Abstract
A growing body of evidence has suggested that reactive oxygen species (ROS) may play an important role in the physiopathology of depression. Evidence has pointed to the β-carboline harmine as a potential therapeutic target for the treatment of depression. The present study we evaluated the effects of acute and chronic administration of harmine (5, 10 and 15 mg/kg) and imipramine (10, 20 and 30 mg/kg) or saline in lipid and protein oxidation levels and superoxide dismutase (SOD) and catalase (CAT) activities in rat prefrontal cortex and hippocampus. Acute and chronic treatments with imipramine and harmine reduced lipid and protein oxidation, compared to control group in prefrontal cortex and hippocampus. The SOD and CAT activities increased with acute and chronic treatments with imipramine and harmine, compared to control group in prefrontal cortex and hippocampus. In conclusion, our results indicate positive effects of imipramine antidepressant and β-carboline harmine of oxidative stress parameters, increasing SOD and CAT activities and decreasing lipid and protein oxidation.
1. Introduction
A growing body of evidence has suggested that reactive oxygen species (ROS) may play an important role in the pathogenesis of neurological and psychiatric diseases including bipolar disorder and major depression [1–9].
ROS are free radicals or reactive anions/molecules containing oxygen atoms, such as hydroxyl radical, superoxide, hydrogen peroxide and peroxynitrite. ROS can cause cell damage by enzyme inactivation, lipid peroxidation and DNA modification [10]. Oxidative stress is well known to contribute to neuronal degeneration in the central nervous system (CNS) in the process of aging, as well, in neurodegenerative diseases.
Studies have consistently reported increase ROS in plasma on patients with major depression, especially with melancholia associated [11]. Recent study showed evidences of oxidative stress in major depression as reflected in increased oxidative stress from frontal regions of patients compared to those of matched controls [12]. Moreover we showed that rats subjected to chronic mild stress (CMS) had increase in superoxide production in hippocampus, prefrontal cortex and cortex brain and thiobarbituric acid reactive in cortex [5]. In addition we demonstrated that stressed rats had increase protein (prefrontal, hippocampus, striatum and cortex) and lipid peroxidation (cerebellum and striatum), increase catalase (cerebellum, hippocampus, striatum and cortex) and a decrease in superoxide dismutase activity (prefrontal cortex, hippocampus, striatum and cortex) [6]. Additionally, oxidative stress is able to affect a number of synaptic functions, resulting in impaired neurotransmission [13].
The monoamine hypothesis posits that depression is caused by decreased monoamine function in brain [14]. Actually, the clinically-used antidepressants increase the extracellular concentrations of monoamines serotonin or norepinephrine either by inhibiting their reuptake from the synapse or by blocking their degradation by inhibiting monoamine oxidase [15–17].
Recently, studies have reported that β-carboline harmine possesses antidepressant properties [18–20]. In fact, harmine interact with monoamine oxidase A (MAO-A) [21] and several cell-surface receptors, including serotonin receptor 2A (5-HT2A), which are involved in antidepressant pharmacotherapy [22, 23].
Because of these findings, we designed the present study to investigate the effects of acute and chronic administration of harmine, imipramine (standard antidepressant) and saline on lipid and protein oxidation levels (markers of oxidative stress) and on superoxide dismutase (SOD) and catalase (CAT) activities (the major antioxidant enzymes) in rat brain.
2. Results
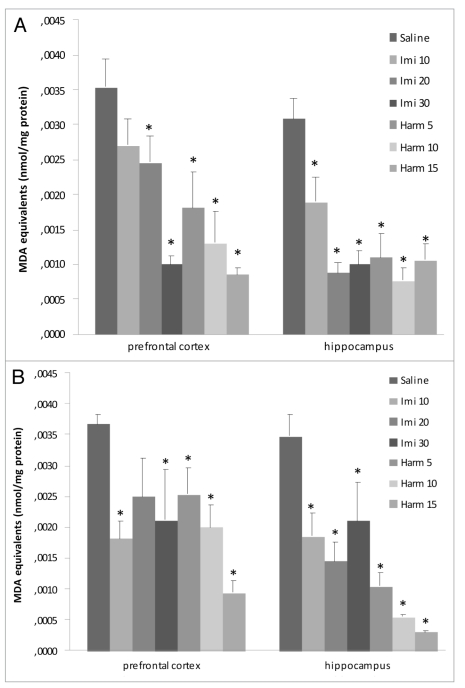
Thiobarbituric acid reactive species (TBARS). In the acute treatment (Fig. 1A), imipramine at doses of 20 and 30 mg/kg and harmine in all doses in prefrontal cortex and both imipramine and harmine in all doses in hippocampus decreased the lipid peroxidation (F = 6.61; p < 0.05). Chronic treatment with imipramine at doses of 10 and 30 mg/kg and harmine in all doses reduced the lipid peroxidation in prefrontal cortex; and in all doses both harmine and imipramine decreased the lipid peroxidation in hippocampus (F = 10.44; p < 0.05; Fig. 1B).
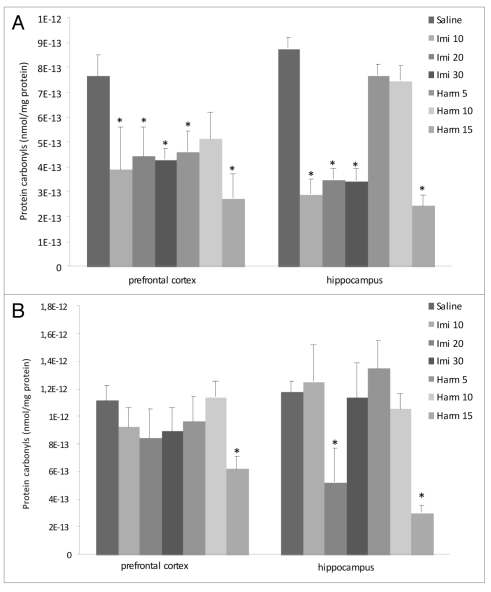
Protein carbonyls. As depicted in Figure 2A, acute administration of imipramine in all doses decreased protein carbonilation in prefrontal cortex and in hippocampus; however, acute treatment with harmine reduced protein carbonilation at doses of 5 and 15 mg/kg in prefrontal cortex and at dose of 15 mg/kg in hippocampus (F = 2.18; p < 0.05). In the chronic treatment only the higher (15 mg/kg) of harmine and decreased the protein carbonilation in prefrontal cortex and hippocampus and imipramine at dose of 20 mg/kg decreases protein carbonilation in hippocampus (F = 28.8; p < 0.05; Fig. 2B).
Figure 2.
Effects of acute (A) and chronic (B) administration of imipramine (10, 20 and 30 mg/kg, i.p.) and harmine (5, 10 and 15 mg/kg, i.p.) on protein peroxidation in rat brain. The carbonyl groups decreased in prefrontal cortex and hippocampus after acute treatment (A) with imipramine and harmine and in prefrontal cortex and hippocampus with harmine and in hippocampus with imipramine after chronic treatment (B), compared to control group. Bars represent means ± S.E.M. of 5 rats. * p <0.05 vs. saline according to ANOVA followed by Tukey post-hoc test.
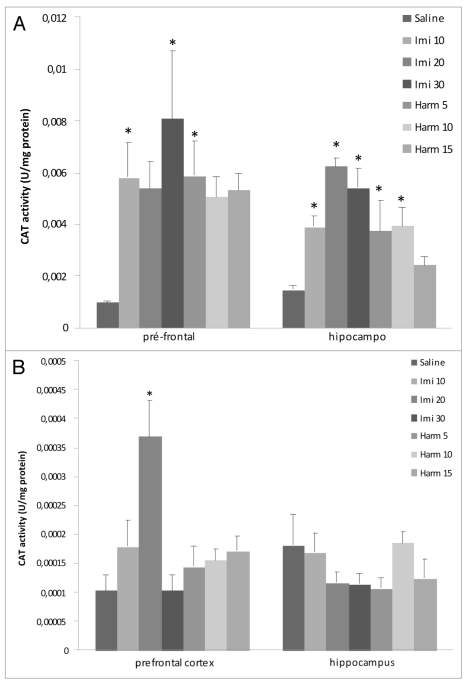
Catalase activity. The intraperitoneal acute treatment with imipramine in doses of 10 and 30 mg/kg and harmine in dose of 5 mg/kg increased catalase activity in prefrontal cortex; treatment with imipramine in all doses and harmine at doses of 5 and 10 mg/kg increased catalase activity in hippocampus (F = 4.9; p < 0.05; Fig. 3A). In the chronic treatment only imipramine at the dose of 20 mg/kg increased catalase activity in prefrontal cortex in comparison with control group (p < 0.05; Fig. 3B).
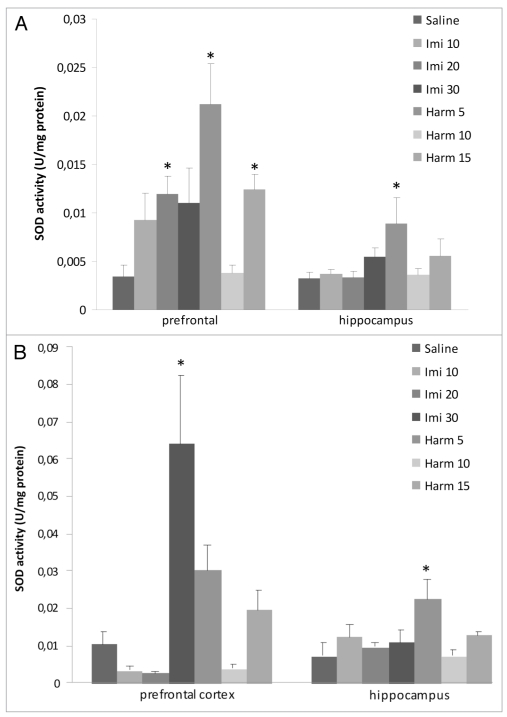
Superoxide dismutase activity. The superoxide dismutase activity increased in prefrontal cortex after acute treatment with imipramine at dose of 20 mg/kg and harmine at doses of 5 and 15 mg/kg. In hippocampus the superoxide dismutase activity increased only harmine at dose of 5 mg/kg (F = 2.45; p < 0.05; Fig. 4A). Figure 4B showed the superoxide dismutase activity after chronic treatment with imipramine and harmine. Only the higher dose (30 mg/kg) of imipramine increased superoxide dismutase activity in prefrontal cortex; and only harmine at dose of 5 mg/kg increased superoxide dismutase activity in hippocampus, compared to the control group (p < 0.05).
Figure 4.
Effects of acute (A) and chronic (B) administration of imipramine (10, 20 and 30 mg/kg, i.p.) and harmine (5, 10 and 15 mg/kg, i.p.) on superoxide dismutase activity in rat brain. The superoxide dismutase activity increased after acute treatment (A) in prefrontal cortex with imipramine and harmine and in hippocampus with harmine and after chronic treatment with imipramine in prefrontal cortex and harmine in hippocampus, compared to control group. Bars represent means ± S.E.M. of 5 rats. * p <0.05 vs. saline according to ANOVA followed by Tukey post-hoc test.
3. Discussion
In the present study we showed that both acute and chronic treatments with imipramine and harmine reduce lipid and protein peroxidation and increased superoxide and catalase activities, compared to saline group in hippocampus and prefrontal cortex.
The hippocampus is one of several limbic structures that have been implicated in mood disorders. In addition, the hippocampus has connections with the prefrontal cortex, region that is more directly involved in emotion and cognition and thereby contributes to other major symptoms of mood disorders [24, 25].
The brain is particularly vulnerable to reactive oxygen species (ROS) production because it metabolizes 20% of total body oxygen and has a limited amount of antioxidant capacity [10].
The oxidative stress in rat brain structures may play a role in the pathogenesis of anxiety [26, 27] and depression [28]. In fact, our group very recently showed that rats subjected to chronic mild stress (CMS) had an increase in superoxide production and thiobarbituric acid reactive protein and lipid peroxidation and catalase activity, and a decrease in superoxide dismutase activity in rat brain [5, 6]. Moreover, a previous study using an animal model of repeated restraint stress showed that this model induced an increase in TBARS levels in hippocampus [29]. In another study, it was demonstrated that animal model of immobilization stress causes significant increases in lipid peroxidation, which was found in cerebral cortex, cerebellum and hippocampus compared to the unstressed controls; significant increases in levels of protein oxidation were also found in cortex, hypothalamus and striatum; oxidative nuclear DNA damage increased after stress in all brain regions, although only the cerebral cortex showed a significant increase [30]. In humans elevated ROS in plasma of patients with major depression, especially in those with melancholic type and increased oxidative stress in depressive woman, was demonstrated [3, 11]. Additionally, Galecki et al. [31] demonstrated that combined fluoxetine antidepressant and acetylsalicylic acid therapy improvement of oxidative stress parameters in patients with depression. Moreover, exogenous administration of 5-hydroxytryptophan prevented depletion of serotonin concentration and antioxidant status induced by p-chlorophenylalanine in rat brain [32].
In this study we showed that acute and chronic treatment with imipramine antidepressant improvement of oxidative stress parameters in rat brain. Several studies have reported the role of imipramine in oxidative stress parameters. In fact, imipramine treatment reversed the lipid peroxidation in brain of Sprague Dawley rats induced by chronic ozone [33]. In addition, acute treatment with imipramine (10 and 20 mg/kg) and venlafaxine (5 and 10 mg/kg) reversed immobilized stress-induced behavioral and biochemical (such as malondialdehyde level, nitrite, glutathione and catalase enzyme) alterations in mice; in some study was showed that l-NAME and/or methylene blue potentiated the effect of both imipramine and venlafaxine, suggesting the involvement of nitric oxide mechanism in the protective effect of imipramine and venlafaxine [34]. In other hand, acute seizure activity promotes lipid peroxidation and increased nitrite levels in frontal cortex and striatum [35]. Imipramine (10 and 20 mg/kg) and trazodone (5 and 10 mg/kg) antidepressants restored depleted reduced glutathione levels and catalase activity and attenuated raised lipid peroxidation and nitrite concentrations in mice sleep-deprived, compared to untreated sleep-deprived [36]. Nevertheless, a significant recovery in the activities of superoxide dismutase, catalase, glutathione S-transferase, glutathione redutase and glutathione levels by fluoxetine, imipramine and venlafaxine treatments following a restraint stressinduced decline of these parameters, and accumulated lipid peroxidation product malondialdehyde and protein carbonyl contents in stressed animal were normalized by some antidepressants [37].
Moreover, we also demonstrated that β-carboline harmine improvement of oxidative stress parameters. Kim et al. [38] reported that harmine has a role in oxidative stress. In fact, they showed that β-carbolines (harmaline, harmalol and harmine) attenuated the dopamine or 6-hydroxydopamine-induced alteration of brain mitochondrial and synaptosomal functions, and viability loss in PC12 cells, by a scavenging action on reactive oxygen species and inhibition of thiol oxidation [38].
Our group has shown antidepressant properties of harmine [19, 20]. In fact, treatment with harmine at doses of 10 and 15 mg/kg and imipramine at doses of 20 and 30 mg/kg decreased immobility time of rats, and increased both climbing and swimming time of rats compared to saline group, and were also showed that imipramine and harmine did not affect spontaneous locomotor activity in the open-field test [19]. In this study were demonstrated that harmine (15 mg/kg) increased brain-derived neurotrophic factor (BDNF) protein levels in rat hippocampus. In other study from our group showed that harmine reverted stress parameters induced by chronic mild stress model [20]. In addition, Farzin et al. [18] demonstrated that treatment with harmane, norharmane and harmine dose-dependently reduced the immobility time in the mouse forced swimming test. These studies suggest antidepressant- like effects of harmine could be due to interactions of harmine with several receptor systems involved in the modulation of behavioral and molecular actions of antidepressants.
In conclusion, considering that oxidative stress is probably involved in the pathophysiology of depression, the modulation by antidepressants could be an important mechanism of action of these drugs and harmine could be a positive effect in oxidative stress parameters, which may play a role in the pathogenesis of depression.
4. Materials and Methods
Animals. Male Adult Wistar rats (60 days old) were obtained from UNESC (Universidade do Extremo Sul Catarinense, Criciuma, Brazil) breeding colony. They were housed five per cage with food and water available ad libitum and were maintained on a 12 h light/dark cycle (lights on at 7:00 AM). All experimental procedures involving animals were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the Brazilian Society for Neuroscience and Behavior (SBNeC) recommendations for animal care and with approval by local Ethics Committee under protocol number 325/2008.
Drugs and treatments. Harmine was obtained from THCPharm/STI-Pharm (Frankfurt, Germany) and imipramine, the standard antidepressant, from Novartis Pharmaceutical Industry (Criciuma, Brazil). Different groups of rats (n = 5 each) were administered intraperitoneally (i.p.) with saline or different doses of harmine (5, 10 and 15 mg/kg) or imipramine (10, 20 and 30 mg/kg) once only (acute treatment) or once a day for 14 days (chronic treatment) [20–23, 39]. Imipramine and harmine were dissolved in saline immediately before the injections. All treatments were administered in a volume of 1 mL/kg. After 60 min acute and chronic treatment, the animals were killed by decapitation. The hippocampus and prefrontal cortex were quickly isolated by hand dissection using a magnifying glass and a thin brush, dissection was based on histological distinctions described by Paxinos and Watson [40]. Samples were stored at 70°C for subsequent analysis of oxidative stress.
Oxidative stress parameters. In order to assess oxidative damage, was measured the formation of thiobarbituric acid reactive species (TBARS) during an acid-heating reaction, as previously described by Esterbauer and Cheeseman [41]. The samples were mixed with 1 mL of trichloroacetic acid (TCA) 10% and 1 mL of thiobarbituric acid 0.67% and were then heated in a boiling water bath for 15 min. TBARS were determined by the absorbance at 535 nm. Oxidative damage to proteins was measured by the quantification of carbonyl groups based on the reaction with dinitrophenylhidrazine (DNPH), as previously described by Levine et al. [42] Proteins were precipitated by the addition of 20% trichloroacetic acid and were redissolved in DNPH; the absorbance was read at 370 nm. To determine CAT activity, the brain tissue was sonicated in 50 mmoL/L phosphate buffer (pH 7.0), and the resulting suspension was centrifuged at 3,000 g for 10 min. The supernatant was used for enzyme assay. CAT activity was measured by the rate of decrease in hydrogen peroxide absorbance at 240 nm [43]. SOD activity was assayed by measuring the inhibition of adrenaline auto-oxidation, as previously described by Bannister and Calabrese [44]. All biochemical measures were normalized to the protein content, with bovine albumin as standard [45].
Statistical analysis. All data are presented as mean ± S.E.M. In the assessment of oxidative stress parameters were determined by one-way ANOVA, followed by Tukey post-hoc test when ANOVA was significant; p values less than 0.05 were considered to be statistical significant.
Figure 1.
Effects of acute (A) and chronic (B) administration of imipramine (10, 20 and 30 mg/kg, i.p.) and harmine (5, 10 and 15 mg/kg, i.p.) on lipidic peroxidation in rat brain. The formation of TBARS decreased in prefrontal cortex and hippocampus after acute (A) and chronic (B) treatments with imipramine and harmine, compared to control group. Bars represent means±S.E.M. of 5 rats. * p <0.05 vs. saline according to ANOVA followed by Tukey post-hoc test.
Figure 3.
Effects of acute (A) and chronic (B) administration of imipramine (10, 20 and 30 mg/kg, i.p.) and harmine (5, 10 and 15 mg/kg, i.p.) on catalase activity in rat brain. The catalase activity increased in prefrontal cortex after acute (A) and chronic (B) treatments with imipramine and in prefrontal cortex and hippocampus after chronic treatment (B) with harmine, compared to control group. Bars represent means ± S.E.M. of 5 rats. * p <0.05 vs. saline according to ANOVA followed by Tukey post-hoc test.
Acknowledgements
This study was supported in part by grants from ‘Conselho Nacional de Desenvolvimento Científico e Tecnológico' (CNPq-Brazil-J.Q., JAC, A.W.Z. J.E.H.), from ‘Fundacao de Amparo a Pesquisa do Estado de Sao Paulo fellowship' (FAPESP-J.A.C., A.W.Z. J.E.H.), FAPESC (J.Q.), and from the Instituto Cerebro e Mente (J.Q.) and UNESC (J.Q. and F.D.P.). J.Q., F.D.P., J.A.C. and A.W.Z. are recipients of CNPq (Brazil) Productivity fellowships. G.Z.R. is holder of a FAPESC/CAPES studentship. This study was also sponsored by THC-Pharm (Frankfurt, Germany) and STI-Pharm (UK) who kindly provided harmine.
References
- 1.Frey BN, Valvassori SS, Réus GZ, et al. Changes in antioxidant defense enzymes after d-amphetamine exposure: implications as an animal model of mania. Neurochemical Research. 2006;31(5):699–703. doi: 10.1007/s11064-006-9070-6. [DOI] [PubMed] [Google Scholar]
- 2.Frey BN, Valvassori SS, Réus GZ, et al. Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania. Journal of Psychiatry and Neuroscience. 2006;31(5):326–332. [PMC free article] [PubMed] [Google Scholar]
- 3.Kodydková J, Vávrová L, Zeman M, et al. Antioxidative enzymes and increased oxidative stress in depressive women. International Journal of Neuropsychopharmacology. 2008;11:851–876. doi: 10.1016/j.clinbiochem.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Kunz M, Gama CS, Andreazza AC, et al. Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in different phases of bipolar disorder and in schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32(7):1677–1681. doi: 10.1016/j.pnpbp.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Lucca G, Comim CM, Valvassori SS, et al. Increased oxidative stress in submitochondrial particles into the brain of rats submitted to the chronic mild stress paradigm. Journal of Psychiatric Research. 2009;43(9):864–869. doi: 10.1016/j.jpsychires.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Lucca G, Comim CM, Valvassori SS, et al. Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochemistry International. 2009;54(5-6):358–362. doi: 10.1016/j.neuint.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. International Journal of Neuropsychopharmacology. 2008;11(6):851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 8.Riegel RE, Valvassori SS, Elias G, et al. Animal model of mania induced by ouabain: evidence of oxidative stress in submitochondrial particles of the rat brain. Neurochemistry International. 2009;55(7):491–495. doi: 10.1016/j.neuint.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Wood SJ, Yücel M, Pantelis C, Berk M. Neurobiology of schizophrenia spectrum disorders: the role of oxidative stress. Annals of the Academy of Medicine Singapore. 2009;38(5):396–406. [PubMed] [Google Scholar]
- 10.Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proceedings of the Society for Experimental Biology and Medicine. 1999;222(3):236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 11.Bilici M, Efe H, Koroglu MA, Uydu HÁ, Bekaroglu M, Deger O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. Journal of Affective Disorders. 2001;64:43–51. doi: 10.1016/s0165-0327(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 12.Michel TM, Frangou S, Thiemeyer D, et al. Evidence for oxidative stress in the frontal cortex in patients with recurrent depressive disorder—a postmortem study. Psychiatry Research. 2007;151(1-2):145–150. doi: 10.1016/j.psychres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Tretter L, Chinopoulos C, Adam-Vizi V. Enhanced depolarization-evoked calcium signal and reduced [ATP]/[ADP] ratio are unrelated events induced by oxidative stress in synaptosomes. Journal of Neurochemistry. 1997;69(6):2529–2537. doi: 10.1046/j.1471-4159.1997.69062529.x. [DOI] [PubMed] [Google Scholar]
- 14.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nature Reviews Neuroscience. 2006;7(2):137–145. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 15.Castrén E. Is mood chemistry? Nature Reviews Neuroscience. 2005;6:241–246. doi: 10.1038/nrn1629. [DOI] [PubMed] [Google Scholar]
- 16.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Archives of General Psychiatry. 1997;54(7):597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 17.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 18.Farzin D, Mansouri N. Antidepressant-like effect of harmane and other β-carbolines in the mouse forced swim test. The European Neuropsychopharmacology. 2006;16(5):324–328. doi: 10.1016/j.euroneuro.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Fortunato JJ, Réus GZ, Kirsch TR, et al. Effects of β-carboline harmine on behavioral and physiological parameters observed in the chronic mild stress model: further evidence of antidepressant properties. Brain Research Bulletin. 2010;81(4-5):491–496. doi: 10.1016/j.brainresbull.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Fortunato JJ, Réus GZ, Kirsch TR, et al. Acute harmine administration induces antidepressive-like effects and increases BDNF levels in the rat hippocampus. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33(8):1425–1430. doi: 10.1016/j.pnpbp.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Sablin SO, Ramsay RR. Inhibition of monoamine oxidase A by beta-carboline derivatives. Archives of Biochemistry and Biophysics. 1997;337:137–142. doi: 10.1006/abbi.1996.9771. [DOI] [PubMed] [Google Scholar]
- 22.Glennon RA, Dukat M, Grella B, et al. Binding of beta-carbolines and related agents at serotonin (5-HT(2) and 5-HT(1A)), dopamine (D(2)) and benzodiazepine receptors. Drug and Alcohol Dependence. 2000;60(2):121–132. doi: 10.1016/s0376-8716(99)00148-9. [DOI] [PubMed] [Google Scholar]
- 23.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. Journal of Clinical Psychopharmacology. 2008;28(6):631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 24.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Hall J, Whalley HC, Marwick K, et al. Hippocampal function in schizophrenia and bipolar disorder. Psychological Medicine. 2009;7:1–10. doi: 10.1017/S0033291709991000. [DOI] [PubMed] [Google Scholar]
- 26.Maiese K. High anxiety: recognizing stress as the stressor. Oxidative Medicine and Cellular Longevity. 2009;2(2):61–62. doi: 10.4161/oxim.2.2.8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouayed J, Rammal H, Soulimani R. Oxidative stress and anxiety: relationship and cellular pathways. Oxidative Medicine and Cellular Longevity. 2009;2(2):63–67. doi: 10.4161/oxim.2.2.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eren I, Naziroğlu M, Demirdas A, et al. Venlafaxine modulates depressioninduced oxidative stress in brain and medulla of rat. Neurochemical Research. 2007;32:497–505. doi: 10.1007/s11064-006-9258-9. [DOI] [PubMed] [Google Scholar]
- 29.Fontella FU, Siqueira IR, Vasconcellos AP, Tabajara AS, Netto CA, Dalmaz C. Repeated restraint stress induces oxidative damage in rat hippocampus. Neurochemical Research. 2005;30(1):105–111. doi: 10.1007/s11064-004-9691-6. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Wang X, Shigenaga MK, Yeo HC, Mori A, Ames BN. Immobilization stress causes oxidative damage to lipid, protein, and DNA in the brain of rats. The FASEB Journal. 1996;10(13):1532–1538. [PubMed] [Google Scholar]
- 31.Gałecki P, Szemraj J, Bieńkiewicz M, Zboralski K, Gałecka E. Oxidative stress parameters after combined fluoxetine and acetylsalicylic acid therapy in depressive patients. Human Psychopharmacology: Clinical and Experimental. 2009;24(4):277–286. doi: 10.1002/hup.1014. [DOI] [PubMed] [Google Scholar]
- 32.Muñoz-Castañeda JR, Montilla P, Padillo FJ, et al. Role of serotonin in cerebral oxidative stress in rats. Acta Neurobiologiae Experimentalis. 2006;66(1):1–6. doi: 10.55782/ane-2006-1581. [DOI] [PubMed] [Google Scholar]
- 33.Mokoena ML, Harvey BH, Oliver DW, Brink CB. Ozone modulates the effects of imipramine on immobility in the forced swim test, and nonspecific parameters of hippocampal oxidative stress in the rat. Metabolic Brain Disease. 2010;25(2):125–133. doi: 10.1007/s11011-010-9189-7. [DOI] [PubMed] [Google Scholar]
- 34.Kumar A, Garg R, Gaur V, Kumar P. Nitric oxide mechanism in protective effect of imipramine and venlafaxine against acute immobilization stress-induced behavioral and biochemical alteration in mice. Neuroscience Letters. 2009;467(2):72–75. doi: 10.1016/j.neulet.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Júnior HV, de França Fonteles MM, Mendes de Freitas R. Acute seizure activity promotes lipid peroxidation, increased nitrite levels and adaptative pathways against oxidative stress in the frontal córtex and striatum. Oxidative Medicine and Cellular Longevity. 2009;2(3):130–137. doi: 10.4161/oxim.2.3.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar A, Garg R. Possible role of trazodone and imipramine in sleep deprivation-induced anxiety-like behavior and oxidative damage in mice. Methods and Findings in Experimental and Clinical Pharmacology. 2009;31(6):383–387. doi: 10.1358/mf.2009.31.6.1386992. [DOI] [PubMed] [Google Scholar]
- 37.Zafir A, Ara A, Banu N. In vivo antioxidant status: a putative target of antidepressant action. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33(2):220–228. doi: 10.1016/j.pnpbp.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Kim DH, Jang YY, Han ES, Lee CS. Protective effect of harmaline and harmalol against dopamineand 6-hydroxydopamine-induced oxidative damage of brain mitochondria and synaptosomes and viability loss of PC12 cells. The European Journal of Neuroscience. 2001;13(10):1861–1872. doi: 10.1046/j.0953-816x.2001.01563.x. [DOI] [PubMed] [Google Scholar]
- 39.Garcia LS, Comim CM, Valvassori SS, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32(1):140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 40.Paxinos G, Watson C. The Rat Brain: Stereotaxic Coordinates. 2nd edition. Sydney, Australia: Academic Press; 1986. [Google Scholar]
- 41.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods in Enzymology. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 42.Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods in Enzymology. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 43.Aebi H. Catalase in vitro. Methods in Enzymology. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 44.Bannister JV, Calabrese L. Assays for superoxide dismutase. Methods of Biochemical Analysis. 1987;32:279–312. doi: 10.1002/9780470110539.ch5. [DOI] [PubMed] [Google Scholar]
- 45.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry. 1951;193(1):265–275. [PubMed] [Google Scholar]