Abstract
Background
Here, we aim to identify defects of apolipoprotein (apo) B lipoprotein metabolism that characterize hypertriglyceridemia, focusing on apoC-III and apoE.
Methods and Results
We studied the transport of plasma apoB within 21 distinct subfractions as separated by anti–apoC-III and anti–apoE immunoaffinity chromatography and ultracentrifugation in 9 patients with moderate hypertriglyceridemia and 12 normotriglyceridemic control subjects. Hypertriglyceridemia was characterized by a 3-fold higher liver secretion of very low-density lipoprotein (VLDL) that had apoC-III but not apoE and a 50% lower secretion of VLDL with both apoC-III and apoE (both P<0.05). This shift in VLDL secretion pattern from apoE to apoC-III resulted in significantly reduced clearance of light VLDL (−39%; P<0.05), compatible with the antagonizing effects of apoC-III on apoE-induced clearance of triglyceride-rich lipoproteins. In addition, rate constants for clearance were reduced for apoE-containing triglyceride-rich lipoproteins in hypertriglyceridemia, associated with increased apoC-III contents of these particles. LDL distribution shifted from light and medium LDL to dense LDL in hypertriglyceridemia through a quartet of kinetic perturbations: increased flux from apoC-III–containing triglyceride-rich lipoproteins, a shift in liver LDL secretion pattern from light to dense LDL, an increased conversion rate from light and medium LDL to dense LDL, and retarded catabolism of dense LDL.
Conclusions
These results support a central role for apoC-III in metabolic defects leading to hypertriglyceridemia. Triglyceride-rich lipoprotein metabolism shifts from an apoE-dominated system in normotriglyceridemic participants characterized by rapid clearance from circulation of VLDL to an apoC-III–dominated system in hypertriglyceridemic patients characterized by reduced clearance of triglyceride-rich lipoproteins and the formation of the dense LDL phenotype.
Keywords: apolipoproteins, lipids, lipoproteins, metabolism
Hypertriglyceridemia is a common form of dyslipidemia that affects 30% of adults in the United States and is frequently associated with increased risk for coronary heart disease (CHD).1 However, unlike the relationship between hypercholesterolemia and CHD, which has been firmly established by observational studies, pathophysiology, and clinical trials, the direct involvement of plasma triglyceride in atherosclerosis and the subsequent development of CHD has been more difficult to prove. Elevated triglyceride levels are thought to increase CHD risk through the atherogenic effects of triglyceride-rich lipoproteins (TRLs) and their remnants.2 However, there is substantial heterogeneity in TRLs; triglyceride can be packaged into a wide variety of lipoprotein particles with potentially varying degrees of atherogenicity. Routine lipoprotein analyses do not identify these particles, so the atherogenic potential of hypertriglyceridemic states may not be adequately appreciated.
Apolipoproteins have long been suspected to play crucial roles in the origin of hypertriglyceridemia in humans. Among them, apolipoprotein (apo) C-III, a small, surface protein found on many TRLs, has been proposed as a key contributor to hypertriglyceridemia on the basis of its inhibitory effects on apoB lipoprotein catabolism. In vitro experiments have demonstrated that apoC-III inhibits lipoprotein lipase and hepatic lipase and retards clearance of very low-density lipoprotein (VLDL) by interfering with binding of apoB100 or apoE to hepatic receptors.3,4 Overexpression of apoC-III in mice causes hypertriglyceridemia5; apoC-III deficiency protects against it.6 In humans, kinetic studies suggest that apoC-III bound to TRL correlates positively with the secretion of VLDL triglyceride7 and negatively with VLDL catabolism.8 In addition, a recent genome-wide association study identifies apoC-III as the major determinant of fasting and postprandial triglyceride levels.9 In contrast to the actions of apoC-III, there is clear evidence that apoE assists in the clearance of apoB lipoproteins by binding to cell-surface receptors and proteoglycans.10 Humans deficient in apoE or homozygous for a defective isoform of apoE have retarded metabolism and defective composition of TRL.11 On the other hand, overexpression of apoE in mice corrects the hypertriglyceridemia produced by overexpression of apoC-III.12
However, it is less clear how closely these findings from in vitro experiments and animal studies map to TRL metabolism in normal and hypertriglyceridemic people. Previously, we reported that a major metabolic defect of hypertriglyceridemic patients is the selective overproduction of a subtype of VLDL containing apoC-III but not apoE and a global reduction in the clearance rate from plasma of all TRLs.13 The present kinetic studies extend these findings to a significantly larger sample to develop a more highly powered kinetic model of plasma apoB lipoprotein metabolic pathways that establish hypertriglyceridemia. We also studied pathways that establish the dense LDL phenotype in hypertriglyceridemia. Our findings support the fundamental involvement of apoC-III as a component of VLDL and intermediate-density lipoprotein (IDL) in abnormal metabolism causing hypertriglyceridemia and the protective role of apoE underlying normal metabolism of apoB lipoproteins. Our findings also reveal 4 major metabolic processes that give rise to the dense LDL phenotype in hypertriglyceridemia, including channeling of apoC-III–containing TRL to LDL, a shift in hepatic secretion of LDL from light to dense particles, increased conversion in plasma of light to dense LDL, and slow clearance of dense LDL.
Methods
A detailed Methods section is available in the online-only Data Supplement. Briefly, 9 patients with moderate hypertriglyceridemia and 12 normotriglyceridemic control subjects participated in the tracer kinetics studies after 3 weeks on a controlled high-carbohydrate, low-fat diet. This type of diet was used because it promotes dense LDL.14 Participants received a 15-hour primed, continuous infusion of [D3]L-leucine and a bolus injection of [D5]L-phenylalanine. Blood samples were collected at baseline, every 20 minutes in the first 2 hours of infusion, and hourly thereafter.
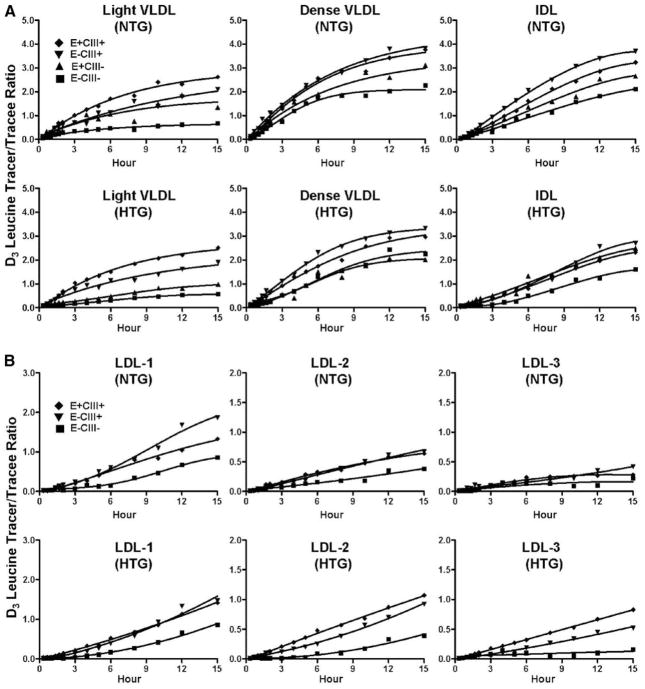
ApoB lipoproteins from these samples were separated by anti–apoC-III and anti-apoE immunoaffinity columns into those containing both apolipoproteins (E+CIII+), either one (E+CIII−, E−CIII+), or none of them (E−CIII−). These 4 immunofractions were further separated according to density into 6 fractions: light LDL (LDL-1), medium LDL (LDL-2), and dense LDL (LDL-3). Thus, 24 distinct apoB lipoprotein fractions were obtained for each sample. ApoB was precipitated from these fractions, and tracer enrichment and tracee mass were measured by gas chromatography–mass spectrometry.13,15 Figure 1 shows leucine enrichment curves in patients with hyperlipidemia and normotriglyceridemic control subjects.
Figure 1.
Tracer/tracee ratios of D3-leucine in hypertriglyceridemic patients (HTG) and normotriglyceridemic control subjects (NTG). Data represent average leucine tracer/tracee ratios and model-fitted curves. LDL-1 indicates light LDL; LDL-2, medium LDL; LDL-3, dense LDL.
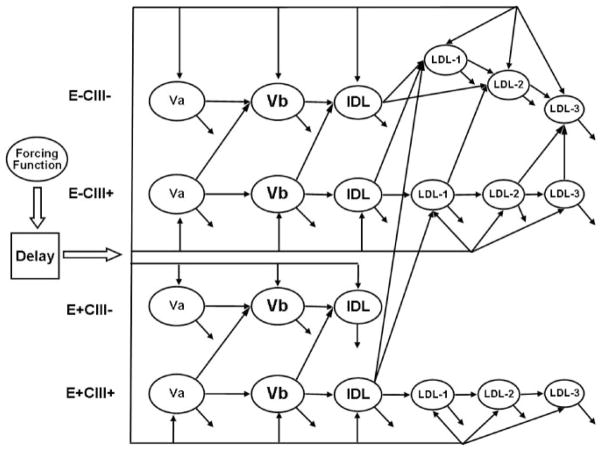
A multicompartment model was used to find the best fit to the observed data with SAAM II software (Figure 2). For each individual, both [D3]L-leucine and [D5]L-phenylalanine tracer data were included in the same model, and the data were solved simultaneously by making the rate constants equal for the leucine and phenylalanine tracer experiments. Thus, for each participant, a single set of rate constants was produced. This model was able to generate excellent fits to the tracer data (Figure 1) and to the apoB pool sizes.
Figure 2.
Structure of the multicompart-mental model. Plasma apoB lipoproteins were separated into 21 compartments by density and apolipoprotein composition. Each plasma apoB compartment represents a physically isolated lipoprotein fraction. See the Methods section in the online-only Data Supplement for model development and validation. LDL-1 indicates light LDL; LDL-2, medium LDL; LDL-3, dense LDL.
Results
Nine hypertriglyceridemic patients and 12 age- and gender-matched normotriglyceridemic control subjects participated in the study. At the screening visit when the participants were eating their usual diet, triglyceride levels were 286±15 mg/dL for hypertriglyceridemic patients versus 93±18 mg/dL for normotriglyceridemic control subjects (P<0.01). Subject characteristics and concentrations of plasma lipids and apolipoproteins after the 3-week controlled low-fat diet are summarized in Tables 1 and 2. Although all subjects were nondiabetic and had normal glucose levels and homeostasis model assessment (HOMA) index, hypertriglyceridemic patients had significantly higher glucose, insulin, HOMA index, and body mass index than normotriglyceridemic control subjects. Hypertriglyceridemic patients also had significantly higher plasma apoC-III and apoE, as well as VLDL apoB, apoC-III, and apoE. The plasma total apoC-III concentration in hypertriglyceridemic patients was double that in normotriglyceridemic control subjects, whereas apoE was higher by only about one-third. In addition, hypertriglyceridemic patients had lower light and medium LDL and higher dense LDL levels, reflecting a dense LDL phenotype.
Table 1.
Characteristics of the Study Participants
| Normotriglyceridemics (n=12) | Hypertriglyceridemics (n=9) | |
|---|---|---|
| Age, y | 50.4±2.6 | 47.9±3.0 |
| Gender, F/M | 6/6 | 5/4 |
| BMI, kg/m2 | 25.8±1.3 | 30.3±1.6* |
| Triglycerides, mg/dL | 80.3±11.8 | 220±34† |
| ApoB, mg/dL | 80.8±5.6 | 88.7±5.6 |
| Cholesterol, mg/dL | 161±11 | 172±10 |
| Glucose, mg/dL | 84.3±4.2 | 95.0±4.3* |
| Insulin, μU/mL | 5.3±1.3 | 9.1±2.6‡ |
| HOMA | 1.1±0.4 | 2.2±0.7* |
BMI indicates body mass index. Values are from plasma samples collected at the time of the tracer studies after an overnight fast. HOMA=glucose (mg/dL)×insulin (μU/mL)/405. Data represent mean±SEM.
P<0.05,
P<0.01 between 2 groups by unpaired t test;
P<0.07.
Table 2.
Plasma Lipids and Lipoproteins
| ApoB
|
Triglyceride
|
Cholesterol
|
ApoE
|
ApoC-III
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NTG | HTG | NTG | HTG | NTG | HTG | NTG | HTG | NTG | HTG | |
| Light VLDL | 1.2±0.2 | 2.2±0.2* | 15.9±4.1 | 86.4±22.9† | 3.5±0.8 | 11.1±3.1* | 0.53±0.09 | 1.52±0.26† | 0.53±0.14 | 2.77±0.44† |
| Dense VLDL | 2.5±0.4 | 3.3±0.3 | 17.0±4.6 | 51.6±7.5† | 6.1±1.5 | 10.2±1.3 | 0.36±0.10 | 1.16±0.31* | 1.02±0.51 | 3.55±0.98* |
| IDL | 8.0±1.8 | 6.6±1.0 | 8.6±1.3 | 18.7±2.6† | 9.9±1.7 | 13.6±1.2 | 0.54±0.09 | 1.01±0.16* | 1.01±0.41 | 3.12±0.87* |
| LDL-1 | 13.5±2.2 | 8.6±1.2 | 4.5±0.7 | 8.1±1.3† | 18.5±2.3 | 14.2±1.1 | 0.24±0.03 | 0.35±0.04* | 0.29±0.11 | 0.64±0.19 |
| LDL-2 | 19.5±1.4 | 12.4±1.8* | 7.6±2.1 | 10.2±2.8* | 30.3±5.1 | 24.5±4.5 | 0.22±0.03 | 0.23±0.03 | 0.21±0.05 | 0.43±0.05* |
| LDL-3 | 36.1±4.9 | 55.6±4.1* | 6.7±1.7 | 17.2±3.1 | 34.1±7.1 | 63.2±5.7* | 0.34±0.04 | 0.34±0.05 | 0.36±0.06 | 0.74±0.09† |
| HDL | 19.9±5.8 | 27.7±4.7 | 58.3±7.0 | 35.3±5.7* | 3.61±0.48 | 3.34±0.65 | 5.38±0.69 | 6.35±1.87 | ||
| Plasma | 80.8±5.6 | 88.7±5.6 | 80.3±11.8 | 220±34† | 161±11 | 172±10 | 5.84±0.47 | 7.94±0.83* | 8.81±1.06 | 17.6±1.8† |
NTG indicates normotriglyceridemic control subjects (n=12); HTG, hypertriglyceridemic patients (n=9); LDL-1, light LDL; LDL-2, medium LDL; LDL-3, dense LDL. Values are from plasma samples at the time of the tracer studies after an overnight fast. Data represent mean±SEM and are expressed in mg/dL.
P<0.05,
P<0.01 by unpaired t test.
Accumulation of E−CIII+ TRL in Hypertriglyceridemia
Compared with control subjects, hypertriglyceridemic patients had significantly higher pool sizes of light VLDL, dense VLDL, and IDL that had apoC-III but not apoE (E−CIII+) (Figure 3). E−CIII+ light VLDL pool size was 3.4-fold higher in hypertriglyceridemic patients (57.0±11.7 versus 16.8±4.5; P<0.05). In contrast, the 2 groups had similar pool sizes of VLDL and IDL that had apoE (E+CIII−, E+CIII+). Hypertriglyceridemic patients also had higher pool sizes of E−CIII− VLDL.
Figure 3.
Plasma apoB pool sizes. Open bars indicate apoB pool sizes among normotriglyceridemic control subjects (NTG); solid bars, hypertriglyceridemic patients (HTG). Data represent mean±SEM (n=12 for NTG, n=9 for HTG). The numbers on the bottom right represent apoB pool sizes in E+CIII+ LDL-3. *P<0.05 between NTG and HTG by unpaired t test.
Increased Liver Secretion of E−CIII+ Light VLDL in Hypertriglyceridemia
Accumulation of E−CIII+ TRL was due at least in part to the fact that hypertriglyceridemic patients had 3-fold higher secretion rates of E−CIII+ light VLDL and 40% lower secretion of E+CIII+ light VLDL (Figure 4). Hypertriglyceridemic patients also had higher secretion of light E−CIII− VLDL. Overall, hypertriglyceridemic patients had lower secretion rates of apoE-containing VLDL and IDL. Total apoB secretion rates were similar between hypertriglyceridemic patients and normotriglyceridemic control subjects (23.7±1.4 versus 26.3±1.5 mg·d−1·kg−1; P=0.56).
Figure 4.
Direct apoB secretion rate. Data represent direct secretion rates into plasma (mg·d−1·kg−1, mean±SEM) of apoB lipoproteins according to density and apoC-III and apoE content. NTG indicates normotriglyceridemic control subjects; HTG, hypertriglyceridemic patients; LDL-1, light LDL; LDL-2, medium LDL; LDL-3, dense LDL. *P<0.05 by unpaired t test.
Slow Metabolism of ApoE-Containing VLDL in Hypertriglyceridemia
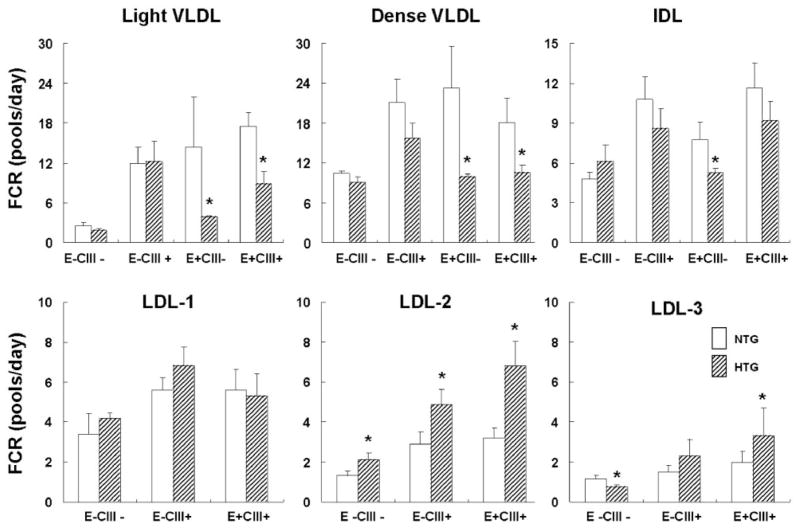
Leucine enrichment curves showed slower rates of appearances for apoE-containing VLDL and LDL among hypertriglyceridemic participants (Figure 1). Indeed, modeling results demonstrated that hypertriglyceridemic patients had significantly lower fractional catabolic rates (FCRs) for apoE-containing VLDL (both E+CIII+ and E+CIII−; Figure 5). The FCR of E+CIII− IDL was also significantly lower in hypertriglyceridemic patients. On the other hand, FCRs of VLDL and IDL without apoE, either E−CIII− or E−CIII+, were similar between the 2 groups.
Figure 5.
FCR of plasma apoB lipoproteins. Data represent mean±SEM. NTG indicates normotriglyceridemic control subjects; HTG, hypertriglyceridemic patients; LDL-1, light LDL; LDL-2, medium LDL; LDL-3, dense LDL. *P<0.05 by unpaired t test.
Hypertriglyceridemia Shifts ApoB Flux From TRL Containing ApoE to Those Without ApoE, Reducing VLDL Clearance and Increasing Synthesis of LDL
As a result of higher secretion rates of E−CIII+ light VLDL (Figure 4), hypertriglyceridemic patients had significantly higher synthetic rates for its products, E−CIII+ dense VLDL and IDL, lipoproteins that almost exclusively underwent further lipolytic processing to IDL and LDL, most of it ending as E−CIII− dense LDL (Figure 6A and Table I of the online-only Data Supplement). In both hypertriglyceridemic patients and normotriglyceridemic control subjects, <7% of E−CIII+ VLDL and IDL were removed from circulation before they were converted to LDL. In addition, >80% of E−CIII+ LDL were converted to E−CIII− LDL. Similarly, hypertriglyceridemic patients also had higher secretion of E−CIII− VLDL, and this was processed in plasma to IDL and LDL. For both hypertriglyceridemic patients and normotriglyceridemic control subjects, the majority of E−CIII− VLDL and IDL (73% in hypertriglyceridemic patients, 90% in normotriglyceridemic control subjects; P=0.43) were hydrolyzed into E−CIII− LDL. In contrast, hypertriglyceridemic patients had much lower secretion of E+CIII+ light VLDL and IDL, resulting in much lower flux of VLDL and IDL out of plasma (Figure 6A). Flux of E+CIII− TRL, a minor fraction coming from E+CIII+TRL, was also similarly reduced among hypertriglyceridemic patients. These changes among hypertriglyceridemic patients resulted in significantly reduced flux rates from faster to slower metabolized types of lipoproteins resulted in significantly reduced flux rates for clearance of light VLDL (2.27±0.4 versus 3.73±0.6 mg·d−1·kg−1; P<0.05) and of total VLDL (5.73±0.7 versus 7.94±0.7 mg·d−1·kg−1; P<0.05) compared with normotriglyceridemic control subjects.
Figure 6.
Changes in flux rates and rate constants of plasma apoB lipoprotein metabolic pathways between hypertriglyceridemic patients and control subjects. Values represent apoB flux rates (A; mg·d−1·kg−1) and rate constants (B; pools per day) in hypertriglyceridemic patients. Numbers inside parentheses indicate percentage changes of these values vs control subjects. Dotted lines represent direct secretion into plasma of each lipoprotein type. Solid arrows connecting 2 plasma lipoprotein compartments represent lipolytic conversion from the originating compartment to the receiving compartment. Arrows exiting a compartment without going into another compartment represent direct removal pathways (ie, clearance from circulation). Numbers inside ovals in A indicate percentage changes in compartment pool sizes. *P<0.05, †P<0.01 between hypertriglyceridemic patients and control subjects by unpaired t test. LDL-1 indicates light LDL; LDL-2, medium LDL; LDL-3, dense LDL.
Retarded Clearance Rate of ApoE-Containing TRL in Hypertriglyceridemia
Rate constants of direct clearance pathways for apoE-containing light and dense VLDL (E+CIII− and E+CIII+) were 50% lower in hypertriglyceridemic patients (P<0.05; Figure 6B). Rate constants for conversion of apoE-containing light VLDL to dense VLDL and further to IDL also showed trends for reduction. Therefore, in addition to reduced liver secretion, apoE-containing TRLs in hypertriglyceridemic patients also had retarded metabolism in plasma.
Shift of LDL Distribution From Light and Medium LDL to Dense LDL in Hypertriglyceridemic Patients
Although total LDL pool sizes were not significantly different between hypertriglyceridemic patients and normotriglyceridemic control subjects (1914±238 versus 1710±205 mg; P=0.52), there was a shift in LDL distribution pattern (Figure 3). In all 3 LDL density subfractions, the predominant type of LDL in both groups by far was E−CIII−, making up >90% of total LDL, and hypertriglyceridemic patients had smaller pool sizes of light and medium LDL (E−CIII− LDL-1, LDL-2) but a larger pool size of dense LDL (E−CIII− LDL-3).
Shift of LDL Secretion Pattern From Light to Dense LDL in Hypertriglyceridemia
Hypertriglyceridemic patients had significantly higher secretion rates of E−CIII− LDL-3, their predominant type of LDL (1.11±0.32 versus 0.72±0.26 mg·d−1·kg−1; P<0.05; Figure 4). In contrast, hypertriglyceridemic patients had much lower secretion of E−CIII− LDL-1 (0.34±0.12 versus 1.03±0.42 mg·d−1·kg−1; P<0.05) and a trend for lower secretion of E−CIII− LDL-2. Liver secretion of LDL-3 E−CIII+ was also higher in hypertriglyceridemic patients.
Accelerated Conversion of Lighter to Denser LDL and Slow Metabolism of Dense LDL in Hypertriglyceridemia
Rate constants for conversion of lighter LDL to denser LDL (LDL-1 to LDL-2, LDL-2 to LDL-3) were significantly higher in hypertriglyceridemic patients for E−CIII−, E−CIII+, and E+CIII+ (Figure 6B). On the other hand, hypertriglyceridemic patients had significantly slower FCRs of E−CIII− LDL-3 (0.75±0.13 versus 1.17±0.13 pools per day; P<0.05; Figure 5). Because >95% of all LDL-3 are E−CIII−, the FCR of total LDL-3 was also significantly lower in hypertriglyceridemic patients (0.72±0.12 versus 1.11±0.12 pools per day; P<0.05).
Plasma Triglyceride Is Predicted by Altered Pattern of ApoB Lipoprotein Secretion
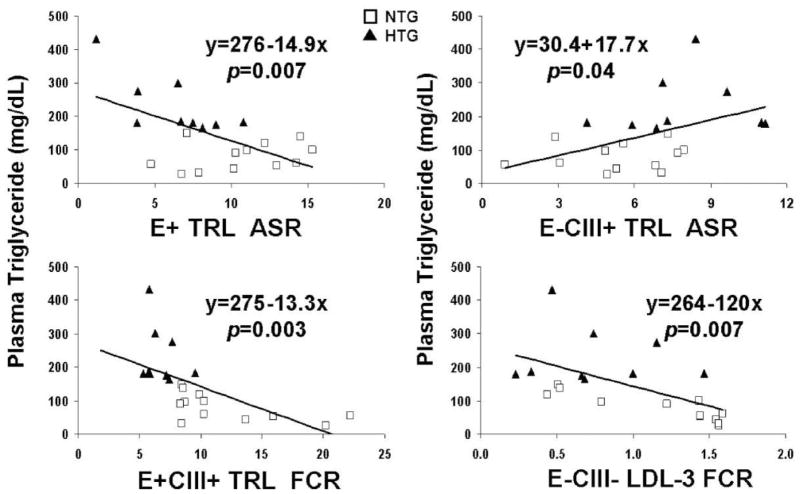
We studied kinetic determinants of plasma triglyceride by linear regression. Secretion rates of apoE-containing TRL negatively predicted plasma triglyceride concentration, whereas secretion rate of TRL with apoC-III but not apoE positively predicted plasma triglyceride (Figure 7, top). These secretion rates still significantly predicted plasma triglyceride in multiple linear regression analysis after adjustment for body mass index and gender or plasma glucose, insulin, or HOMA index (data not shown).
Figure 7.
Relationship between kinetic parameters and plasma triglyceride concentration. Linear regression was performed on baseline plasma triglyceride concentration (dependent variable) and the secretion rate of apoE-containing TRL (top left), the secretion rate of E−CIII+ TRL (top right), the FCR of E+CIII+ TRL (bottom left), or the FCR of E−CIII− dense LDL (bottom right). Regression equations represent plasma triglyceride as a function of these kinetic parameters. P values represent the probability that the regression coefficient (slope) is different from 0. TRL includes VLDL and IDL. ▲ Indicates hypertriglyceridemic patients (HTG); □, normotriglyceridemic controls (NTG); and ASR, absolute liver secretion rate.
Plasma Triglyceride Is Dependent on ApoB Lipoprotein FCRs
Linear regression analysis also showed that the FCR of E+CIII+ TRL (Figure 7, bottom left) or FCR of total VLDL negatively predicted plasma triglyceride, indicating that reduced VLDL FCR contributed to hypertriglyceridemia. In addition, because of the close association between hypertriglyceridemia and the small, dense LDL phenotype, we explored the relationship between plasma triglyceride and the kinetics of E−CIII− LDL-3, the smallest and predominant LDL type, and found that E−CIII− LDL-3 FCR negatively predicted plasma triglyceride (Figure 7, bottom right). Furthermore, in multiple linear regression analysis after adjustment for body mass index and gender or plasma glucose, insulin, or HOMA index, the FCRs of E+CIII+ TRL or E−CIII− LDL-3 still significantly predicted plasma triglyceride (data not shown).
Enrichment of ApoCs but Not apoE in TRL in Hypertriglyceridemia
ApoE-containing TRL had on average ≈10 to 30 molecules of apoE per particle, measured as molar ratio of apoE to apoB, similar in hypertriglyceridemic patients and normotriglyceridemic control subjects (Table 3). In contrast, hypertriglyceridemic patients had on average more than twice as many apoC-III molecules per particle as control subjects. TRLs were also enriched in apoC-I and apoC-II in hypertriglyceridemic patients.
Table 3.
Apolipoprotein Contents of TRL
| ApoE
|
ApoC-III
|
ApoC-I
|
ApoC-II
|
|||||
|---|---|---|---|---|---|---|---|---|
| NTG | HTG | NTG | HTG | NTG | HTG | NTG | HTG | |
| Light VLDL | ||||||||
| E−CIII− | … | … | … | … | 2.9±0.5 | 5.3±1.2* | 3.8±1.2 | 11±5* |
| E+CIII− | 18±5 | 20±5 | … | … | 15±5 | 20±9 | 13.3±5 | 35±13* |
| E−CIII+ | … | … | 45±12 | 123±24* | 24±6 | 30±7 | 37±15 | 104±24* |
| E+CIII+ | 16±3 | 23±4 | 42±11 | 90±21* | 38±8 | 59±8 | 35±13 | 125±12* |
| Dense VLDL | ||||||||
| E−CIII− | … | … | … | … | 1.9±0.5 | 2.9±0.6 | 3.3±1.0 | 11±4* |
| E+CIII− | 23±6 | 26±14 | … | … | 6.2±2.2 | 15±7* | 8.6±2.3 | 62±21* |
| E−CIII+ | … | - | 54±22 | 82±20* | 24±9 | 36±7 | 27±13 | 45±8* |
| E+CIII+ | 23±6 | 20±7 | 65±14 | 106±42* | 63±12 | 68±21 | 59±20 | 84±12* |
| IDL | ||||||||
| E−CIII− | … | … | … | … | 0.5±0.1 | 0.7±0.1 | 0.5±0.1 | 1.9±0.8* |
| E+CIII− | 11±2 | 21±3* | … | … | 2.7±0.5 | 6.7±1.4* | 2.1±0.6 | 16±5* |
| E−CIII+ | … | … | 37±15 | 68±18* | 14±5 | 15±4 | 22±12 | 39±13* |
| E+CIII+ | 23±4 | 18±4 | 43±14 | 75±25* | 40±12 | 35±8 | 36±10 | 54±16 |
NTG indicates normotriglyceridemic control subjects; HTG, hypertriglyceridemic patients. Data represent the number of apolipoproteins per particle in TRL as calculated by molar ratios of corresponding apolipoprotein vs apoB based on plasma concentrations of these apolipoproteins and apoB. Data represent mean±SEM.
P<0.05 by unpaired t test.
Discussion
We explored apoB lipoprotein metabolism in a group of patients with moderate hypertriglyceridemia but normal plasma LDL cholesterol levels. Our results reveal major perturbations in both TRL and LDL metabolism that involve apoC-III and apoE. The defects in TRL metabolism in hypertriglyceridemia are 2-fold: (1) a shift of secretion pattern from apoE-containing TRL, lipoproteins that are quickly removed from the circulation before reaching LDL, to TRLs that do not contain apoE and are retained in the circulation longer while being converted to denser lipoproteins, and (2) reduced rate constants for clearance in apoE-containing TRL. These 2 metabolic defects result in reduced clearance and thus accumulation of TRLs, hence hypertriglyceridemia. In addition, hypertriglyceridemic patients also witness a shift in LDL distribution to dense particles, a phenotype that is caused by a quartet of factors: high rate of formation in plasma of LDL from apoC-III– containing triglyceride-rich VLDL via stepwise delipidation, shift in hepatic secretion from light to dense LDL, increased conversion in plasma of light to dense LDL, and slow clearance rate of dense LDL. We illustrated these metabolic defects in Figure 8.
Figure 8.
Major metabolic pathways for apoB lipoproteins in normotriglyceridemia (NTG) and their alteration in hypertriglyceridemia (HTG) leading to the formation of the dense LDL phenotype. Shown are the apoB lipoprotein metabolic pathways in NTG (A) and changes in HTG (B). Width of the arrows indicates the relative amount of flux through each pathway. The plus and minus signs indicate significant changes in flux in corresponding pathways in HTG vs NTG. Liver secretion of TRL (VLDL and IDL) shifts from apoE-containing TRL to TRL without apoE in hypertriglyceridemia, resulting in reduced TRL clearance and increased conversion of TRL to LDL. Accumulation of dense LDL is caused by a quartet of metabolic disturbances: increased flux from apoC-III-containing TRL to dense LDL, increased conversion of light and medium LDL to dense LDL, increased direct liver secretion of dense LDL E−CIII− and E−CIII+, and reduced clearance of dense LDL. E+CIII− apoB lipoproteins make up only a minor fraction of plasma apoB lipoproteins and thus are not included.
Consistent with our earlier observations,13,15 this study demonstrates again that apoC-III and apoE are crucial regulators of apoB lipoprotein metabolism. Possession of apoE is key for direct clearance of TRLs. ApoE-containing TRLs mostly undergo direct removal and have a low tendency for LDL formation, whereas apoC-III antagonizes the action of apoE, channeling particles to lipolysis and LDL formation instead. TRLs with apoC-III but not apoE have minimal direct clearance and are metabolized quickly to LDL. TRLs without apoE or apoC-III are also nearly exclusively metabolized into LDL but with slower rates. This fundamental structure of apoB lipoprotein metabolism not only is at work under normal physiology13 but also explains metabolic changes during dietary intervention15 and in patients with hypertriglyceridemia.
Results of this study combined with our previous findings indicate that dietary carbohydrate could elicit changes in apoB lipoprotein metabolism in a manner parallel to endogenous hypertriglyceridemia. Previously, we have demonstrated that isocaloric substitution of dietary mono-unsaturated fat with carbohydrates significantly increases plasma levels of TRL with apoC-III but not apoE while reducing TRL with apoE.15 These phenotypic changes are qualitatively similar to what we observed in the present study in hypertriglyceridemic patients compared with normotriglyceridemic control subjects. Moreover, the dietary carbohydrate–induced TRL redistribution in plasma from apoE containing to those without apoE is caused by a corresponding shift in the liver secretion pattern, just as what the present study finds in hypertriglyceridemia compared with normotriglyceridemia. Therefore, hypertriglyc-eridemia is associated with changes in apoB lipoprotein metabolism that mimic the effects of dietary carbohydrates.
The observation that dietary intervention and hypertriglyceridemia induce similar phenotypic and mechanistic changes in apoB lipoprotein metabolism has clear physiological and interventional implications. For hypertriglyceridemic patients, consumption of a carbohydrate-rich diet seems to exacerbate an already unfavorable TRL secretion profile; on top of increased secretion of TRLs that have long circulation time and little direct removal (E−CIII+ and E−CIII−), dietary carbohydrates also reduce the secretion of TRL that have short circulation time and high tendency for removal (E+CIII+ and E+CIII−), resulting in reduced clearance and increased accumulation of TRLs. Conversely, for hypertriglyceridemic patients, replacing dietary carbohydrates with monounsaturated fat may help remedy the unfavorable domination by anticlearance apoC-III to a more normal proclearance apoE-mediated apoB lipoprotein system. This is exactly what we have observed: Hypertriglyceridemic patients eating a monounsaturated fat-rich diet do not have low secretion of apoE-containing TRLs,13 in contrast with findings of the present study, which has been carried out in subjects consuming a high-carbohydrates diet.
In addition to macronutrients and hypertriglyceridemia, other physiological or environmental conditions may also affect TRL and LDL subfraction metabolism through similar mechanisms that are closely regulated by apoC-III and apoE. Ginsberg and colleagues16 elegantly studied the effects of weight loss on VLDL and LDL kinetics in a group of hypertriglyceridemic patients. One major finding is that normalization of plasma triglyceride by weight loss is associated with reduced flux from VLDL to LDL and proportionately increased liver secretion of LDL, similar to what we find in normotriglyceridemic compared with hypertriglyceridemic subjects. In addition, we have previously studied the effects of estrogen treatment on apoB lipoprotein metabolism in a group of healthy postmenopausal women.17 It is interesting that oral estrogen, presumably having a totally different mode of action than dietary carbohydrates or endogenous hypertriglyceridemia, induces secretion of light VLDL and channels its flux through dense VLDL to IDL to dense LDL, similar to what the present study finds. In other words, it is possible that hyperproduction of TRL, whatever the instigator, causes this flux alteration to produce dense LDL. ApoC-III and apoE may play important roles in these conditions.
This study sheds new light on the complex mechanisms behind the formation of dense LDL. Hypertriglyceridemia is usually associated with a shift of the LDL distribution from lighter to denser particles, and this pattern of elevated plasma triglyceride, a predominance of dense LDL, and low high-density lipoprotein cholesterol is proposed as a distinct form of dyslipidemia called the atherogenic lipoprotein phenotype.18 Fisher et al,19 Packard and colleagues,20 and Vega et al21 hypothesized from kinetic studies that dense LDL is formed from the substantial light VLDL production, heightened activity of cholesteryl ester transfer protein-mediated exchange of lipids between light VLDL and LDL, and finally the conversion of the triglyceride-enriched, cholesterol ester– depleted light LDL to dense LDL via the function of hepatic lipase.22 They and others demonstrated a major flux pathway from light VLDL to dense LDL with little removal of intermediates, whereas in contrast, normotriglyceridemic subjects have much more direct LDL production and removal of dense VLDL and IDL.22 This study suggests that apoC-III and apoE are fundamental elements that produce this pathway. In hypertriglyceridemia, we observe a high secretion rate of apoC-III– containing light VLDL that does not have apoE. The high apoC-III and low apoE together channel these light VLDL to smaller lipoproteins all the way to dense LDL because apoC-III blocks clearance pathways mediated by apoB and apoE. Consistent with these results, Mauger and colleagues23 found that a high secretion rate of apoC-III into plasma is associated with a preponderance of dense LDL. The relative rarity of apoE in VLDL and IDL of hypertriglyceridemic patients excludes a major clearance mechanism. Further enhancing dense LDL formation is increased conversion rate in plasma of light to dense LDL presumably mediated by the typically high hepatic lipase activity in patients with high triglyceride levels.24 We have also found additional novel metabolic perturbations that increase dense LDL in hypertriglyceridemia, ie, a shift in hepatic LDL secretion from light to dense particles, and selectively reduced clearance rate of the predominant dense LDL.
Obesity and insulin resistance are potential confounders of our results because the hypertriglyceridemic patients had higher body mass index and HOMA index. However, after these factors are controlled for, the strong relationships between apoB kinetics parameters and plasma triglyceride remain significant. Therefore, we believe that our kinetics findings in the present study are relevant to typical hypertriglyceridemic patients with obesity or insulin resistance, and perhaps with type 2 diabetes.
One limitation of the present study is that our patients have only moderately elevated fasting plasma triglyceride. They have normal plasma cholesterol and apoB. However, normal apoB concentrations are estimated to be present in two thirds of hypertriglyceridemic patients,25 and participants of this study represent this common subgroup of hypertriglyceridemia. This study does not include patients suffering mixed hyperlipidemia, hyper-apoB, fatty liver, or familial hypertriglyceridemia. In these patients, additional mechanisms may be involved to support hypertriglyceridemia. However, because elevated plasma and TRL apoC-III are common features underlying various hypertriglyceridemic conditions,7 we suspect that key findings of the present study, ie, a shift of TRL metabolism from apoE to apoC-III and mechanisms for the formation of the dense LDL phenotype, could also be at play.
Our detailed separation of plasma apoB lipoproteins into 24 subfractions led to the generation of many kinetic parameters, ie, rate constants and flux rates. Special care is necessary when reporting multiple outcomes because a large number of unadjusted tests increases the susceptibility to type I error. However, tracer kinetic studies are unique in that the main outcomes, ie, kinetics parameters, are often interdependent. Computation of kinetic parameters during multicompartmental modeling is constrained by the metabolic connections between each lipoprotein compartment; thus, these parameters are dependent on each other. Furthermore, comparisons of these outcomes are preplanned, are previously published for other studies,13,15 and are not opportunistic. These considerations mitigate the risk of type I error.
Our study supports a central and causal role of apoC-III in the pathogenesis of hypertriglyceridemia. Plasma apoC-III levels in hypertriglyceridemic patients were on average twice as high as in control subjects. ApoC-III transgenic mice overproduce VLDL triglyceride.12 In humans, production of VLDL apoC-III correlates strongly with secretion of VLDL triglyceride.7 It has also been reported recently that apoC-III can stimulate the secretion of triglyceride-rich VLDL at the cellular level.26 In addition to altered secretion, a main defect of TRL metabolism in hypertriglyceridemia is the reduced catabolism of particles containing apoE. One likely mechanism could be the enrichment of apoC-III because we show that hypertriglyceridemic patients have on average twice as many apoC-III molecules on every apoE-containing VLDL. There is abundant evidence that apoC-III inhibits VLDL clearance.3 Taken together, these results indicate that apoC-III affects multiple major steps of triglyceride metabolism from secretion to clearance and may be an attractive pharmaceutical target to treat hypertriglyceridemia.
Finally, independently of its deleterious effects on apoB lipoprotein metabolism, our recent findings suggest that apoC-III exerts direct proatherogenic and proinflammatory effects on vascular cells, alone or as a component of apoB lipoproteins.27 Thus, the accumulation of plasma apoC-III and apoC-III-containing apoB lipoproteins in hypertriglyceridemic patients could expose them to greater risk for CHD. Therefore, elevated apoC-III may be a major mechanism linking hypertriglyceridemia to atherosclerosis and CHD.
Conclusions
ApoC-III and apoE play a central role in the pathogenesis of hypertriglyceridemia. In normotriglyceridemic participants, the majority of nascent TRL is secreted together with apoE and undergoes fast removal as a result of the strong proclearance functions of apoE. In contrast, in patients with moderate hypertriglyceridemia, TRL metabolism shifts from an apoE-dominated system to an apoC-III– dominated system as the majority of nascent lipoproteins are secreted without apoE but with apoC-III. ApoC-III antagonizes apoE- and apoB-induced lipoprotein clearance, an action that results in reduced clearance and consequently accumulation of atherogenic TRLs and their remnants that contain apoC-III. Formation of dense LDL, a feature of hypertriglyceridemia, is also linked to apoC-III via increased flux of the overproduced apoC-III– containing VLDL through lipolytic pathways to dense LDL. These adverse changes in apoB lipoprotein metabolism, in addition to the newly established proatherogenic effects of apoC-III itself, directly link hypertriglyceridemia to increased risk for CHD.
Supplementary Material
CLINICAL PERSPECTIVE.
Hypertriglyceridemia is a common form of dyslipidemia that is frequently linked to premature coronary artery disease. Hypertriglyceridemic patients often exhibit a small, dense low-density lipoprotein (LDL) phenotype. Our previous investigations demonstrate that apolipoprotein (apo) C-III and E, surface protein components of very low-density lipoprotein (VLDL) and LDL, play a dominant role in regulating the metabolism of these lipoproteins. In this work, we studied VLDL and LDL metabolism by the kinetics of its principal protein component, apoB, in 9 patients with moderate hypertriglyceridemia and 12 normotriglyceridemic control subjects using stable isotope labeling. Our results support a central role for apoC-III in VLDL and LDL metabolic defects leading to hypertriglyceridemia. Triglyceride-rich lipoprotein metabolism shifts from an apoE-dominated system in normolipidemic participants characterized by rapid clearance from the circulation of VLDL to an apoC-III-dominated system in hypertriglyceridemic patients characterized by reduced clearance of triglyceride-rich lipoproteins that are channeled to formation of dense LDL in plasma. In addition, apoC-III contributes to the formation of the dense LDL phenotype through a quartet of kinetic perturbations. These results indicate that the action of apoC-III to retard clearance of triglyceride-rich lipoproteins is a central metabolic feature underlying major changes in VLDL and LDL metabolism in hypertriglyceridemia. These adverse changes in apoB lipoprotein metabolism caused by apoC-III, in addition to the newly established proatherogenic effects of apoC-III itself, directly link moderate hypertriglyceridemia to increased risk for coronary heart disease. Therefore, modulating apoC-III may not only improve lipid profiles but also prevent the development of atherosclerotic plaques and their acute thrombotic complications.
Acknowledgments
We thank Tae Kim, Janis Swain, and Helen Judge-Ellis for their technical assistance.
Sources of Funding
This work was supported by National Institutes of Health grants R01-HL-34980, R01-HL-56210, and RR02635.
Footnotes
Disclosures
Dr Sacks is a consultant to ISIS Pharmaceuticals and Genzyme and previously received a research grant from ISIS on apoC-III. The other authors report no conflicts.
References
- 1.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V. Triglycerides and the risk of coronary heart disease: 10 158 incident cases among 262 525 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 2.Zilversmit DB. Atherogenic nature of triglycerides, postprandial lipidemia, and triglyceride-rich remnant lipoproteins. Clin Chem. 1995;41:153–158. [PubMed] [Google Scholar]
- 3.Sehayek E, Eisenberg S. Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway. J Biol Chem. 1991;266:18259–18267. [PubMed] [Google Scholar]
- 4.Wang CS, McConathy WJ, Kloer HU, Alaupovic P. Modulation of lipoprotein lipase activity by apolipoproteins: effect of apolipoprotein C-III. J Clin Invest. 1985;75:384–390. doi: 10.1172/JCI111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito Y, Azrolan N, O’Connell A, Walsh A, Breslow JL. Hypertriglyceridemia as a result of human apo CIII gene expression in transgenic mice. Science. 1990;249:790–793. doi: 10.1126/science.2167514. [DOI] [PubMed] [Google Scholar]
- 6.Maeda N, Li H, Lee D, Oliver P, Quarfordt SH, Osada J. Targeted disruption of the apolipoprotein C-III gene in mice results in hypotriglyceridemia and protection from postprandial hypertriglyceridemia. J Biol Chem. 1994;269:23610–23616. [PubMed] [Google Scholar]
- 7.Cohn JS, Patterson BW, Uffelman KD, Davignon J, Steiner G. Rate of production of plasma and very-low-density lipoprotein (VLDL) apolipoprotein C-III is strongly related to the concentration and level of production of VLDL triglyceride in male subjects with different body weights and levels of insulin sensitivity. J Clin Endocrinol Metab. 2004;89:3949–3955. doi: 10.1210/jc.2003-032056. [DOI] [PubMed] [Google Scholar]
- 8.Chan DC, Watts GF, Nguyen MN, Barrett PH. Apolipoproteins C-III and A-V as predictors of very-low-density lipoprotein triglyceride and apolipoprotein B-100 kinetics. Arterioscler Thromb Vasc Biol. 2006;26:590–596. doi: 10.1161/01.ATV.0000203519.25116.54. [DOI] [PubMed] [Google Scholar]
- 9.Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, Post W, McLenithan JC, Bielak LF, Peyser PA, Mitchell BD, Miller M, O’Connell JR, Shuldiner AR. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–1705. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowal RC, Herz J, Weisgraber KH, Mahley RW, Brown MS, Goldstein JL. Opposing effects of apolipoproteins E and C on lipoprotein binding to low density lipoprotein receptor-related protein. J Biol Chem. 1990;265:10771–10779. [PubMed] [Google Scholar]
- 11.Schaefer EJ, Gregg RE, Ghiselli G, Forte TM, Ordovas JM, Zech LA, Brewer HB., Jr Familial apolipoprotein E deficiency. J Clin Invest. 1986;78:1206–1219. doi: 10.1172/JCI112704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Silva HV, Lauer SJ, Wang J, Simonet WS, Weisgraber KH, Mahley RW, Taylor JM. Overexpression of human apolipoprotein C-III in transgenic mice results in an accumulation of apolipoprotein B48 remnants that is corrected by excess apolipoprotein E. J Biol Chem. 1994;269:2324–2335. [PubMed] [Google Scholar]
- 13.Zheng C, Khoo C, Ikewaki K, Sacks FM. Rapid turnover of apolipoprotein C-III-containing triglyceride-rich lipoproteins contributing to the formation of LDL subfractions. J Lipid Res. 2007;48:1190 –1203. doi: 10.1194/jlr.P600011-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Krauss RM, Dreon DM. Low-density-lipoprotein subclasses and response to a low-fat diet in healthy men. Am J Clin Nutr. 1995;62:478S– 487S. doi: 10.1093/ajcn/62.2.478S. [DOI] [PubMed] [Google Scholar]
- 15.Zheng C, Khoo C, Furtado J, Ikewaki K, Sacks FM. Dietary monounsaturated fat activates metabolic pathways for triglyceride-rich lipoproteins that involve apolipoproteins E and C-III. Am J Clin Nutr. 2008;88:272–281. doi: 10.1093/ajcn/88.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginsberg HN, Le NA, Gibson JC. Regulation of the production and catabolism of plasma low density lipoproteins in hypertriglyceridemic subjects: effect of weight loss. J Clin Invest. 1985;75:614–623. doi: 10.1172/JCI111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campos H, Walsh BW, Judge H, Sacks FM. Effect of estrogen on very low density lipoprotein and low density lipoprotein subclass metabolism in postmenopausal women. J Clin Endocrinol Metab. 1997;82:3955–3963. doi: 10.1210/jcem.82.12.4437. [DOI] [PubMed] [Google Scholar]
- 18.Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype: a proposed genetic marker for coronary heart disease risk. Circulation. 1990;82:495–506. doi: 10.1161/01.cir.82.2.495. [DOI] [PubMed] [Google Scholar]
- 19.Fisher WR, Zech LA, Bardalaye P, Warmke G, Berman M. The metabolism of apolipoprotein B in subjects with hypertriglyceridemia and polydisperse LDL. J Lipid Res. 1980;21:760–774. [PubMed] [Google Scholar]
- 20.Packard CJ, Demant T, Stewart JP, Bedford D, Caslake MJ, Schwertfeger G, Bedynek A, Shepherd J, Seidel D. Apolipoprotein B metabolism and the distribution of VLDL and LDL subfractions. J Lipid Res. 2000;41:305–318. [PubMed] [Google Scholar]
- 21.Vega GL, Grundy SM. Kinetic heterogeneity of low density lipoproteins in primary hypertriglyceridemia. Arteriosclerosis. 1986;6:395–406. doi: 10.1161/01.atv.6.4.395. [DOI] [PubMed] [Google Scholar]
- 22.Packard CJ, Shepherd J. Lipoprotein heterogeneity and apolipoprotein B metabolism. Arterioscler Thromb Vasc Biol. 1997;17:3542–3556. doi: 10.1161/01.atv.17.12.3542. [DOI] [PubMed] [Google Scholar]
- 23.Mauger JF, Couture P, Bergeron N, Lamarche B. Apolipoprotein C-III isoforms: kinetics and relative implication in lipid metabolism. J Lipid Res. 2006;47:1212–1218. doi: 10.1194/jlr.M500455-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Tan CE, Foster L, Caslake MJ, Bedford D, Watson TD, McConnell M, Packard CJ, Shepherd J. Relations between plasma lipids and post-heparin plasma lipases and VLDL and LDL subfraction patterns in normolipemic men and women. Arterioscler Thromb Vasc Biol. 1995;15:1839 –1848. doi: 10.1161/01.atv.15.11.1839. [DOI] [PubMed] [Google Scholar]
- 25.Sniderman AD, Wolfson C, Teng B, Franklin FA, Bachorik PS, Kwiterovich PO., Jr Association of hyperapobetalipoproteinemia with endogenous hypertriglyceridemia and atherosclerosis. Ann Intern Med. 1982;97:833–839. doi: 10.7326/0003-4819-97-6-833. [DOI] [PubMed] [Google Scholar]
- 26.Sundaram M, Zhong S, Khalil MB, Links PH, Zhao Y, Iqbal J, Hussain MM, Parks RJ, Wang Y, Yao Z. Expression of apolipoprotein C-III in McA-RH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions. J Lipid Res. 51:150–161. doi: 10.1194/jlr.M900346-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawakami A, Osaka M, Tani M, Azuma H, Sacks FM, Shimokado K, Yoshida M. Apolipoprotein CIII links hyperlipidemia with vascular endothelial cell dysfunction. Circulation. 2008;118:731–742. doi: 10.1161/CIRCULATIONAHA.108.784785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.