Abstract
Anxiety, a sustained state of heightened apprehension in the absence of immediate threat, becomes severely debilitating in disease states1. Anxiety disorders represent the most common of psychiatric diseases (28% lifetime prevalence)2, and contribute to the etiology of major depression and substance abuse3,4. Although it has been proposed that the amygdala, a brain region important for emotional processing5–8, has a role in anxiety9–13, neural mechanisms that control anxiety remain unclear. Here we explore neural circuits underlying anxiety-related behaviors by using optogenetics with two-photon microscopy, anxiety assays in freely moving mice, and electrophysiology. With the capability of optogenetics14–16 to control not only cell types but also specific connections between cells, we observed that temporally precise optogenetic stimulation of basolateral amygdala (BLA) terminals in the central nucleus of the amygdala (CeA-achieved by viral transduction of the BLA with a codon-optimized channelrhodopsin followed by restricted illumination in downstream CeA - exerted an acute, reversible anxiolytic effect. Conversely, selective optogenetic inhibition of the same projection with a third-generation halorhodopsin15 (eNpHR3.0) increased anxiety-related behaviors. Importantly, these effects were not observed with direct optogenetic control of BLA somata, possibly owing to recruitment of antagonistic downstream structures. Together, these results implicate specific BLA-CeA projections as critical circuit elements for acute anxiety control in the mammalian brain, and demonstrate the importance of optogenetically targeting defined projections, beyond simply targeting cell types, in the study of circuit function relevant to neuropsychiatric disease.
Despite the high prevalence1,2 of anxiety disorders, the underlying neural circuitry is incompletely understood. Available treatments are inconsistently effective or, in the case of benzodiazepines, addictive and linked to significant side-effects including cognitive impairment and respiratory suppression17, pointing to the need for deeper understanding of anxiety control mechanisms in the mammalian brain.
Although amygdala microcircuitry for conditioned fear has been optogenetically dissected18,19, the causal underpinnings of unconditioned anxiety11 have not yet been investigated with cellular precision. Pointing to the need for precise optogenetic exploration, the amygdala is composed of functionally and morphologically heterogeneous subnuclei with complex interconnectivity. The BLA is primarily glutamatergic (~90%)20, 21 while the CeA, which encompasses the centrolateral (CeL) and centromedial (CeM) nuclei, consists of ~95% GABAergic medium spiny neurons22. The primary output region of the amygdala is the CeM23,24 which (when chemically or electrically excited) mediates autonomic and behavioral responses associated with fear and anxiety via projections to the brainstem25. Because patients with generalized anxiety disorder may have abnormal activity arising from the BLA and CeM11, and since BLA neurons excite GABAergic CeL neurons26 that provide feed-forward inhibition onto CeM “output” neurons6,18,19, we considered that the BLA-CeL-CeM circuit could be causally involved in anxiety. However, BLA pyramidal neurons as a whole could have varied and antagonistic roles in diverse projections throughout the brain, with targets including the bed nucleus of the stria terminalis (BNST), nucleus accumbens, hippocampus and cortex26.
We therefore developed a method to selectively control BLA terminals in the CeA (Supplementary Methods). BLA glutamatergic projection neurons were transduced with an adeno-associated virus serotype 5 (AAV5) carrying codon-optimized channelrhodopsin (ChR2)-eYFP under control of the CaMKIIαeYFP promoter followed by unilateral implantation of a bevelled guide cannula to allow preferential illumination of the non-transduced CeL (Supplementary Figs. 1,2). In vivo electrophysiological recordings were used to determine illumination parameters for selective control of those BLA terminals in the CeA without nonspecific control of all BLA somata (Supplementary Fig. 3).
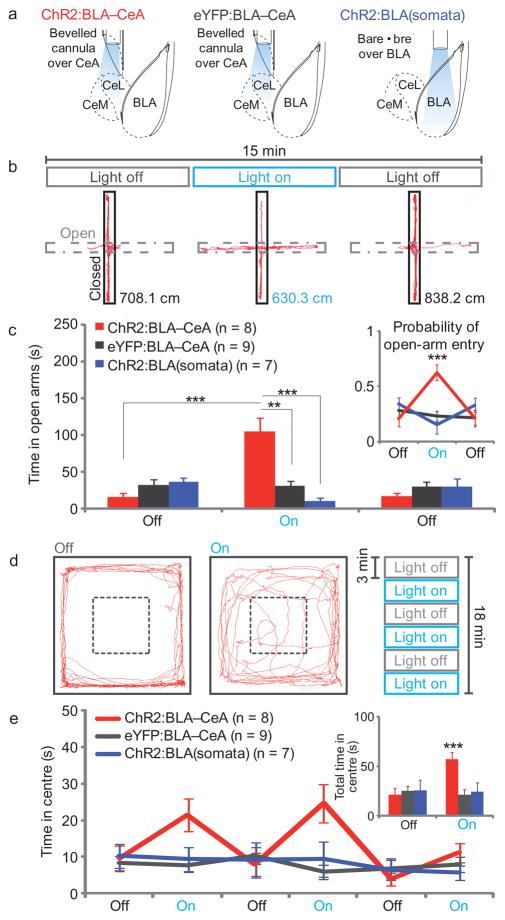
To investigate the functional role of the BLA-CeA pathway in anxiety, we probed freely moving mice under projection-specific optogenetic control in two well-validated27 anxiety assays: the elevated-plus maze (EPM) and the open-field test (OFT; Fig. 1a–e). Mice display anxiety-related behaviors in open spaces; therefore increased time spent in EPM open arms or in the OFT center is interpreted as reduced anxiety27. To test whether anxiety-related behaviors could be related to activation of the BLA-CeA projection, and not all BLA somata as a whole, we compared mice receiving projection-specific photostimulation (ChR2:BLA-CeA; Fig. 1a) to a group with identical illumination parameters transduced with a control virus (eYFP:BLA-CeA), and to another control group expressing ChR2 in the BLA receiving direct illumination of the BLA (ChR2:BLA (somata)). Photostimulation of BLA terminals in the CeA (ChR2:BLA-CeA) increased open-arm time (F1,42=69.09, p<0.00001; Fig. 1b,c) and probability of open-arm entry from the maze center (F1,42=24.69, p<0.00001: Fig. 1c inset; Supplementary Movie) on the EPM, as well as increased center time in the OFT (F1,105=24.46, p<0.00001; Fig. 1d,e), reflecting anxiety reduction, without impaired locomotion (Supplementary Fig. 4). In contrast, the ChR2:BLA(somata)group showed reduced open arm time (F1,42=6.20, p=0.02; Fig. 1b,c) and probability of open arm entry (F1,42=5.15, p=0.03) during photostimulation relative to eYFP:BLA-CeA controls, reflecting a distinct anxiogenic effect. Thus, selective illumination of BLA projections to the CeA, but not of BLA somata nonspecifically, produced an acute, rapidly-reversible anxiolytic effect.
Figure 1. Projection-specific excitation of BLA terminals in the CeA induces acute reversible anxiolysis.
a) Mice were housed in a high-stress environment before behavioral manipulations and receive 5-ms light pulses at 20Hz for all light-on conditions. (b–c) ChR2:BLA-CeA mice (n=8) received selective illumination of BLA terminals in the CeA during the light-on epoch on the EPM; see ChR2:BLA-CeA representative path (b), which induced an increase in open-arm time upon photostimulation relative to eYFP:BLA-CeA (n=9) and ChR2:BLA(somata) (n=7) controls (c), and an increase in probability of open arm entry (see inset). (d–e) ChR2:BLA-CeA mice also increased center time on the OFT, as seen in a representative path (d), during light-on epochs relative to light-off epochs and eYFP:BLA-CeA and ChR2:BLA(somata) controls (e).
Next we investigated the physiological basis of this light-induced anxiolysis. We considered that preferential photostimulation of BLA terminals in the CeL could activate CeL neurons and exert feed-forward inhibition onto brainstem-projecting CeM output neurons18,19 to implement anxiolysis. To test this, we undertook in vivo experiments, with light delivery protocols matched to those delivered in the behavioral experiments, using activity-dependent immediate early gene (c-fos) expression to track neuronal activation. We quantified the proportion of neurons in the BLA, CeL and CeM (Supplementary Fig. 5) within each group that expressed eYFP or showed c-fos immunoreactivity. Opsin expression was specific to BLA glutamatergic neurons, and was not observed in intercalated cells (Supplementary Fig. 6). No group differences were detected in the proportion of eYFP-positive cells within each region (Supplementary Fig. 5). We found a significantly higher proportion of c-fos-positive BLA cells in the ChR2:BLA(somata) group (F2,9=10.12, p<0.01), relative to ChR2:BLA-CeA or eYFP:BLA-CeA groups (p<0.01 and p<0.05, respectively), but no detectable difference between the ChR2:BLA-CeA and eYFP:BLA-CeA groups, indicating that the bevelled cannula shielding effectively prevented BLA somata photostimulation. A higher proportion of CeL neurons expressed c-fos in the ChR2:BLA-CeA group relative to the eYFP:BLA-CeA group (F2,9=4.54, p=0.04), but not the ChR2:BLA(somata)group (Supplementary Fig. 5). Thus, the in vivo illumination of BLA-CeA projections that triggered acute anxiolysis was found to excite CeL neurons without activating all BLA somata nonspecifically.
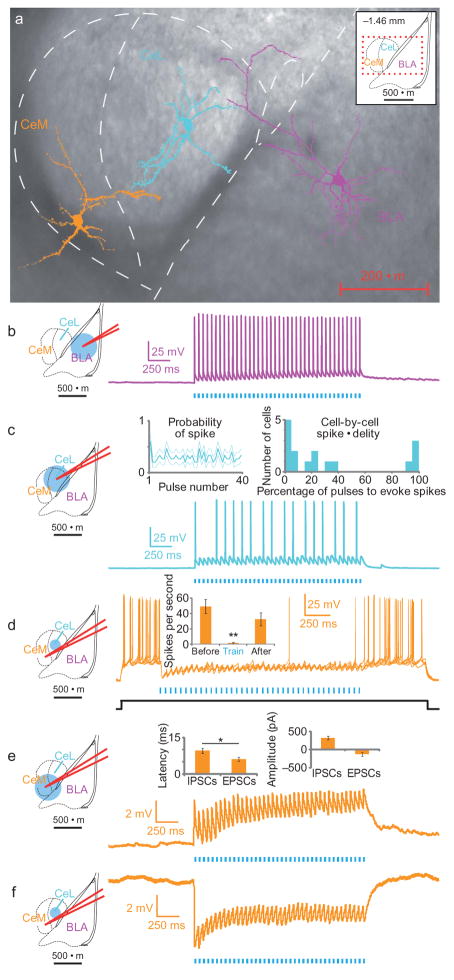
To test the hypothesis that preferential illumination of BLA-CeL terminals induced feed-forward inhibition of CeM output neurons, we combined whole-cell patch-clamp electrophysiology with two-photon imaging to visualize the microcircuit while simultaneously probing the functional relationships among these cells during projection-specific optogenetic control (Fig. 2a–f). BLA neurons showed high-fidelity spiking to direct illumination (Fig. 2b). Illumination of BLA terminals in CeL elicited excitatory responses in both weakly- and strongly-connected CeL cells (n=16; Fig. 2c).
Figure 2. Projection-specific excitation of BLA terminals in the CeA activates CeL neurons and elicits feed-forward inhibition of CeM neurons.
a) Two-photon images of representative BLA, CeL and CeM cells imaged from the same slice, overlaid on a brightfield image. (b–f) Schematics of the recording and illumination sites for the associated representative current-clamp traces (Vm=~−70 mV. b) Representative BLA pyramidal neuron trace expressing ChR2, all of which spiked for every pulse (n=4). c) Representative trace from a CeL neuron in the terminal field of BLA projection neurons, showing both sub- and supra-threshold excitatory responses upon photostimulation (n=16). Inset left, population summary of mean probability of spiking for each pulse in a 40-pulse train at 20Hz, dotted lines indicate s.e.m. Inset right, frequency histogram showing individual cell spiking fidelity; y-axis is the number of cells per each 5% bin. d) Six sweeps from a CeM neuron spiking in response to a current step (~60 pA; indicated in black) and inhibition of spiking upon 20Hz illumination of BLA terminals in the CeL. Inset, spike frequency was significantly reduced during light stimulation of CeL neurons (n=4; spikes/second before (49±9.0), during (1.5±0.87), and after (33±8.4) illumination; mean±s.e.m.). (e–f) Upon broad illumination of the CeM, voltage-clamp summaries show that the latency of excitatory postsynaptic currents (EPSCs) is significantly shorter than the latency of inhibitory postsynaptic currents (IPSCs), whereas there was a non-significant difference in the amplitude of EPSCs and IPSCs (n=11; *p=0.04, see insets). The same CeM neurons (n=7) showed either net excitation when receiving illumination of the CeM (e) or net inhibition upon selective illumination of the CeL (f).
To test whether illumination of BLA-CeL synapses could block CeM spiking via feed-forward inhibition from CeL neurons, we recorded from CeM neurons while selectively illuminating BLA-CeL synapses (Fig. 2d). Indeed, we observed potent spiking inhibition in CeM cells upon illumination of BLA-CeL terminals (Fig. 2d; F2,11=15.35, p=0.004). Figure 2e shows CeM responses recorded during illumination of ChR2-expressing BLA neurons in the CeM; importantly, the very same CeM neurons (n=7) showed net excitation upon broad illumination of BLA inputs to the CeM (Fig. 2e), but showed net inhibition upon selective illumination of BLA inputs to the CeL (Fig. 2f). These data from a structurally- and functionally-identified microcircuit25 illustrate that the balance of direct and indirect inputs from the BLA to the CeM can modulate CeM activity. We then examined whether overlapping or distinct populations of BLA neurons projected to the CeL and CeM in the mouse (Supplementary Fig. 7) by two-photon imaging in 350 μm-thick coronal slices. Of the BLA neurons sampled (n=18, Supplementary Fig. 7); 44% projected to the CeL alone and 17% projected to the CeM alone, with only one cell observed to project to both the CeL and the CeM.
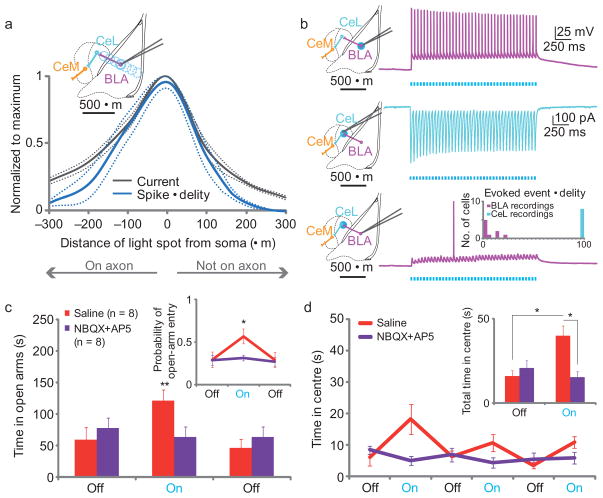
Since in vivo c-fos assays had indicated that illumination of BLA terminals in the CeL sufficed to drive excitation of postsynaptic CeL neurons but not to recruit efficiently BLA neurons as a whole, we next investigated the properties of optogenetically-driven terminal stimulation in this microcircuit using whole-cell recordings. We first recorded from BLA pyramidal neurons expressing ChR2 and moved a restricted light spot (~125 μm-diameter) in 100 μm steps from the cell soma, both in a direction over the visually-identified axon and in a direction where there was no axon collateral, illustrating the spatial properties of light scattering in this circuit (Fig. 3a,b); spiking fidelity in the BLA neuron and evoked inward currents are summarized (Fig. 3a). Next, we found that typical photostimulation parameters drove reliable transmission when delivered to BLA-CeA synapses (assayed with recordings from postsynaptic CeL neurons; Fig. 3b; Supplementary Fig. 8); in contrast, when recording from the BLA somata instead ~300 μm from the light spot, we did not observe reliable antidromically-driven action potential firing (only ~5% reliability) despite use of the very same BLA-CeA synapse illumination conditions that elicited 100% reliable transmitter release from the illuminated terminals and the same cells known to spike robustly in response to somatic illumination (Fig. 3b). These results were consistent with the c-fos immunoreactivity and behavioral data (Fig. 1) and held even upon bath application of GABA and glutamate receptor antagonists (n=7) to eliminate local circuitry effects. The marked difference between effective synaptic transmission and antidromic spiking fidelity (p=0.0039; Fig. 3b, inset) reveals that optogenetically-driven vesicle release may occur in the absence of reliable antidromic drive, a potentially useful property that may relate to projection parameters such as axon caliber and myelination status (optogenetic stimuli will recruit thinner axons more efficiently than electrical stimuli), as well as experimental light intensity and spatial restriction properties.
Figure 3. Light-induced anxiolytic effects are attributable to activation of BLA-CeL synapses.
(a–b) Schematic of the recording site and illumination positions, as whole-cell recordings were performed at each illumination location, in 100 μm increments away from the cell soma both over a visualized axon and in a direction that was not over an axon (inset). Normalized summary of spike fidelity and depolarizing current (a) to a 20Hz train delivered at various distances from the soma. (b) Representative traces upon ~125 μm diameter illumination at various locations within each slice (n=7). Illumination of BLA somata elicits high-fidelity spiking (top). Illumination of BLA terminals in CeL elicits strong excitatory responses shown in voltage-clamp in the postsynaptic CeL neuron (middle), but does not elicit reliable antidromic spiking in the BLA neuron itself (bottom), summarized in a frequency histogram (inset). (c,d) A separate group of ChR2:BLA-CeA mice (n=8) performed the EPM and OFT twice, one session preceded with intra-CeA infusions of saline (red) and the other session with glutamate receptor antagonists NBQX and AP5 (purple), counter-balanced for order. Glutamate receptor blockade in the CeA attenuated light-induced increases in both open arm time (c) and probability of open arm entry (inset) on the EPM and center time on the OFT (d, inset shows pooled summary), without altering baseline performance.
To further confirm that the anxiolytic effect was due to the selective activation of BLA-CeL projections alone, and not BLA axons passing through the CeA, nor back-propagation of action potentials to BLA cell bodies that would then innervate all BLA projection target regions, we tested whether local glutamate receptor antagonism would attenuate light-induced anxiolytic effects. This distinction is critical, since previous reports that CeA lesions that alter anxiety are confounded by the ablation of BLA projections to the BNST passing through CeA28. In a separate group of mice, we selectively illuminated BLA-CeA terminals as before (n=8; Supplementary Fig. 1), but infused glutamate antagonists or saline via the fiberoptic guide cannula, before testing on the EPM and OFT. Confirming a local synaptic mechanism rather than control of fibers of passage, intra-CeA glutamate receptor antagonism abolished light-induced reductions in anxiety as measured by open-arm time (F1,35=8.61; p=0.008) and probability of open-arm entry on the EPM (F1,35=5.92, p=0.02), and center time during the OFT (F1,77=13.99; p=0.0006; Fig. 3c,d). Importantly, drug treatment did not impair locomotor activity (Supplementary Fig. 9), and in acute slices time-locked light-evoked excitatory responses were abolished upon bath application of 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f] quinoxaline-2,3-dione (NBQX) and (2R)-amino-5-phosphonopentanoate (AP5) (Supplementary Fig. 10). These data demonstrate that the light-induced anxiolytic effects were caused by the activation of BLA-CeA synapses, and not attributable to BLA projections to distal targets passing through the CeA.
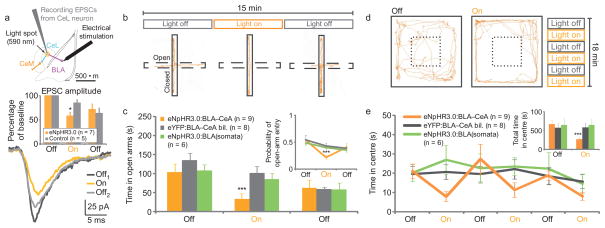
Finally, to test whether basal anxiety-reducing processes could be blocked by selectively inhibiting the BLA-CeA pathway, we bilaterally transduced either eNpHR3.0 -which hyperpolarizes neuronal membranes upon illumination with amber light15 - or eYFP alone, under the CaMKIIα promoter in the BLA, and implanted bilateral bevelled guide cannulae to allow selective illumination of BLA terminals in the CeA (Supplementary Fig. 11). eNpHR3.0 expression was restricted to glutamatergic neurons in the BLA, and the eNpHR3.0:BLA-CeA group showed elevated levels of c-fos expression, relative to the eYFP:BLA-CeA and eNpHR3.0:BLA(somata) groups, in the CeM (p<0.05; Supplementary Fig. 12), consistent with the hypothesis that inhibition of BLA-CeL synapses suppresses feed-forward inhibition from CeL neurons to CeM neurons, thereby increasing CeM excitability and the downstream processes leading to increased anxiety phenotypes. Selective illumination of eNpHR3.0-expressing axon terminals reduced the probability of both spontaneously occurring (frequency: F1,8=32.99, p=0.00024; amplitude: F1,8=21.96, p=0.001; Supplementary Fig. 13) and electrically-evoked (F1,10=10.79, p=0.006; Fig. 4a) vesicle release, without preventing spiking at the soma (Supplementary Fig. 14). BLA somata inhibition did not induce an anxiogenic response, perhaps owing to the simultaneous decrease in direct BLA-CeM excitatory input. We also found that the eNpHR3.0:BLA-CeA group showed reduced open-arm time and probability of open-arm entry on EPM (F1,40=21.08, p<0.00001; and F1,40=19.93, p<0.00001, respectively; Fig. 4b,c) and center-time in the OFT (F1,100=18.919, p<0.00001; Fig. 4d,e) during photostimulation when compared to the eYFP and BLA(somata) groups, without altering locomotor activity (Supplementary Fig. 15). These data demonstrate that preferential inhibition of BLA-CeL synapses acutely increases anxiety-like behaviors.
Figure 4. Selective inhibition of BLA terminals in the CeA induces an acute and reversible increase in anxiety.
(a–e) Mice were group-housed in a low-stress environment and received bilateral constant 594 nm light during light-on epochs. a) Selective illumination of eNpHR3.0-expressing BLA terminals suppresses vesicle release evoked by electrical stimulation in the BLA. Schematic indicates the locations of the stimulating electrode, the recording electrode and the ~125 μm diameter light spot. Representative CeL EPSCs before (Off1), during (On) and after (Off2) selective illumination of eNpHR3.0-expressing BLA terminals. Normalized EPSC amplitude summary data from sections containing BLA neurons expressing eNpHR3.0 (n=7) and non-transduced controls (n=5) show that selectively illuminating BLA-CeL terminals reduces (*p=0.006) electrically-evoked EPSC amplitude in postsynaptic CeL neurons relative to non-transduced control slice preparations (inset). (b–c) Representative eNpHR3.0:BLA-CeA path (b) indicates reduced open arm time (c) and probability of open arm entry (inset) during illumination, relative to controls. (d–e) Representative eNpHR3.0:BLA-CeA path (d) reflects reduced center time on the OFT (e) for the eNpHR3.0:BLA-CeA group during light-on, but not light-off, epochs as compared to controls (inset shows pooled data).
Here, we have identified the BLA-CeL pathway as a neural substrate for real-time bidirectional modulation of the unconditioned expression of anxiety. The observation that selective illumination of specific BLA terminals produces distinct, and even opposite, behavioral responses from illumination of all glutamatergic BLA somata nonspecifically, points to the essential value of optogenetic control in causally dissecting intact neural circuitry, and suggests that multiple subpopulations or projections of BLA neurons can act in opposition (e.g. direct excitation of CeM along with feed-forward inhibition of CeM). Neural circuitry arranged in this way provides many opportunities for modulation of expression of anxiety phenotypes; for example, this microcircuit is well-positioned to be influenced by top-down cortical control from regions important for processing fear and anxiety, including the prelimbic, infralimbic and insular cortices that provide robust innervation to the BLA and CeL.
These data are consistent with reports implicating CeA involvement in anxiety9,11,12, but it is important to note that our findings do not exclude downstream or parallel circuits including the BNST28, the insular and prefrontal cortices29, and the septal-hippocampal circuit30; for example, stress induces CeL release of corticotropin releasing hormone (CRH) in the BNST28. In the course of providing insight into native anxiogenic and anxiolytic processes, these findings demonstrate that anxiety is continuously regulated by balanced antagonistic pathways within the amygdala, and illustrate the importance of resolving specific projections in the study of neural circuit function relevant to psychiatric disease.
Methods Summary
Virus-mediated opsin gene expression
The pAAV-CaMKIIα-hChR2(H134R)-eYFP, pAAV-CaMKIIα-eYFP and pAAV-CaMKIIα-eNpHR3.0-eYFP plasmids were designed and constructed by standard methods and packaged as AAV5. 0.5 μl of virus was injected into the BLA. Maps and clones are available at www.optogenetics.org.
In vivo projection-specific targeting
To investigate the role of the BLA-CeL pathway in modulating anxiety, we performed viral transduction and surgical implantation of bevelled guide cannulae to allow selective illumination of BLA fibers in the CeA under stereotaxic guidance. Behavioral, electrophysiological and imaging data were collected 4–6 weeks following surgery.
Two-photon imaging and functional mapping using ex vivo electrophysiology
Acute slices were collected for two-photon imaging and ex vivo electrophysiological recordings. While light-stimulation parameters used in vivo were delivered via fiberoptic and light in ex vivo experiments was delivered onto coronal sections, we matched light power density at our target region ~6mW/mm2. Whole-cell recordings were made from BLA pyramidal neurons simultaneously during two-photon visualization of neuronal processes with Alexa Fluor dye. We visually tracked axonal projections from BLA neurons to the CeL nucleus. We recorded from CeL neurons upon illumination with an aperture-restricted light spot (~125 μm diameter), mimicking the preferential illumination of BLA terminals, but not BLA somata, delivered in vivo. Two-photon imaging allowed axonal tracking to the CeM, where whole-cell recordings were collected from CeM neurons in the terminal field of CeL axons, with aperture-restricted illumination over the CeL to allow selective illumination of BLA terminals in the CeL whilst recording from the CeM neuron.
Opsin expression validation and immunohistochemistry
To validate specificity, sensitivity and spatial distribution of opsin expression as well as neuronal activity, brain slices were prepared for optical microscopy and immunohistochemistry. Coronal sections were stained for DAPI and immunoreactivity for c-fos. Quantitative analyses of confocal images were performed with both staining and analysis blind to experimental condition.
Supplementary Material
Acknowledgments
We would like to thank Drs. P. Janak, H. Fields, G. Stuber, E. Thomas, F. Zhang, I. Witten, V. Sohal, T. Davidson and M. Warden as well as J. Mattis, R. Durand, M. Mogri, J. Mirzabekov and E. Steinberg for helpful discussion, and the entire Deisseroth lab for their support. All viruses were packaged at UNC vector Core. Supported by NIMH (1F32MH088010-01, K.M.T), NARSAD (K.R.T), Samsung Scholarship (S-Y.K.), NSF IGERT Award 0801700 (L.G.) and McKnight Foundations, as well as NIDA, NIMH and the NIH Pioneer Award (K.D.).
Footnotes
Author Contributions
K.M.T., R.P., S-Y. K., L.E.F. and K.D. contributed to study design and data interpretation. K.M.T., R.P., S-Y. K. and L.E.F. contributed to data collection and K.M.T. coordinated data collection and analysis. K.M.T., S-Y.K., H.Z. and K.R.T. contributed to immunohistochemical processing, fluorescence imaging and quantitative analyses. K.M.T. and L.G. performed the behavioral and ex vivo electrophysiology statistical analyses. V.G. and C.R. contributed to the design of eNpHR 3.0. C.R. cloned all constructs and managed viral packaging processes. K.D. supervised all aspects of the work. All authors contributed to writing the paper.
References
- 1.Lieb R. Anxiety disorders: clinical presentation and epidemiology. Handb Exp Pharmacol. 2005:405–432. doi: 10.1007/3-540-28082-0_14. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 7.Tye KM, Stuber GD, de Ridder B, Bonci A, Janak PH. Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature. 2008;453:1253–1257. doi: 10.1038/nature06963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J Comp Physiol Psychol. 1956;49:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- 9.Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lesscher HM, et al. Amygdala protein kinase C epsilon regulates corticotropin-releasing factor and anxiety-like behavior. Genes Brain Behav. 2008;7:323–333. doi: 10.1111/j.1601-183X.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- 11.Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66:1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyons AM, Thiele TE. Neuropeptide Y conjugated to saporin alters anxiety-like behavior when injected into the central nucleus of the amygdala or basomedial hypothalamus in BALB/cJ mice. Peptides. 2010 doi: 10.1016/j.peptides.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009 doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 14.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 15.Gradinaru V, et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deisseroth K. Optogenetics: controlling the brain with light. Scientific American. 2010;303:48–55. doi: 10.1038/scientificamerican1110-48. [DOI] [PubMed] [Google Scholar]
- 17.Woods JH, Katz JL, Winger G. Benzodiazepines: use, abuse, and consequences. Pharmacol Rev. 1992;44:151–347. [PubMed] [Google Scholar]
- 18.Ciocchi S, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- 19.Haubensak W, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlsen J. Immunocytochemical localization of glutamate decarboxylase in the rat basolateral amygdaloid nucleus, with special reference to GABAergic innervation of amygdalostriatal projection neurons. J Comp Neurol. 1988;273:513–526. doi: 10.1002/cne.902730407. [DOI] [PubMed] [Google Scholar]
- 21.Smith Y, Pare D. Intra-amygdaloid projections of the lateral nucleus in the cat: PHA-L anterograde labeling combined with postembedding GABA and glutamate immunocytochemistry. J Comp Neurol. 1994;342:232–248. doi: 10.1002/cne.903420207. [DOI] [PubMed] [Google Scholar]
- 22.McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol. 1982;208:401–418. doi: 10.1002/cne.902080409. [DOI] [PubMed] [Google Scholar]
- 23.Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol. 1978;178:255–280. doi: 10.1002/cne.901780205. [DOI] [PubMed] [Google Scholar]
- 24.Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol. 1978;178:225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- 25.Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: JP A, editor. The Amygdala. Oxford University Press; Oxford, UK: 2000. pp. 213–288. [Google Scholar]
- 26.Pitkanen A. Connectivity of the rat amygdaloid complex. In: JP A, editor. The Amygdala. Oxford University Press; Oxford, UK: 2000. pp. 31–99. [Google Scholar]
- 27.Carola V, D’Olimpio F, Brunamonti E, Mangia F, Renzi P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res. 2002;134:49–57. doi: 10.1016/s0166-4328(01)00452-1. [DOI] [PubMed] [Google Scholar]
- 28.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–35. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gray JA, McNaughton N. The neuropsychology of anxiety: reprise. Nebr Symp Motiv. 1996;43:61–134. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.