03)00107-x. [DOI] [PubMed] [Google Scholar]
- 6.a) Rao CB, Rao DV, Raju VSN, Sullivan BW, Faulkner DJ. Heterocycles. 1989;28:103–106. [Google Scholar]; b) Venkateswarlu Y, Reddy NS, Ramesh P, Reddy PS, Jamil K. Heterocycl. Commun. 1998;4:575–580. [Google Scholar]
- 7.a) Yamaguchi K, Yada M, Tsuji T, Kuramoto M, Uemura D. Biol. Pharm. Bull. 1999;22:920–928. doi: 10.1248/bpb.22.920. [DOI] [PubMed] [Google Scholar]; b) Kuramoto M, Hayashi K, Yamaguchi K, Yada M, Tsuji T, Uemura D. Bull. Chem. Soc. Jpn. 1998;71:771–779. [Google Scholar]; c) Kinugawa M, Fukuzawa S, Tachibana K. J. Bone Miner. Metab. 2009;27:303–314. doi: 10.1007/s00774-009-0049-7. [DOI] [PubMed] [Google Scholar]; c) Kuramoto M, Arimoto M, Uemura D. Mar. Drugs. 2004;2:39–54. [Google Scholar]
- 8.For a comprehensive review of the chemistry and biology of zoanthamines, see: Behenna DC, Stockdill JL, Stoltz BM. Angew. Chem., Int. Ed. 2008;47:2365–2386. doi: 10.1002/anie.200703172.
- 9.For representative publications from each group see: Juhl M, Monrad R, Sotofte I, Tanner D. J. Org. Chem. 2007;72:4644–4654. doi: 10.1021/jo070165r. Irifune T, Ohashi T, Ichino T, Sakai E, Suenaga K, Uemura D. Chem. Lett. 2005;34:1058–1059. Rivas F, Ghosh S, Theodorakis EA. Tetrahedron Lett. 2005;46:5281–5284. Williams DR, Patnaik S, Cortez GS. Heterocycles. 2007;72:213–219. Hirai G, Oguri H, Hirama M. Chem. Lett. 1999:141–142. Behenna DC, Stockdill JL, Stoltz BM. Angew. Chem. Int. Ed. 2007;46:4077–4080. doi: 10.1002/anie.200700430.
- 10.a) Miyashita M, Sasaki M, Hattori I, Sakai M, Tanino K. Science. 2004;305:495–499. doi: 10.1126/science.1098851. [DOI] [PubMed] [Google Scholar]; b) Miyashita M. Pure Appl. Chem. 2007;79:651–665. [Google Scholar]; c) Yoshimura F, Sasaki M, Hattori I, Komatsu K, Sakai M, Tanino K, Miyashita M. Chem. Eur. J. 2009;15:6626–6644. doi: 10.1002/chem.200900310. [DOI] [PubMed] [Google Scholar]
- 11.a) Murata Y, Yamashita D, Kitahara K, Minasako Y, Nakazaki A, Kobayashi S. Angew. Chem. Int. Ed. 2009;48:1400–1403. doi: 10.1002/anie.200804544. [DOI] [PubMed] [Google Scholar]; b) Yamashita D, Murata Y, Hikage N, Takao KI, Nakazaki A, Kobayashi S. Angew. Chem. Int. Ed. 2009;48:1404–1406. doi: 10.1002/anie.200804546. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi Y, Yoshimutra F, Tanino K, Miyashita M. Angew. Chem. Int. Ed. 2009;48:8905–8908. doi: 10.1002/anie.200904537. [DOI] [PubMed] [Google Scholar]
- 13.For a review of annulation chemistry, see: Jung ME. Tetrahedron. 1976;32:3–31.
- 14.Ghosh S, Rivas F, Fischer D, Gonzalez MA, Theodorakis EA. Org. Lett. 2004;6:941–944. doi: 10.1021/ol036492c. [DOI] [PubMed] [Google Scholar]
- 15.a) Ling T, Kramer BA, Palladino MA, Theodorakis EA. Org. Lett. 2000;2:2073–2076. doi: 10.1021/ol0060578. [DOI] [PubMed] [Google Scholar]; b) Ling T, Chowdhury C, Kramer BA, Vong BG, Palladino MA, Theodorakis EA. J. Org. Chem. 2001;66:8843–8853. doi: 10.1021/jo0159035. [DOI] [PubMed] [Google Scholar]
- 16.Iyengar R, Schildknegt K, Morton M, Aubé J. J. Org. Chem. 2005;70:10645–10652. doi: 10.1021/jo051212n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a) Tamai Y, Mizutani Y, Hagiwara H, Uda H, Harada N. J. Chem. Res. Synop. 1985:148–149. [Google Scholar]; b) Hagiwara H, Uda H. J. Org. Chem. 1988;53:2308–2311. [Google Scholar]
- 18.a) Goering HL, Eikenberry JN, Koermer GS. J. Am. Chem. Soc. 1971;93:5913–5914. [Google Scholar]; b) Corey EJ, Virgil SC. J. Am. Chem. Soc. 1990;112:6429–6431. [Google Scholar]; c) Calmes M, Daunis J, Jacquier R, Verducci J. Tetrahedron. 1987;43:2285–2292. [Google Scholar]
- 19.Spencer TA, Smith RAJ, Storm DL, Villarica RM. J. Am. Chem. Soc. 1971;93:4856–4864. [Google Scholar]
- 20.CCDC-820554 (17) and CCDC-820555 (5) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/const/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge, CB21EZ, UK; fax: (+44)1223-336-033; or deposit@ccdc.cam.ac.uk).
- 21.Alcaide B, Tarazona MP, Fernandez F. J. Chem. Soc. Perkin Trans. I. 1982:2117–2122. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.