Abstract
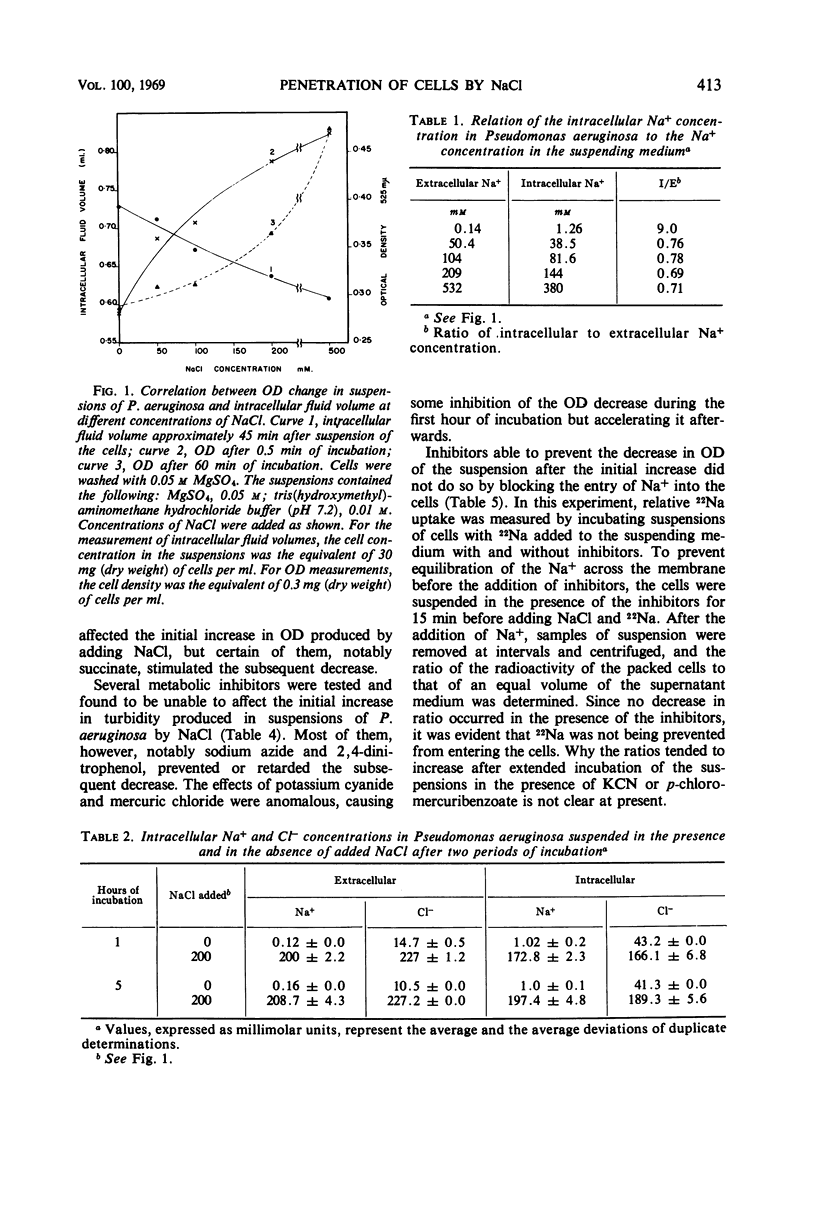
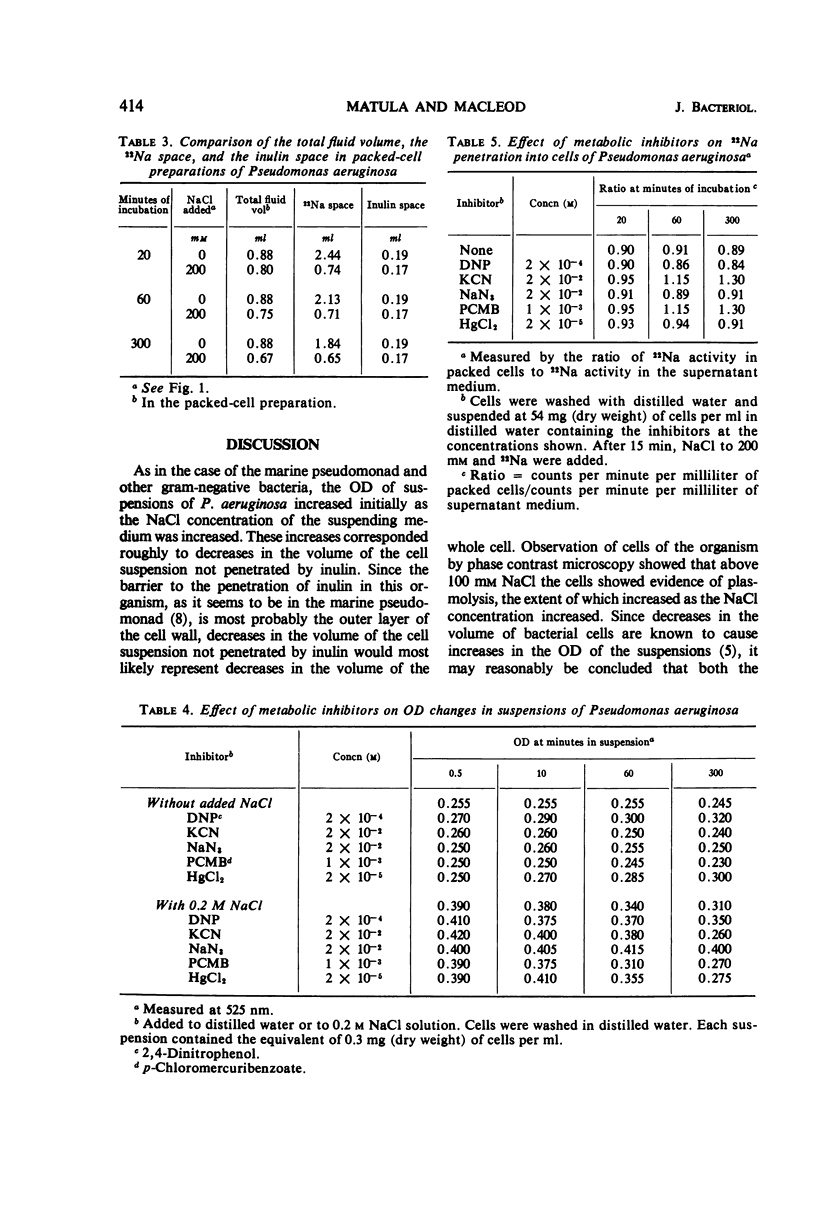
When cells of Pseudomonas aeruginosa were suspended in solutions containing increasing concentrations of NaCl, the optical density (OD) of the suspensions measured within 30 sec was found to increase in proportion to the increase in salt concentration. Measurement of intracellular fluid volumes indicated that the volume of the cells decreased roughly in proportion to the increase in salt concentration. After the initial increase in optical density, there was a slow decrease at all concentrations of NaCl tested except the highest, 500 mm. Metabolic inhibitors such as sodium azide and 2,4-dinitrophenol prevented the decrease. Direct analysis showed that the Na+ and Cl− concentrations in the cells were 86 and 77%, respectively, of the concentrations of the ions in the suspending medium after 1 hr. Measurement of the 22Na space in packed cells showed that Na+ penetrated the total fluid space in the packed cells. The penetration of 22Na was not prevented by the presence of metabolic inhibitors or by 500 mm NaCl in the suspending medium. The results indicate that the OD increases produced in suspensions of P. aeruginosa by NaCl are not due to the osmotic action of the salt. The subsequent optical density decreases observed are under metabolic control.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVI-DOR Y., KUCZYNSKI M., SCHATZBERG G., MAGER J. Turbidity changes in bacterial suspensions: kinetics and relation to metabolic state. J Gen Microbiol. 1956 Feb;14(1):76–83. doi: 10.1099/00221287-14-1-76. [DOI] [PubMed] [Google Scholar]
- BERNHEIM F. Factors which affect the size of the organisms and the optical density of suspensions of Pseudomonas aeruginosa and Escherichia coli. J Gen Microbiol. 1963 Jan;30:53–58. doi: 10.1099/00221287-30-1-53. [DOI] [PubMed] [Google Scholar]
- Eagon R. G. Cell wall-associated inorganic substances from Pseudomonas aeruginosa. Can J Microbiol. 1969 Feb;15(2):235–236. doi: 10.1139/m69-039. [DOI] [PubMed] [Google Scholar]
- HENNEMAN D. H., UMBREIT W. W. FACTORS WHICH MODIFY THE EFFECT OF SODIUM AND POTASSIUM ON BACTERIAL CELL MEMBRANES. J Bacteriol. 1964 Jun;87:1266–1273. doi: 10.1128/jb.87.6.1266-1273.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH A. L. Some calculations on the turbidity of mitochondria and bacteria. Biochim Biophys Acta. 1961 Aug 19;51:429–441. doi: 10.1016/0006-3002(61)90599-6. [DOI] [PubMed] [Google Scholar]
- MAGER J., KUCZYNSKI M., SCHATZBERG G., AVI-DOR Y. Turbidity changes in bacterial suspensions in relation to osmotic pressure. J Gen Microbiol. 1956 Feb;14(1):69–75. doi: 10.1099/00221287-14-1-69. [DOI] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Permeability of the envelopes of Staphylococcus aureus to some salts, amino acids, and non-electrolytes. J Gen Microbiol. 1959 Apr;20(2):434–441. doi: 10.1099/00221287-20-2-434. [DOI] [PubMed] [Google Scholar]
- Marquis R. E. Salt-induced contraction of bacterial cell walls. J Bacteriol. 1968 Mar;95(3):775–781. doi: 10.1128/jb.95.3.775-781.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matula T. I., MacLeod R. A. Mechanism of optical effects in suspensions of a marine pseudomonad. J Bacteriol. 1969 Oct;100(1):403–410. doi: 10.1128/jb.100.1.403-410.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



